Coatings and Surface Modification of Alloys for Tribo-Corrosion Applications
Abstract
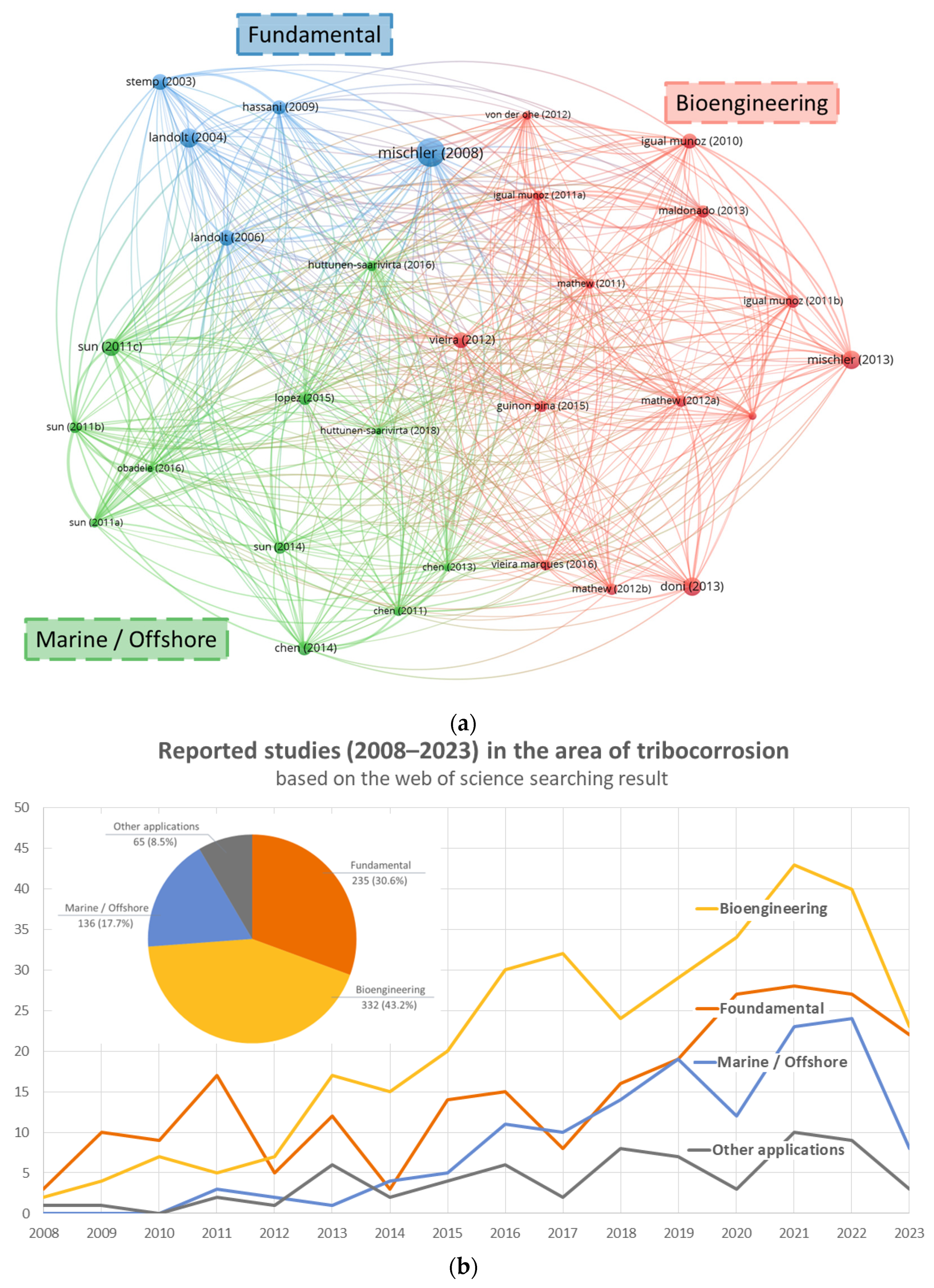
1. Introduction and Signpost of Reviews
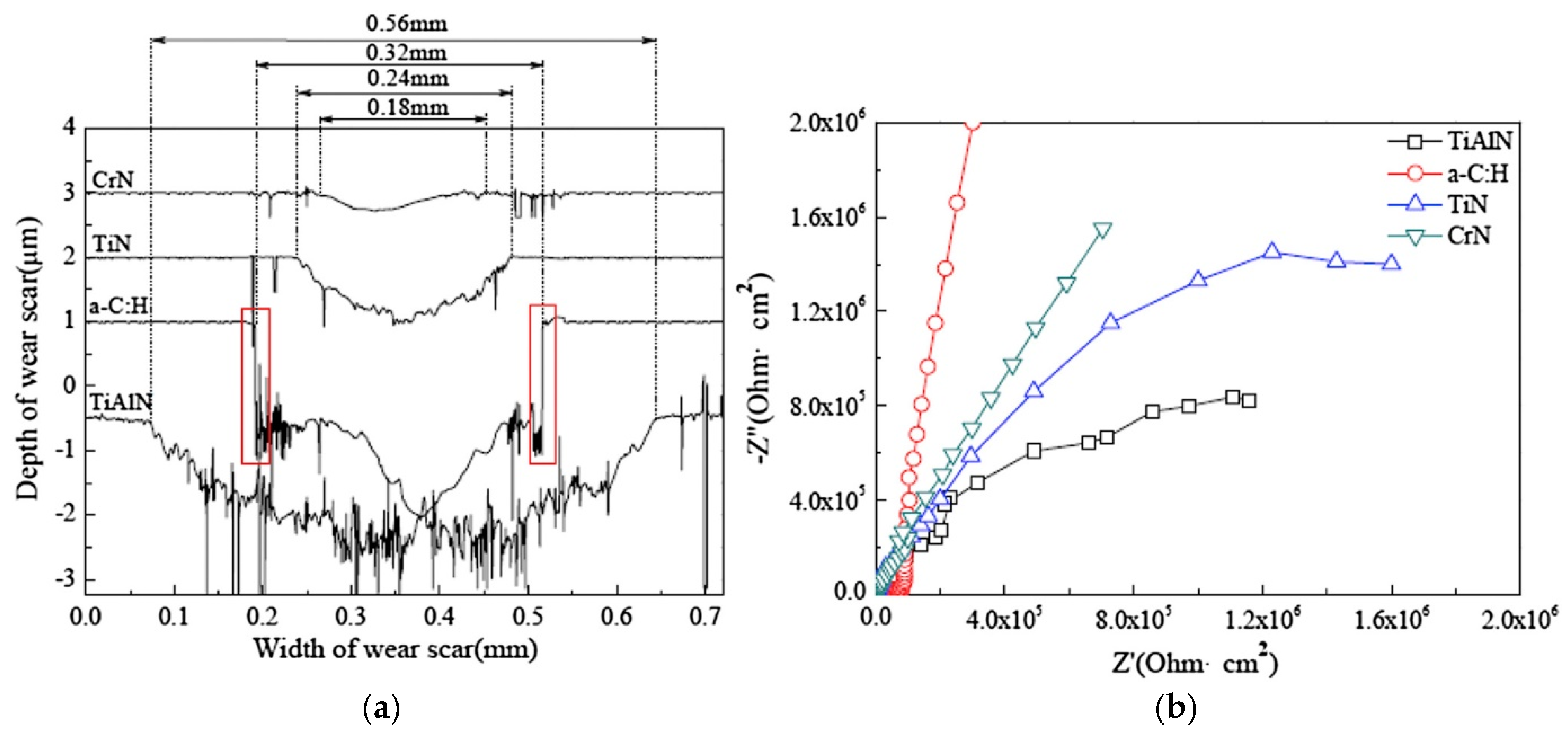
2. Coating Development
2.1. Coating Techniques (Monolayers)
2.1.1. Molten Deposition
2.1.2. Vapor Deposition
2.1.3. Deposition from Solutions
| Substrate | Tech | Solution | Test | Findings | Ref. |
|---|---|---|---|---|---|
| AZ31B Mg alloy | PEO with pullulan | SBF | EIS, OCP, PDP | An optimal pullulan concentration is determined in the silicate-based electrolyte solution for achieving the best anti-corrosion properties in the PEO-treated alloys. Specifically, the corrosion resistance improved as the pullulan concentration increased from 0 to 1.0 g/L. | [89] |
| AZ31B Mg alloy | PEO + CS dip | SBF | EIS, OCP, PDP | The PEO-treated AZ31B Mg alloy is porous and has multiple micro-cracks. Using hydrothermal treatment (HT) leads to the precipitation of Mg(OH)2 crystals over the PEO sample. When a chitosan (CS) layer was applied on top of these treated samples, it effectively sealed the pores and cracks. As a result, the combination of PEO, HT and CS coatings significantly enhanced the corrosion resistance of the Mg alloy, as evidenced by the order of anti-corrosion properties: PEO/HT/CS > PEO/CS > PEO/ HT > PEO > substrate. | [90] |
| Zr-2.5 Nb alloy | PEO (ZrO2-Al2O3) | PBS | EIS, OCP | The main wear mechanism for the uncoated Zr-2.5 Nb alloy substrate was identified as adhesion, with wear particles adhering to the surface locally. In contrast, for the coated surfaces, delamination was the predominant wear mechanism, signifying a significant improvement in wear resistance for the coatings. | [91] |
| AZ31B Mg alloy | PEO with multi-walled carbon nanotubes | SBF | OCP, PDP | The presence of MWCNTs induced the formation of Mg2SiO4 in the coating, in addition to the already existing MgO. The reinforcement with MWCNTs led to a 15% reduction in the coefficient of friction and decreased wear-related damage by 60%. Moreover, the corrosion resistance of the coating was enhanced, especially against pitting corrosion. | [92] |
| AZ31B Mg alloy | MAO Ca/P coating | SBF | EIS, OCP, PDP | The magnesium alloy, when treated with ultrasonic cold forging technology (UCFT) followed by micro-arc oxidation (MAO) containing calcium phosphate, demonstrated superior tribocorrosion resistance. When friction was applied to the samples, there was a significant increase in corrosion. | [93] |
| AZ31B Mg alloy | Si, Si + P, Si + B | SBF | EIS, OCP, PDP | The microstructure and surface morphology of the MAO coatings were found to be similar, with all having an outer porous layer and an inner dense layer. Among the various coatings, the one produced with both Si and B (Si + B) in the electrolyte was the thickest and exhibited the highest hardness. | [94] |
3. Modelling
| Model | Technique | Validation | Ref. |
|---|---|---|---|
| Wear maps | Rough asperities are represented as a system of adjacent cuboids. Each cuboid refers to one asperity to explore levels of deformation and stress. Two mechanisms of asperity wear are considered: micro-shearing and low cycle fatigue. | Repassivation of AISI 304 steel in a 0.5 M solution of H2SO4 | [114] |
| Modelling the evolution of the electrochemical current in the potentiostatic condition using an asperity-scale model of tribocorrosion | This is an asperity-scale electrochemical model coupled to a computational contact mechanics solution. It models the real-time evolution of surface topography, wear and corrosion. | The model determines the micro-scale current, and the summation of these currents defines the macro-scale quantity of the current. This model was validated with experiments using a ball-on-plate tribometer. | [103] |
| A New Asperity-Scale Mechanistic Model of Tribocorrosive Wear: Synergistic Effects of Mechanical Wear and Corrosion | A corrosive wear model is considered at the asperity scale and coupled with a traditional Archard-type mechanical wear model. The geometry of the surface asperities is modified in a contact mechanics model. This deterministic model calculates the corrosion-enhanced wear. | This work studied CoCrMo as the working electrode (WE) and the Si3N4 ball as the counter body in a reciprocating configuration. Contributions of total mechanical wear and corrosion suggest that wear-enhanced corrosion was a significant contribution in the overall degradation of the material. | [112] |
| Predictive wear model based on a mechanistic approach of tribocorrosion and lubrication and taking into account clinically relevant parameters such as normal load, velocity and clearance | Passive metals are considered that allow for plastic deformation at asperity contacts, combining mechanical wear (Archard’s law), chemical wear (wear-accelerated corrosion) and hydrodynamic lubrication. | Good agreement with wear rates observed in tribometers and the running-in wear rate of artificial hip joints tested in simulators | [105] |
| Time dependence of the material degradation mechanisms | BEM/Archard | AISI316L in a 3% NaCl solution | [95] |
| Tribocorrosion mechanisms of NiCrMo-625alloy: electrochemical modelling | Archard plus electrochemical model | Experimentally validated | [96] |
| Hip joint stem interface modelling | An adaptive finite-element-based model using a combination of Landolt’s passive film equation and Faraday’s law, in parallel with Archard’s wear model to simulate fretting corrosion | This model was able to predict the damage pattern at the retrieved head–neck interface. | [104] |
| Finite element-based simulation of tribocorrosion at the head–neck junction of hip implants | Finite element (FE) model for the head–neck junction of hip implants. Archard wear equation and Maldonado electrochemical model used [118] | The FE-predicted wear patterns were comparable with those observed in retrieved tapers. | [98] |
| In situ determinations of the wear surfaces, volumes and kinetics of repassivation: contribution to the understanding of the tribocorrosion behavior of a ferritic stainless steel at various pH levels | Wear model using Archard’s law; however, a non-linear dependence of Archard’s coefficient with pH is seen. The role of pH in influencing the film regrowth kinetics is shown. | The film reactivity depends on pH; tribocorrosion experiments were performed on ferritic stainless steels in sulphate solution (pH 1.5, 6.5 or 12.5). | [103] |
| Electrochemical Simulation of the Current and Potential Response in Sliding Tribocorrosion | Combined Tafel, galvanic (wear/surrounding area) and Archard equations | Excellent agreement of experimental wear data and the experimental OCP curves | [99] |
| Area-dependent impedance-based voltage shifts during tribocorrosion of Ti-6Al-4V biomaterials | An area-dependent Randle’s circuit is used with the tribocorrosion current equation. The potential drop over time depends on the surface impedance characteristics, the area of the electrode, the material and the mechanical conditions, giving rise to the oxide film abrasion. It accounts for the effects of oxide film onset potential, the voltage dependence of the oxide film properties and repassivation, and galvanic effects. The model also incorporated voltage-dependent circuit elements such as the Tafel and the Mott–Schottky elements to account for the changes in these properties with voltage. | Experiments use titanium pin-on-disc fretting corrosion | [101,102] |
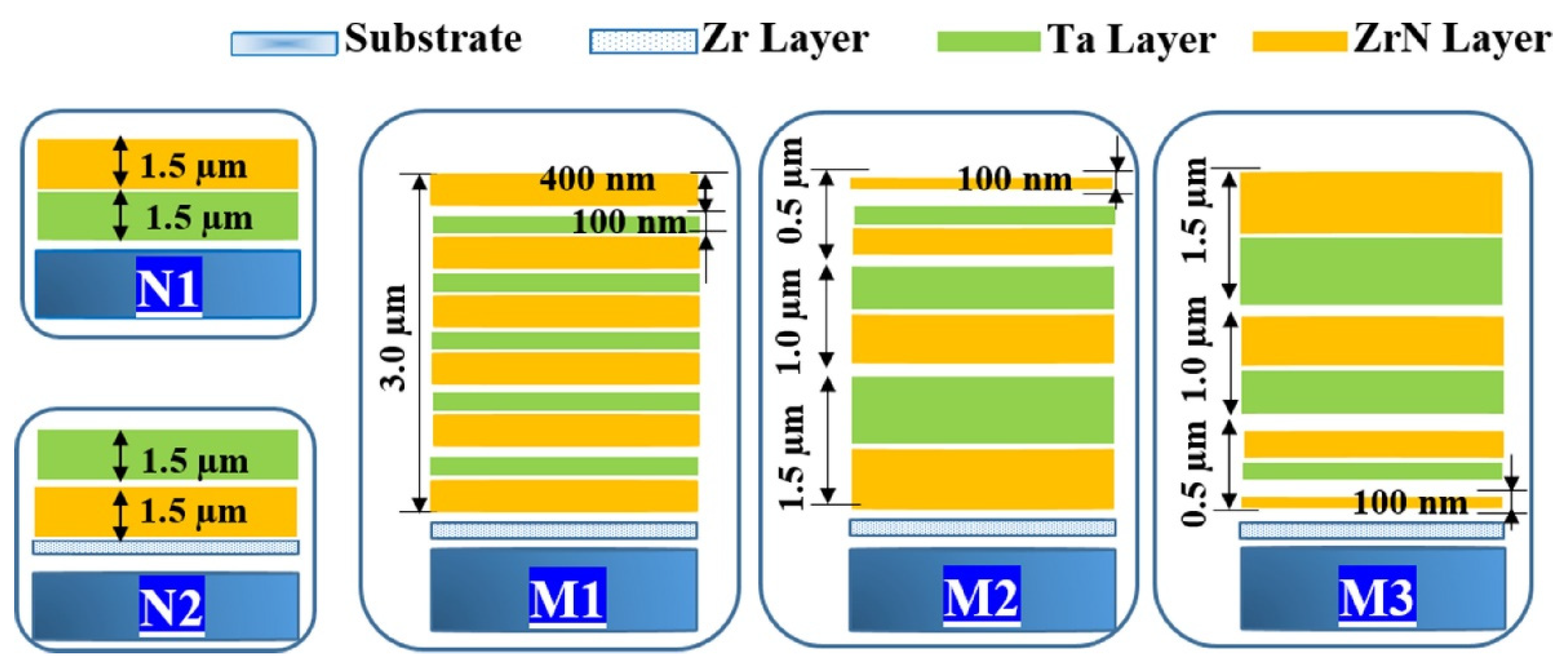
| Modelling nanostructured metallic multilayers | A finite element (FE)-based computational model and density functional theory (DFT) calculations. | Nanomechanical and electrochemical measurements were coupled with advanced material characterization tools. | [109] |
| Multiphysics modeling and uncertainty quantification of tribocorrosion in aluminum alloys | Finite element-based multiphysics model. Two surrogate models, Gaussian process and neural network with dropout, were used for uncertainty quantification of the finite element model. | Validated by existing tribocorrosion experiments of two Al alloys | [97] |
| Tribocorrosion of CoCr Alloy and Ti with Galvanic Coupling in Simulated Body Fluid | Numerical simulation of current density distributions conducted with COMSOL Multiphysics 5.3 software | Experimental results | [106] |
| Modelling Current Transients in a Reciprocal Motion Tribocorrosion Experiment | Kinetics for interface-limited film growth were used to find an analytical expression for the current transients. This included the conductivity of the electrolyte as well as the contribution from the confined geometry within the mechanical contact. | Good experimental fit over a range of electrolyte conductivities and with electrochemical impedance spectroscopy | [110] |
| Growth mechanism and repassivation kinetic determinations on stainless steel under sliding: Role of the solution pH and dissolved oxygen concentration | Higher wear rates were measured in low pH and aerated solution. Temporal analysis of the transients showed faster passivation of the wear track in acidic solution. | The degradation rate was correlated to the passivation kinetic. | [111] |
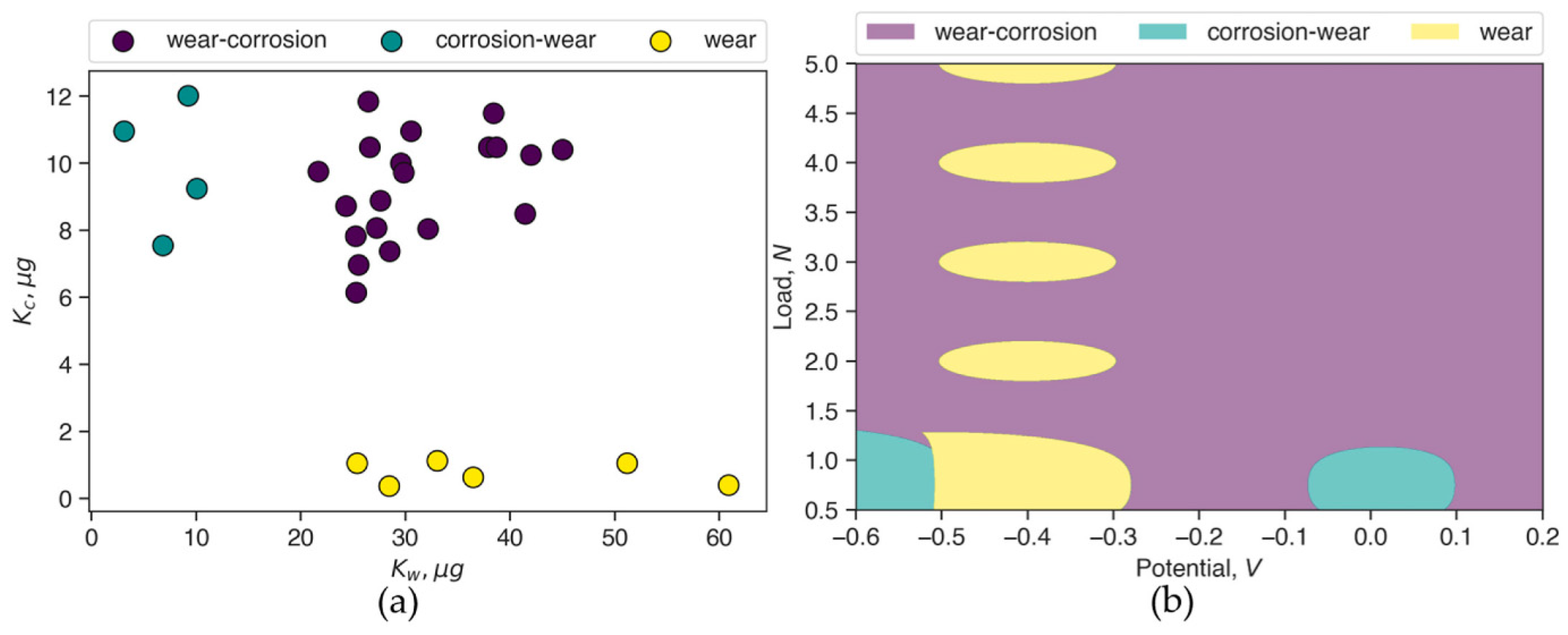
| Machine Learning Model to Map Tribocorrosion Regimes in Feature Space | Unsupervised machine learning is used to identify and label clusters from tribocorrosion data. The clusters are used to train a support vector classification model, which is used to generate tribocorrosion maps. | The generated maps are compared with those from the literature. | [115] |
| Applicability of a recently proposed tribocorrosion model for CoCr alloys with different carbide content | Dowson model for mechanical and electrochemical wear [119] | Validated by tests on Stellite 6 and Stellite 21 | [100] |
| Fully Coupled Tribocorrosion Simulation Method for Anchor Chain, Considering Mechano-Electrochemical Interaction | Fortran model, where both stress-accelerated corrosion and corrosion-accelerated wear can be considered to predict the time-variant damage morphology. | Simulation results were compared to theoretical values. | [108] |
| Modelling the multi-degradation mechanisms of combined tribocorrosion interacting with static and cyclic loaded surfaces of passive metals exposed to seawater | Model coupling corrosion fatigue (CF) and stress corrosion cracking (SCC) mechanisms. The multi-degradation model is based on surface hardness and corrosion resistance. | Further experiments needed to validate model | [107] |
4. Environmental Effects on Tribocorrosion
4.1. pH (Non-Bio and Marine)
4.2. Biomedical Sectors
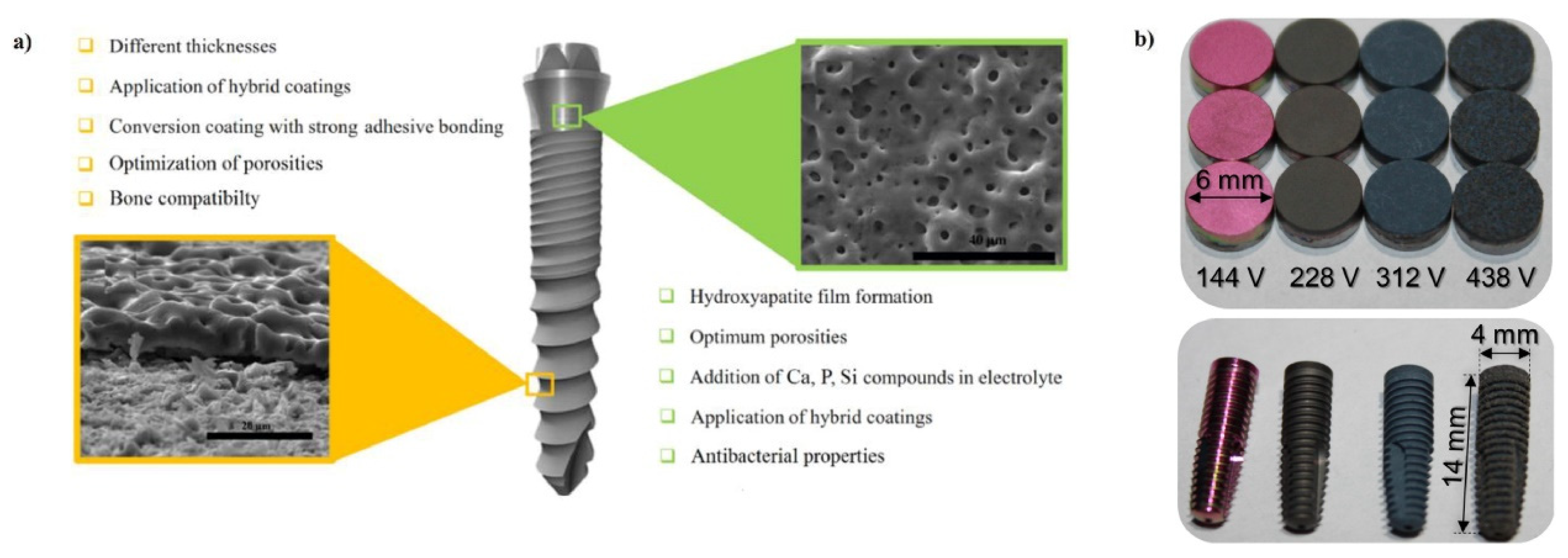
4.2.1. Dental
4.2.2. Joint Prostheses
| Substrate | Tech | Solution | Test | Findings | Ref. |
|---|---|---|---|---|---|
| Ti-6Al-4V | Thermal sprayed Alumina, Alumina-Zirconia, Alumina-titania | bovine calf serum (BCS) | OCP | The ceramic coatings primarily suffered from mechanical wear rather than corrosion. The nanostructured bilayered IDZAT coating demonstrated the best performance. | [191] |
| 316L | Dipping polyurethane (PU)–polydimethylsiloxane (PDMS) blended coatings | Ringer’s solution | OCP | The wear volume and the friction coefficient of PU–PDMS coatings decreased with the increased PU concentration in the blend. Polymer-coated samples containing up to 50% PDMS prevented corrosive wear. The PU25–PDMS75 coating could not protect the surface due to its weaker mechanical properties. | [192] |
| 304 | Electrodeposition Co/nano-CeO2 | Hank’s solution | EIS | The inclusion of nano-CeO2 particles into the cobalt matrix by electroco-deposition significantly reduces the friction coefficient, wear weight loss and corrosion damage. | [193] |
| 304 | PVD calcium phosphate-calcium titanate | Hank’s solution | Polarization OCP | Fretting: Calcium titanate coatings showed reduced wear of the bone compared to bare stainless steel, with wear decreasing as the calcium titanate content increased. Calcium titanate coatings are potentially more effective candidate materials than calcium phosphate coatings for bone tissue replacement in hip prostheses. | [194] |
| Ti-6Al-4V (additive manufactured) | PVD TiN | 25%(v/v) newborn bovine serum (17 g/L total protein content) diluted by PBS | OCP | Duplex-TiN coatings were applied on both ALM and traditionally wrought manufactured Ti-6Al-4V substrates. The coatings enhanced hardness (from 3.5 GPa to 14–15 GPa), improved corrosion resistance and showcased excellent wear resistance (wear depth of 0.3 μm against a coating thickness of 3–6 μm). The coated materials displayed the ability to rapidly recover their protective layer after wear tests. | [195] |
| Ti | plasma spraying Ti-6Al-4V-TiB-TiN | Hank’s solution | PDP OCP | The tests also revealed domination of synergistic effects, i.e., corrosion affects wear more than wear affects corrosion. Increasing the deposition speed and/or decreasing the plasma power led to an increase in mechanical wear and corrosion loss. | [196] |
| Ti | double-glow plasma alloying Nb | Ringer’s solution | OCP | DG-Nb wear rate is merely 4.92% of that of Pure Ti. | [197] |
| Ti, Ti-6Al-4V, Ti13Nb13Zr | CVDNanocrystalline diamond (NCD) coatings | 9 g/L NaCl | OCP | NCD coatings showed promise in corrosion resistance; their response under combined wear and corrosion was mixed. Notably, the occurrence of blistering could hinder the long-term performance of such implants. | [198] |
4.3. Marine and Offshore Sector
| Substrate | Tech | Interlayer | Solution | Tests | Findings | Ref. |
|---|---|---|---|---|---|---|
| AISI 1045 | PVD Cr/CrN | Nitrided | 3.5 wt% NaCl | OCP, PDP | A clear synergy effect of over 30% between friction and corrosion; the synergy effect is caused by the fact that corrosion processes create local cavities which, at successive time intervals, are most likely to intensify mechanical wear. The area around the cavities facilitates plastic deformation, the initiation of cracking of cyclically deformed layers and the tearing off of larger fragments of material (especially at higher unit pressures in the frictional contact zones). | [236] |
| 316L, TC4, and H65 copper | PVD Si-Doped TiSiN-Ag | artificial seawater | OCP, PDP | TiSiN-Ag composite coatings with 8 wt.% Si doping exhibit superior characteristics. These coatings’ network structure effectively hinders the generation and growth of cracks. Si doping content can help mitigate the presence of defects such as pinholes and cracks. | [237] | |
| Ti-6Al-4V | W-DLC CrN + a-C:H:W HVOF Cr3C2 in Ni/Cr Ion implantation nitriding | artificial seawater | OCP, PDP | The ion implantation treatment on Ti6Al4V substrate yielded the best static corrosion resistance. The W-DLC-coated Ti6Al4V substrate exhibited the most stable and lowest friction, leading to a commendable wear and corrosion resistance in artificial seawater. Both the HVOF coating- and ion implantation-treated substrates had noticeable crack initiation, increasing susceptibility to localized corrosion. | [238] | |
| F22 steel | Thermal spray Fe-based amorphous | artificial seawater | OCP, PDP | Synergy effect: Wear-induced material loss (W) is the major material damage under the current experimental conditions. Seawater aided in lubrication and reducing friction. | [239] | |
| SS316 | Thermal spray WC/Ni60 | artificial seawater | The WC phases in the coating help resist wear, while the Ni-based materials improve corrosion resistance. | [240] | ||
| SS316 | Laser cladding titanium carbonitride | 3 M NaCl saturated solution | OCP, PDP | 316L laser claddings reinforced with titanium carbonitride grains improved tribocorrosion performance by over 10-fold in certain conditions. The optimized addition of (Ti,Mo)(C,N)-Ni powder led to homogeneously distributed reinforcements, enhancing durability. Inappropriate quantities of (Ti,Mo)(C,N)-Ni powder can cause either high agglomerations or uneven dispersion. | [241] | |
| SS304 | High-Intensity Pulsed Ion Beam (HIPIB) CrN/TiN | 3.5 wt% NaCl | OCP | Coated surfaces present excellent tribocorrosion properties in seawater, showcasing a high open circuit potential, low coefficient of friction and low specific tribocorrosion rate, all without pitting corrosion due to the high surface integrity, stable interfaces and dense microstructure. | [242] | |
| R4 mooring chain | pack cementation method FeAl | 3.5 wt% NaCl | OCP, PDP | Improved corrosion resistance was offered by the aluminized coating, with the oxide layer on the surface playing a key role in this improvement. However, its wear resistance decreased due to the presence of brittle phases. | [243] |
5. Conclusions and Future Challenges
Funding
Conflicts of Interest
Abbreviation
| a-C | Amorphous carbon |
| ALTR | adverse local tissue reactions |
| AP | aluminum phosphate |
| ARMD | adverse reactions to metal debris |
| CBPCC | chemically bonded phosphate ceramic coating |
| CF | corrosion fatigue |
| CVD | Chemical Vapor Deposition |
| DLC | Diamond-Like Carbon |
| EIS | Electrochemical Impedance Spectroscopy |
| ESD | electro-spark deposition |
| FDA | Food and Drug Administration |
| GSR | gross slip regime |
| H/E | hardness to elastic modulus |
| HA | hydroxyapatite |
| HEA | High-entropy alloy |
| HVOF | High-Velocity Oxygen Fuel |
| IBAD | Ion Beam-Assisted Deposition |
| MAO | Micro-Arc Oxidation |
| MOCVD | Metal Organic Chemical Vapor Deposition |
| MoM | metal-on-metal |
| NAB | nickel aluminum bronze |
| OCP | open circuit potential |
| PAE | pulsed arc evaporation |
| PDMS | polydimethylsiloxane |
| PDP | Potentiodynamic Polarization |
| PECVD | plasma-enhanced chemical vapor deposition |
| PEO | Plasma Electrolytic Oxidation |
| PU | polyurethane |
| PVP | polyurethane and polyvinylpyrrolidone |
| PWRs | Pressurized Water Reactors |
| SCC | stress corrosion cracking |
| THR | total hip replacement |
| TO | Thermal Oxidation |
| TSA | Thermally Sprayed Aluminum |
| UCFT | ultrasonic cold forging technology |
| UHMWPE | Ultra-High Molecular Weight Polyethylene |
| UNSM | Ultrasonic nanocrystal surface modification |
| XPS | X-Ray Photoelectron Spectroscopy |
References
- Kumar, V.; Agarwal, A.K.; Jena, A.; Upadhyay, R.K. (Eds.) Advances in Engine Tribology; Springer: Singapore, 2022; ISBN 9789811683367. [Google Scholar]
- López-Ortega, A.; Arana, J.L.; Bayón, R. Tribocorrosion of Passive Materials: A Review on Test Procedures and Standards. Int. J. Corros. 2018, 2018, 7345346. [Google Scholar] [CrossRef]
- Blau, P.J.; Wood, R.; Stack, M.M.; Mischler, S.; Jiang, J.; Drees, D.; Rocha, L.A.; Wimmer, M.A.; Celis, J.-P.; Cowan, R. Future Needs and Challenges in Tribo-Corrosion Research and Testing. In Tribo-Corrosion: Research, Testing, and Applications; Blau, P.J., Celis, J.-P., Drees, D., Eds.; ASTM International: West Conshohocken, PA, USA, 2013; pp. 214–226. ISBN 978-0-8031-7549-5. [Google Scholar]
- Mathew, M.T.; Cheng, K.; Sun, Y.; Barao, V.A.R. The Progress in Tribocorrosion Research (2010–2021): Focused on the Orthopedics and Dental Implants. J. Bio-Tribo-Corros. 2023, 9, 48. [Google Scholar] [CrossRef]
- Oluwatosin Abegunde, O.; Titilayo Akinlabi, E.; Philip Oladijo, O.; Akinlabi, S.; Uchenna Ude, A. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Pinto, G.; Silva, F.; Porteiro, J.; Mínguez, J.; Baptista, A. Numerical Simulation Applied to PVD Reactors: An Overview. Coatings 2018, 8, 410. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Kovaci, H.; Yetim, A.; Celik, A. Tribocorrosion properties and mechanism of a shot peened AISI 4140 low-alloy steel. Surf. Coat. Technol. 2022, 440, 128444. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, S.; Lin, N.; Zeng, Q.; Wang, Z.; Wu, Y. Application of ultrasonic nanocrystal surface modification (UNSM) technique for surface strengthening of titanium and titanium alloys: A mini review. J. Mater. Res. Technol. 2021, 11, 351–377. [Google Scholar] [CrossRef]
- Wood, R.J.K. Tribo-corrosion of coatings: A review. J. Phys. Appl. Phys. 2007, 40, 5502–5521. [Google Scholar] [CrossRef]
- Zeisel, H.; Durst, F. Computations of Erosion–Corrosion Processes in Separated Two-Phase Flows NACE Corrosion; NACE: Phoenix, AZ, USA, 1990. [Google Scholar]
- Vignal, V.; Mary, N.; Ponthiaux, P.; Wenger, F. Influence of friction on the local mechanical and electrochemical behaviour of duplex stainless steels. Wear 2006, 261, 947–953. [Google Scholar] [CrossRef]
- Zhou, S.; Stack, M.M.; Newman, R.C. Characterization of synergistic effects between erosion and corrosion in an aqueous environment using electrochemical techniques. Corrosion 1996, 52, 934–946. [Google Scholar] [CrossRef]
- Matsumura, M. Erosion-corrosion of metallic materials in slurries. Corros. Rev. 1994, 12, 321–340. [Google Scholar] [CrossRef]
- Neville, A.; Hodgkiess, T.; Xu, H. An electrochemical and microstructural assessment of erosion–corrosion of cast iron. Wear 1999, 233, 523–534. [Google Scholar] [CrossRef]
- Mathew, M.T.; Srinivasa Pai, P.; Pourzal, R.; Fischer, A.; Wimmer, M.A. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Adv. Tribol. 2009, 2009, 250986. [Google Scholar] [CrossRef]
- Skjöldebrand, C.; Tipper, J.L.; Hatto, P.; Bryant, M.; Hall, R.M.; Persson, C. Current status and future potential of wear-resistant coatings and articulating surfaces for hip and knee implants. Mater. Today Bio 2022, 15, 100270. [Google Scholar] [CrossRef] [PubMed]
- Puthillam, U.; Selvam, R.E. Tribocorrosion in biomaterials and control techniques: A review. Corros. Rev. 2023. [Google Scholar] [CrossRef]
- Wood, R.J.K. Marine wear and tribocorrosion. Wear 2017, 376–377, 893–910. [Google Scholar] [CrossRef]
- Wood, R.J.K. Erosion–corrosion interactions and their effect on marine and offshore materials. Wear 2006, 261, 1012–1023. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Zucchi, F. Corrosion and tribocorrosion behaviour of thermally sprayed ceramic coatings on steel. Surf. Coat. Technol. 2011, 205, 3683–3691. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Zucchi, F. Corrosion and tribocorrosion behaviour of cermet and cermet/nanoscale multilayer CrN/NbN coatings. Surf. Coat. Technol. 2010, 204, 1452–1460. [Google Scholar] [CrossRef]
- Ge, Y.; Cheng, J.; Yan, C.; Xue, L.; Zhang, B.; Liang, X. Devitrification and sliding wear behaviors of AlFeSi metallic glass coatings. J. Mater. Res. Technol. 2021, 15, 7022–7032. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Wan, Y.; Pu, J. Corrosion and tribocorrosion behavior of W doped DLC coating in artificial seawater. Diam. Relat. Mater. 2020, 109, 108019. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, J.; Liao, B.; Zhang, X.; Zhao, Y.; Zeng, X.; Chen, L.; Pang, P.; Bao, F. Tribocorrosion and tribological behavior of Ti-DLC coatings deposited by filtered cathodic vacuum arc. Diam. Relat. Mater. 2022, 125, 108985. [Google Scholar] [CrossRef]
- Yasir, M.; Zhang, C.; Wang, W.; Xu, P.; Liu, L. Wear behaviors of Fe-based amorphous composite coatings reinforced by Al2O3 particles in air and in NaCl solution. Mater. Des. 2015, 88, 207–213. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Sheveyko, A.N.; Manakova, O.S.; Sidorenko, D.A.; Shtansky, D.V. Comparative investigation of single-layer and multilayer Nb-doped TiC coatings deposited by pulsed vacuum deposition techniques. Surf. Coat. Technol. 2020, 385, 125422. [Google Scholar] [CrossRef]
- Hu, K.; Liu, X.; Zhang, S.; Xue, Z.; Yang, Y.; Yang, K. Tribocorrosion behavior of HVOF sprayed WC-based cermet coatings in sodium chloride solution environment in relation to binder phases. Surf. Coat. Technol. 2022, 435, 128248. [Google Scholar] [CrossRef]
- Pileggi, R.; Tului, M.; Stocchi, D.; Lionetti, S. Tribo-corrosion behaviour of chromium carbide based coatings deposited by HVOF. Surf. Coat. Technol. 2015, 268, 247–251. [Google Scholar] [CrossRef]
- Rodrigues, I.; Nunes, B.; Almeida, A.; Vilar, R.; Figueiredo-Pina, C.G. Are Cr3C2-25(80Ni20Cr) coatings deposited by HVOF suitable for reciprocating sliding contacts under aqueous media? Tribol. Int. 2020, 149, 105825. [Google Scholar] [CrossRef]
- Caha, I.; Alves, A.; Affonco, L.; Lisboa, P.; da Silva, J.; Rocha, L.; Pinto, A.; Toptan, F. Corrosion and tribocorrosion behaviour of titanium nitride thin films grown on titanium under different deposition times. Surf. Coat. Technol. 2019, 374, 878–888. [Google Scholar] [CrossRef]
- Merl, D.; Panjan, P.; Cekada, M.; Gselman, P.; Paskvale, S. Tribocorrosion degradation of protective coatings on stainless steel. Mater. Tehnol. 2013, 47, 435–439. [Google Scholar]
- Cui, W.; Niu, F.; Tan, Y.; Qin, G. Microstructure and tribocorrosion performance of nanocrystalline TiN graded coating on biomedical titanium alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1026–1035. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.; Liu, Z. Bio-tribocorrosion behavior of a nanocrystalline TiZrN coating on biomedical titanium alloy. Surf. Coat. Technol. 2019, 369, 79–86. [Google Scholar] [CrossRef]
- Sáenz de Viteri, V.; Barandika, G.; Bayón, R.; Fernández, X.; Ciarsolo, I.; Igartua, A.; Pérez Tanoira, R.; Moreno, J.E.; Peremarch, C.P.-J. Development of Ti–C–N coatings with improved tribological behavior and antibacterial properties. J. Mech. Behav. Biomed. Mater. 2016, 55, 75–86. [Google Scholar] [CrossRef]
- Tijerina-Gonzalez, J.L.; Hernandez-Rodriguez, M.A.L.; Lozano, D.E.; Martinez-Cazares, G.M.; Bedolla-Gil, Y. Tribological Characterization of AlCrN, AlTiN, AlTiON, and AlCrON Coatings on CoCrMo Alloy. Tribol. Trans. 2021, 64, 119–125. [Google Scholar] [CrossRef]
- Pana, I.; Vladescu, A.; Constantin, L.; Sandu, I.; Dinu, M.; Cotrut, C. In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings. Coatings 2020, 10, 654. [Google Scholar] [CrossRef]
- de Oliveira, S.M.M.; da Silva, N.S.; Sene, A.; Gandra, R.F.; Junges, D.S.B.; Ramos, M.A.R.; Vieira, L. Comparative Study of Candida albicans Inactivation by Nonthermal Plasma on Stainless Steel with and without Diamond-like Carbon Film. ACS Omega 2019, 4, 6891–6902. [Google Scholar] [CrossRef]
- Bayon, R.; Igartua, A.; Gonzalez, J.; de Gopegui, U. Influence of the carbon content on the corrosion and tribocorrosion performance of Ti-DLC coatings for biomedical alloys. Tribol. Int. 2015, 88, 115–125. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wu, B.; Zhang, T.; Leng, Y.; Huang, N. Tribocorrosion behavior of DLC-coated CoCrMo alloy in simulated biological environment. Vacuum 2013, 92, 39–43. [Google Scholar] [CrossRef]
- Azzi, M.; Paquette, M.; Szpunar, J.; Klemberg-Sapieha, J.; Martinu, L. Tribocorrosion behaviour of DLC-coated 316L stainless steel. Wear 2009, 267, 860–866. [Google Scholar] [CrossRef]
- Oliveira, S.M.M.; Barzotto, I.L.M.; Vieira, L.; Sene, A.; Radi, P.A.; Fraga, S.; Bessa, M.J.; Teixeira, J.P.; Carvalho, I.C.S.; da Silva, N.S. Tribocorrosion studies on diamond-like carbon film deposited by PECVD on 304 stainless steel in simulated body fluid. Biomed. Phys. Eng. Express 2019, 5, 045012. [Google Scholar] [CrossRef]
- Gracia-Escosa, E.; Garcia, I.; Sanchez-Lopez, J.; Abad, M.; Mariscal, A.; Arenas, M.; de Damborenea, J.; Conde, A. Tribocorrosion behavior of TiBxCy/a-C nanocomposite coating in strong oxidant disinfectant solutions. Surf. Coat. Technol. 2015, 263, 78–85. [Google Scholar] [CrossRef]
- Wu, J.; Wu, G.; Kou, X.; Lu, Z.; Zhang, G.; Wu, Z. Probing Tribological Behaviors of Cr-DLC in Corrosion Solution by Tailoring Sliding Interface. Tribol. Lett. 2020, 68, 95. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, J.; Liao, B.; Chen, L.; Zhang, X.; Zhao, Y.; Pang, P.; Zeng, X. Enhanced Anti-Tribocorrosion Performance of Ti-DLC Coatings Deposited by Filtered Cathodic Vacuum Arc with the Optimization of Bias Voltage. Coatings 2022, 12, 697. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Y.; Xu, X.; Li, Z.; Su, F. Synthesis of Nitrogen-Doped Diamond-Like Carbon Films Produced by Plasma-Enhanced Chemical Vapor Deposition and their Tribocorrosion Behavior in Hanks’ Solution. J. Mater. Eng. Perform. 2022, 31, 8334–8345. [Google Scholar] [CrossRef]
- Cui, M.; Pu, J.; Liang, J.; Wang, L.; Zhang, G.; Xue, Q. Corrosion and tribocorrosion performance of multilayer diamond-like carbon film in NaCl solution. RSC Adv. 2015, 5, 104829–104840. [Google Scholar] [CrossRef]
- Pu, J.; Wang, J.; He, D.; Wan, S. Corrosion and tribocorrosion behaviour of super-thick diamond-like carbon films deposited on stainless steel in NaCl solution. Surf. Interface Anal. 2016, 48, 360–367. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Wang, C.; Yuen, M.-F.; Wang, M.; Qian, T.; Matsumoto, M.; Yan, J. Comparison of tribological and electrochemical properties of TiN, CrN, TiAlN and a-C:H coatings in simulated body fluid. Mater. Chem. Phys. 2015, 158, 74–81. [Google Scholar] [CrossRef]
- Lou, B.-S.; Rahmadtulloh, I.; Wang, C.-J.; Wang, W.-H.; Lee, J.-W. Tribocorrosion behaviors of VNbMoTaWCr high entropy alloy coatings. Surf. Coat. Technol. 2023, 476, 130250. [Google Scholar] [CrossRef]
- Tasdemir, M.; Senaslan, F.; Celik, A. Wear, Corrosion and Tribocorrosion Behavior of Polyurethane and Polyvinylpyrrolidone Blends as Coating for Corrosion Protection of AISI 316L Stainless Steel. Int. J. Electrochem. Sci. 2021, 16, 210510. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, X.; Cai, M.; Yan, H.; Zhao, Z.; Fan, X.; Zhu, M. Enhancement of Si3N4@MoS2 core–shell structure on wear/corrosion resistance of epoxy resin/polyacrylate IPN composite coating. Appl. Surf. Sci. 2021, 568, 150938. [Google Scholar] [CrossRef]
- Lajevardi, S.A.; Shahrabi, T.; Szpunar, J.A. Tribological Properties of Functionally Graded Ni-Al2O3 Nanocomposite Coating. J. Electrochem. Soc. 2017, 164, D275–D281. [Google Scholar] [CrossRef]
- Offoiach, R.; Lekka, M.; Lanzutti, A.; Martínez-Nogués, V.; Vega, J.M.; García-Lecina, E.; Fedrizzi, L. Tribocorrosion study of Ni/B electrodeposits with low B content. Surf. Coat. Technol. 2019, 369, 1–15. [Google Scholar] [CrossRef]
- Lee, H.B. Synergy Between Corrosion and Wear of Electrodeposited Ni–W Coating. Tribol. Lett. 2013, 50, 407–419. [Google Scholar] [CrossRef]
- Hassani, S.; Raeissi, K.; Azzi, M.; Li, D.; Golozar, M.; Szpunar, J. Improving the corrosion and tribocorrosion resistance of Ni-Co nanocrystalline coatings in NaOH solution. Corros. Sci. 2009, 51, 2371–2379. [Google Scholar] [CrossRef]
- Malfatti, C.; Veit, H.; Santos, C.; Metzner, M.; Hololeczek, H.; Bonino, J. Heat Treated NiP-SiC Composite Coatings: Elaboration and Tribocorrosion Behaviour in NaCl Solution. Tribol. Lett. 2009, 36, 165–173. [Google Scholar] [CrossRef][Green Version]
- Benea, L. Electrodeposition and tribocorrosion behaviour of ZrO2-Ni composite coatings. J. Appl. Electrochem. 2009, 39, 1671–1681. [Google Scholar] [CrossRef]
- Chun-Ying, L.; Wei-Ti, M.; Ming-Der, G.; Hung-Bin, L. A study on the corrosion and wear behavior of nanocrystalline Ni-Mo electrodeposited coatings. Surf. Coat. Technol. 2019, 366, 286–295. [Google Scholar] [CrossRef]
- Benea, L.; Basa, S.; Danaila, E.; Caron, N.; Raquet, O.; Ponthiaux, P.; Celis, J. Fretting and wear behaviors of Ni/nano-WC composite coatings in dry and wet conditions. Mater. Des. 2015, 65, 550–558. [Google Scholar] [CrossRef]
- Fathollahzade, N.; Raeissi, K. A tribocorrosion study of cobalt–tungsten electrodeposited coating with a mixed amorphous/nanocrystalline structure. Int. J. Surf. Eng. Coat. 2016, 94, 328–335. [Google Scholar] [CrossRef]
- Rajabi, M.; Tahmasebi, K.; Ehteshamzadeh, M.; Soroushian, S. Tribological and magnetic behaviour of Ni-P coating modified with NiO nanoparticles. Tribol. Mater. Surf. Interfaces 2021, 15, 243–251. [Google Scholar] [CrossRef]
- Salicio-Paz, A.; Dalmau, A.; Grande, H.; Iriarte, A.; Sort, J.; Pellicer, E.; Fornell, J.; García-Lecina, E. Impact of the multilayer approach on the tribocorrosion behaviour of nanocrystalline electroless nickel coatings obtained by different plating modes. Wear 2020, 456–457, 203384. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Effect of temperature on ultrasound-assisted electroless nickel-boron plating. Ultrason. Sonochem. 2019, 56, 327–336. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Raeissi, K.; Saatchi, A.; Kazmanlı, K.; Ürgen, M. Effect of heat treatment on tribocorrosion of nanostructure Ni–P coatings. Surf. Eng. 2013, 29, 671–676. [Google Scholar] [CrossRef]
- Wang, J.; Bian, D.; Liu, Y.; Zhao, Y.; Tang, H. Influence of aluminum phosphate on the tribocorrosion performance of chemically bonded phosphate ceramic coatings. Mater. Corros. Werkst. Korros. 2021, 72, 1677–1686. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Qi, J.; Chen, F. Corrosion protection of Ti6Al4V by a composite coating with a plasma electrolytic oxidation layer and sol-gel layer filled with graphene oxide. Prog. Org. Coat. 2020, 144, 105632. [Google Scholar] [CrossRef]
- Da, B.; Yaxuan, L.; Vasu, A.T.; Yongxin, G.; Hao, T.; Yongwu, Z.; Yongguang, W. Improving tribocorrosion performance of chemically bonded ceramic phosphate coating reinforced by GO-ZnO. Ceram. Int. 2021, 47, 15722–15731. [Google Scholar] [CrossRef]
- Ripoll, M.R.; Torres, H. Chapter 6–Tribocorrosion of hard coatings and thin films. In Tribocorrosion; Siddaiah, A., Ramachandran, R., Menezes, P.L., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 127–171. ISBN 978-0-12-818916-0. [Google Scholar]
- Łapaj, Ł.; Wendland, J.; Markuszewski, J.; Mróz, A.; Wiśniewski, T. Retrieval analysis of titanium nitride (TiN) coated prosthetic femoral heads articulating with polyethylene. J. Mech. Behav. Biomed. Mater. 2016, 55, 127–139. [Google Scholar] [CrossRef]
- Pougoum, F.; Qian, J.; Martinu, L.; Klemberg-Sapieha, J.; Zhou, Z.; Li, K.Y.; Savoie, S.; Lacasse, R.; Potvin, E.; Schulz, R. Study of corrosion and tribocorrosion of Fe3Al-based duplex PVD/HVOF coatings against alumina in NaCl solution. Surf. Coat. Technol. 2019, 357, 774–783. [Google Scholar] [CrossRef]
- Kovacı, H.; Bozkurt, Y.B.; Yetim, A.F.; Baran, Ö.; Çelik, A. Corrosion and tribocorrosion properties of duplex surface treatments consisting of plasma nitriding and DLC coating. Tribol. Int. 2021, 156, 106823. [Google Scholar] [CrossRef]
- Bayon, R.; Nevshupa, R.; Zubizarreta, C.; de Gopegui, U.; Barriga, J.; Igartua, A. Characterisation of tribocorrosion behaviour of multilayer PVD coatings. Anal. Bioanal. Chem. 2010, 396, 2855–2862. [Google Scholar] [CrossRef]
- Bai, W.Q.; Li, L.L.; Xie, Y.J.; Liu, D.G.; Wang, X.L.; Jin, G.; Tu, J.P. Corrosion and tribocorrosion performance of M (M Ta, Ti) doped amorphous carbon multilayers in Hank’s solution. Surf. Coat. Technol. 2016, 305, 11–22. [Google Scholar] [CrossRef]
- Çomaklı, O. Improved structural, mechanical, corrosion and tribocorrosion properties of Ti45Nb alloys by TiN, TiAlN monolayers, and TiAlN/TiN multilayer ceramic films. Ceram. Int. 2021, 47, 4149–4156. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.C.; Affonço, L.J.; da Silva, J.H.D.; Rodrigues, I.R.; Grandini, C.R.; Rocha, L.A.; Pinto, A.M.P.; Lisboa-Filho, P.N.; Toptan, F. Degradation behaviour of Ti-12Nb alloy coated with ZnO/TiN double layer. Surf. Coat. Technol. 2021, 413, 127104. [Google Scholar] [CrossRef]
- Sun, Y.; Dearnley, P.A.; Mallia, B. Response of duplex Cr(N)/S and Cr(C)/S coatings on 316L stainless steel to tribocorrosion in 0.89% NaCl solution under plastic contact conditions: Response of Duplex Cr(N)/S and Cr(C)/S Coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1503–1513. [Google Scholar] [CrossRef]
- Mazzonello, A.; Buhagiar, J.; Chetcuti, R.; Dearnley, P.; Valsesia, A.; Colpo, P.; Mallia, B. A tribocorrosion appraisal of a dual layer PVD coated CoCrMo alloy tribopair. Surf. Coat. Technol. 2022, 442, 128341. [Google Scholar] [CrossRef]
- Chetcuti, R.; Dearnley, P.; Mazzonello, A.; Buhagiar, J.; Mallia, B. Tribocorrosion response of duplex layered CoCrMoC/CrN and CrN/CoCrMoC coatings on implant grade 316LVM stainless steel. Surf. Coat. Technol. 2020, 384, 125313. [Google Scholar] [CrossRef]
- Naghibi, S.; Raeissi, K.; Fathi, M. Corrosion and tribocorrosion behavior of Ti/TiN PVD coating on 316L stainless steel substrate in Ringer’s solution. Mater. Chem. Phys. 2014, 148, 614–623. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Yuan, Z.; Li, J. Structure and tribocorrosion behavior of Ti/TiN multilayer coatings in simulated body fluid by arc ion plating. Surf. Coat. Technol. 2020, 403, 126399. [Google Scholar] [CrossRef]
- Beliardouh, N.; Ramoul, C.; Nouveau, C.; Kaleli, E.; Montagne, A. Synthesis and tribocorrosion performances of multilayer (Ta/ZrN) n coatings. Thin Solid Film. 2022, 749, 139184. [Google Scholar] [CrossRef]
- de Frutos, A.; Arenas, M.; Fuentes, G.; Rodriguez, R.; Martinez, R.; Avelar-Batista, J.; de Damborenea, J. Tribocorrosion behaviour of duplex surface treated AISI 304 stainless steel. Surf. Coat. Technol. 2010, 204, 1623–1630. [Google Scholar] [CrossRef]
- Zhao, G.-H.; Aune, R.E.; Espallargas, N. Tribocorrosion studies of metallic biomaterials: The effect of plasma nitriding and DLC surface modifications. J. Mech. Behav. Biomed. Mater. 2016, 63, 100–114. [Google Scholar] [CrossRef]
- Uzun, Y. Tribocorrosion properties of plasma nitrided, Ti-DLC coated and duplex surface treated AISI 316L stainless steel. Surf. Coat. Technol. 2022, 441, 128587. [Google Scholar] [CrossRef]
- Grabarczyk, J.; Gaj, J.; Pazik, B.; Kaczorowski, W.; Januszewicz, B. Tribocorrosion behavior of Ti6Al4V alloy after thermo-chemical treatment and DLC deposition for biomedical applications. Tribol. Int. 2021, 153, 106560. [Google Scholar] [CrossRef]
- Lopez-Ortega, A.; de Viteri, V.; Alves, S.; Mendoza, G.; Fuentes, E.; Mitran, V.; Cimpean, A.; Dan, I.; Vela, A.; Bayon, R. Multifunctional TiO2 coatings developed by plasma electrolytic oxidation technique on a Ti20Nb20Zr4Ta alloy for dental applications. Biomater. Adv. 2022, 138, 212875. [Google Scholar] [CrossRef]
- Rahmatian, B.; Ghasemi, H.M.; Sohi, M.H.; De Baets, P. Insight into tribocorrosion resistance and tribofilm formation on titanium boride coatings in a phosphate buffer saline solution. J. Mater. Res. Technol. 2023, 27, 6847–6862. [Google Scholar] [CrossRef]
- Zhai, H.; Cui, S.; Li, S.; He, D.; Cheng, B.; Zhang, X.; Li, W.; Viktor, Z.; Seniuts, U. Tribocorrosion behaviors of Ti-based bulk metallic glass via laser shock peening in 3.5 wt % NaCl solutions. Intermetallics 2024, 164, 108127. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, D.; Su, H.; Yu, P.; Wan, Y.; Sun, H. Improving the tribocorrosion performance of plasma electrolytic oxidized coatings on AZ31B magnesium alloy using pullulan as an electrolyte additive. Surf. Coat. Technol. 2022, 446, 128754. [Google Scholar] [CrossRef]
- Xu, L.; Fu, X.; Su, H.; Sun, H.; Li, R.; Wan, Y. Corrosion and tribocorrosion protection of AZ31B Mg alloy by a hydrothermally treated PEO/chitosan composite coating. Prog. Org. Coat. 2022, 170, 107002. [Google Scholar] [CrossRef]
- Sourani, F.; Raeissi, K.; Enayati, M.; Kharaziha, M.; Hakimizad, A.; Blugan, G.; Salimijazi, H. Corrosion and tribocorrosion behavior of ZrO2-Al2O3 composite coatings developed by plasma electrolytic oxidation for load-bearing implants. J. Alloys Compd. 2022, 920, 165856. [Google Scholar] [CrossRef]
- Daavari, M.; Atapour, M.; Mohedano, M.; Arrabal, R.; Matykina, E.; Taherizadeh, A. Biotribology and biocorrosion of MWCNTs-reinforced PEO coating on AZ31B Mg alloy. Surf. Interfaces 2021, 22, 100850. [Google Scholar] [CrossRef]
- Yang, J.; Gu, Y.; Zhou, X.; Zhang, Y. Tribocorrosion behavior and mechanism of micro-arc oxidation Ca/P coating on nanocrystallized magnesium alloys. Mater. Corros. Werkst. Korros. 2018, 69, 749–759. [Google Scholar] [CrossRef]
- Xu, L.; Liu, N.; Cao, L.; Wan, Y. Influences of electrolytes on tribocorrosion performance of MAO coating on AZ31B magnesium alloy in simulated body fluid. Int. J. Appl. Ceram. Technol. 2021, 18, 1657–1669. [Google Scholar] [CrossRef]
- Dalmau, A.; Buch, A.R.; Rovira, A.; Navarro-Laboulais, J.; Muñoz, A.I. Wear model for describing the time dependence of the material degradation mechanisms of the AISI 316L in a NaCl solution. Wear 2018, 394–395, 166–175. [Google Scholar] [CrossRef]
- Papageorgiou, N.; von Bonin, A.; Espallargas, N. Tribocorrosion mechanisms of NiCrMo-625 alloy: An electrochemical modeling approach. Tribol. Int. 2014, 73, 177–186. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Yue, X.; Cai, W. Multiphysics modeling and uncertainty quantification of tribocorrosion in aluminum alloys. Corros. Sci. 2021, 178, 109095. [Google Scholar] [CrossRef]
- Fallahnezhad, K.; Feyzi, M.; Ghadirinejad, K.; Hashemi, R.; Taylor, M. Finite element based simulation of tribocorrosion at the head-neck junction of hip implants. Tribol. Int. 2022, 165, 107284. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Mischler, S. Electrochemical Simulation of the Current and Potential Response in Sliding Tribocorrosion. Tribol. Lett. 2012, 48, 271–283. [Google Scholar] [CrossRef]
- Guadalupe, S.; Cao, S.; Cantoni, M.; Chitty, W.-J.; Falcand, C.; Mischler, S. Applicability of a recently proposed tribocorrosion model to CoCr alloys with different carbides content. Wear 2017, 376–377, 203–211. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Mali, S.A.; Liu, Y. Area-dependent impedance-based voltage shifts during tribocorrosion of Ti-6Al-4V biomaterials: Theory and experiment. Surf. Topogr. Metrol. Prop. 2016, 4, 034002. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Zhu, D. A metallic biomaterial tribocorrosion model linking fretting mechanics, currents, and potentials: Model development and experimental comparison. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3174–3189. [Google Scholar] [CrossRef]
- Dalbert, V.; Mary, N.; Normand, B.; Verdu, C.; Saedlou, S. In situ determinations of the wear surfaces, volumes and kinetics of repassivation: Contribution in the understanding of the tribocorrosion behaviour of a ferritic stainless steel in various pH. Tribol. Int. 2020, 150, 106374. [Google Scholar] [CrossRef]
- Fallahnezhad, K.; Feyzi, M.; Hashemi, R.; Taylor, M. The Role of the Assembly Force in the Tribocorrosion Behaviour of Hip Implant Head-Neck Junctions: An Adaptive Finite Element Approach. Bioengineering 2022, 9, 629. [Google Scholar] [CrossRef]
- Cao, S.; Maldonado, S.; Mischler, S. Tribocorrosion of passive metals in the mixed lubrication regime: Theoretical model and application to metal-on-metal artificial hip joints. Wear 2015, 324, 55–63. [Google Scholar] [CrossRef]
- Miyabe, S.; Fujii, N.; Fujimoto, S. Numerical Simulation of Tribocorrosion of CoCr Alloy and Ti with Galvanic Coupling in Simulated Body Fluid. Mater. Trans. 2021, 62, 1489–1494. [Google Scholar] [CrossRef]
- von der Ohe, C.B.; Johnsen, R.; Espallargas, N. Modeling the multi-degradation mechanisms of combined tribocorrosion interacting with static and cyclic loaded surfaces of passive metals exposed to seawater. Wear 2010, 269, 607–616. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Zhang, Y.; Zhu, Y.; Liu, F.; Wang, T. A Fully Coupled Tribocorrosion Simulation Method for Anchor Chain Considering Mechano-Electrochemical Interaction. Lubricants 2022, 10, 330. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Zhang, Z.; Chen, J.; Mou, T.; Michel, F.; Xin, H.; Cai, W. Ultrahigh tribocorrosion resistance of metals enabled by nano-layering. ACTA Mater. 2021, 206, 116609. [Google Scholar] [CrossRef]
- Olsson, C.-O.A.; Munoz, A.N.I.; Cao, S.; Mischler, S. Modeling Current Transients in a Reciprocal Motion Tribocorrosion Experiment. J. Electrochem. Soc. 2021, 168, 031503. [Google Scholar] [CrossRef]
- Mary, N.; Ter-Ovanessian, B.; Normand, B. Growth mechanism and repassivation kinetic determinations on stainless steel under sliding: Role of the solution pH and dissolved oxygen concentration. Wear 2020, 460–461, 203478. [Google Scholar] [CrossRef]
- Ghanbarzadeh, A.; Salehi, F.M.; Bryant, M.; Neville, A. A New Asperity-Scale Mechanistic Model of Tribocorrosive Wear: Synergistic Effects of Mechanical Wear and Corrosion. J. Tribol. 2019, 141, 021601. [Google Scholar] [CrossRef]
- Stachowiak, A.; Zwierzycki, W. Analysis of the tribocorrosion mechanisms in a pin-on-plate combination on the example of AISI304 steel. Wear 2012, 294, 277–285. [Google Scholar] [CrossRef]
- Stachowiak, A.; Tyczewski, P.; Zwierzycki, W. The application of wear maps for analyzing the results of research into tribocorrosion. Wear 2016, 352–353, 146–154. [Google Scholar] [CrossRef]
- Ramachandran, R. Machine learning model to cluster and map tribocorrosion regimes in feature space. arXiv 2020, arXiv:2006.06252. [Google Scholar] [CrossRef]
- Ramachandran, R. Machine Learning Model to Map Tribocorrosion Regimes in Feature Space. Coatings 2021, 11, 450. [Google Scholar] [CrossRef]
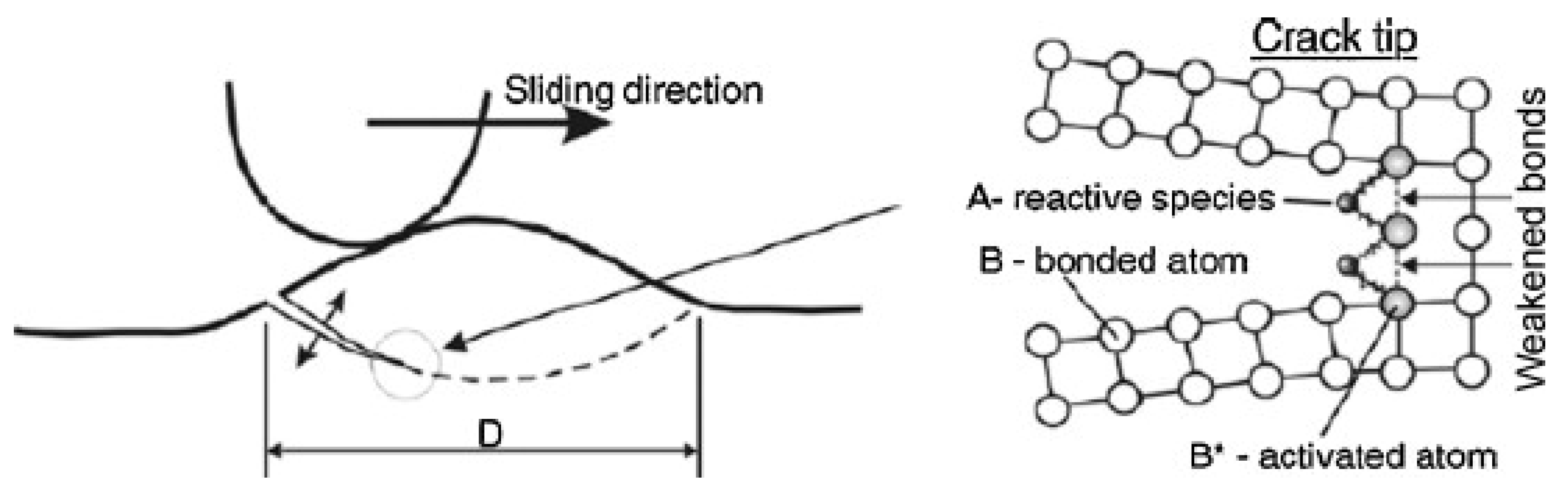
- Jiang, J.; Stack, M.M.; Neville, A. Modelling the tribo-corrosion interaction in aqueous sliding conditions. Tribol. Int. 2002, 35, 669–679. [Google Scholar] [CrossRef]
- Maldonado, S.G.; Mischler, S.; Cantoni, M.; Chitty, W.J.; Falcand, C.; Hertz, D. Mechanical and chemical mechanisms in the tribocorrosion of a Stellite type alloy. Wear 2013, 308, 213–221. [Google Scholar] [CrossRef]
- Dowson, D. Tribological principles in metal-on-metal hip joint design. Proc. Inst. Mech.Eng. Part H J. Eng. Med. 2006, 220, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Soltanahmadi, S.; Morina, A.; van Eijk, M.C.; Nedelcu, I.; Neville, A. Tribochemical study of micropitting in tribocorrosive lubricated contacts: The influence of water and relative humidity. Tribol. Int. 2017, 107, 184–198. [Google Scholar] [CrossRef]
- Wang, W.; Mraied, H.; Diyatmika, W.; Chu, J.; Li, L.; Cai, W. Effects of nanoscale chemical heterogeneity on the wear, corrosion, and tribocorrosion resistance of Zr-based thin film metallic glasses. Surf. Coat. Technol. 2020, 402, 126324. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Z.; Ma, Y.; Yan, Y.; Qiao, L. Role of gradient nano-structured surface in collapsed pitting corrosion on AISI 316L stainless steel during tribocorrosion. Corros. Sci. 2022, 197, 110043. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, E.; Du, S.; Zhang, J.; Wang, L.; Du, H.; Cai, H. Tribocorrosion Behavior of Typical Austenitic, Martensitic, and Ferritic Stainless Steels in 3.5% NaCl Solution. J. Mater. Eng. Perform. 2021, 30, 6284–6296. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Z.; Ma, Y. Tribocorrosion Behavior and Degradation Mechanism of 316L Stainless Steel in Typical Corrosive Media. Acta Metall. Sin. Engl. Lett. 2021, 34, 813–824. [Google Scholar] [CrossRef]
- Sousa, J.M.; Alves, A.C.; Toptan, F.; Ariza, E.; Guedes, A. Corrosion and Tribocorrosion Behavior of Ti-B4C Composites Joined with TiCuNi Brazing Alloy. J. Mater. Eng. Perform. 2019, 28, 4972–4982. [Google Scholar] [CrossRef]
- Mraied, H.; Cai, W. The effects of Mn concentration on the tribocorrosion resistance of Al–Mn alloys. Wear 2017, 380–381, 191–202. [Google Scholar] [CrossRef]
- Obadele, B.A.; Andrews, A.; Shongwe, M.B.; Olubambi, P.A. Tribocorrosion behaviours of AISI 310 and AISI 316 austenitic stainless steels in 3.5% NaCl solution. Mater. Chem. Phys. 2016, 171, 239–246. [Google Scholar] [CrossRef]
- Sun, Y.; Rana, V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5M NaCl solution. Mater. Chem. Phys. 2011, 129, 138–147. [Google Scholar] [CrossRef]
- Kossman, S.; Coelho, L.B.; Mejias, A.; Montagne, A.; Van Gorp, A.; Coorevits, T.; Touzin, M.; Poorteman, M.; Olivier, M.-G.; Iost, A.; et al. Impact of industrially applied surface finishing processes on tribocorrosion performance of 316L stainless steel. Wear 2020, 456–457, 203341. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.; Yan, Y.; Wang, J.; Yan, F. Tribocorrosion behaviors of 304SS: Effect of solution pH. RSC Adv. 2015, 5, 17676–17682. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Z.; Ma, Y.; Tan, L. Tribocorrosion Behavior and Degradation Mechanism of 316L Stainless Steel in Alkaline Solution: Effect of Tribo-Film. ACTA Metall. Sin. Engl. Lett. 2022, 35, 1365–1375. [Google Scholar] [CrossRef]
- Xin, L.; Jiang, L. Effects of Normal Force on the Tribocorrosion Behavior of a Nickel-Based Superalloy in Alkaline Solution: An Electrochemical Study. Materials 2020, 13, 3959. [Google Scholar] [CrossRef]
- Du, J.; Cao, S.; Igual Munoz, A.; Mischler, S. Tribological and tribocorrosion behavior of nickel sliding against oxide ceramics. Wear 2019, 426–427, 1496–1506. [Google Scholar] [CrossRef]
- Espallargas, N.; Mischler, S. Tribocorrosion behaviour of overlay welded Ni-Cr 625 alloy in sulphuric and nitric acids: Electrochemical and chemical effects. Tribol. Int. 2010, 43, 1209–1217. [Google Scholar] [CrossRef]
- Balla, V.K.; Das, M. Advances in Wear and Tribocorrosion Testing of Artificial Implants and Materials: A Review. Trends Biomater. Artif. Organs 2017, 31, 150. [Google Scholar]
- Zheng, Y.; Bashandeh, K.; Shakil, A.; Jha, S.; Polycarpou, A.A. Review of dental tribology: Current status and challenges. Tribol. Int. 2022, 166, 107354. [Google Scholar] [CrossRef]
- Zindani, D.; Kumar, K.; Paulo Davim, J. Metallic biomaterials—A review. In Mechanical Behaviour of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–99. ISBN 978-0-08-102174-3. [Google Scholar]
- Siddaiah, A. Understanding the Tribocorrosion Behavior of Engineered Surfaces. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2020; 163p. [Google Scholar]
- Ferreira, D.; Almeida, S.; Soares, R.; Juliani, L.; Bracarense, A.; Lins, V.; Junqueira, R. Synergism between mechanical wear and corrosion on tribocorrosion of a titanium alloy in a Ringer solution. J. Mater. Res. Technol. 2019, 8, 1593–1600. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Sun, Y.; Yuan, J.C.-C.; Mathew, M.T.; Sukotjo, C.; Takoudis, C.G. In vitro corrosion behavior of coated Ti6Al4V with TiO2, ZrO2, and TiO2/ZrO2 mixed nanofilms using atomic layer deposition for dental implants. Surf. Coat. Technol. 2022, 444, 128686. [Google Scholar] [CrossRef]
- Alves, S.A.; Patel, S.B.; Sukotjo, C.; Mathew, M.T.; Filho, P.N.; Celis, J.-P.; Rocha, L.A.; Shokuhfar, T. Synthesis of calcium-phosphorous doped TiO2 nanotubes by anodization and reverse polarization: A promising strategy for an efficient biofunctional implant surface. Appl. Surf. Sci. 2017, 399, 682–701. [Google Scholar] [CrossRef]
- Tobin, E.J. Recent coating developments for combination devices in orthopedic and dental applications: A literature review. Adv. Drug Deliv. Rev. 2017, 112, 88–100. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Gemini-Piperni, S.; Travassos, R.; Lemgruber, L.; Silva, R.C.; Rossi, A.L.; Farina, M.; Anselme, K.; Shokuhfar, T.; Shahbazian-Yassar, R.; et al. Trojan-Like Internalization of Anatase Titanium Dioxide Nanoparticles by Human Osteoblast Cells. Sci. Rep. 2016, 6, 23615. [Google Scholar] [CrossRef]
- Yang, R.; Hao, Y.; Li, S. Development and application of low-modulus biomedical titanium alloy Ti2448. Biomed. Eng. Trends 2011, 10, 225–247. [Google Scholar]
- Mastnak, T.; Maver, U.; Finšgar, M. Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 2786. [Google Scholar] [CrossRef]
- Brooks, E.K.; Brooks, R.P.; Ehrensberger, M.T. Effects of simulated inflammation on the corrosion of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 200–205. [Google Scholar] [CrossRef]
- Rafiq, N.M.; Wang, W.; Liew, S.L.; Chua, C.S.; Wang, S. A review on multifunctional bioceramic coatings in hip implants for osteointegration enhancement. Appl. Surf. Sci. Adv. 2023, 13, 100353. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 11. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Zhang, G.; Hang, R.; Huang, X.; Tang, B.; Zhang, X. Electrochemical corrosion, wear and cell behavior of ZrO2/TiO2 alloyed layer on Ti-6Al-4V. Bioelectrochemistry 2018, 121, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Alhamad, M.; Barão, V.A.R.; Sukotjo, C.; Cooper, L.F.; Mathew, M.T. Ti-Ions and/or Particles in Saliva Potentially Aggravate Dental Implant Corrosion. Materials 2021, 14, 5733. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, M.; Vijayalakshmi, U. Improved corrosion resistant and mechanical behavior of distinct composite coatings (silica/titania/zirconia) on Ti–6Al–4V deposited by EPD. J. Asian Ceram. Soc. 2017, 5, 326–333. [Google Scholar] [CrossRef][Green Version]
- Romanos, G.; Fischer, G.; Delgado-Ruiz, R. Titanium Wear of Dental Implants from Placement, under Loading and Maintenance Protocols. Int. J. Mol. Sci. 2021, 22, 1067. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; Resende, C.R.S.; Roestel, J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. Dent. Mater. 2015, 31, e1–e13. [Google Scholar] [CrossRef]
- Ji, X.; Luo, C.; Jin, J.; Zhang, Y.; Sun, Y.; Fu, L. Tribocorrosion performance of 316L stainless steel enhanced by laser clad 2-layer coating using Fe-based amorphous powder. J. Mater. Res. Technol. 2022, 17, 612–621. [Google Scholar] [CrossRef]
- Ji, X.; Luo, C.; Sun, Y.; Zhao, J. Corrosive wear of multi-layer Fe-based coatings laser cladded from amorphous powders. Wear 2019, 438–439, 203113. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Ji, C.; Zhang, Y.; Wang, H. Tribocorrosion of Fe-Based Amorphous Coating in Simulated Body Fluids. Lubricants 2018, 6, 37. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef]
- Costa, R.C.; Abdo, V.L.; Mendes, P.H.C.; Mota-Veloso, I.; Bertolini, M.; Mathew, M.T.; Barão, V.A.R.; Souza, J.G.S. Microbial Corrosion in Titanium-Based Dental Implants: How Tiny Bacteria Can Create a Big Problem? J. Bio- Tribo-Corros. 2021, 7, 136. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. On the mechanical integrity of retrieved dental implants. J. Mech. Behav. Biomed. Mater. 2015, 49, 290–299. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Richard, C.; Kowandy, C.; Landoulsi, J.; Geetha, M.; Ramasawmy, H. Corrosion and wear behavior of thermally sprayed nano ceramic coatings on commercially pure Titanium and Ti–13Nb–13Zr substrates. Int. J. Refract. Met. Hard Mater. 2010, 28, 115–123. [Google Scholar] [CrossRef]
- Visentin, F.; Galenda, A.; Fabrizio, M.; Battiston, S.; Brianese, N.; Gerbasi, R.; Zin, V.; El Habra, N. Assessment of synergistic effects of LP-MOCVD TiO2 and Ti surface finish for dental implant purposes. Appl. Surf. Sci. 2019, 490, 568–579. [Google Scholar] [CrossRef]
- Mezger, P.R.; Creugers, N.H.J. Titanium nitride coatings in clinical dentistry. J. Dent. 1992, 20, 342–344. [Google Scholar] [CrossRef]
- Alves, S.; Bayon, R.; Igartua, A.; de Viteri, V.; Rocha, L. Tribocorrosion behaviour of anodic titanium oxide films produced by plasma electrolytic oxidation for dental implants. Lubr. Sci. 2014, 26, 500–513. [Google Scholar] [CrossRef]
- Alves, A.C.; Oliveira, F.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Rocha, L.A. Tribocorrosion behaviour of anodic treated titanium surfaces intended for dental implants. J. Phys. Appl. Phys. 2013, 46, 404001. [Google Scholar] [CrossRef]
- Toptan, F.; Alves, A.; Pinto, A.; Ponthiaux, P. Tribocorrosion behavior of bio-functionalized highly porous titanium. J. Mech. Behav. Biomed. Mater. 2017, 69, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.I.; Viana, F.; Toptan, F. Preliminary tribocorrosion evaluation of bio-functionalized Ti doped with Ca-P-Sr. Mater. Lett. 2021, 283, 128775. [Google Scholar] [CrossRef]
- Rodrigues, N.; Alves, A.; Toptan, F.; Rocha, L. Preliminary investigation on the tribocorrosion behaviour of nanotubular structured Ti6Al4V surfaces. Mater. Lett. 2018, 213, 214–217. [Google Scholar] [CrossRef]
- Costa, A.I.; Sousa, L.; Alves, A.C.; Toptan, F. Tribocorrosion behaviour of bio-functionalized porous Ti surfaces obtained by two-step anodic treatment. Corros. Sci. 2020, 166, 108467. [Google Scholar] [CrossRef]
- Sousa, L.; Mendes, A.; Pinto, A.; Toptan, F.; Alves, A. Influence of Calcium Acetate Concentration in Electrolyte on Tribocorrosion Behaviour of MAO Treated Titanium. Metals 2021, 11, 1985. [Google Scholar] [CrossRef]
- Caha, I.; Alves, A.; Chirico, C.; Pinto, A.; Tsipas, S.; Gordo, E.; Toptan, F. A promising method to develop TiO2-based nanotubular surfaces on Ti-40Nb alloy with enhanced adhesion and improved tribocorrosion resistance. Appl. Surf. Sci. 2021, 542, 148658. [Google Scholar] [CrossRef]
- Caha, I.; Alves, A.; Chirico, C.; Pinto, A.; Tsipas, S.; Gordo, E.; Toptan, F. Tribocorrosion-Resistant Ti40Nb-TiN Composites HavingTiO2-Based Nanotubular Surfaces. ACS Biomater. Sci. Eng. 2022, 8, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Çaha, I.; Alves, A.C.; Chirico, C.; Pinto, A.M.; Tsipas, S.; Gordo, E.; Toptan, F. Improved tribocorrosion behavior on bio-functionalized β-type titanium alloy by the pillar effect given by TiN reinforcements. Surf. Coat. Technol. 2021, 415, 127122. [Google Scholar] [CrossRef]
- Garcia-Cabezón, C.; Rodríguez-Méndez, M.L.; Borrás, V.A.; Bayón, R.; Salvo-Comino, C.; Garcia-Hernandez, C.; Martin-Pedrosa, F. Improvements in tribological and anticorrosion performance of porous Ti-6Al-4V via PEO coating. Friction 2021, 9, 1303–1318. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, T.; Jiang, X.; Wu, M.; Zhang, Y.; Chen, F. Tribocorrosion behavior of Ca-P MAO coatings on Ti6Al4V alloy at various applied voltages. J. Mater. Res. 2020, 35, 444–453. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, T.; Yu, P.; Zhao, Z.; Chen, X.; Zhang, Y.; Chen, F. Effect of graphene oxide additive on tribocorrosion behavior of MAO coatings prepared on Ti6Al4V alloy. Appl. Surf. Sci. 2019, 480, 26–34. [Google Scholar] [CrossRef]
- He, B.; Xin, C.; Chen, Y.; Xu, Y.; Zhao, Q.; Hou, Z.; Tang, Y.; Liu, H.; Su, X.; Zhao, Y. Biological performance and tribocorrosion behavior of in-situ synthesized CuxO/TiO2 coatings. Appl. Surf. Sci. 2022, 600, 154096. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Satchithananda, K.; Liddle, A.D.; Sabah, S.A.; McRobbie, D.; Henckel, J.; Cobb, J.P.; Skinner, J.A.; Mitchell, A.W. Pseudotumors in Association with Well-Functioning Metal-on-Metal Hip Prostheses: A Case-Control Study Using Three-Dimensional Computed Tomography and Magnetic Resonance Imaging. J. Bone Jt. Surg. Am. 2012, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of Wear Particles to the Liver, Spleen, and Abdominal Lymph Nodes of Patients with Hip or Knee Replacement*. J. Bone Jt. Surg. Am. 2000, 82, 457. [Google Scholar] [CrossRef] [PubMed]
- Concerns about Metal-on-Metal Hip Implants. In US Food & Drug Administration. Available online: https://www.fda.gov/medical-devices/metal-metal-hip-implants/concerns-about-metal-metal-hip-implants (accessed on 12 November 2023).
- Boulila, A.; Bouzid, L.; Ayadi, M. Chapter 6—Failure of total hip arthroplasty (THA): State of the art. In Medical and Healthcare Robotics; Boubaker, O., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 157–181. ISBN 978-0-443-18460-4. [Google Scholar]
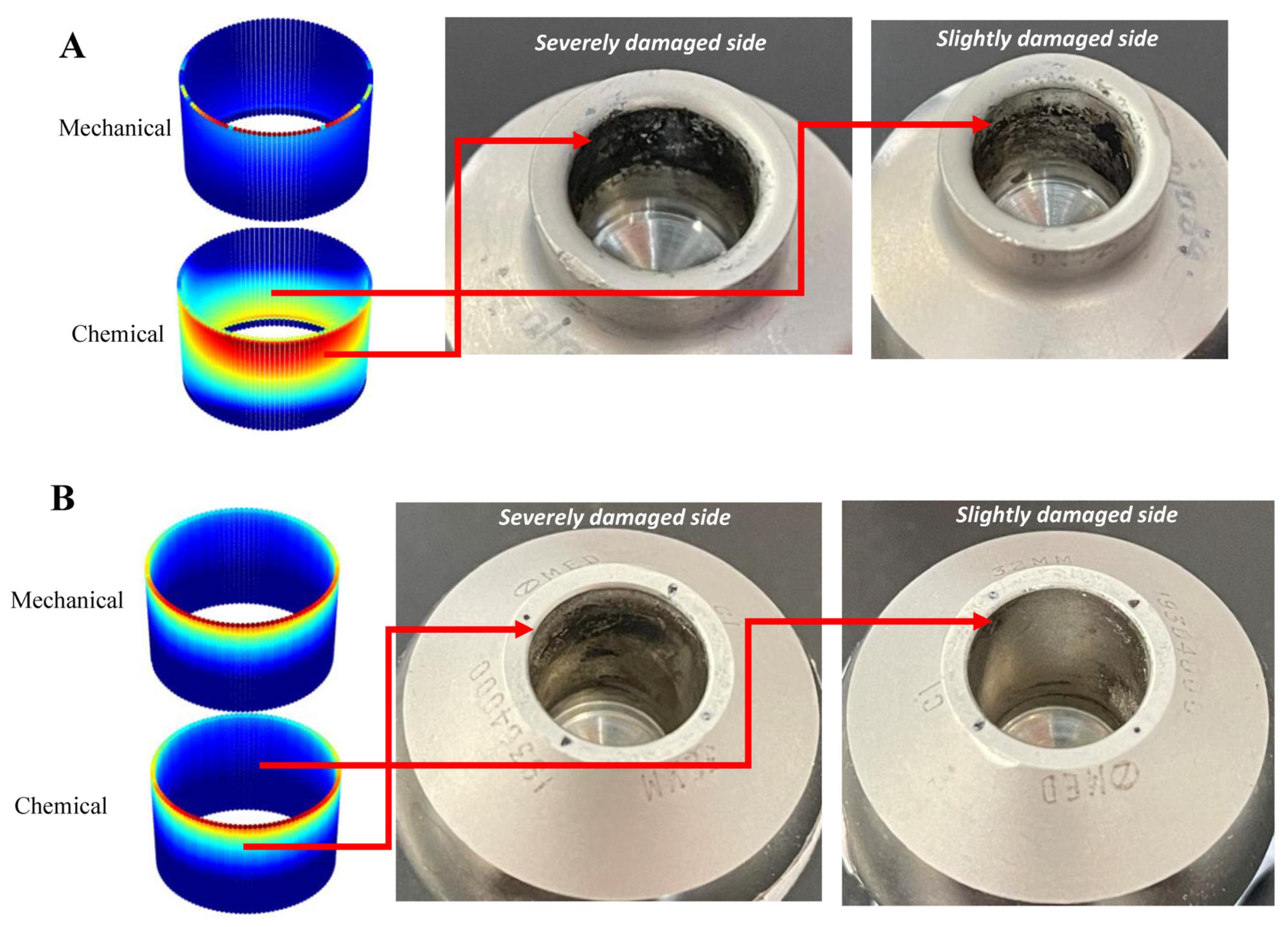
- Cheng, K.-Y.; Bijukumar, D.; Runa, M.; McNallan, M.; Mathew, M. Chapter 5–Tribocorrosion aspects of implant coatings: Hip replacements. In Tribocorrosion; Siddaiah, A., Ramachandran, R., Menezes, P.L., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 93–126. ISBN 978-0-12-818916-0. [Google Scholar]
- Swaminathan, V.; Gilbert, J. Fretting corrosion of CoCrMo and Ti6Al4V interfaces. Biomaterials 2012, 33, 5487–5503. [Google Scholar] [CrossRef]
- Norman, T.L.; Denen, J.E.; Land, A.J.; Kienitz, D.M.; Fehring, T.A. Taper-Trunnion Interface Stress Varies Significantly with Head Size and Activity. J. Arthroplast. 2019, 34, 157–162. [Google Scholar] [CrossRef]
- Harman, M.K.; Banks, S.A.; Hodge, W.A. Wear analysis of a retrieved hip implant with titanium nitride coating. J. Arthroplast. 1997, 12, 938–945. [Google Scholar] [CrossRef]
- Teresa Raimondi, M.; Pietrabissa, R. The in-vivo wear performance of prosthetic femoral heads with titanium nitride coating. Biomaterials 2000, 21, 907–913. [Google Scholar] [CrossRef]
- Taeger, G.; Podleska, L.E.; Schmidt, B.; Ziegler, M.; Nast-Kolb, D. Comparison of Diamond-Like-Carbon and Alumina-Oxide articulating with Polyethylene in Total Hip Arthroplasty. Mater. Werkst. 2003, 34, 1094–1100. [Google Scholar] [CrossRef]
- Cheng, K.; Gopal, V.; McNallan, M.; Manivasagam, G.; Mathew, M.T. Enhanced Tribocorrosion Resistance of Hard Ceramic Coated Ti-6Al-4V Alloy for Hip Implant Application: In-Vitro Simulation Study. ACS Biomater. Sci. Eng. 2019, 5, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, M.; Senaslan, F.; Celik, A. Investigation of corrosion and thermal behavior of PU-PDMS-coated AISI 316L. e-Polymers 2021, 21, 355–365. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu, N.; Celis, J.P. Electro-codeposition of CeO2 nanoparticles into cobalt matrix to improve the tribocorrosion performances of Co/nano CeO2 composite layers in biological solution for medical applications. J. Mech. Behav. Biomed. Mater. 2020, 101, 103443. [Google Scholar] [CrossRef] [PubMed]
- Esguerra-Arce, J.; Castañeda, A.B.; Esguerra-Arce, A.; Aguilar, Y.; Mischler, S. Fretting corrosion between bone and calcium phosphate-calcium titanate coatings. Wear 2018, 414–415, 366–375. [Google Scholar] [CrossRef]
- Esfahani, E.; Bukuaghangin, O.; Banfield, S.; Vangolu, Y.; Yang, L.; Neville, A.; Hall, R.; Bryant, M. Surface engineering of wrought and additive layer manufactured Ti-6Al-4V alloy for enhanced load bearing and bio-tribocorrosion applications. Surf. Coat. Technol. 2022, 442, 128139. [Google Scholar] [CrossRef]
- Anand, A.; Das, M.; Kundu, B.; Balla, V.K.; Bodhak, S.; Gangadharan, S. Tribocorrosion characteristics of Ti6Al4V-TiB-TiN in-situ composite coatings prepared using plasma spraying. J. Compos. Mater. 2021, 55, 1935–1946. [Google Scholar] [CrossRef]
- Zhu, X.; Dang, B.; Li, F.; Wei, D.; Zhang, P.; Li, S. Tribocorrosion behavior of Nb coating deposited by double-glow plasma alloying. Mater. Res. Express 2021, 8, 016411. [Google Scholar] [CrossRef]
- Gopal, V.; Chandran, M.; Rao, M.S.R.; Mischler, S.; Cao, S.; Manivasagam, G. Tribocorrosion and electrochemical behaviour of nanocrystalline diamond coated Ti based alloys for orthopaedic application. Tribol. Int. 2017, 106, 88–100. [Google Scholar] [CrossRef]
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of protective coatings for offshore applications. Corrosion and tribocorrosion behavior in synthetic seawater. Surf. Coat. Technol. 2018, 349, 1083–1097. [Google Scholar] [CrossRef]
- Sun, X.; Huang, D.; Wu, G. The current state of offshore wind energy technology development. Energy 2012, 41, 298–312. [Google Scholar] [CrossRef]
- ISO 12944-5:2019; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 5: Protective Paint Systems. International Organization for Standardization: Geneva, Switzerland, 2019.
- NORSOK M-501:2022; Surface Preparation and Protective Coating. Standards Norway: Lysaker, Norway, 2022.
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials 2019, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cai, M.; Song, S.; Huang, Y.; Fan, X.; Zhu, M. Expounding the interaction of ultraviolet irradiation and tribocorrosion on soap fiber enhanced epoxy coating. Prog. Org. Coat. 2022, 163, 106604. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Guo, P.; Sun, L.; Wei, J.; Liu, Y.; Li, S.; Wang, S.; Lee, K.; Ke, P.; et al. Long-term tribocorrosion resistance and failure tolerable of multilayer carbon-based coatings. Friction 2022, 10, 1707–1721. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, H.; Ma, G.; Sun, L.; Guo, P.; Ke, P.; Lee, K.; Wang, A. Controllable defect engineering to enhance the corrosion resistance of Cr/GLC multilayered coating for deep-sea applications. Corros. Sci. 2022, 199, 110175. [Google Scholar] [CrossRef]
- Xu, X.; Guo, P.; Zuo, X.; Sun, L.; Li, X.; Lee, K.; Wang, A. Understanding the effect of Al/Ti ratio on the tribocorrosion performance of Al/Ti co-doped diamond-like carbon films for marine applications. Surf. Coat. Technol. 2020, 402, 126347. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Antonyuk, M.N.; Bondarev, A.V.; Sheveyko, A.N.; Shtansky, D.V. Electrospark deposition of wear and corrosion resistant Ta(Zr)C-(Fe,Mo,Ni) coatings to protect stainless steel from tribocorrosion in seawater. Wear 2021, 486–487, 204094. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Wang, C.; Sui, X.; Wang, Y.; Zhou, H.; Hao, J. Tailoring the microstructure, mechanical and tribocorrosion performance of (CrNbTiAlV)N-x high-entropy nitride films by controlling nitrogen flow. J. Mater. Sci. Technol. 2022, 107, 172–182. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, C.; Sui, X.; Lu, X.; Zhang, X.; Wang, C.; Hao, J.; Shi, Z. Microstructure, mechanical properties and tribo-corrosion mechanism of (CrNbTiAlVMo)xN1−x coated 316 L stainless steel in 3.5 wt% NaCl solution. Tribol. Int. 2022, 173, 107638. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, C.; Guo, W.; Lu, X.; Sui, Y.; Lan, J. Inhibiting tribocorrosion damage of Cr/CrxN coatings by multi-layer design. Ceram. Int. 2021, 47, 842–850. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhou, S.; Wang, Y.; Wang, C.; Wang, Y.; Sui, Y.; Lan, J.; Xue, Q. Improvement in the tribocorrosion performance of CrCN coating by multilayered design for marine protective application. Appl. Surf. Sci. 2020, 528, 147061. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Shan, L.; Liu, Y.; Zhang, P.; Jia, Y. Preparation and tribocorrosion performance of CrCN coatings in artificial seawater on different substrates with different bias voltages. Ceram. Int. 2019, 45, 9901–9911. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Zhang, Y.; Zhang, Q.; Xue, Q. Tribocorrosion behaviors of PVD CrN coated stainless steel in seawater. Wear 2016, 362–363, 97–104. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Xu, Z.; Li, Z.; Su, F. Microstructure, electrochemical and tribocorrosion behaviors of CrCN nanocomposite coating with various carbon content. Surf. Coat. Technol. 2021, 411, 126997. [Google Scholar] [CrossRef]
- Wang, H.; Ou, Y.; Zhang, X.; Liao, B.; Ou, X.; Luo, J.; Pang, P.; Chen, L.; Hua, Q.; Bao, M. Tribocorrosion behaviors of TiSiCN nanocomposite coatings deposited by high power impulse magnetron sputtering. Mater. Res. Express 2020, 7, 076407. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Y. A comparative study on the tribocorrosion behaviors of AlFeCrNiMo high entropy alloy coatings and 304 stainless steel. Mater. Today Commun. 2020, 24, 101261. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Tribocorrosion behavior in artificial seawater and anti-microbiologically influenced corrosion properties of TiSiN-Cu coating on F690 steel. J. Mater. Sci. Technol. 2019, 35, 448–459. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Structural, mechanical and tribocorrosion behaviour in artificial seawater of CrN/AlN nano-multilayer coatings on F690 steel substrates. Appl. Surf. Sci. 2018, 428, 404–414. [Google Scholar] [CrossRef]
- Alkan, S.; Gök, M.S. Influence of plasma nitriding pre-treatment on the corrosion and tribocorrosion behaviours of PVD CrN, TiN and AlTiN coated AISI 4140 steel in seawater. Lubr. Sci. 2022, 34, 67–83. [Google Scholar] [CrossRef]
- Cheng, J.; Ge, Y.; Wang, B.; Zhang, L.; Hu, X.; Hong, S.; Liang, X.; Zhang, X. Microstructure and Tribocorrosion Behavior of Al2O3/Al Composite Coatings: Role of Al2O3 Addition. J. Therm. Spray Technol. 2020, 29, 1741–1751. [Google Scholar] [CrossRef]
- López-Ortega, A.; Arana, J.L.; Rodríguez, E.; Bayón, R. Corrosion, wear and tribocorrosion performance of a thermally sprayed aluminum coating modified by plasma electrolytic oxidation technique for offshore submerged components protection. Corros. Sci. 2018, 143, 258–280. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, H.; Hua, Q.; Liao, B.; Ouyang, X. Tribocorrosion behaviors of superhard yet tough Ti-C-N ceramic coatings. Surf. Coat. Technol. 2022, 439, 128448. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, Q.; Yu, Q.; Guan, Z.; Deng, Y.; Liu, Y. Influence of high hydrostatic pressure on tribocorrosion behavior of HVOF WC-10Co-4Cr coating coupled with Si3N4 in artificial seawater. Int. J. Refract. Met. Hard Mater. 2022, 108, 105936. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Sheveyko, A.N.; Sidorenko, D.A.; Shtansky, D.V. Electro-spark deposition in vacuum using graphite electrode at different electrode polarities: Peculiarities of microstructure, electrochemical and tribological properties. Appl. Surf. Sci. 2021, 566, 150722. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, F.; Zhang, M.; Wang, Q.; Zhou, Z. Structural, mechanical and tribocorrosion performances of CrMoSiN coatings with various Mo contents in artificial seawater. Appl. Surf. Sci. 2020, 525, 146629. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, F.; Wang, Q.; Fu, Y.; Zhou, Z. Tribocorrosion characteristics of CrMoSiCN/Ag coatings on Ti6Al4V alloys in seawater. Ceram. Int. 2021, 47, 31780–31797. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, F.; Zhang, M.; Wang, Q.; Zhou, Z. Structure and tribocorrosion behavior of CrMoSiCN nanocomposite coating with low C content in artificial seawater. Friction 2021, 9, 1599–1615. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, F.; Wang, Q.; Zhang, M.; Zhou, Z. Electrochemical and tribocorrosion performances of CrMoSiCN coating on Ti-6Al-4V titanium alloy in artificial seawater. Corros. Sci. 2020, 165, 108385. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dang, C.; Wang, Y.; Zhu, Y. Influence of bias voltage on structure and tribocorrosion properties of TiSiCN coating in artificial seawater. Mater. Charact. 2017, 127, 198–208. [Google Scholar] [CrossRef]
- Liu, K.; Ma, F.; Lou, M.; Dong, M.; Zhu, Y.; Wang, Y.; Wu, X.; Liu, X.; Li, J. Structure and tribocorrosion behavior of TiAlCN coatings with different Al contents in artificial seawater by multi-arc ion plating. Surf. Topogr. Metrol. Prop. 2021, 9, 045004. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, M.; Li, J.; Wang, L. The improved corrosion and tribocorrosion properties of TiSiN/Ag by thermal treatment. Surf. Coat. Technol. 2020, 385, 125437. [Google Scholar] [CrossRef]
- Dong, M.; Zhu, Y.; Xu, L.; Ren, X.; Ma, F.; Mao, F.; Li, J.; Wang, L. Tribocorrosion performance of nano-layered coating in artificial seawater. Appl. Surf. Sci. 2019, 487, 647–654. [Google Scholar] [CrossRef]
- Dong, M.; Zhu, Y.; Wang, C.; Shan, L.; Li, J. Structure and tribocorrosion properties of duplex treatment coatings of TiSiCN/nitride on Ti6Al4V alloy. Ceram. Int. 2019, 45, 12461–12468. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dang, C.; Wang, Y.; Zhu, Y. Influence of carbon contents on the structure and tribocorrosion properties of TiSiCN coatings on Ti6Al4V. Tribol. Int. 2017, 109, 285–296. [Google Scholar] [CrossRef]
- Kowalski, M.; Stachowiak, A. Tribocorrosion Performance of Cr/CrN Hybrid Layer as a Coating for Machine Components Used in a Chloride Ions Environment. Coatings 2021, 11, 242. [Google Scholar] [CrossRef]
- Cai, K.; Jiang, B.; Zhang, J.; Su, X. Preparation and Tribocorrosion Performance of Different Si-Doped TiSiN-Ag Coatings on Different Substrates in Seawater. Coatings 2021, 11, 459. [Google Scholar] [CrossRef]
- Totolin, V.; Pejaković, V.; Csanyi, T.; Hekele, O.; Huber, M.; Rodríguez Ripoll, M. Surface engineering of Ti6Al4V surfaces for enhanced tribocorrosion performance in artificial seawater. Mater. Des. 2016, 104, 10–18. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, Q.; Zhu, S.; Tao, X.; Wang, Z.; Zhang, B. Exploration of tribocorrosion behavior of Fe-based amorphous coating in simulated seawater. J. Adhes. Sci. Technol. 2021, 37, 997–1009. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, Y.; Wang, X.; Zeng, Z.; Du, H.; Qin, H. Tribocorrosion behaviors of thermal spraying WC/Ni60 coated 316L stainless steel in artificial seawater. Ind. Lubr. Tribol. 2019, 71, 741–748. [Google Scholar] [CrossRef]
- Pejakovic, V.; Berger, L.; Thiele, S.; Rojacz, H.; Ripoll, M. Fine grained titanium carbonitride reinforcements for laser deposition processes of 316L boost tribocorrosion resistance in marine environments. Mater. Des. 2021, 207, 109847. [Google Scholar] [CrossRef]
- Ou, Y.X.; Wang, H.Q.; Liao, B.; Lei, M.K.; Ouyang, X.P. Tribological behaviors in air and seawater of CrN/TiN superlattice coatings irradiated by high-intensity pulsed ion beam. Ceram. Int. 2019, 45, 24405–24412. [Google Scholar] [CrossRef]
- Alkan, S. Enhancement of marine corrosion and tribocorrosion resistance of offshore mooring chain steel by aluminizing process. Brodogradnja 2022, 73, 131–159. [Google Scholar] [CrossRef]









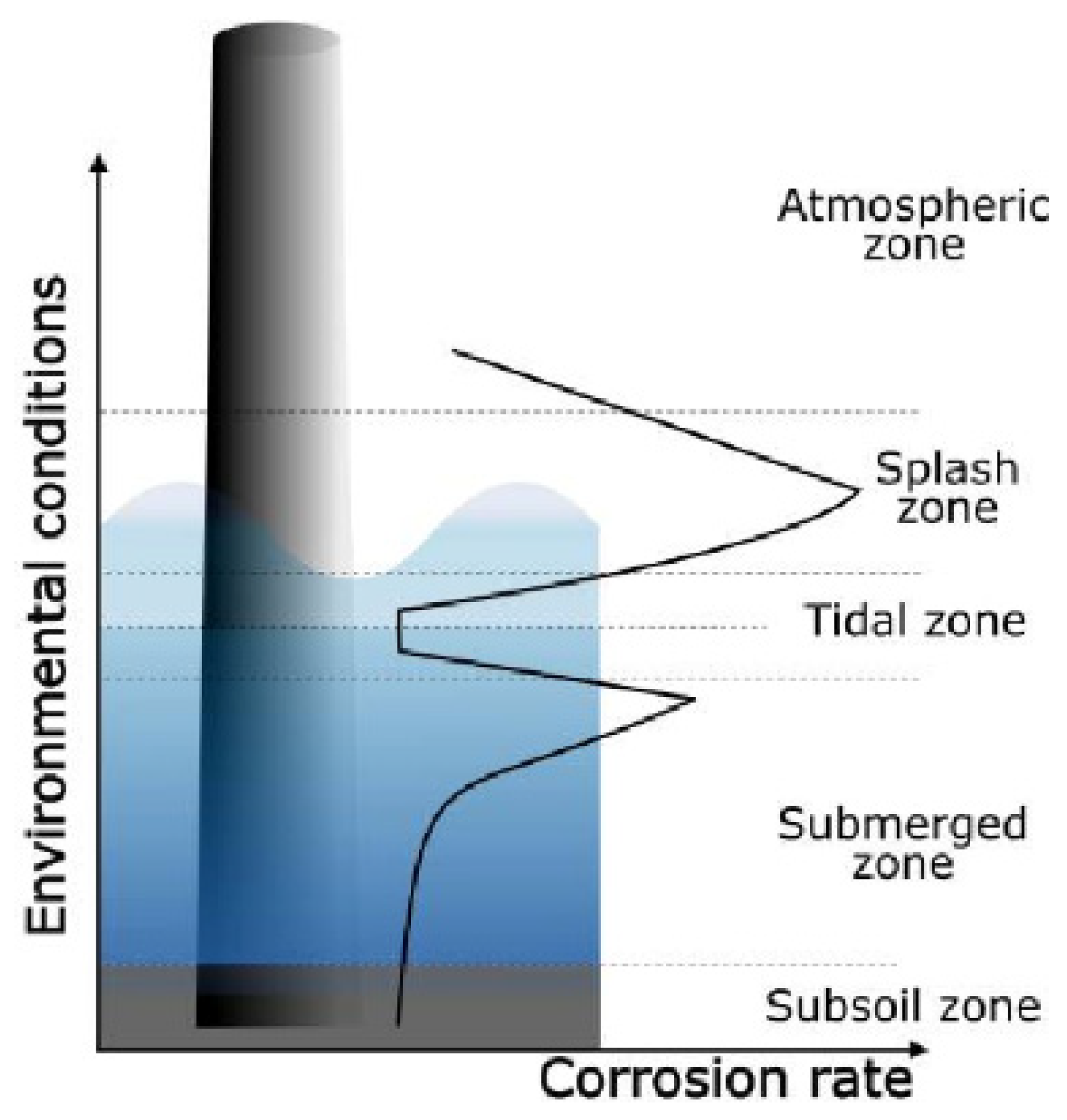
| Exposure Zone | Category (ISO 12944) | Coating System | Desirable Coating Properties |
|---|---|---|---|
| Atmospheric | C5-M | Zinc-rich epoxy primer (60–100 µm) Epoxy intermediate layer (100–120 µm) Polyurethan topcoat (50–80 µm) | Corrosion-resistant, Erosion-resistant, anti-icing, UV-resistant |
| Splash and tidal | C-M and Im2 | Two or three epoxy-based coats (>1000 µm in total) Polyurethane topcoat (50–80 µm) | Combination of atmospheric and submerged coatings’ properties |
| Submerged | Im2 | Two or three epoxy-based coats (>450 µm in total) | Corrosion-resistant, antifouling, wear-resistant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, R.J.K.; Lu, P. Coatings and Surface Modification of Alloys for Tribo-Corrosion Applications. Coatings 2024, 14, 99. https://doi.org/10.3390/coatings14010099
Wood RJK, Lu P. Coatings and Surface Modification of Alloys for Tribo-Corrosion Applications. Coatings. 2024; 14(1):99. https://doi.org/10.3390/coatings14010099
Chicago/Turabian StyleWood, Robert J. K., and Ping Lu. 2024. "Coatings and Surface Modification of Alloys for Tribo-Corrosion Applications" Coatings 14, no. 1: 99. https://doi.org/10.3390/coatings14010099
APA StyleWood, R. J. K., & Lu, P. (2024). Coatings and Surface Modification of Alloys for Tribo-Corrosion Applications. Coatings, 14(1), 99. https://doi.org/10.3390/coatings14010099






