Phototuning of Multi-Color Emission in PMMA Composite Films for Information Encryption Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of R-1/PMMA Composite Films
2.3. Characterization
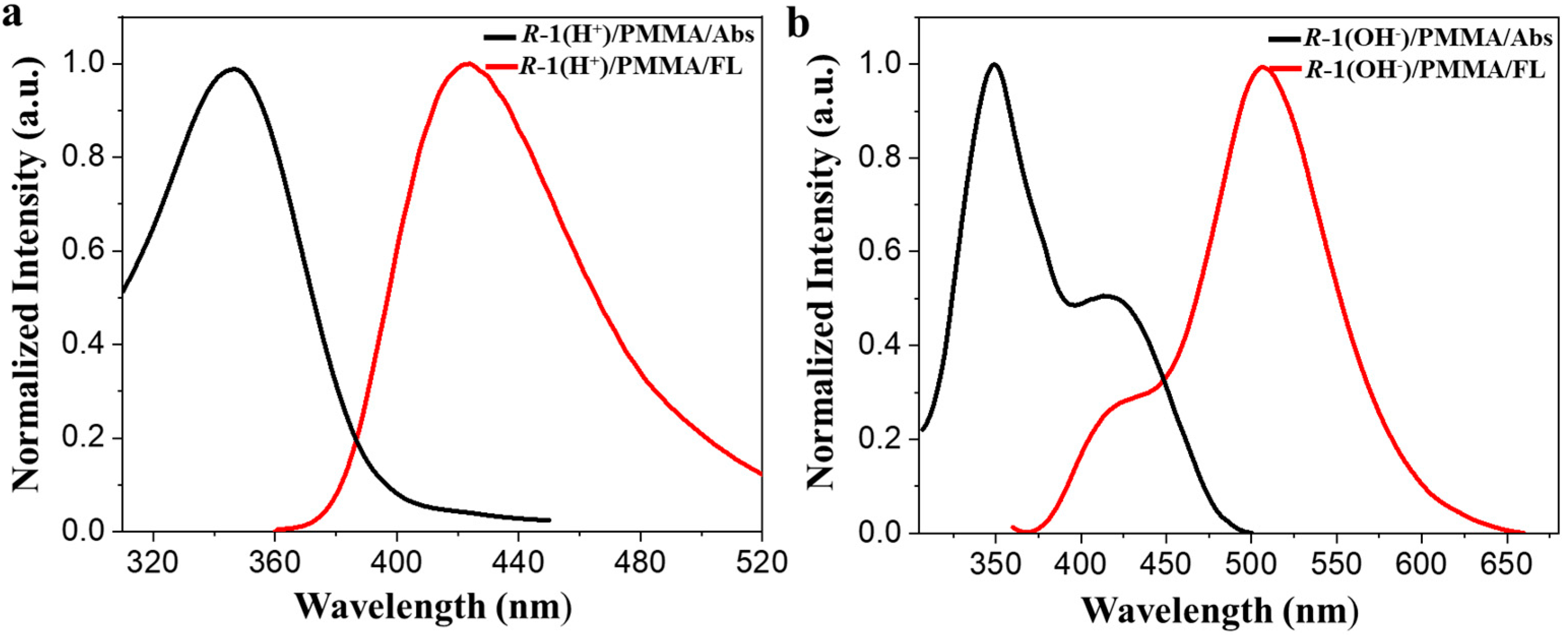
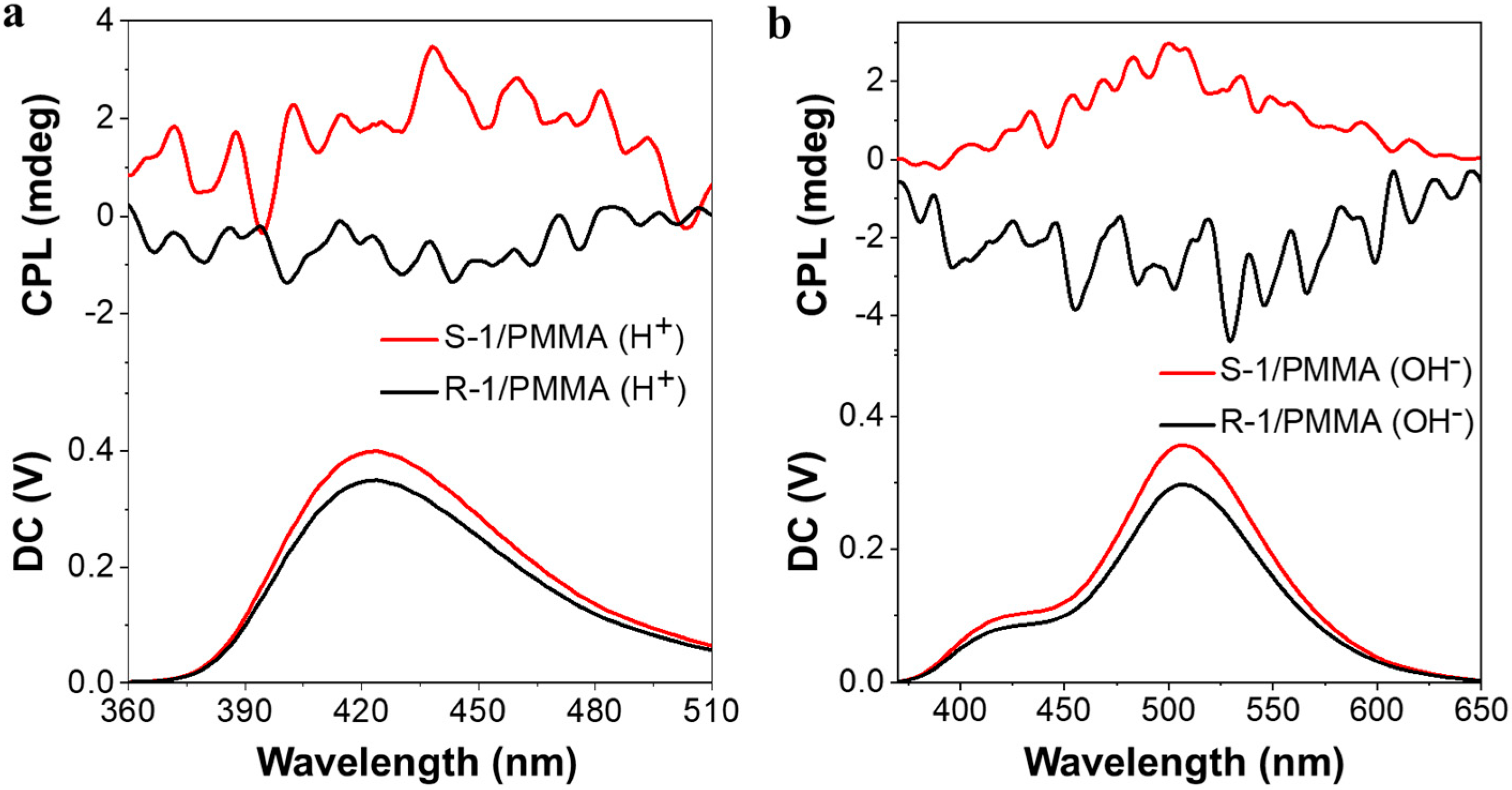
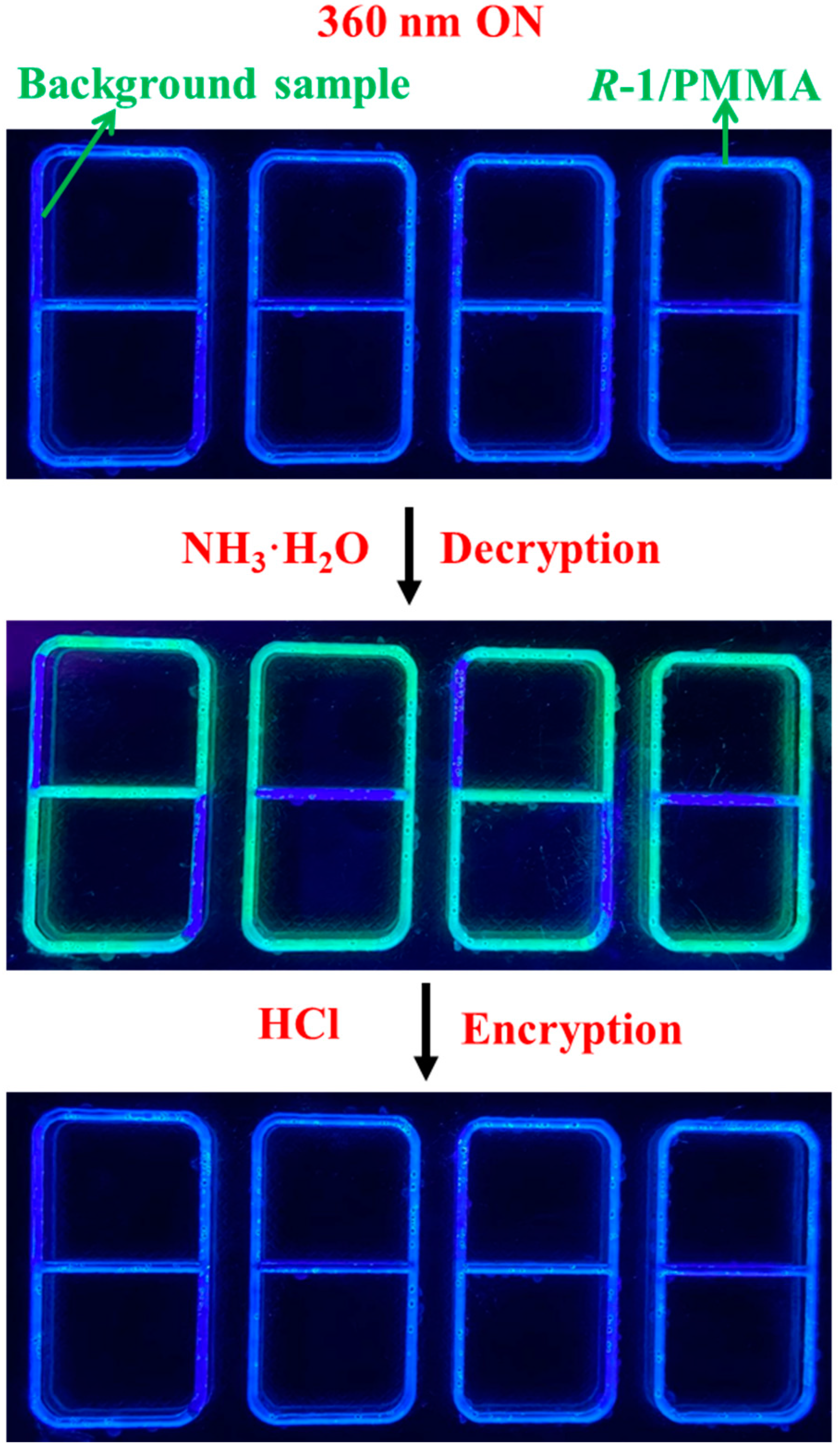
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, H.; Li, Z.; Gong, J.; Hu, L.; Alam, P.; Ji, X.; Hu, Y.; Chau, J.H.C.; Lam, J.W.Y.; Kwok, R.T.K.; et al. Phototriggered Aggregation-Induced Emission and Direct Generation of 4D Soft Patterns. Adv. Mater. 2021, 33, 2105113. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-W.; Chen, Y.; Lu, Y.-L.; Qin, Y.-X.; Zhang, H.; Xu, X.; Liu, Y. Tunable Second-Level Room-Temperature Phosphorescence of Solid Supramolecules between Acrylamide-Phenylpyridium Copolymers and Cucurbit 7 uril. Angew. Chem. Int. Ed. 2022, 61, e202115265. [Google Scholar]
- Yao, X.; Wang, J.; Jiao, D.; Huang, Z.; Mhirsi, O.; Lossada, F.; Chen, L.; Haehnle, B.; Kuehne, A.J.C.; Ma, X.; et al. Room-Temperature Phosphorescence Enabled through Nacre-Mimetic Nanocomposite Design. Adv. Mater. 2021, 33, 2005973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Hu, W.; Stoddart, J.F. Color-Tunable Supramolecular Luminescent Materials. Adv. Mater. 2022, 34, 2105405. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Li, M.-D.; Li, Z.A.; Peng, H.; Xie, X. Crosstalk-Free Patterning of Cooperative-Thermoresponse Images by the Synergy of the AIEgen with the Liquid Crystal. Angew. Chem. Int. Ed. 2020, 59, 10066–10072. [Google Scholar] [CrossRef]
- Tan, J.; Li, Q.; Meng, S.; Li, Y.; Yang, J.; Ye, Y.; Tang, Z.; Qu, S.; Ren, X. Time-Dependent Phosphorescence Colors from Carbon Dots for Advanced Dynamic Information Encryption. Adv. Mater. 2021, 33, 2006781. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Yang, Y.; Ji, X.; Sessler, J.L. Unlocking Chemically Encrypted Information Using Three Types of External Stimuli. J. Am. Chem. Soc. 2021, 143, 18635–18642. [Google Scholar] [CrossRef]
- Yang, Y.-D.; Ji, X.; Lu, Z.-H.; Yang, J.; Gao, C.; Zhang, H.; Tang, B.Z.; Sessler, J.L.; Gong, H.-Y. Time-dependent solid-state molecular motion and colour tuning of host-guest systems by organic solvents. Nat. Commun. 2020, 11, 77. [Google Scholar] [CrossRef]
- Ren, W.; Lin, G.; Clarke, C.; Zhou, J.; Jin, D. Optical Nanomaterials and Enabling Technologies for High-Security-Level Anticounterfeiting. Adv. Mater. 2020, 32, 1901430. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, G.; Li, B.; Chen, H.; Li, H.; Zhao, Y. Photoresponsive supramolecular coordination polyelectrolyte as smart anticounterfeiting inks. Nat. Commun. 2021, 12, 1363. [Google Scholar] [CrossRef]
- Song, Y.; Lu, M.; Mandl, G.A.; Xie, Y.; Sun, G.; Chen, J.; Liu, X.; Capobianco, J.A.; Sun, L. Energy Migration Control of Multimodal Emissions in an Er3+-Doped Nanostructure for Information Encryption and Deep-Learning Decoding. Angew. Chem. Int. Ed. 2021, 60, 23790–23796. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sheng, L.; Xu, Y.; Chen, Q.; Gu, C.; Li, M.; Zhang, S.X.-A. Printable Off-On Thermoswitchable Fluorescent Materials for Programmable Thermally Controlled Full-Color Displays and Multiple Encryption. Adv. Mater. 2021, 33, 2008055. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Sauve, E.R.; Tonge, C.M.; Hudson, Z.M. Aggregation-Induced Energy Transfer in Color-Tunable Multiblock Bottlebrush Nanofibers. J. Am. Chem. Soc. 2019, 141, 16422–16431. [Google Scholar] [CrossRef]
- Klajn, R.; Wesson, P.J.; Bishop, K.J.M.; Grzybowski, B.A. Writing Self-Erasing Images using Metastable Nanoparticle “Inks”. Angew. Chem. Int. Ed. 2009, 48, 7035–7039. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Guo, Y.; Yang, Z.; Li, H.; Liu, H. Paper-Structure Inspired Multiresponsive Hydrogels with Solvent-Induced Reversible Information Recording, Self-Encryption, and Multidecryption. Adv. Funct. Mater. 2022, 32, 2201009. [Google Scholar] [CrossRef]
- Kundu, P.K.; Samanta, D.; Leizrowice, R.; Margulis, B.; Zhao, H.; Boerner, M.; Udayabhaskararao, T.; Manna, D.; Klajn, R. Light-controlled self-assembly of non-photoresponsive nanoparticles. Nat. Chem. 2015, 7, 646–652. [Google Scholar] [CrossRef]
- Gao, Z.; Han, Y.; Wang, F. Cooperative supramolecular polymers with anthracene. Endoperoxide photo-switching for fluorescent anti-counterfeiting. Nat. Commun. 2018, 9, 3977. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, S.-J.; Park, S.Y. Highly Fluorescent Chameleon Nanoparticles and Polymer Films: Multicomponent Organic Systems that Combine FRET and Photochromic Switching. J. Am. Chem. Soc. 2012, 134, 12091–12097. [Google Scholar] [CrossRef]
- Naren, G.; Hsu, C.-W.; Li, S.; Morimoto, M.; Tang, S.; Hernando, J.; Guirado, G.; Irie, M.; Raymo, F.M.; Sunden, H.; et al. An all-photonic full color RGB system based on molecular photoswitches. Nat. Commun. 2019, 10, 3996. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, P.; Krishnan, B.P.; Wang, H.; Gao, Y.; Chen, S.; Zeng, R.; Cui, J.; Chen, J. From a Molecular Toolbox to a Toolbox for Photoswitchable Fluorescent Polymeric Nanoparticles. Adv. Funct. Mater. 2018, 28, 1804759. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, H.; Yu, M.; Guo, X.; Zhang, C.; Deng, H.; Zhang, P.; Chen, S.; Zeng, R.; Cui, J.; et al. Photoswitchable ultrahigh-brightness red fluorescent polymeric nanoparticles for information encryption, anti-counterfeiting and bioimaging. J. Mater. Chem. C 2019, 7, 11515–11521. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, J.E.; Park, S.Y. Is Color-Specific Photoswitching in Dual-Color Fluorescence Systems Possible? Manipulating Intermolecular Energy Transfer among Two Different Fluorophores and One Photoswitch. Adv. Opt. Mater. 2016, 4, 790–797. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, J.E.; Park, S.Y. Fully Reversible Multistate Fluorescence Switching: Organogel System Consisting of Luminescent Cyanostilbene and Turn-On Diarylethene. Adv. Funct. Mater. 2018, 28, 1706213. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Jin, B.; Liu, X.; Fu, H.; Shangguan, D. A guanidine derivative of naphthalimide with excited-state deprotonation coupled intramolecular charge transfer properties and its application. J. Mater. Chem. C 2013, 1, 4427–4436. [Google Scholar] [CrossRef]
- Mansueto, E.S.; Wight, C.A. Excited-state proton-transfer polymerization of amorphous formaldehyde. J. Am. Chem. Soc. 1989, 111, 1900–1901. [Google Scholar] [CrossRef]
- Kamioka, M.; Ishikawa, Y.; Kaetsu, H.; Isomura, S.; Arai, S. Isotope-selective infrared multiple photon decomposition of hexafluorodisilane. J. Phys. Chem. 1986, 90, 5727–5730. [Google Scholar] [CrossRef]
- Erez, Y.; Presiado, I.; Gepshtein, R.; Huppert, D. Excited-State Intermolecular Proton Transfer of Firefly Luciferin IV. Temperature and pH Dependence. J. Phys. Chem. A 2011, 115, 1617–1626. [Google Scholar] [CrossRef]
- Presiado, I.; Erez, Y.; Huppert, D. Excited-State Intermolecular Proton Transfer of Firefly Luciferin III. Proton Transfer to a Mild Base. J. Phys. Chem. A 2010, 114, 13337–13346. [Google Scholar] [CrossRef]
- Han, D.; Li, C.; Jin, X.; Zhou, J.; Xu, Y.; Jiao, T.; Duan, P. Tunable Circularly Polarized Luminescence of Excited-State-Proton-Transfer-Based Chiral Guanidine. Adv. Photonics Res. 2022, 3, 2100287. [Google Scholar] [CrossRef]



| State | τ(R-1)+*/ns | τ(R-1)*/ns | Φf (%) |
|---|---|---|---|
| R-1/PMMA(H+) | τ = 7.33 (425 nm) | 52.3 | |
| R-1/PMMA(OH−) | τ = 7.34 (425 nm) | τ1 = 5.01, τ2 = 19.55 (506 nm) | 54.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Han, D.; Cui, S.; Peng, L. Phototuning of Multi-Color Emission in PMMA Composite Films for Information Encryption Applications. Coatings 2024, 14, 1360. https://doi.org/10.3390/coatings14111360
Chen G, Han D, Cui S, Peng L. Phototuning of Multi-Color Emission in PMMA Composite Films for Information Encryption Applications. Coatings. 2024; 14(11):1360. https://doi.org/10.3390/coatings14111360
Chicago/Turabian StyleChen, Guang, Dongxue Han, Songya Cui, and Liang Peng. 2024. "Phototuning of Multi-Color Emission in PMMA Composite Films for Information Encryption Applications" Coatings 14, no. 11: 1360. https://doi.org/10.3390/coatings14111360
APA StyleChen, G., Han, D., Cui, S., & Peng, L. (2024). Phototuning of Multi-Color Emission in PMMA Composite Films for Information Encryption Applications. Coatings, 14(11), 1360. https://doi.org/10.3390/coatings14111360






