Research Progress in the Corrosion Mechanisms and Anticorrosion Technologies of Waste-to-Energy Plant Boilers
Abstract
1. Introduction
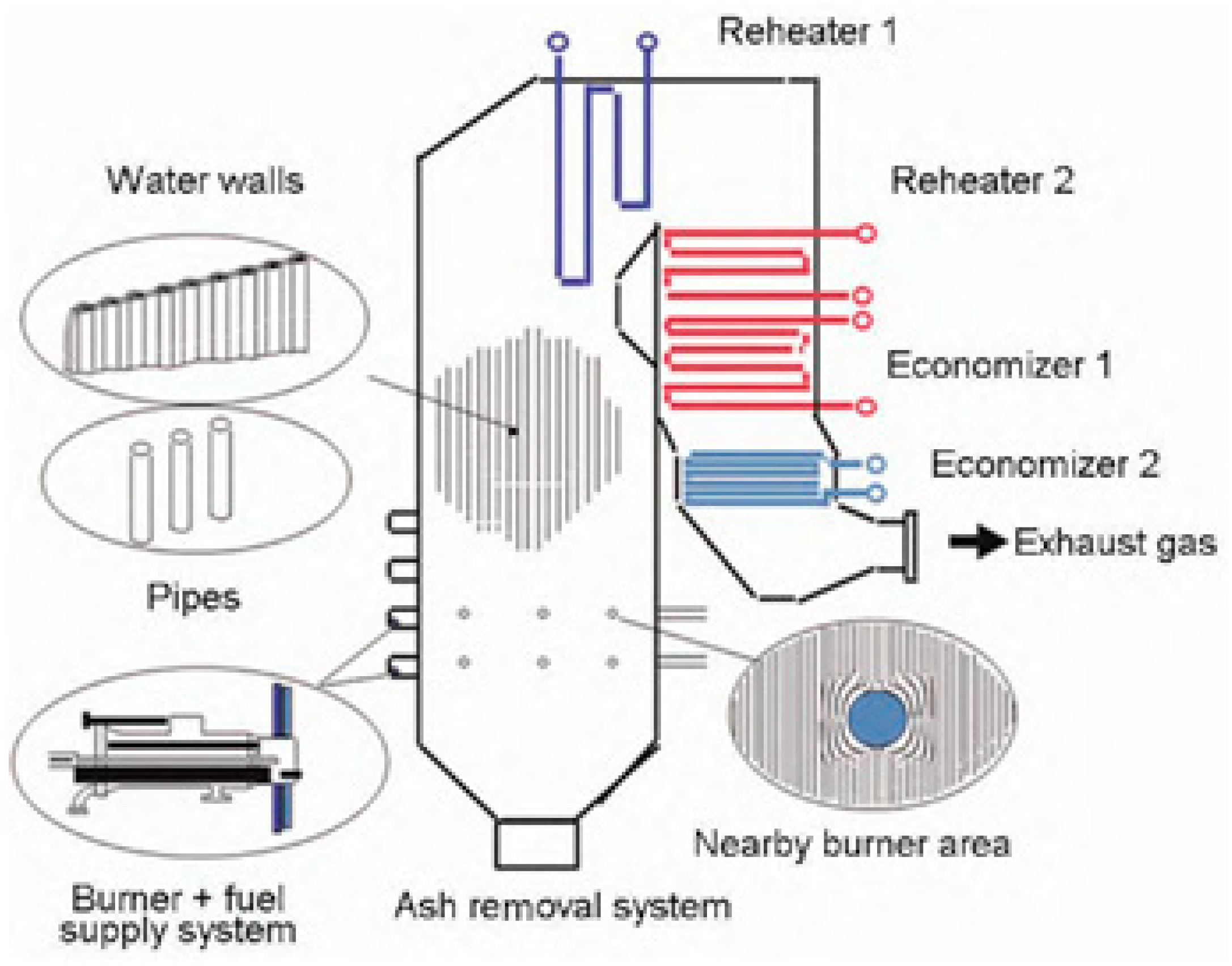
2. Corrosion Mechanism of Waste Power Plant Boiler
2.1. Activated Oxidation Theory
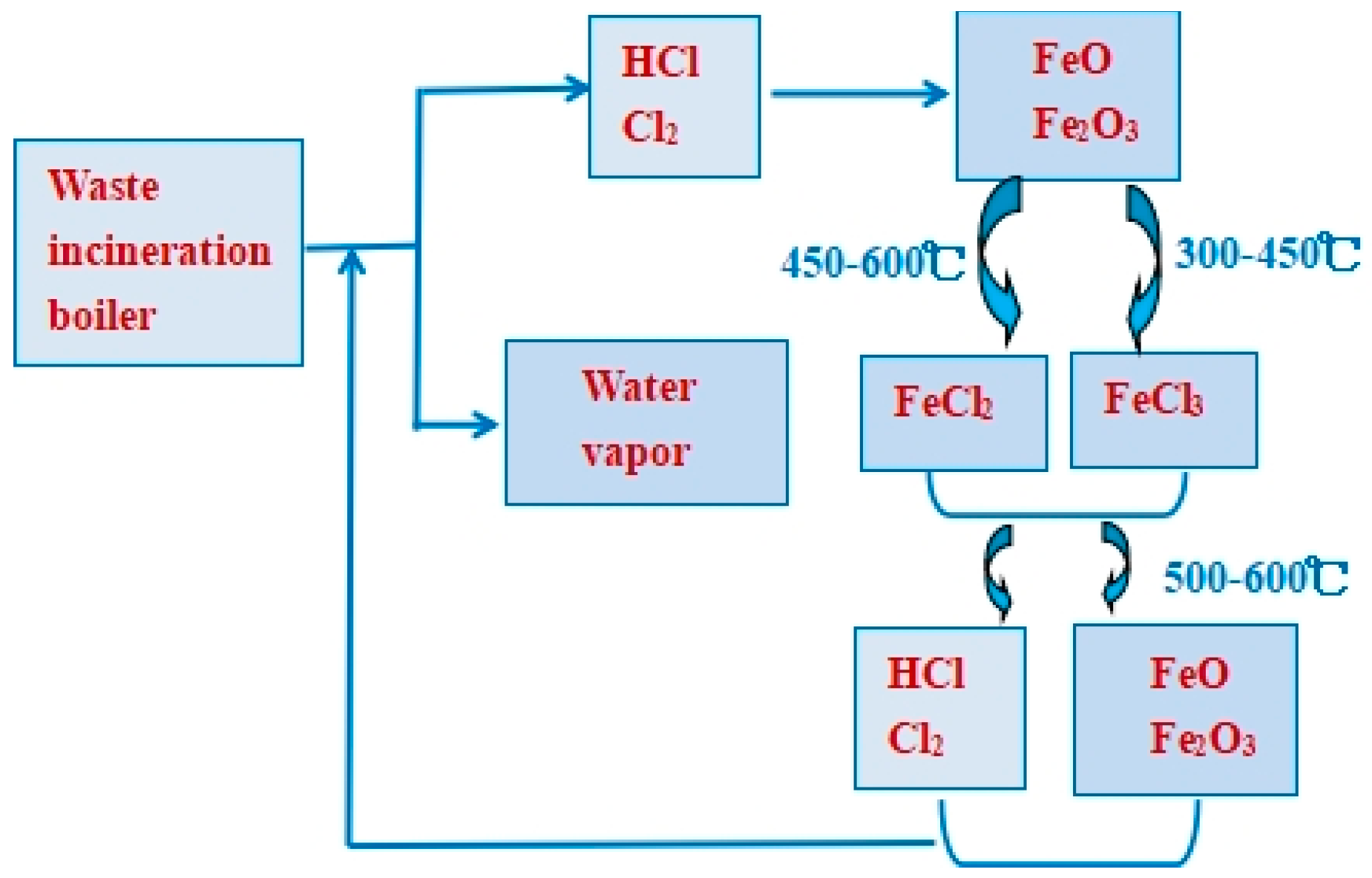
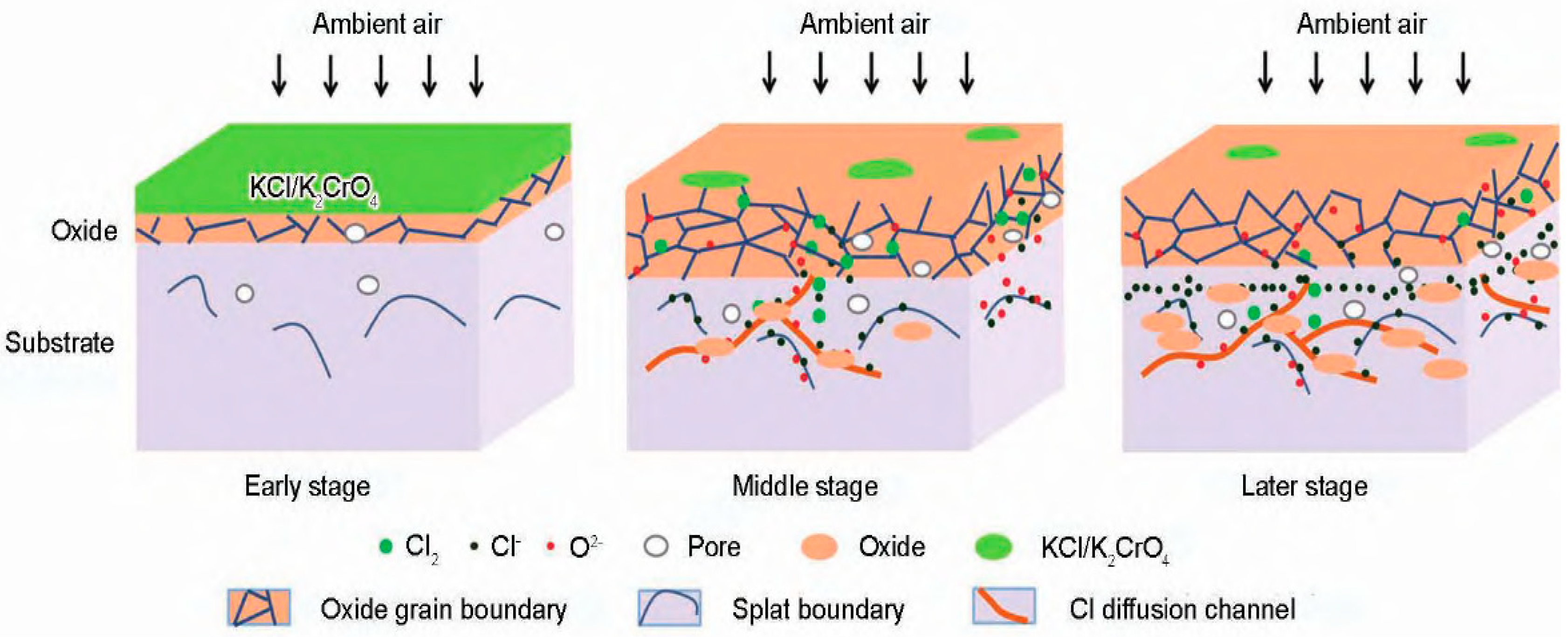
2.2. Alkali-Metal-Chloride-Induced Corrosion
2.3. Sulfide-Induced Corrosion
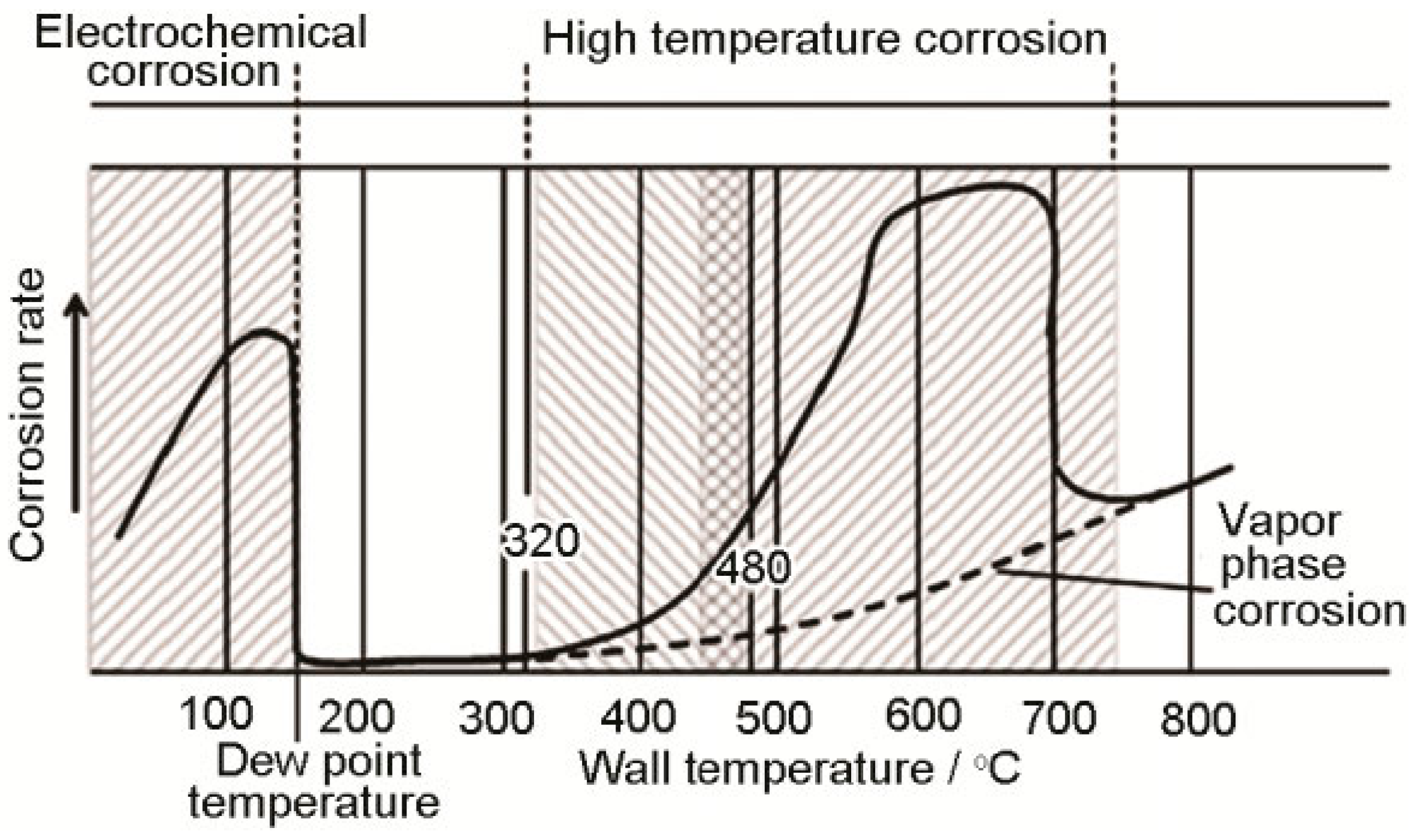
2.4. Influence of Wall Temperature on Corrosion Rate
2.5. Influence of Flue Gas Temperature on Corrosion Rate
3. Anticorrosion Technology for Waste Power Plant Boilers
3.1. Substrate Materials for Tubes
3.2. Protective Coating Technology
3.2.1. Coating Materials
3.2.2. Coating Preparation Methods
- (1)
- Thermal spraying technology
- (2)
- High-velocity oxygen fuel technology
- (3)
- Inconel 625 surfacing welding technology
- (4)
- Induction welding technology
- (5)
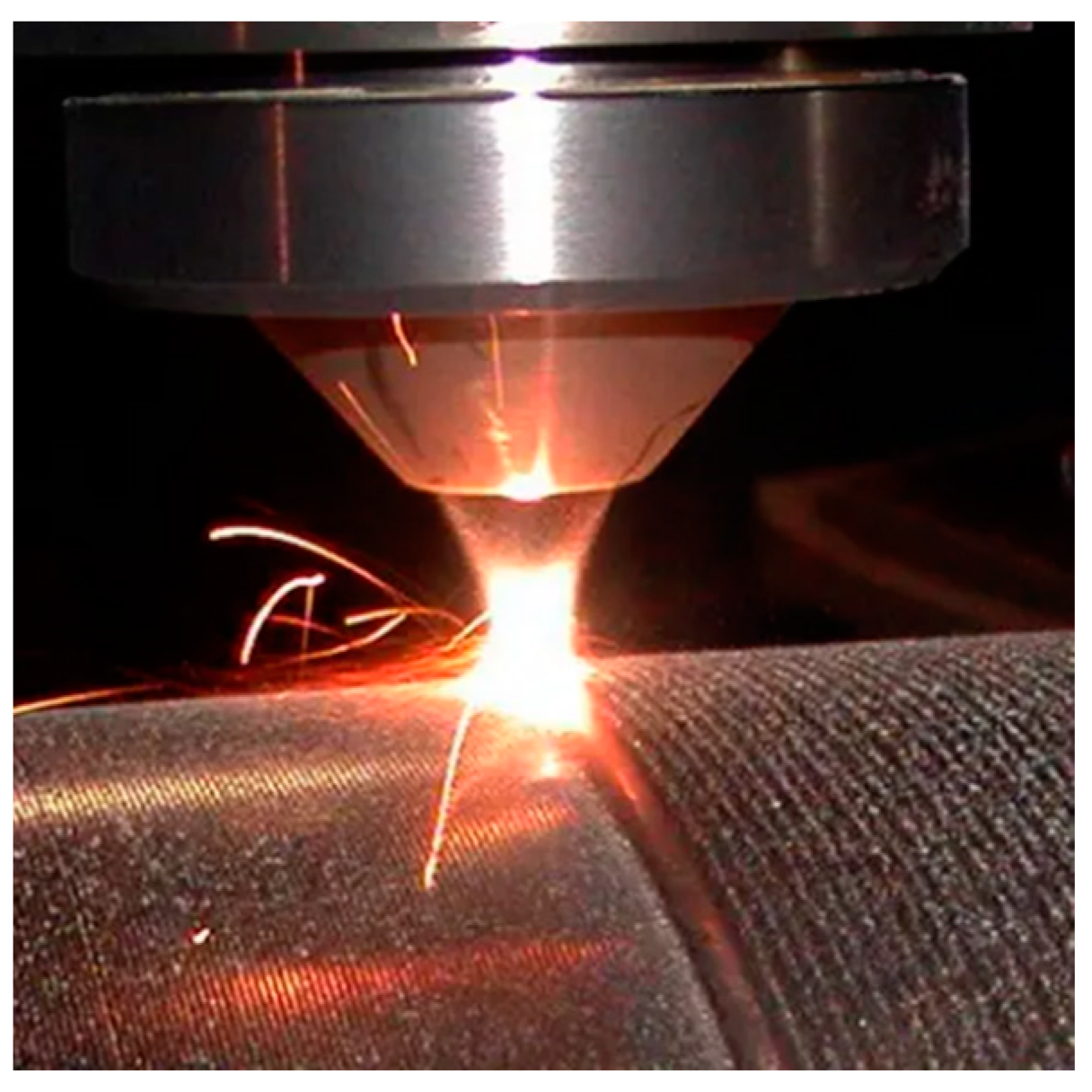
- Laser cladding technology
- (6)
- Thermally cured nanoceramic coating technology
- (7)
- Aluminizing coating technology
4. Challenges and Prospects for Future Research
4.1. Corrosion Mechanisms
4.2. Protective Coating Technology
- (1)
- Technological advancements in coating materials
- (2)
- Proactive development of high-temperature corrosion protection technologies
- (3)
- Addressing challenges encountered by high-parameter boilers
5. Conclusions
- (1)
- Typical chlorine-induced corrosion mechanisms in waste incinerators can be classified into gaseous corrosion and molten-salt corrosion, which coexist and mutually enforce each other, thus accelerating the overall chlorine-induced corrosion process within waste boilers. Chloride gases, particularly Cl2 and HCl, are primarily responsible for corrosion in waste boilers, causing metal material loss through active oxidation. Solid-salt chlorides, particularly alkali metal chlorides and heavy metal chlorides, also contribute to this corrosion, owing to their relatively low melting points and their ability to form eutectic salts featuring even lower melting points. The study of the chlorine/sulfur ratio could be an important aspect of further research and development of this topic. Furthermore, both excessively high and low chlorine/sulfur ratios can lead to severe corrosion, primarily owing to the significant impact of molten-salt corrosion triggered by low-melting-point chlorides and sulfates.
- (2)
- The correlation between corrosion and boiler tube wall temperatures indicates that high-temperature corrosion zones begin forming at wall temperatures of >320 °C. Within the 320–480 °C range, mild corrosion effects are prevalent, owing to the gradual formation of FeCl3 and basic sulfates. The most active high-temperature corrosion reactions occur within the 480–700 °C range, primarily owing to the decomposition and melting of FeCl3 and basic sulfates. Meanwhile, academic opinions regarding the impact of flue gas temperature on corrosion rate vary widely. One perspective suggests that high flue gas temperatures are primarily responsible for tube wall corrosion, mainly affecting water walls and superheaters by raising wall temperatures. Meanwhile, another perspective suggests that the magnitude of the impact varies across temperature ranges, as flue gas temperatures fundamentally influence the formation sequence and stability of corrosive media. Finally, based on years of engineering practical experience in operating waste boilers, the effect of flue gas temperature on wall temperature is believed to be limited, with the working fluid temperature predominantly influencing the wall temperature.
- (3)
- Corrosion protection technologies for waste boilers include various methods such as thermal spraying, surfacing, induction welding, laser cladding, thermoset reaction nanoceramic coating, and aluminizing coating. Conventional thermal spraying is less frequently adopted owing to the high porosity and low bond strength of the resulting coatings, while HVOF is somewhat more prevalent. Laser cladding remains in the research stage, and thermoset reaction nanoceramic coatings are limited in their applications owing to thin coating layers and brief service lives. Research on aluminizing coatings for waste boilers is relatively recent, and the technology is yet to completely mature. At present, surfacing and induction welding are predominantly used for waste incineration boiler protection in China.
- (4)
- To impart systems with resistance against high-temperature chlorine-induced corrosion, high-Cr content FeCr and NiCr alloys are adopted as the primary material systems. Notably, improving existing coating corrosion protection technologies primarily involves developing more suitable high-performance, low-cost, and low-dilution-rate surfacing materials and processes to address the uneven heating resulting from high-frequency remelting in water wall tube arrays. Existing research reveals that superfast aluminizing and similar processes can be implemented on stainless steel surfaces by inducing electromigration effects through electric eddy currents. In the future, the development of new technologies for high-temperature corrosion protection primarily focuses on introducing new coating technologies with excellent protective performance and longer service life, along with competitive production efficiency and lower preparation costs; onsite preparation technologies for protective boiler coatings; novel Ni–Al coating technologies; and strategies to actively address the challenges posed by the rapid development of high-parameter boilers.
Author Contributions
Funding
Conflicts of Interest
References
- Yu, X.F. Analysis and prospect of China’s municipal waste incineration power generation situation. China Power Enterp. Manag. 2018, 7, 57–90. [Google Scholar]
- Szymaski, K.; Hernas, A.; Moskal, G.; Myalska, H. Thermally sprayed coatings resistant to erosion and corrosion for power plant boilers A review. Surf. Coat. Technol. 2015, 268, 153. [Google Scholar] [CrossRef]
- Gu, W.L. Analysis on chlorine-corrosion issue from waste-incineration boilers. Appl. Energy Technol. 2020, 5, 52–54. [Google Scholar]
- Bai, X.X.; Zhang, Y.G. Research on treatment of water-wall high temperature corrosion of waste heat boiler in municipal solid waste incinerator. Environ. Sanit. Eng. 2018, 26, 68. [Google Scholar]
- Pan, C.Y.; Jiang, X.G.; Shang, N.; Yan, J.; Chi, C. Research and development of high temperature corrosion of HCl in flue gas from MSW incineration. Boil. Technol. 2003, 34, 72. [Google Scholar]
- Yang, B.; Zhong, Z.Q.; Huang, Q.X.; Huang, G.J.; Zhang, S.H.; Li, S.P. Research and development of high temperature chlorine corrosion in waste incineration boilers. Guangdong Electr. Power 2016, 29, 5. [Google Scholar]
- Jiang, X.G.; Liu, X.B. Research progress and direction thinking on corrosion of key heat transfer components in waste incineration boilers. J. Chin. Soc. Corros. Prot. 2020, 40, 205–214. [Google Scholar]
- Zhang, S.H.; Hu, K.; Liu, M.; Yang, Y. Corrosion-erosion mechanism and research prospect of bare materials and protective coatings for power generation boiler. Acta Metall. Sin. 2022, 58, 272–294. [Google Scholar]
- Li, Y.S.; Niu, Y.; Spiegel, M. High temperature interaction of Al/Si-modified FeCr alloys with KCl. Corros. Sci. 2007, 49, 1799. [Google Scholar] [CrossRef]
- Kawahara, Y. Controlling factors of localized corrosion in boiler combustion gas environments. Oxid. Met. 2016, 85, 127. [Google Scholar] [CrossRef]
- Pan, C.Y. Study on High Temperature Corrosion of HCl in Superheater Curvature in MSW Boiler. Master’s Thesis, Zhejiang University, Hangzhou, China, 2004. [Google Scholar]
- Ma, H.T. Corrosion of Metallic Materials in High-Temperature Chloride Salt Environments. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2003. [Google Scholar]
- Bankiewicz, D.; Yrjas, P.; Lindberg, D.; Hupa, M. Determination of the corrosivity of Pb-containing salt mixtures. Corros. Sci. 2013, 66, 225. [Google Scholar] [CrossRef]
- Galetz, M.C.; Bauer, J.T.; Schütze, M.; Noguchi, M.; Takatoh, C.; Cho, H. The influence of Copper in ash deposits on the corrosion of boiler tube alloys for waste-to-energy plants. Mater. Corros. 2014, 65, 778. [Google Scholar] [CrossRef]
- Henderson, P.J.; Szakálos, P.; Pettersson, R.; Andersson, C.; Högberg, J. Reducing superheater corrosion in wood-fired boilers. Mater. Corros. 2006, 57, 128. [Google Scholar] [CrossRef]
- Karlsson, S.; Jonsson, T.; Hall, J.; Svensson, J.E.; Liske, J. Mitigation of fireside corrosion of stainless steel in power plants: A laboratory study of the influences of SO2 and KCl on initial stages of corrosion. Energy Fuels 2014, 28, 3102. [Google Scholar] [CrossRef]
- Aho, M.; Vainikka, P.; Taipale, R.; Yrjas, P. Effective new chemicals to prevent corrosion due to Chlorine in power plant superheaters. Fuel 2008, 87, 647. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhang, X.W.; Chang, X.P.; Li, Y. Experimental study on influence of temperature for high temperature corrosion on waterwall of supercritical boiler. Ind. Furn. 2019, 41, 26–31. [Google Scholar]
- Pan, T.J.; Zeng, C.L.; Niu, Y. Corrosion of three commercial steels under ZnCl2-KCl deposits in a reducing atmosphere containing HCl and H2S at 400–500 °C. Oxid. Met. 2007, 67, 107. [Google Scholar] [CrossRef]
- Paneru, M.; Stein-Brzozowska, G.; Maier, J.; Scheffknecht, G. Corrosion mechanism of alloy 310 austenitic steel beneath NaCl deposit under varying SO2 concentrations in an Oxy fuel combustion atmosphere. Energy Fuels 2013, 27, 5699. [Google Scholar] [CrossRef]
- Schaal, E.; David, N.; Panteix, P.J.; Rapin, C.; Brossard, J.M.; Maad, F. Effect of chloride content in ash in oxidation kinetics of Ni-Based and Fe-based alloys. Oxid. Met. 2015, 84, 307. [Google Scholar] [CrossRef]
- Ijiri, M.; Okada, N.; Kanetou, S.; Yamamoto, M.; Nakagawa, D.; Tanaka, K.; Yoshimura, T. Thermal stress relaxation and high-temperature corrosion of Cr-Mo steel processed using multifunction cavitation. Materials 2018, 11, 2291. [Google Scholar] [CrossRef]
- Li, X.G. Thermal spraying technology for corrosion protection of power station boiler tubes. Surf. Eng. 1995, 11, 7–11. [Google Scholar]
- Wang, H.R. Application of thermal spray coating on boiler tube of waste incineration power station. Heat Treat. Technol. Equip. 2002, 23, 46–48. [Google Scholar]
- Fukudaand, Y.; Kumon, M. Application of high velocity flame spraying for the heat exchanger tubes in coal fired boilers. In Proceedings of the ITSC’95: 14th International Thermal Spraying Conference, Kobe, Japan, 22–26 May 1995; pp. 107–110. [Google Scholar]
- Wang, L.; Zhou, Z.; Wang, G.; Wu, X.; Jin, T.; He, D. Surface protection methods for high-temperature corrosion of waste incinerators. Therm. Spray Technol. 2017, 9, 1–6. [Google Scholar]
- Sun, H.H.; Liu, A.G.; Meng, F.L. Microstructure and properties of the membrane water-walls of boiler with inconel 625 alloy surfacing layer. Trans. Mater. Heat Treat. 2013, 34, 96–99. [Google Scholar]
- Qiu, L.L.; Bian, H.J. Technical analysis of CMT surfacing layer inconel 625 nickel base material on the heating surface of boiler in waste power plant. Technol. Innov. Appl. 2018, 18, 162–163. [Google Scholar]
- Fan, H.P. A Brief introduction of high temperature alloy technology for waste incinerator membrane water cooled wall. J. Henan Sci. Technol. 2018, 634, 25–26. [Google Scholar]
- Notomi, A. Application of self-fused alloy coating by HF induction heating to boiler tubes. Jpn. Weld. Soc. 1995, 54, 94. [Google Scholar]
- Wu, M.; Pan, L.; Tong, X.Y.; Sun, W. Induction remelting technology and its application in modern industry. Inf. Surf. Eng. 2017, 17, 29–31. [Google Scholar]
- Gao, X.S.; Tian, Z.J.; Liu, Z.D.; Shen, L.D. Interface characteristics of Al2O3-13%TiO2 ceramic coatings prepared by laser cladding. Trans. Nonferrous Met. Soc. China 2012, 22, 2498–2503. [Google Scholar] [CrossRef]
- Ren, C.M. Study on High Temperature Corrosion Mechanism and Protection Technology of Waste Incineration Power Plant. Master’s Thesis, North China Electric Power University, Beijing, China, 2018. [Google Scholar]
- Cheng, H.S.; Liu, G.; Lei, G.; Tan, J.; Chen, C.L.; Liang, Y.; Su, Y.L.; Wu, K.Y.; Du, Y.B. Research progress of high temperature corrosion protection coating technology for coal-burning boiler heating surface. Mater. Rep. 2020, 34, 433–435+447. [Google Scholar]
- Zahs, A.; Spiegel, M.; Grabke, H. The influence of alloying elements on the chlorine-induced high temperature corrosion of Fe-Cr alloys in oxidizing atmospheres. Mater. Corros. 1999, 50, 561–578. [Google Scholar] [CrossRef]
- Grabke, H.J.; Spiegel, M.; Zahs, A. Role of alloying elements and carbides in the chlorine-induced corrosion of steels and alloys. Mater. Res. 2004, 7, 89–95. [Google Scholar] [CrossRef]
- Kawahara, Y. An overview on corrosion-resistant coating technologies in biomass/waste-to-energy plants in recent decades. Coatings 2016, 6, 34. [Google Scholar] [CrossRef]
- Matsubara, Y.; Sochi, Y.; Tanabe, M.; Takeya, A. Advanced coatings on furnace wall tubes. J. Therm. Spray Technol. 2007, 16, 195–201. [Google Scholar] [CrossRef]
- Masaki, U.; Kamei, Y.; Fujii, K. Effect of static stress on high temperature corrosion behavior of boiler tubes in waste incineration environment. J. Jpn. Inst. Met. 2002, 66, 576. [Google Scholar]
- Wang, H.; Li, G.Z.; Li, Y.N. Application of high temperature resistant materials in coal-fired power plants and research progress of aluminizing coating preparation technology. Mater. China 2020, 39, 487–495. [Google Scholar]
- Wu, D.L. Important progress in the study of biomass high temperature corrosion mechanism of Ni2Al3 coatings. Lubr. Eng. 2020, 10, 27. [Google Scholar]
- Wang, Y.W. Corrosion of heating surface of high-parameter refuse incinerator. Boil. Technol. 2021, 4, 56–60. [Google Scholar]
- Qu, Z.P.; Zhong, R.G.; Wang, L.; Zhao, W.B.; Tian, X.L.; Wang, H.J. Research progress on high-temperature corrosion treatment schemes of waste to energy boilers. China Surf. Eng. 2020, 33, 50–60. [Google Scholar]
- Xu, L.; Tan, W.; Zhu, Q.X.; Lu, Q.Q.; Ruan, X.Y.; Zhu, Q.; Qin, E.W.; Wu, S.H. Corrosion resistance for high temperature chlorine of arc-sprayed Ni-based coatings applied in waste incineration boilers. Mater. Prot. 2018, 51, 138–142. [Google Scholar]
- Xi, Q.Q. Analysis on the anticorrosion at high temperature in the waste incineration grate boiler. China Resour. Compr. Util. 2013, 31, 32–34. [Google Scholar]
- Xi, Q.Q. Analysis on the anticorrosion of high temperature superheater in the waste incinerator. Energy Energy Conserv. 2013, 8, 44–66. [Google Scholar]
- Zhou, Z.; Bobzin, K.; He, D.Y.; Zhao, L.D.; Zhao, X.Z.; Kopp, N.; Zhao, Q.Y.; Li, R. Wear and high-temperature corrosion behavior of a wire-arc sprayed Ni Cr B coating. Therm. Spray Bull. 2013, 6, 48–54. [Google Scholar]
- Håkan, K.; Linda, B.; Åmand, L.E. The importance of SO2 and SO3 for sulphation of gaseous KCl-An experimental investigation in a biomass fired CFB boiler. Combust. Flame 2010, 157, 1649. [Google Scholar]
- Sure, J.; Shankar, A.R.; Mudali, U.K. Surface modification of plasma sprayed Al2O3-40wt%TiO2 coatings by pulsed Nd:YAG laser melting. Opt. Laser Technol. 2013, 48, 366–374. [Google Scholar] [CrossRef]
- Cui, C.; Ye, F.; Song, G. Laser surface remelting of Fe-based alloy coatings deposited by HVOF. Surf. Coat. Technol. 2012, 206, 2388–2395. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, J.; Song, R.; Bai, L.L.; Shao, J.Z.; Qu, C.C. Effect of the scanning speed on microstructural evolution and wear behaviors of laser cladding NiCrBSi composite coatings. Opt. Laser Technol. 2015, 72, 86–99. [Google Scholar] [CrossRef]
- Israelsson, N.; Unocic, K.A.; Hellström, K.; Jonsson, T.; Norell, M.; Svensson, J.E.; Johansson, L.G. A microstructural and kinetic investigation of the KCl-induced corrosion of an FeCrAl alloy at 600 °C. Oxid. Met. 2015, 84, 105. [Google Scholar] [CrossRef]
- Okoro, S.C.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. Influence of preoxidation on high temperature corrosion of a Ni-based alloy under conditions relevant to biomass firing. Surf. Coat. Technol. 2017, 319, 76. [Google Scholar] [CrossRef]
- Klein, L.; Killian, M.S.; Virtanen, S. The effect of nickel and silicon addition on some oxidation properties of novel Co-based high temperature alloys. Corros. Sci. 2013, 69, 43. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.J.; Wan, K.; Chen, C. The influence of Nb on hot corrosion behavior of Ni-based superalloy at 800 °C in a mixture of Na2SO4-NaCl. J. Mater. Res. 2014, 29, 2596. [Google Scholar] [CrossRef]
- Schütze, M.; Malessa, M.; Rohr, V.; Weber, T. Development of coatings for protection in specific high temperature environments. Surf. Coat. Technol. 2006, 201, 3872. [Google Scholar] [CrossRef]
- Trindade, V.; Christ, H.J.; Krupp, U. Grain-size effects on the high-temperature oxidation behaviour of chromium steels. Oxid. Met. 2010, 73, 551. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Huhtakangas, M.; Joshi, S. Isothermal oxidation of HVAF-sprayed Ni-based chromia, alumina and mixed-oxide scale forming coatings in ambient air. Surf. Coat. Technol. 2017, 316, 10. [Google Scholar] [CrossRef]
- Davis, J.R. Handbook of Thermal Spray Technology; ASM International: Materials Park, OH, USA, 2004; Volume 54. [Google Scholar]
- Wang, G.; Liu, H.; Chen, T.; Zhang, X.; Li, H.; Yu, Y.; Yao, H. Comparative investigation on thermal corrosion of alloy coatings in simulated waste incinerator environment. Corros. Sci. 2021, 189, 109592. [Google Scholar] [CrossRef]
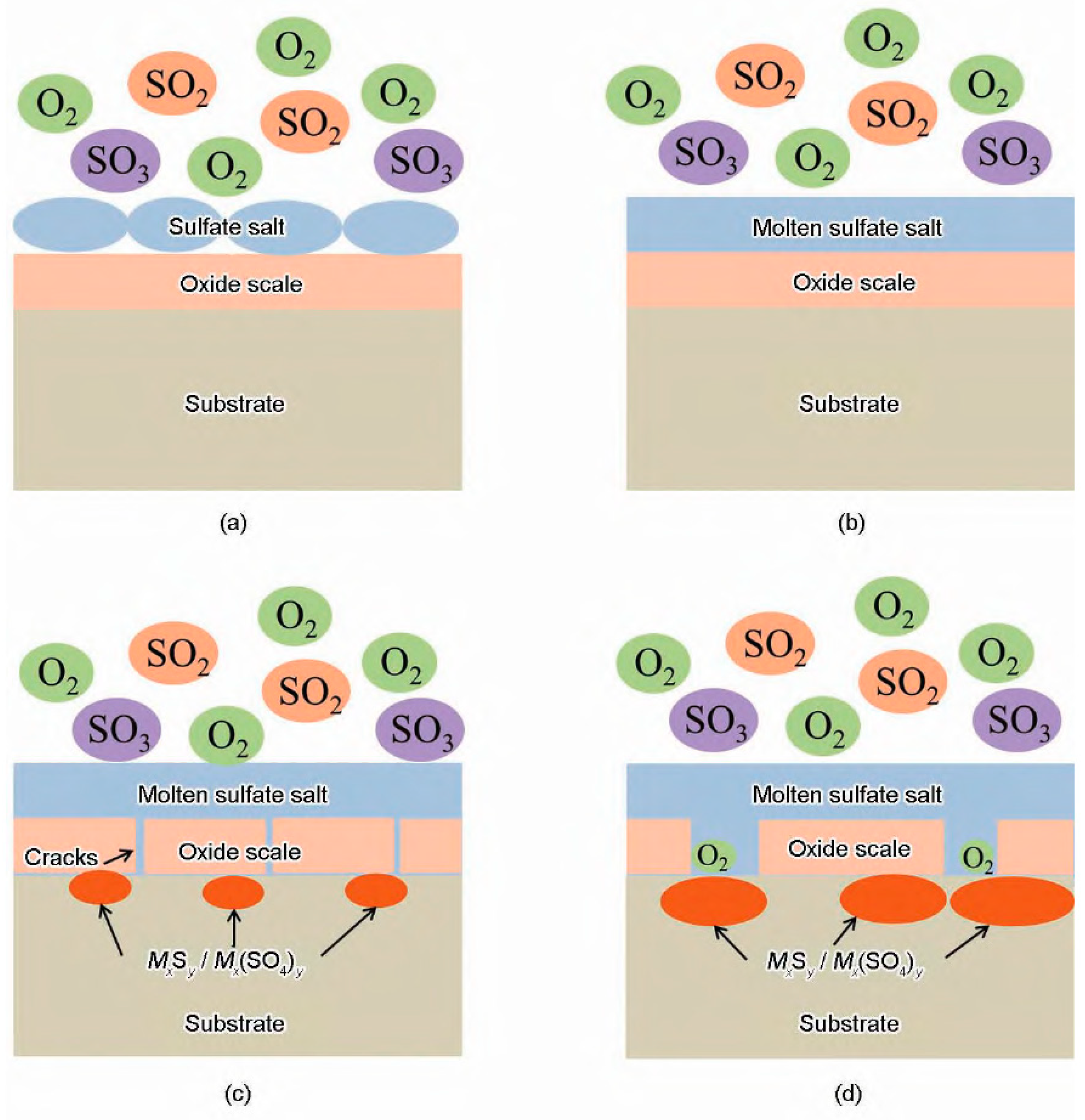


| Tubing Materials | Performance Characteristics | Applicable Locations | Thermal Conductivity (W/m k) | Temperature Resistance (K) | Element Content (%) |
|---|---|---|---|---|---|
| 20G | Exhibits higher levels of temperature resistance compared to ordinary carbon steel tubes, with superior plasticity and toughness. | Water walls | 45–50 | <450 | C: 0.17–0.23 Si: 0.17–0.37 Mn: 0.35–0.65 |
| 15CrMoG | Demonstrates commendable overall performance below 500 °C; however, mechanical properties gradually diminish with increasing temperature. | Water walls or medium- and low-temperature superheaters | 150–165 | <500 | C: 0.12–0.18 Si: 0.17–0.37 Mn: 0.40–0.70 |
| 12Cr1MoVG | Demonstrates better high-temperature performance than 15CrMoG, particularly in terms of its oxidation resistance. | High- and medium-temperature superheaters | 150–160 | <550 | C: 0.08~0.15 Si: 0.17~0.37 Mn: 0.40~0.70 |
| TP310S | Exhibits excellent oxidation resistance, corrosion resistance, acid and alkali resistance, and high-temperature performance, but undergoes softening at temperatures above 800 °C. | Medium- and high-temperature superheaters | 14–19 | <700 | Ni: 19.00–22.00 Cr: 24.00–26.00 S ≤ 1.50 Mn ≤ 2.00 |
| TP347H | Demonstrates superior high-temperature performance compared to TP310S, with strong resistance to high-temperature acid and intergranular corrosion. | High-temperature superheaters | 14–19 | <750 | C ≤ 0.08 Si ≤ 1.00 Mn ≤ 2.00 |
| Thermal Spray Technology (THSP) | Spraying Temperature/K | Spraying Velocity/m s−1 | Porosity/% | Bond Strength/MPa | Coating Lifespan/Years |
|---|---|---|---|---|---|
| Arc spraying (AS) | >6000 | 50–100 | 10–20 | 25–30 | 1–3 |
| Plasma spraying (PS) | 10,000–15,000 | 300–500 | 5–10 | 20–50 | 2–3 |
| Flame spraying (FST) | 3000 | 100–200 | 10–15 | 20–30 | 1–2 |
| High-velocity oxygen fuel (HVOF) spraying | 2500–5500 | 1000–1200 | <2 | 50–70 | >5 |
| Cold spraying | 0–700 | 300–1200 | <5 | >50 | >3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Tian, X. Research Progress in the Corrosion Mechanisms and Anticorrosion Technologies of Waste-to-Energy Plant Boilers. Coatings 2024, 14, 1391. https://doi.org/10.3390/coatings14111391
Qu Z, Tian X. Research Progress in the Corrosion Mechanisms and Anticorrosion Technologies of Waste-to-Energy Plant Boilers. Coatings. 2024; 14(11):1391. https://doi.org/10.3390/coatings14111391
Chicago/Turabian StyleQu, Zuopeng, and Xinli Tian. 2024. "Research Progress in the Corrosion Mechanisms and Anticorrosion Technologies of Waste-to-Energy Plant Boilers" Coatings 14, no. 11: 1391. https://doi.org/10.3390/coatings14111391
APA StyleQu, Z., & Tian, X. (2024). Research Progress in the Corrosion Mechanisms and Anticorrosion Technologies of Waste-to-Energy Plant Boilers. Coatings, 14(11), 1391. https://doi.org/10.3390/coatings14111391