Investigation on Microstructure and Properties of Cold-Sprayed Ni-Mo-Al2O3 Composite Coating
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Coating Preparation
2.2. Coating Characterization
2.3. Corrosion Resistance Test
3. Results and Discussion
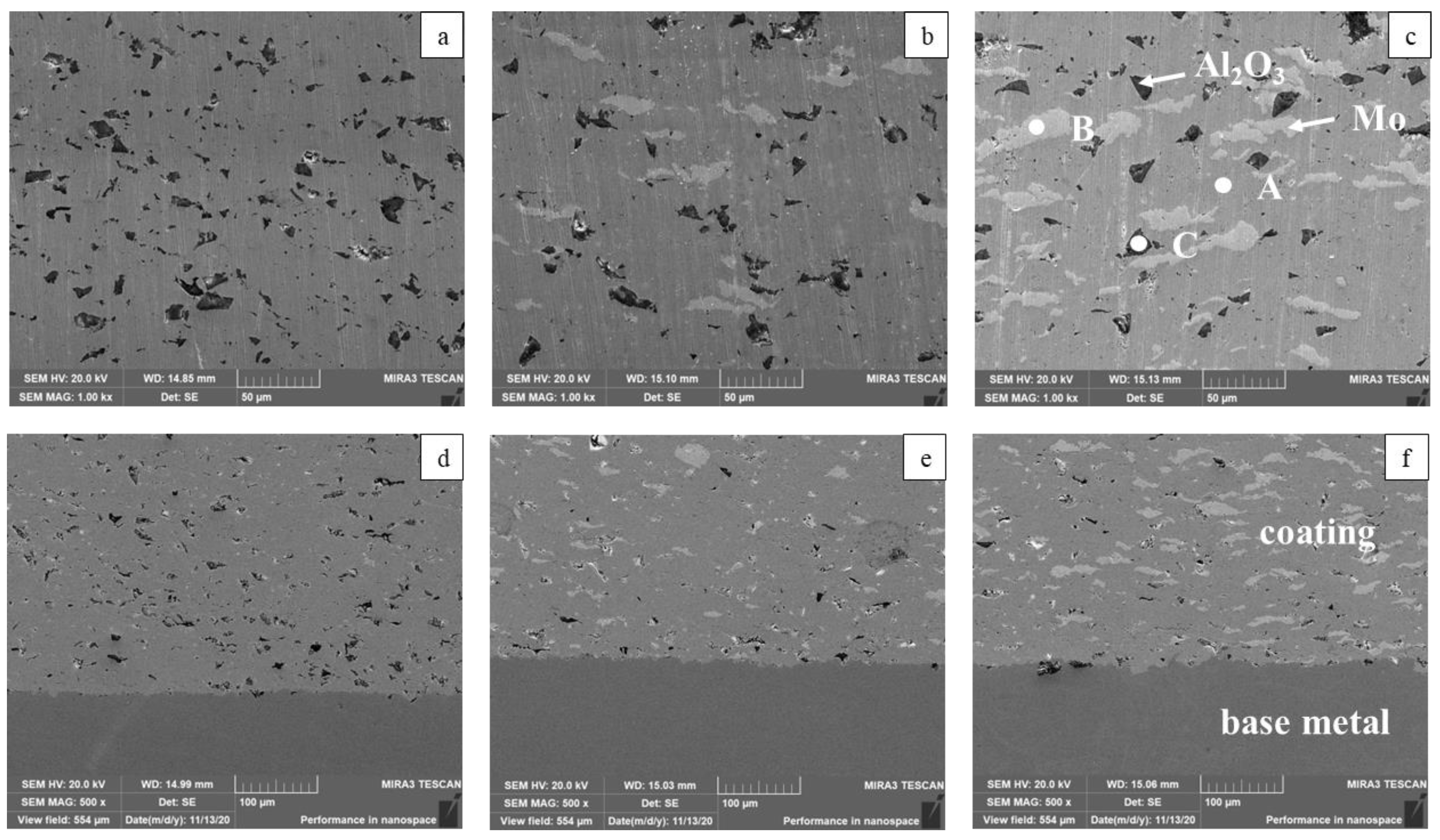
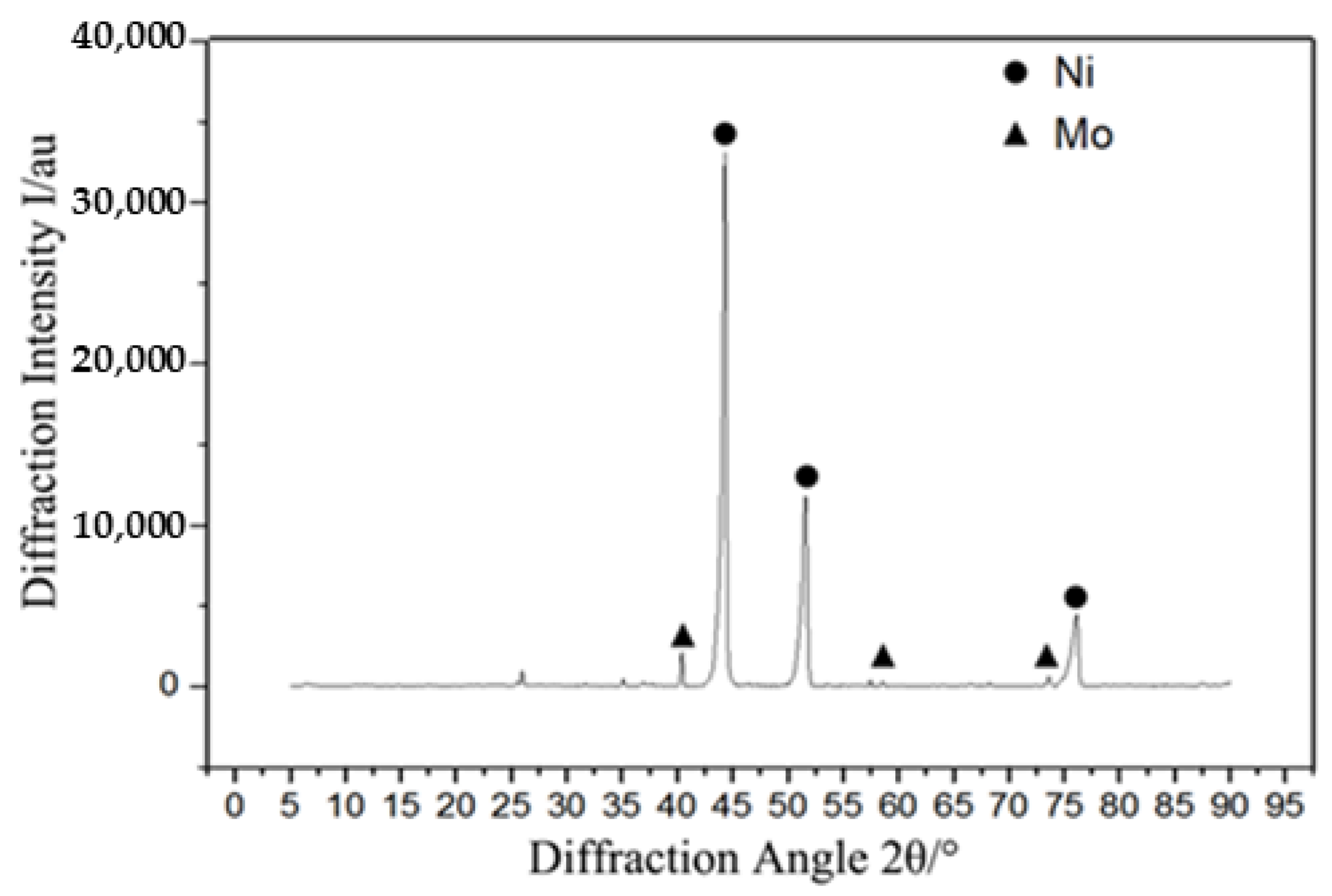
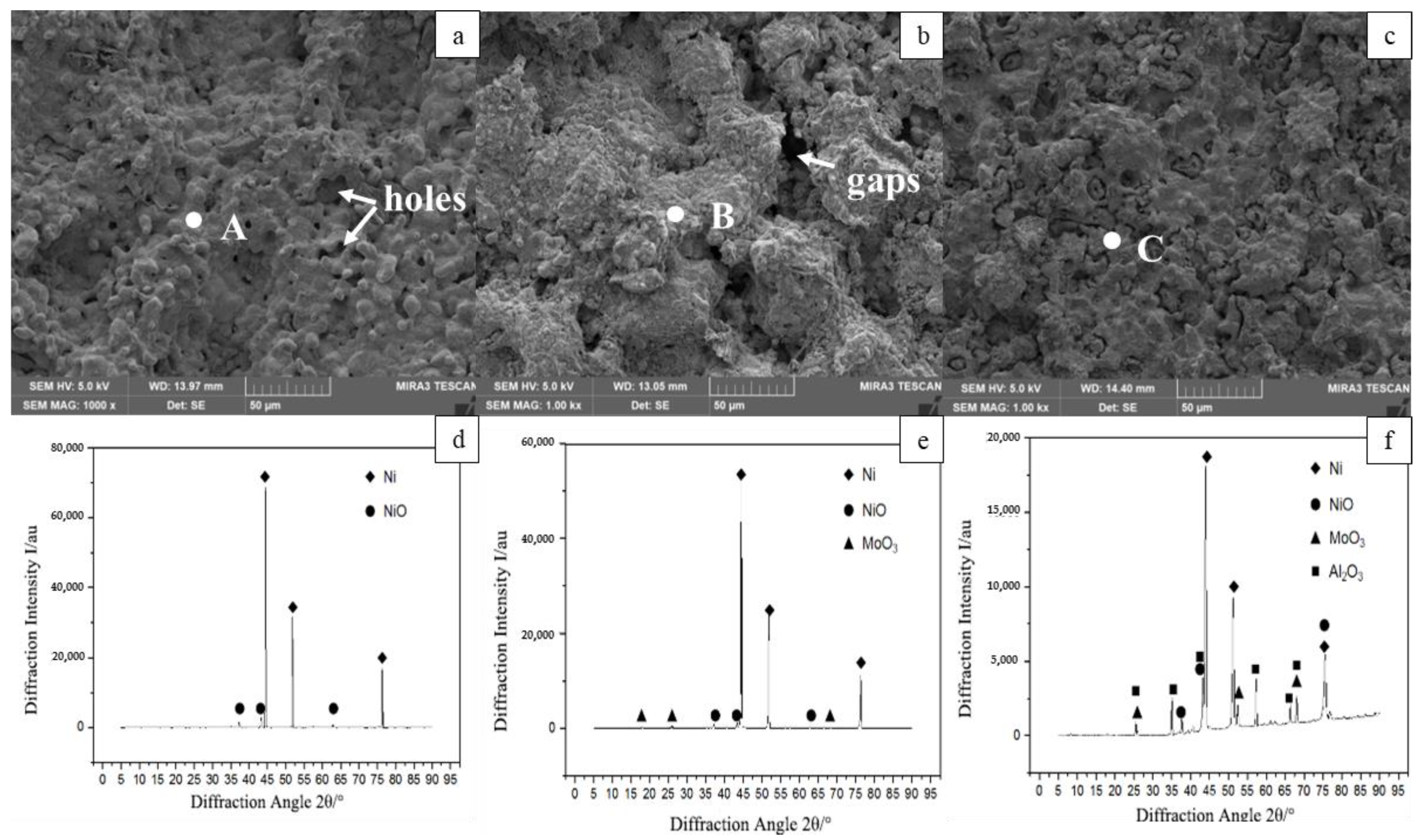
3.1. Microstructure and Composition
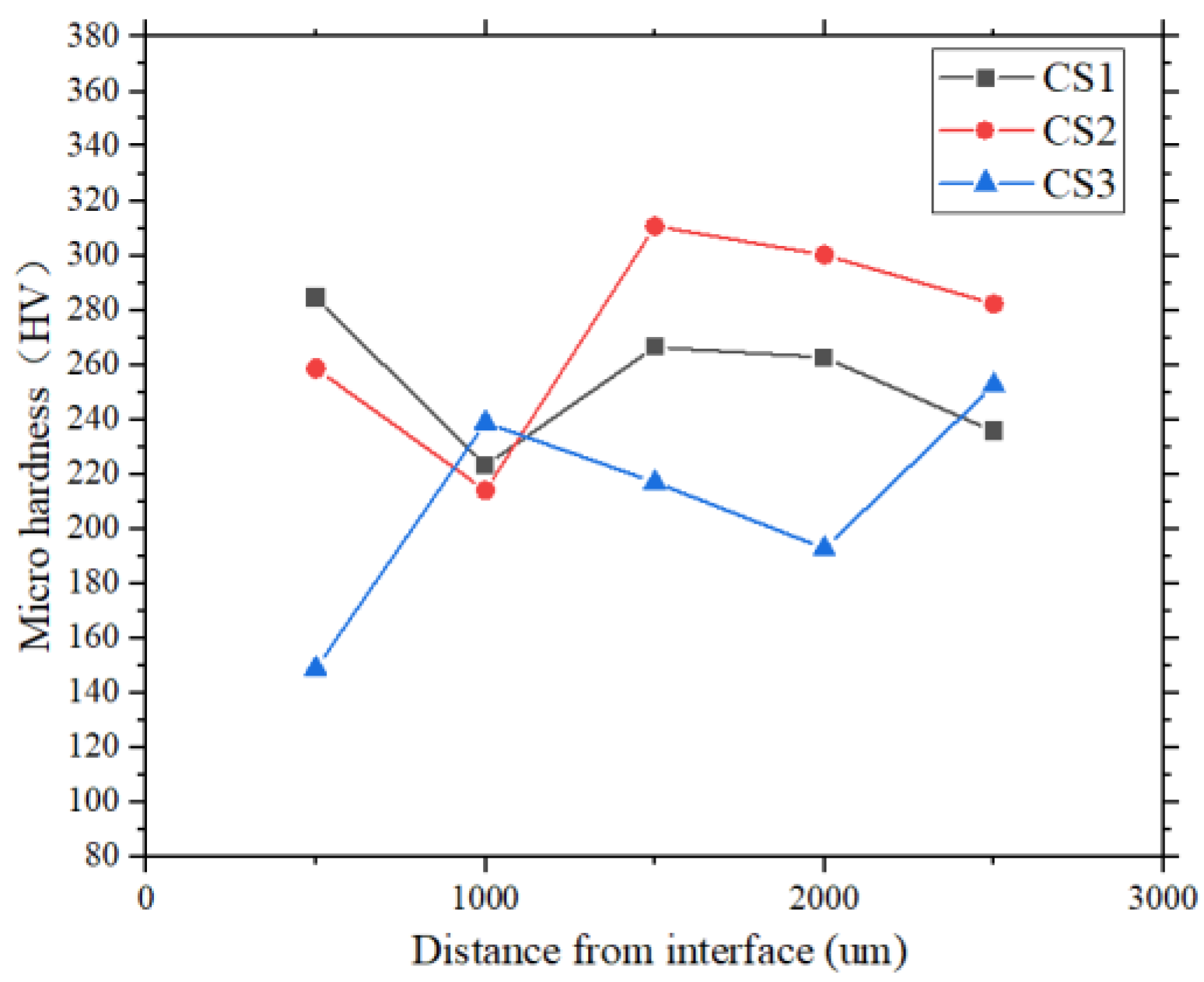
3.2. Hardness
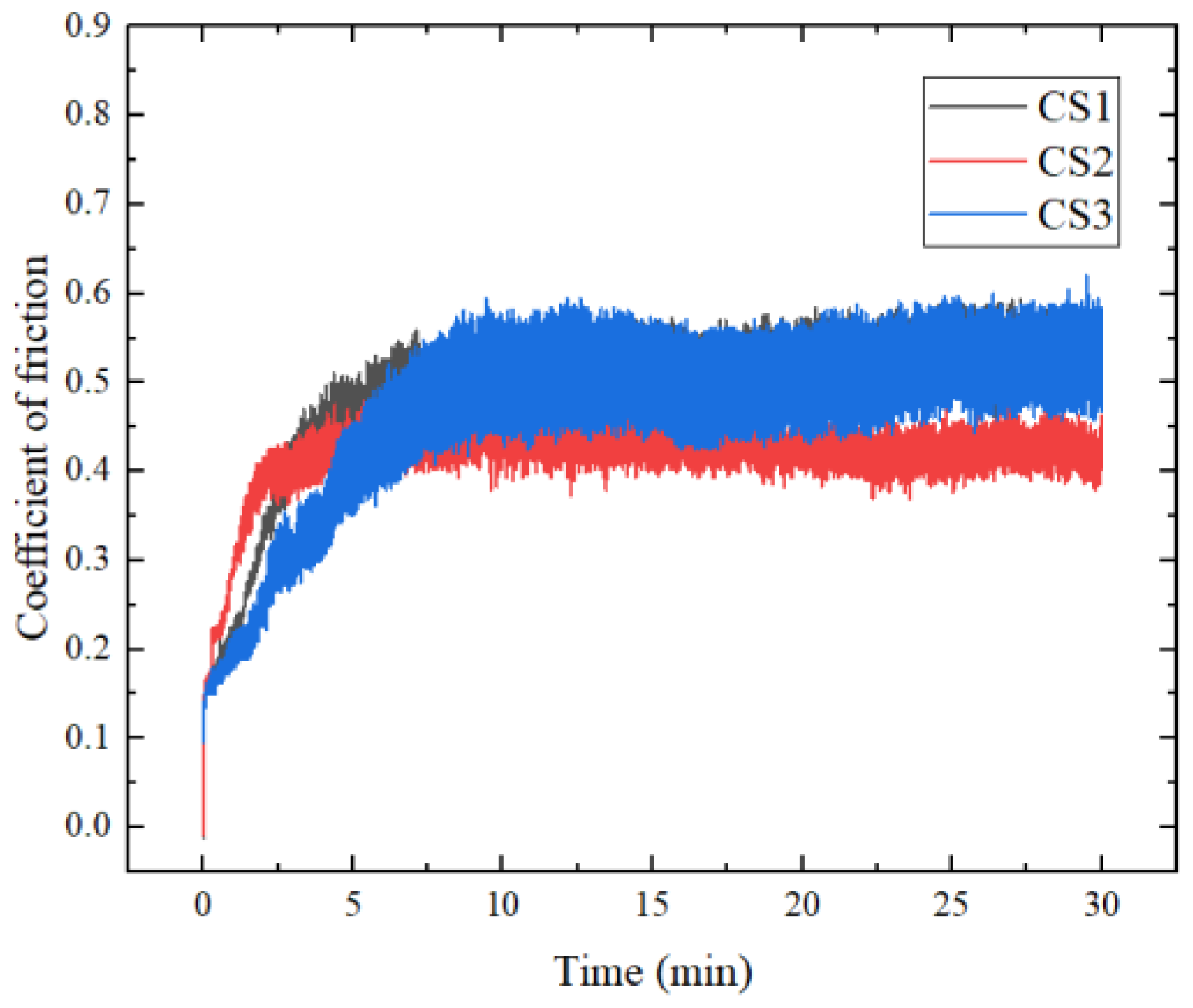
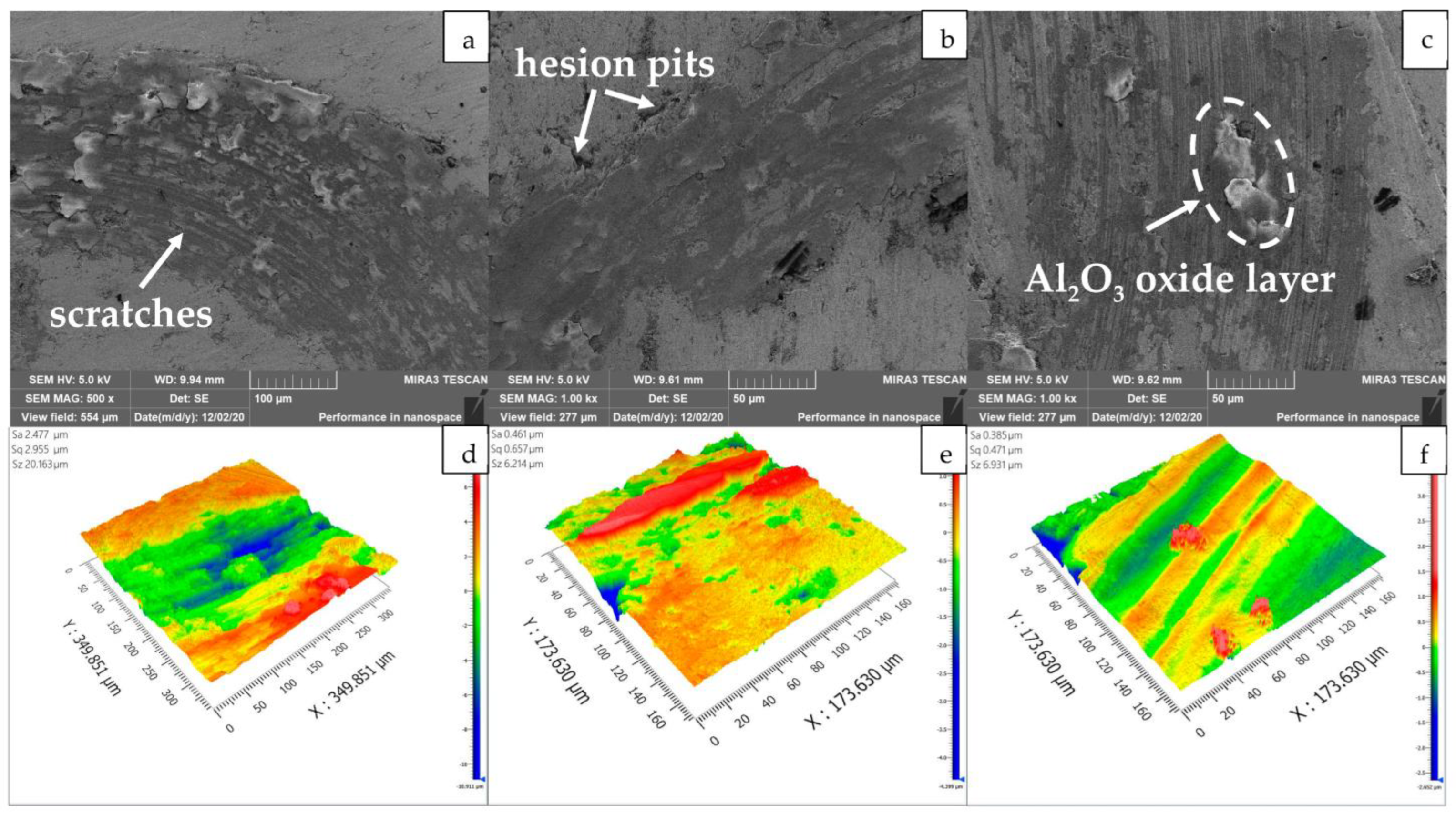
3.3. Friction and Wear Performance
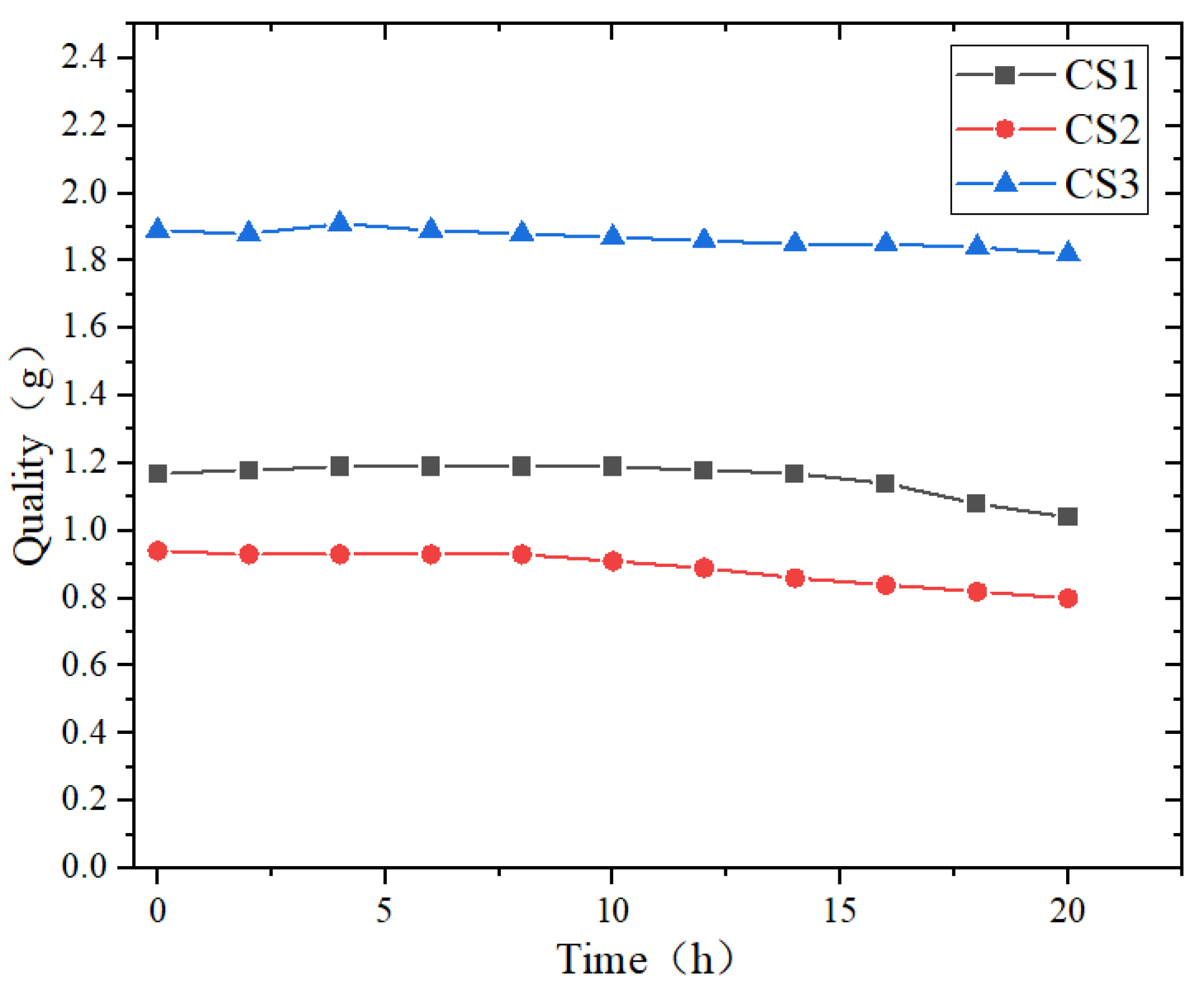
3.4. Corrosion Resistance Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, X.-T.; Li, Y.-J.; Li, C.-J. A comparison of cold spray deposition behavior between gas atomized and dendritic porous electrolytic Ni powders under the same spray conditions. Mater. Lett. 2016, 163, 58–60. [Google Scholar] [CrossRef]
- Qiu, X.; Tariq, N.u.H.; Qi, L.; Tang, J.-R.; Cui, X.-Y.; Du, H.; Wang, J.-Q. Effects of Dissimilar Alumina Particulates on Microstructure and Properties of Cold-Sprayed Alumina/A380 Composite Coatings. Acta Metall. Sin. Engl. Lett. 2019, 32, 1449–1458. [Google Scholar] [CrossRef]
- Aghasibeig, M.; Monajatizadeh, H.; Bocher, P.; Dolatabadi, A.; Wuthrich, R.; Moreau, C. Cold spray as a novel method for development of nickel electrode coatings for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 227–238. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Q.; Liu, X.; Lou, S.; Li, Q.; Wang, Z. Microstructures and high-temperature wear behavior of NiAl/WC-Fe x coatings on carbon steel by plasma cladding. J. Alloys Compd. 2020, 842, 155850. [Google Scholar] [CrossRef]
- Xu, X.; Dehghani, G.; Ning, J.; Li, P. Basic properties of eutectic chloride salts NaCl-KC -ZnCl2 and NaCl-KCl-MgCl2 as HTFs and thermal storage media measured using simultaneous DSC-TGA. Sol. Energy 2018, 162, 431–441. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Corrosion behavior of metallic alloys in molten chloride salts for thermal energy storage in concentrated solar power plants: A review. Front. Chem. Sci. Eng. 2018, 12, 564–576. [Google Scholar] [CrossRef]
- Mehos, M.; Jorgenson, J.; Denholm, P.; Turchi, C. An Assessment of the Net Value of CSP Systems Integrated with Thermal Energy Storage. Energy Procedia 2015, 69, 2060–2071. [Google Scholar] [CrossRef]
- Loureiro, T.; Sterling, R.; Testani, C.; Torralba-Calleja, E.; Turchetti, L.; Blanco, M.; Ferriere, A.; Perrotta, F. Next Generation of Concentrated Solar Power Technologies. Proceedings 2019, 20, 361–371. [Google Scholar]
- Sarvghad, M.; Maher, S.D.; Collard, D.; Tassan, M.; Will, G.; Steinberg, T.A. Materials compatibility for the next generation of Concentrated Solar Power plants. Energy Storage Mater. 2018, 14, 179–198. [Google Scholar] [CrossRef]
- Yin, Y. Theoretical Studies on the Surface Behaviors of Nickel-Based Alloys Under Molten Salts Environment; University of Chinese Academy of Sciences (Shanghai Institute of Applied Physics, Chinese Academy of Sciences): Shanghai, China, 2019. [Google Scholar]
- Liu, B.; Wei, X.; Wang, W.; Song, M.; Ding, J. Corrosion behavior of In625 alloy and 316L stainless steel in NaCl-CaCl2-MgCl2 ternary eutectic molten salt. CIESC J. 2017, 68, 3202–3210. [Google Scholar]
- Zhao, Z.; Wang, Y.; Liu, B.; Wei, G. Experimental Study on Corrosion Characteristics of Ternary Mixed Chloride Salt NaCl-KCl-MgCl2. Power Gener. Technol. 2018, 39, 561–565. [Google Scholar]
- Ma, H.; Zhu, M.; Zhao, Y.; Xia, J. Corrosion Behaviors of Two Kinds of Alloys in Chloride Molten Salts. Mater. Rev. 2014, 28, 109–113. [Google Scholar]
- Zhang, X.; Li, H.; Li, S. Research Progress on Corrosion and Damage of Stainless Steel in High Temperature Molten Salts. Corros. Sci. Prot. Technol. 2019, 31, 349–354. [Google Scholar]
- Ambrosek, J.W. Molten Chloride Salts for Heat Transfer in Nuclear Systems; University of Wisconsin-Madison: Madison, WI, USA, 2011. [Google Scholar]
- Wang, L.; Li, B.; Shen, M.; Li, S.-Y.; Yu, J.-G. Corrosion resistance of steel materials in LiCl-KCl melts. Int. J. Min. Met. Mater 2012, 19, 930–933. [Google Scholar] [CrossRef]
- Chen, L.Y.; Lan, H.; Huang, C.B.; Yang, B.; Du, L.Z.; Zhang, W.G. Hot corrosion of Ni, Cr, and 80Ni20Cr in the presence of NaCl and water vapor at 750 °C. Mater. Corros. 2017, 68, 1172–1179. [Google Scholar] [CrossRef]
- Chen, L.; Lan, H.; Huang, C.; Yang, B.; Du, L.; Zhang, W. Hot corrosion behavior of porous nickel-based alloys containing molybdenum in the presence of NaCl at 750 degrees C. Eng. Fail. Anal. 2017, 79, 245–252. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, S.; Xu, B.; Zhang, G. Fabrication and characterization of Ni-Coated Graphite/Al–Zn coatings by cold spraying. Surf. Eng. 2020, 36, 1032–1039. [Google Scholar] [CrossRef]
- Rokni, M.R.; Widener, C.A.; Ozdemir, O.C.; Crawford, G.A. Microstructure and mechanical properties of cold sprayed 6061 Al in As-sprayed and heat treated condition. Surf. Coat. Technol. 2017, 309, 641–650. [Google Scholar] [CrossRef]
- Hiratsuka, K.; Muramoto, K. Role of wear particles in severe–mild wear transition. Wear 2005, 259, 467–476. [Google Scholar] [CrossRef]
- da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, G.; Guilemany, J.M.; Benedetti, A.V. Influence of cold gas spray parameters on the corrosion resistance of Al-Al2O3 coatings sprayed on carbon steel. Corros. Eng. Sci. Technol. 2019, 54, 567–574. [Google Scholar] [CrossRef]
- Zong, L.; Zhou, J.; Yang, Y.; Wang, M. Effect of Mo on the microstructure and properties of Fe-Cr-Mo-C hardfacing layers. Weld. Technol. 2021, 50, 15–18+105. [Google Scholar]
- Gao, R. The Behavior of Alloy Oxides in High-Temperature Molten Salts Application Environment; University of Chinese Academy of Sciences (Shanghai Institute of Applied Physics, Chinese Academy of Sciences): Shanghai, China, 2019. [Google Scholar]


| Sample | Ni | Mo | Al2O3 |
|---|---|---|---|
| CS1 | Bal. | 0 | 10 |
| CS2 | Bal. | 10 | 10 |
| CS3 | Bal. | 20 | 10 |
| Element | Ni | Mo | Al | O |
|---|---|---|---|---|
| A | 98.28 | 1.12 | 0.05 | 0.55 |
| B | 1.64 | 97.59 | 0.20 | 0.57 |
| C | 2.36 | 0.67 | 51.33 | 45.64 |
| Element | Ni | Mo | O | Al | Cl | Mg | Na |
|---|---|---|---|---|---|---|---|
| A | 71.05 | — | 21.19 | 4.65 | 3.12 | — | — |
| B | 68.79 | 4.46 | 16.61 | 4.03 | 1.96 | 3.19 | 1.05 |
| C | 52.81 | 13.26 | 27.61 | 1.32 | 2.97 | 2.02 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Xiao, C.; Hu, S.; Zhou, Y.; Li, C.; Yang, B.; Zhang, J.; Zhang, G. Investigation on Microstructure and Properties of Cold-Sprayed Ni-Mo-Al2O3 Composite Coating. Coatings 2024, 14, 205. https://doi.org/10.3390/coatings14020205
Gong Y, Xiao C, Hu S, Zhou Y, Li C, Yang B, Zhang J, Zhang G. Investigation on Microstructure and Properties of Cold-Sprayed Ni-Mo-Al2O3 Composite Coating. Coatings. 2024; 14(2):205. https://doi.org/10.3390/coatings14020205
Chicago/Turabian StyleGong, Yinqing, Cong Xiao, Shunjie Hu, Yicheng Zhou, Chenglin Li, Bing Yang, Jianqiang Zhang, and Guodong Zhang. 2024. "Investigation on Microstructure and Properties of Cold-Sprayed Ni-Mo-Al2O3 Composite Coating" Coatings 14, no. 2: 205. https://doi.org/10.3390/coatings14020205
APA StyleGong, Y., Xiao, C., Hu, S., Zhou, Y., Li, C., Yang, B., Zhang, J., & Zhang, G. (2024). Investigation on Microstructure and Properties of Cold-Sprayed Ni-Mo-Al2O3 Composite Coating. Coatings, 14(2), 205. https://doi.org/10.3390/coatings14020205






