Effect of Vacancy Defects on the Electronic Structure and Optical Properties of Bi4O5Br2: First-Principles Calculations
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Crystal Structure
3.2. Vacancy Formation Energy
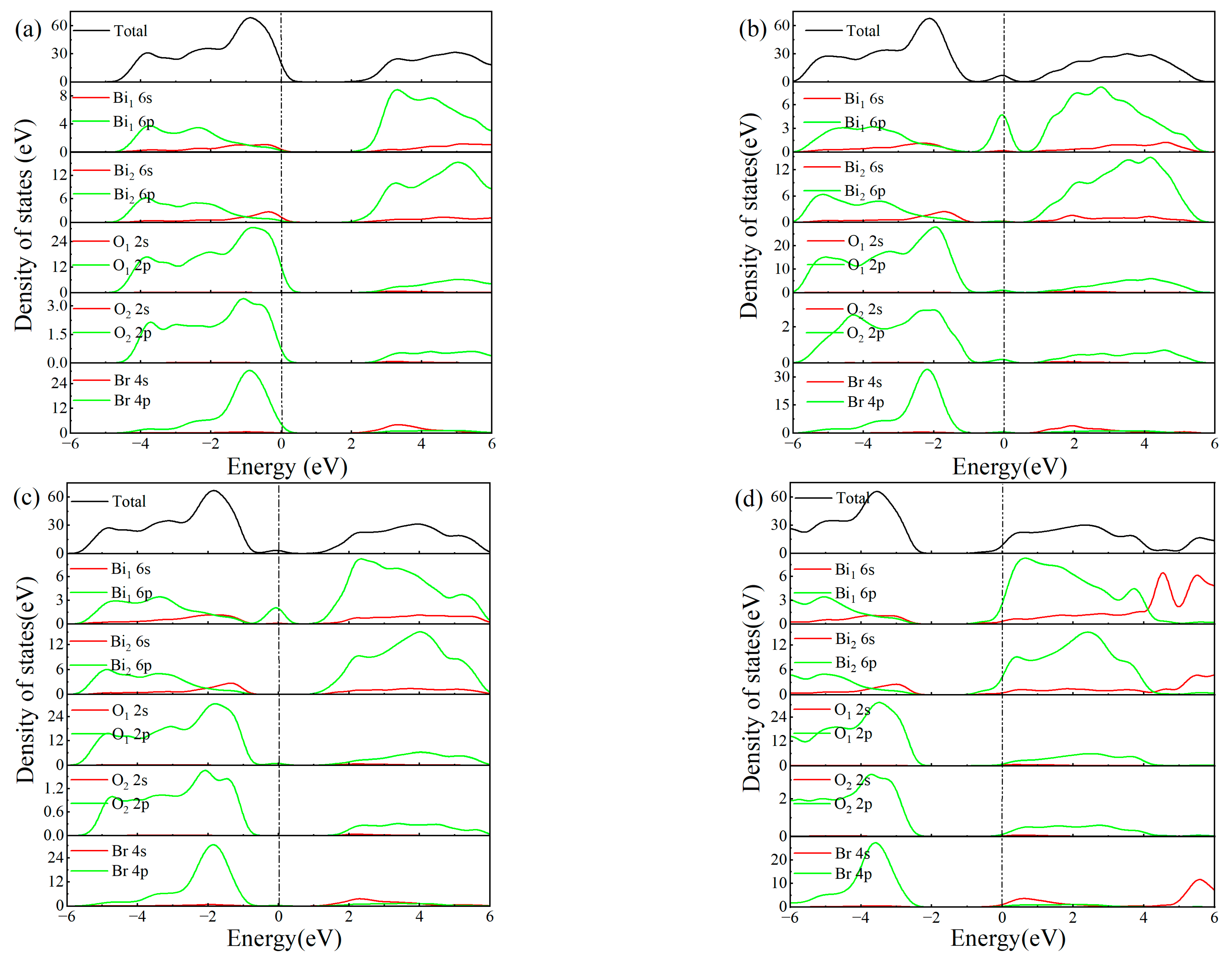
3.3. Electronic Structure
3.4. The Effective Mass Changes in Charge Carriers
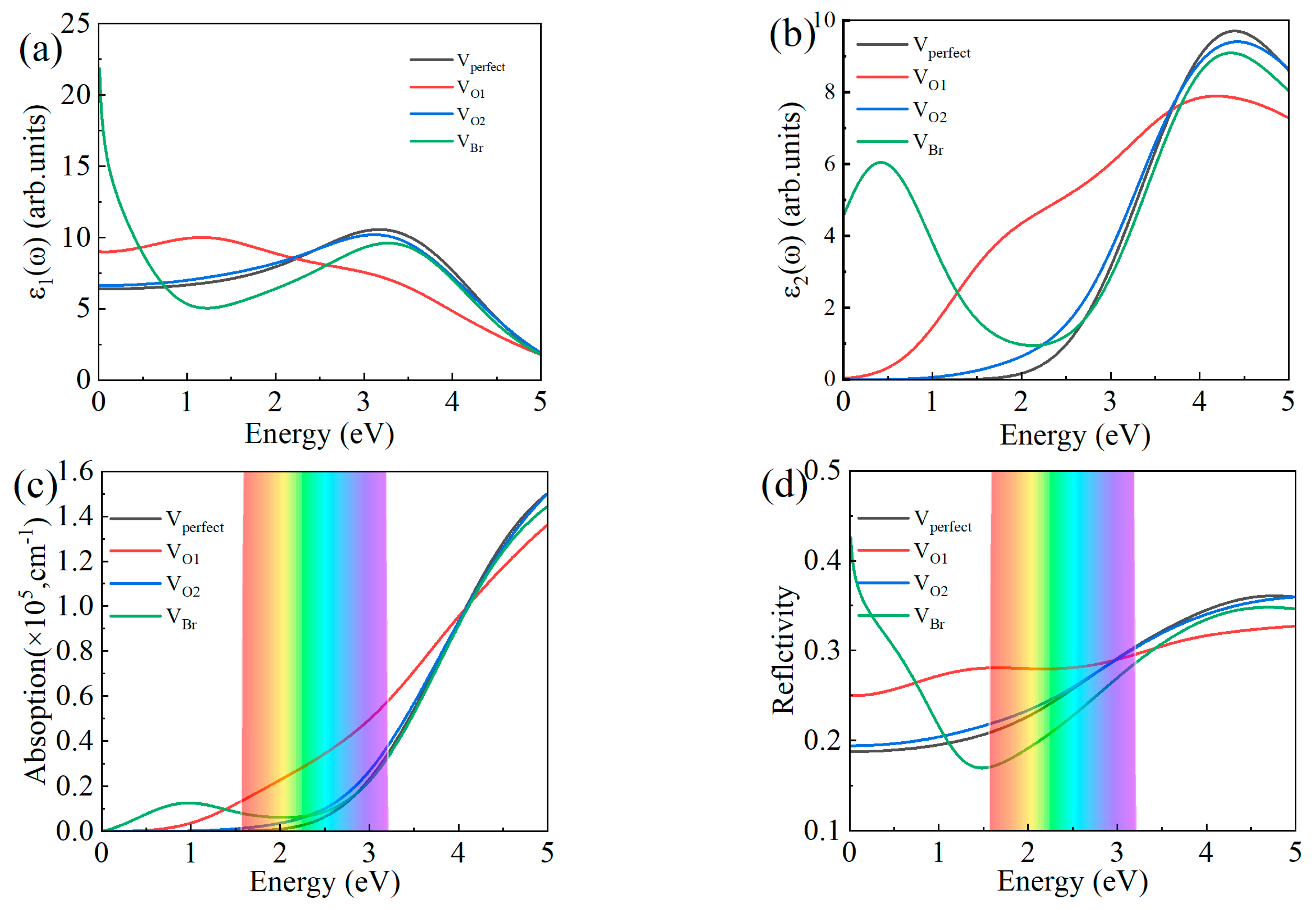
3.5. The Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, U.; Khan, A.; Raza, W.; Khan, A.; Bahnemann, D.; Muneer, M. Highly efficient Y and V co-doped ZnO photocatalyst with enhanced dye sensitized visible light photocatalytic activity. Catal. Today 2017, 284, 169–178. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Vadivel, S.; Fujii, M.; Rajendran, S. Novel S-scheme 2D/2D Bi4O5Br2 nanoplatelets/g-C3N5 heterojunctions with enhanced photocatalytic activity towards organic pollutants removal. Environ. Res. 2022, 213, 113736. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ye, L.; Xie, H.; Chen, G. Bismuth-rich bismuth oxyhalides for environmental and energy photocatalysis. Coord. Chem. Rev. 2017, 349, 84–101. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Meng, J.; Ban, C.; Duan, Y.; Feng, Y.; Jing, S.; Ma, J.; Yu, D.; Gan, L.; et al. Constructing atomic surface concaves on Bi5O7Br nanotube for efficient photocatalytic CO2 reduction. Nano Energy 2024, 119, 109067. [Google Scholar] [CrossRef]
- Yang, W.J.; Sun, K.L.; Wan, J.; Ma, Y.A.; Liu, J.Q.; Zhu, B.C.; Liu, L.; Fu, F. Oosting Holes Generation and O2 Activation by Bifunctional Nicop Modified Bi4O5Br2 for Efficient Photocatalytic Aerobic Oxidation. Appl. Catal. B Environ. 2023, 320, 121978. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Fang, W.L.; Meng, X.C.; Wang, L.; Bai, H.C.; Li, C.H. In-Situ Synthesis of Metal Bi to Improve the Stability of Oxygen Vacancies and Enhance the Photocatalytic Activity of Bi4O5Br2 in H2 Evolution. J. Alloys Compd. 2022, 910, 164883. [Google Scholar] [CrossRef]
- Sun, J.M.; Li, X.L.; Li, J.M.; Mu, M.; Yin, X.H. Fabrication of Bi4O5Br2-Decorated Rod-Like Mof-Derived MoS2 Hierarchical Heterostructures for Boosting Photocatalytic CO2 Reduction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129940. [Google Scholar] [CrossRef]
- Dong, X.A.; Cui, Z.; Shi, X.; Yan, P.; Wang, Z.; Co, A.C.; Dong, F. Insights into Dynamic Surface Bromide Sites in Bi4O5Br2 for Sustainable N2 Photofixation. Angew. Chem. Int. Ed. Engl. 2022, 61, 937. [Google Scholar] [CrossRef]
- Peng, H.; Deng, X.; Li, G.; Wang, Q.; Song, M.; Chen, P.; Yin, S. Oxygen vacancy and Van der Waals heterojunction modulated interfacial chemical bond over Mo2C/Bi4O5Br2 for boosting photocatalytic CO2 reduction. Appl. Catal. B Environ. 2022, 318, 121866. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, X.; Xin, S.H.; Shi, H.; Liu, G.H.; Wu, Q.; Xue, S.Q.; Wang, X.Y.; Shao, T.T.; Liu, Y.; et al. First-Principles Study of Electronic Structure and Optical Properties of Ni-Doped Bi4O5Br2. Coatings 2024, 14, 67. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Xiao, X.; Wu, Q.-F.; Fan, Q.; Chen, S.; Yang, W.-X.; Zhang, F.-C. Facile synthesis of novel Mn-doped Bi4O5Br2 for enhanced photocatalytic NO removal activity. J. Alloys Compd. 2020, 826, 154204. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Cheng, H.; Qiu, H.; Sun, W.; Fang, X.; Zhu, J.; Zheng, Y. Bamboo-charcoal-loaded graphitic carbon nitride for photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2022, 47, 3733–3740. [Google Scholar] [CrossRef]
- Ding, S.; Meng, L.; Sun, C.; Yang, S.; Dai, Y. One-pot microemulsion-mediated synthesis of Bi-rich Bi4O5Br2 with controllable morphologies and excellent visible-light photocatalytic removal of pollutants. Appl. Catal. B Environ. Int. J. Devoted Catal. Sci. Appl. 2017, 207, 153–165. [Google Scholar] [CrossRef]
- Rahman, M.H.; Yang, J.; Sun, Y.; Mannodi-Kanakkithodi, A. Defect engineering in ZnIn2X4 (X = S, Se, Te) semiconductors for improved photocatalysis. Surf. Interfaces 2023, 39, 102960. [Google Scholar] [CrossRef]
- Komsa, H.-P.; Krasheninnikov, A.V. Native defects in bulk and monolayer MoS2 from first principles. Phys. Rev. B. 2015, 91, 125304. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Y.; Gao, L.; Yin, H.; Zhang, M.; Zhang, H.; Ju, P.; Dou, K.; Ai, S. Ascorbic Acid Induced the Improved Oxygen Vacancy Defects of Bi4O5Br2 and Its Application on Photoelectrochemical Detection of DNA Demethylase MBD2 with Improved Detection Sensitivity. Small 2023, 20, 2306365. [Google Scholar] [CrossRef]
- Xiong, J.; Song, P.; Di, J.; Li, H. Bismuth-rich bismuth oxyhalides: A new opportunity to trigger high-efficiency photocatalysis. J. Mater. Chem. A 2020, 8, 21434–21454. [Google Scholar] [CrossRef]
- Chen, W.; Pasquarello, A. First-principles determination of defect energy levels through hybrid density functionals and GW. J. Phys. Condens. Matter. 2015, 27, 133202. [Google Scholar] [CrossRef]
- Zhang, L.; Zhai, T.; Yang, M.; Hu, C. Few-layered Bi. J. Hazard. Mater. 2022, 437, 129274. [Google Scholar] [CrossRef]
- Cai, H.; Chen, F.; Hu, C.; Ge, W.; Li, T.; Zhang, X.; Huang, H. Oxygen vacancies mediated ultrathin Bi4O5Br2 nanosheets for efficient piezocatalytic peroxide hydrogen generation in pure water. Chin. J. Catal. 2024, 57, 123–132. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Li, X.; Yang, Y.; Yang, S. Nanoarchitectonics of Oxygen-Vacancy BiOCl@Bi4O5Br2 Z-Type Heterojunctions with Efficient Photocatalytic Degradation Performance. Nano 2023, 18, 2350067. [Google Scholar] [CrossRef]
- Vinoth, S.; Ong, W.-J.; Pandikumar, A. Defect engineering of BiOX (X = Cl, Br, I) based photocatalysts for energy and environmental applications: Current progress and future perspectives. Coord. Chem. Rev. 2022, 464, 214541. [Google Scholar] [CrossRef]
- Dreyer, C.E.; Alkauskas, A.; Lyons, J.L.; Janotti, A.; Van de Walle, C.G. First-Principles Calculations of Point Defects for Quantum Technologies. Annu. Rev. Mater. Res. 2018, 48, 1–26. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Für Krist.—Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- John PPerdew, K.B.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wang, J.; Zhang, J.; Huang, Y.; Wei, X. Theoretical investigation of the photocatalytic mechanism of single Au adsorption on the Bi4O5Br2 (−1 0 1) surface. Chem. Phys. Lett. 2020, 757, 137851. [Google Scholar] [CrossRef]
- Keller, E.; Ketterer, J.; Krämer, V. Crystal structure and twinning of Bi4O5Br2. Z. Für Krist.—Cryst. Mater. 2001, 216, 595–599. [Google Scholar] [CrossRef]
- Keller, E.; Krämer, V.; Schmidt, M.; Oppermann, H. The crystal structure of Bi4O5I2 and its relation to the structure of Bi4O5Br2. Z. Für Krist.—Cryst. Mater. 2002, 217, 256–264. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zhang, W.; Dang, F.; Zhang, S.; Du, Y. First-principles studies on cation point defects in LiTi2O4. Phys. B Condens. Matter. 2022, 639, 413959. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Wang, F.; Yan, Y. First Principles Study of Electronic Structure and Optical Properties of ZnNb2O6 with Vacancy Defects. J. Inorg. Mater. 2021, 36, 271–276. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L. Bismuth-rich Bi4O5X2 (X = Br, and I) nanosheets with dominant {101} facets exposure for photocatalytic H2 evolution. Chem. Eng. J. 2016, 304, 454–460. [Google Scholar] [CrossRef]
- Opoku, F.; Krishna, K.G.; Cornelia, G.C.; Penny, P.G. Enhancing Charge Separation and Photocatalytic Activity of Cubic SrTiO3 with perovskite-Type Materials MTaO3 (M = Na, K) for Environmental Remediation: A First-Principles Study. Chem. Select. 2017, 2, 6304. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; He, M.; Zhu, G.; Yang, W.; Tian, Y.; Zhang, Z.; Zhang, S.; Zhang, F.; Wu, Q. Enhanced photocatalytic properties of Bi4O5Br2 by Mn doping: A first principles study. Mater. Res. Express 2018, 5, 075512. [Google Scholar] [CrossRef]
- Thulin, L.; Guerra, J. Calculations of strain-modified anatase TiO2 band structures. Phys. Rev. B 2008, 77, 195112. [Google Scholar] [CrossRef]
- Li, F.Y.; Yang, Z.X.; Cheng, X.; Zeng, L.Y.; Ouyang, F.P. First-principles study of electronic structure and optical properties of monolayer defective tellurene. Acta Phys. Sin. 2021, 70, 166301. [Google Scholar] [CrossRef]
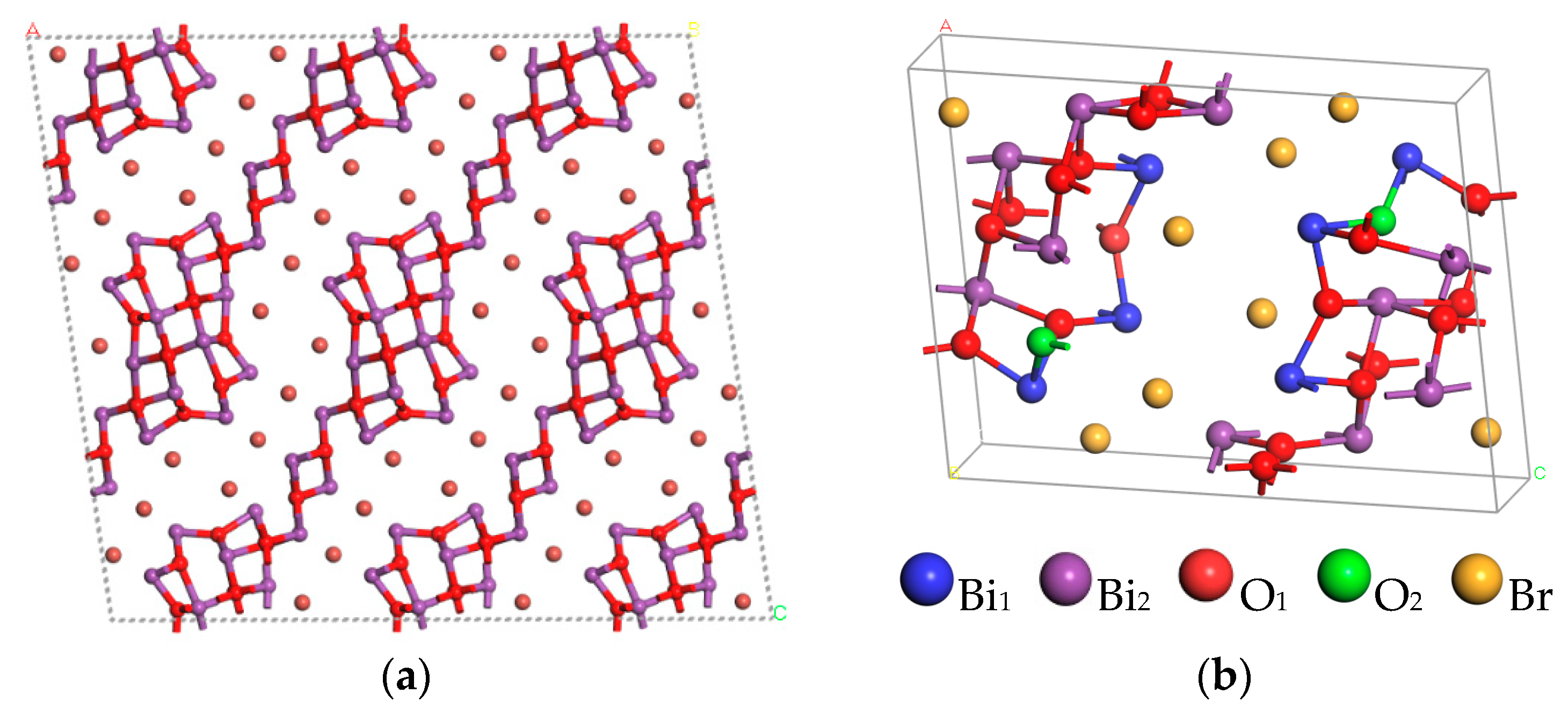

| Model | a/Å | b/Å | c/Å | α | β | γ |
|---|---|---|---|---|---|---|
| Experiment [29] | 14.539 | 5.605 | 10.782 | 90.00 | 97.75 | 90.00 |
| Unit cell | 14.595 | 5.651 | 10.938 | 90.00 | 97.93 | 90.00 |
| VBi1 | 14.606 | 5.645 | 10.943 | 90.00 | 98.00 | 90.00 |
| VBi2 | 14.594 | 5.651 | 10.941 | 90.00 | 98.01 | 90.00 |
| VO1 | 14.621 | 5.660 | 10.902 | 90.00 | 97.79 | 90.00 |
| VO2 | 14.600 | 5.647 | 10.943 | 90.16 | 98.00 | 90.10 |
| VBr | 14.592 | 5.651 | 10.939 | 89.99 | 97.90 | 89.98 |
| Model | Ebulk/eV | Edefect/eV | Ef/eV |
|---|---|---|---|
| VBi1 (1 × 1 × 1) | −18,819.348 | −18,020.219 | 9.03 |
| VBi2 (1 × 1 × 1) | −18,819.348 | −18,016.572 | 12.68 |
| VO1 (1 × 1 × 1) | −18,819.348 | −18,383.048 | 2.26 |
| VO1 (2 × 1 × 1) | −37,570.360 | −37,134.076 | 2.24 |
| VO1 (2 × 2 × 1) | −75,140.223 | −74,703.941 | 2.24 |
| VO2 (1 × 1 × 1) | −18,819.348 | −18,379.705 | 5.60 |
| VBr (1 × 1 × 1) | −18,819.348 | −18,353.409 | 2.91 |
| Species | D | ||
|---|---|---|---|
| Perfect | 0.140 | 0.432 | 3.086 |
| VBi1 | 0.638 | 0.921 | 1.444 |
| VBi2 | 0.225 | 0.881 | 3.916 |
| VO1 | 0.172 | 0.668 | 3.884 |
| VO2 | 0.184 | 0.482 | 2.620 |
| VBr | 0.147 | 1.010 | 6.871 |
| g-C3N4 [34] | 3.9 | 29 | 7.4 |
| TiO2 [36] | 0.62 | 1.70 | 2.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Liu, Y.; Zhang, Y.; Zhang, F.; Yang, Y.; Li, J. Effect of Vacancy Defects on the Electronic Structure and Optical Properties of Bi4O5Br2: First-Principles Calculations. Coatings 2024, 14, 1361. https://doi.org/10.3390/coatings14111361
Huang B, Liu Y, Zhang Y, Zhang F, Yang Y, Li J. Effect of Vacancy Defects on the Electronic Structure and Optical Properties of Bi4O5Br2: First-Principles Calculations. Coatings. 2024; 14(11):1361. https://doi.org/10.3390/coatings14111361
Chicago/Turabian StyleHuang, Baorui, Yeqi Liu, Yanni Zhang, Fuchun Zhang, Yanning Yang, and Jiaxin Li. 2024. "Effect of Vacancy Defects on the Electronic Structure and Optical Properties of Bi4O5Br2: First-Principles Calculations" Coatings 14, no. 11: 1361. https://doi.org/10.3390/coatings14111361
APA StyleHuang, B., Liu, Y., Zhang, Y., Zhang, F., Yang, Y., & Li, J. (2024). Effect of Vacancy Defects on the Electronic Structure and Optical Properties of Bi4O5Br2: First-Principles Calculations. Coatings, 14(11), 1361. https://doi.org/10.3390/coatings14111361






