Facile Synthesis and Characterization of TiO2/SnS Nanocomposites by Eco-Friendly Methods
Abstract
1. Introduction
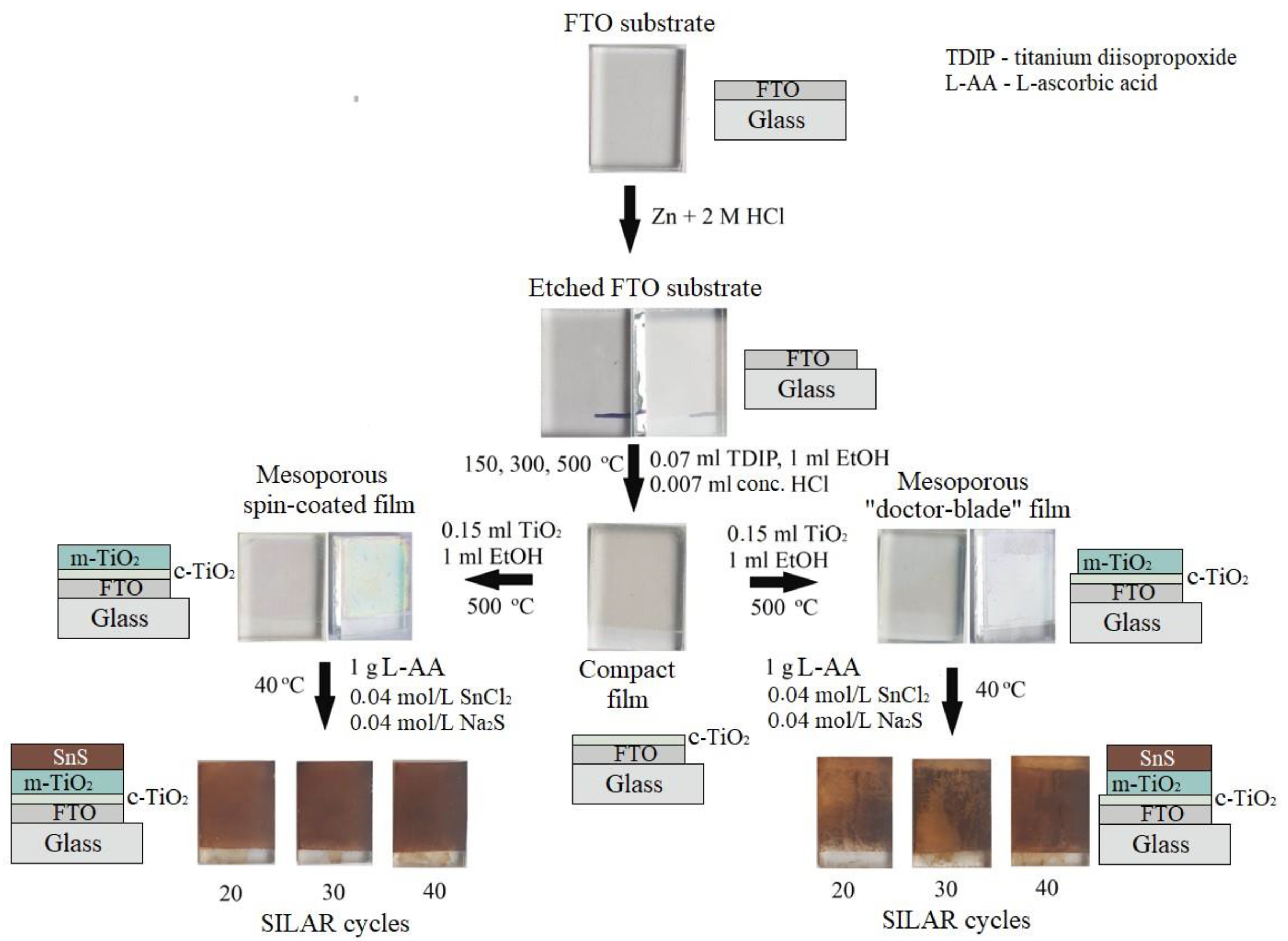
2. Materials and Methods
3. Results and Discussion
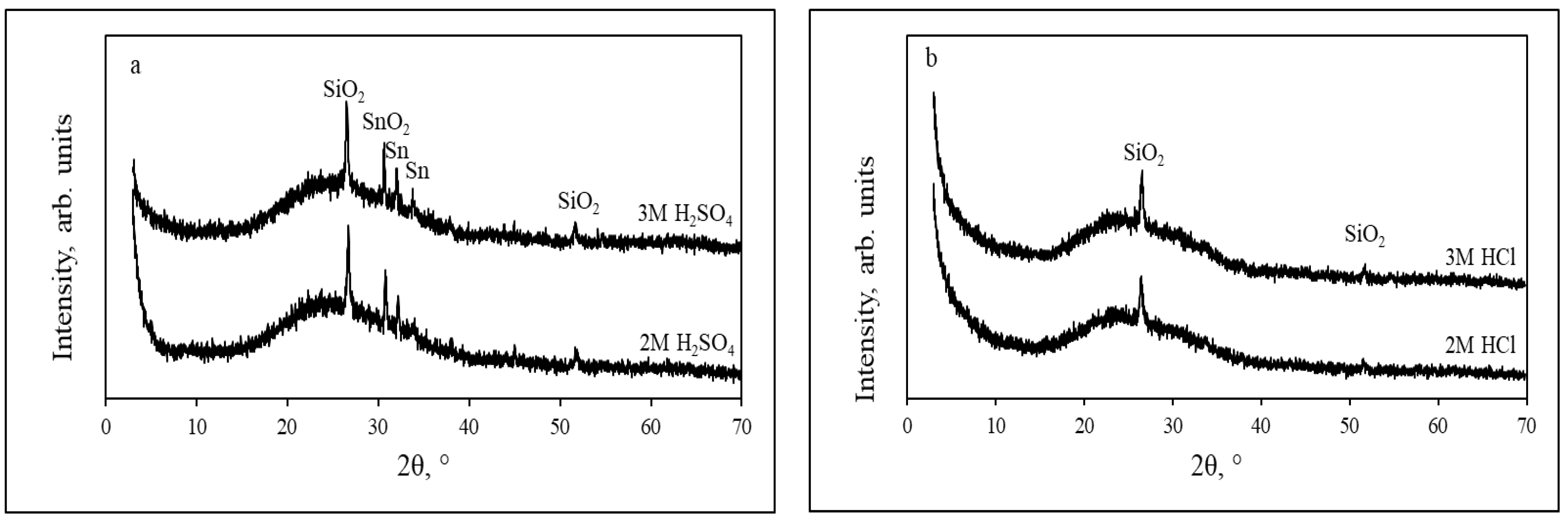
3.1. Etching of FTO Glass Substrates
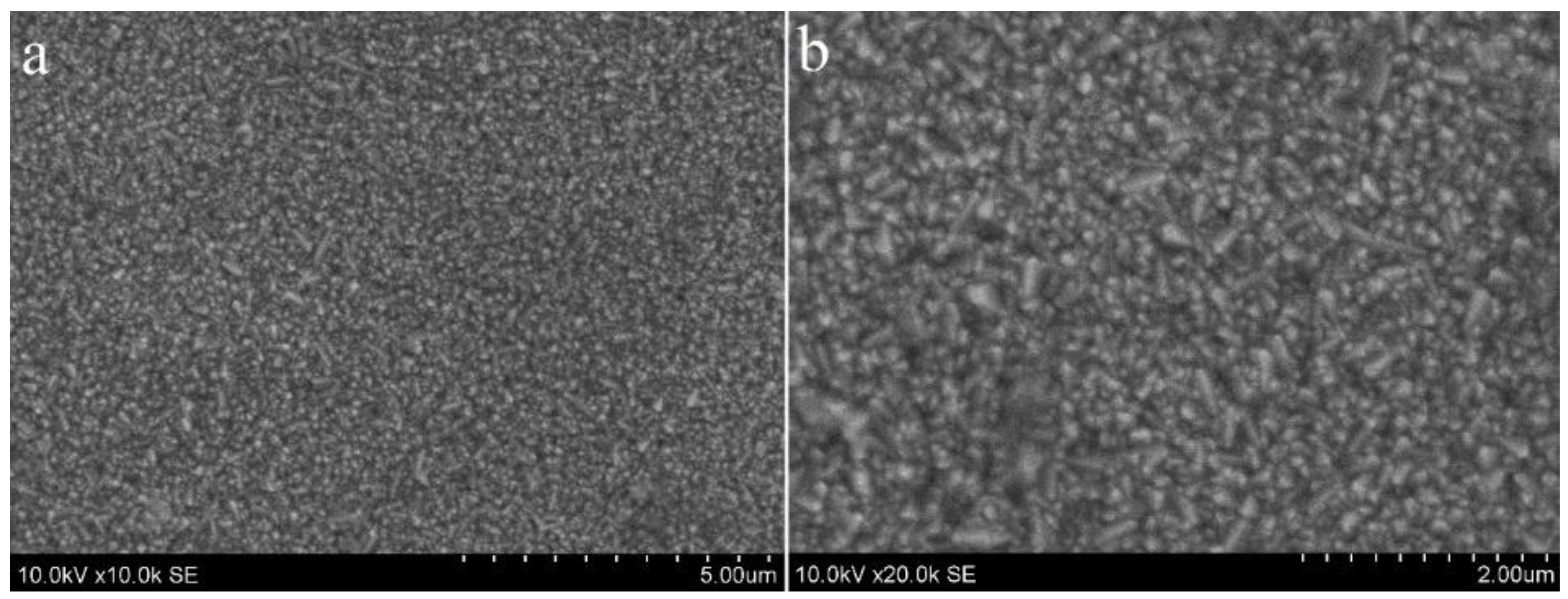
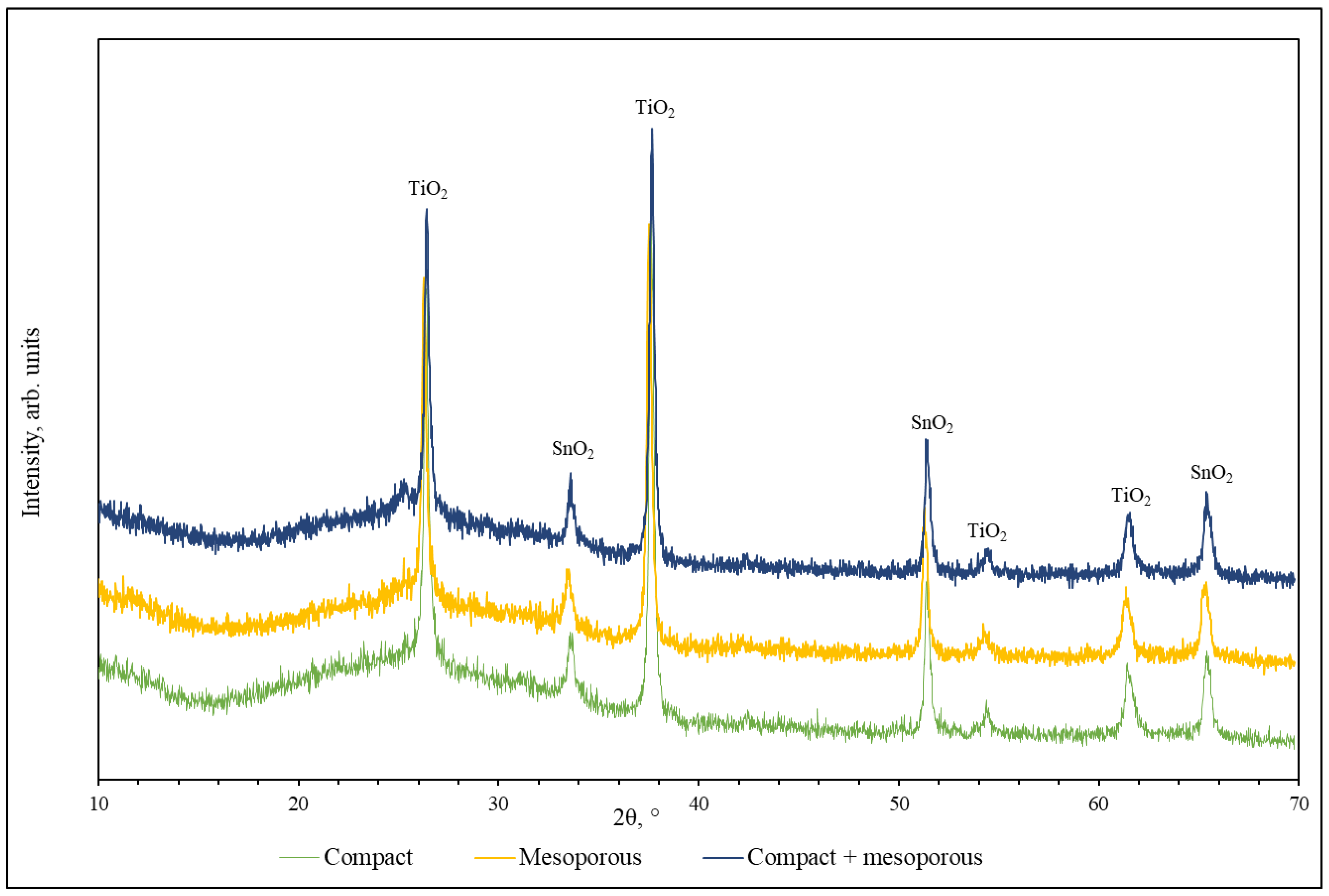
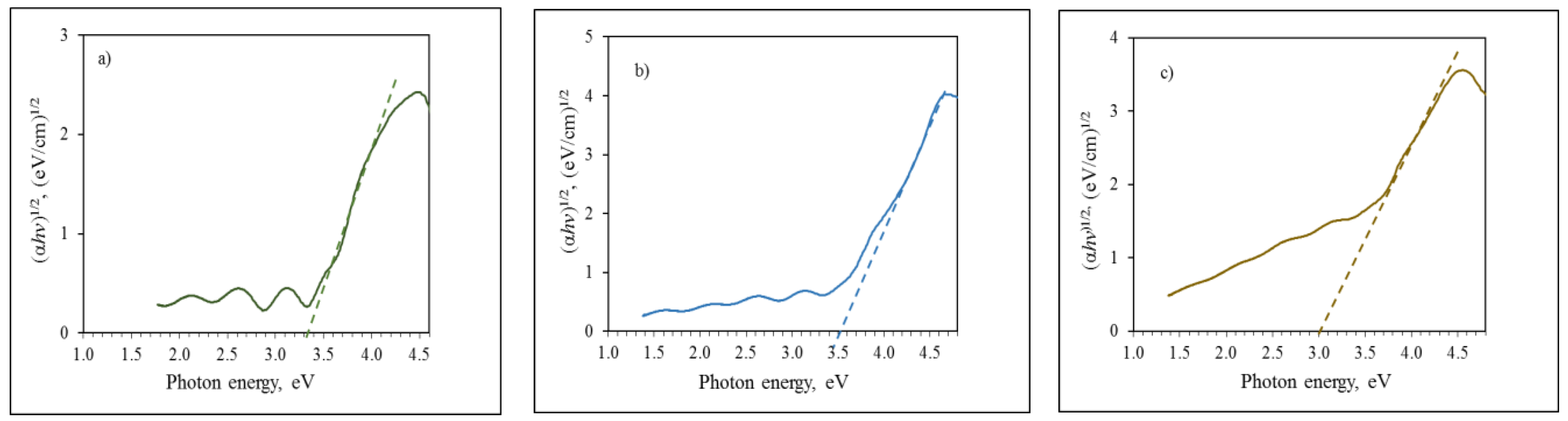
3.2. Compact and Mesoporous TiO2 Films
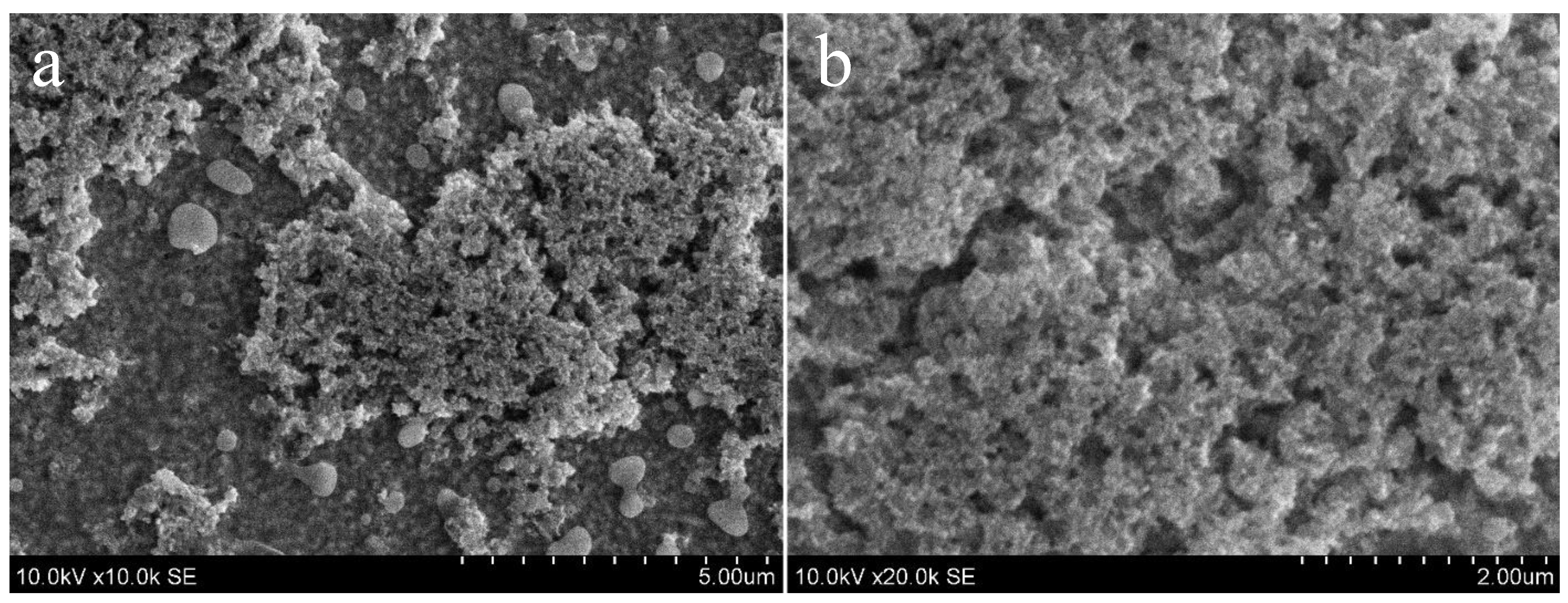
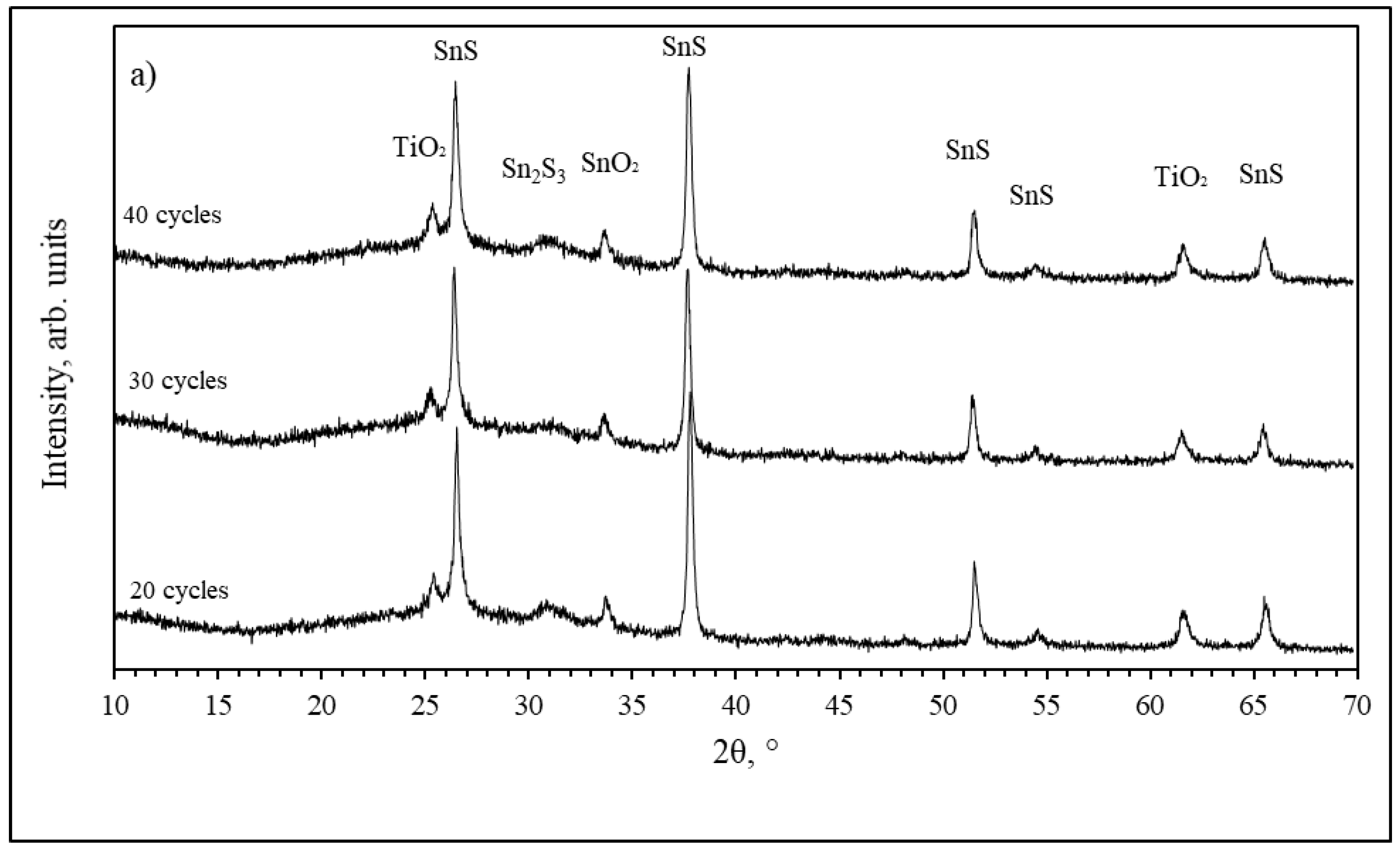
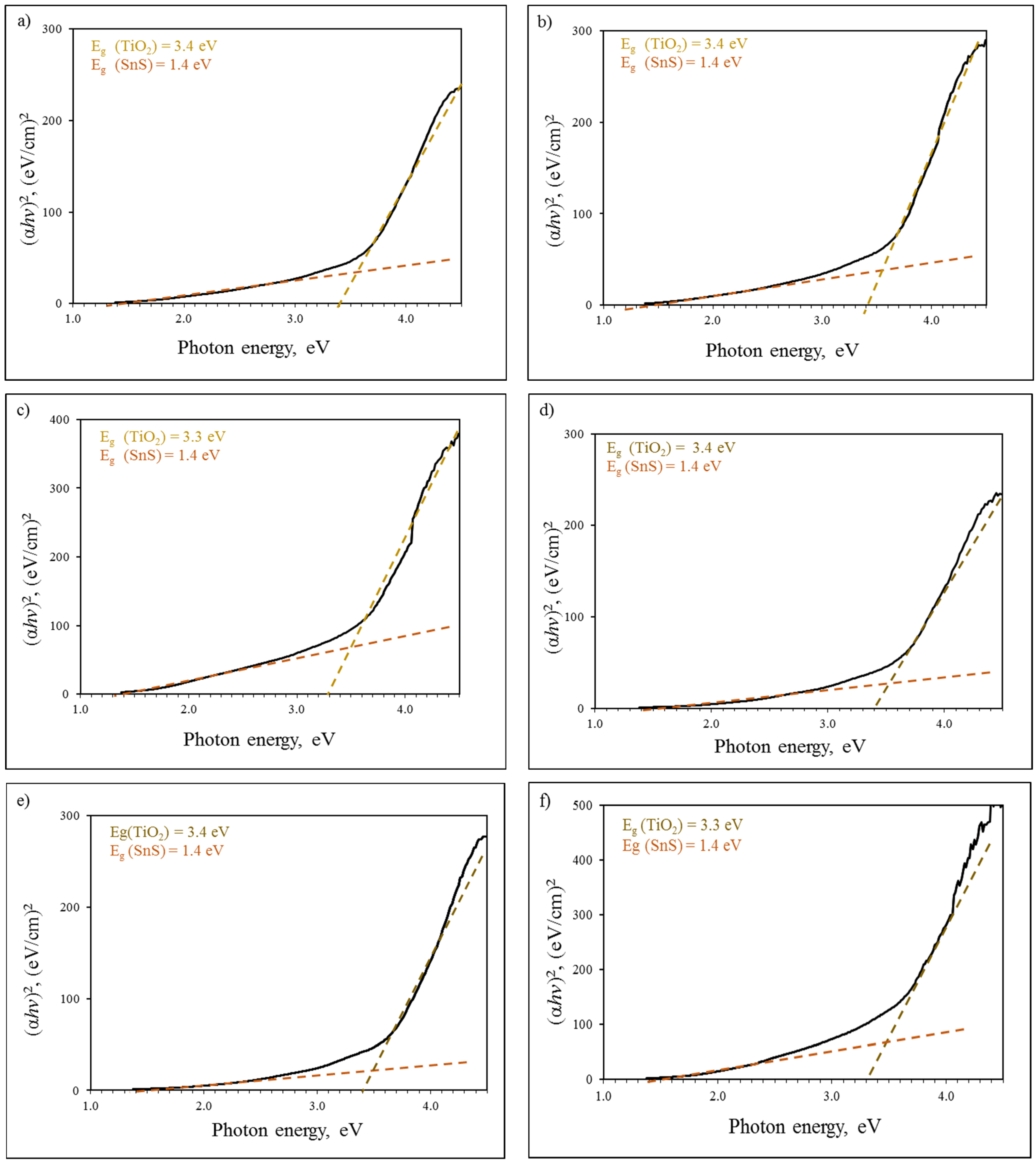
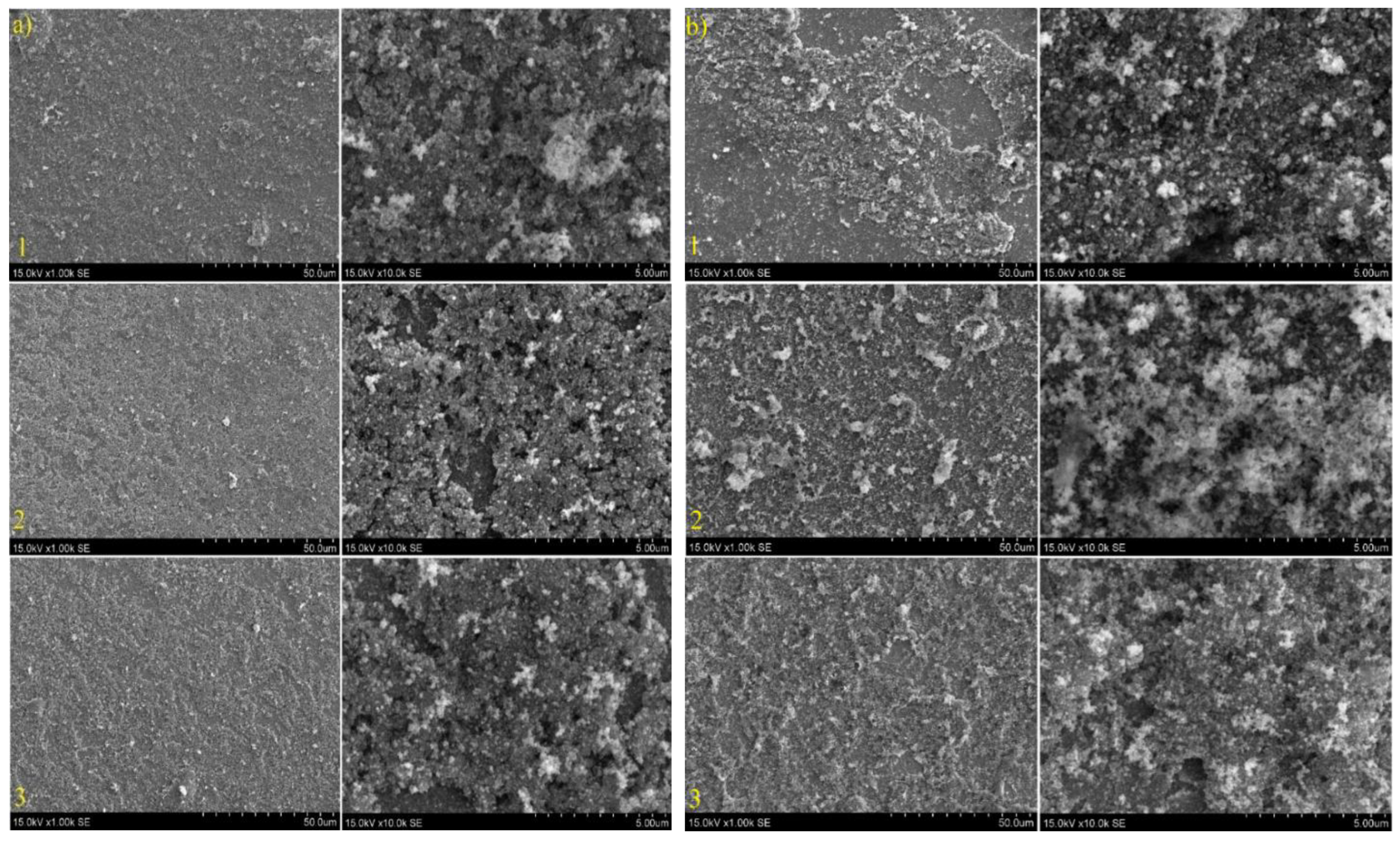
3.3. TiO2/SnS Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abutbul, R.E.; Golan, Y. Chemical Epitaxy of π-Phase Cubic Tin Monosulphide. CrystEngComm 2020, 22, 6170. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, M.J.; Heo, H.; Sung, J.H.; Kim, K.; Hwang, H.; Jo, M.H. Deterministic Two-Dimensional Polymorphism Growth of Hexagonal n-Type SnS2 and Orthorhombic p-Type SnS Crystals. Nano Lett. 2015, 15, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Energy Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Chauhan, H.; Singh, M.K.; Hashmi, S.A.; Deka, S. Synthesis of Surfactant-Free SnS Nanorods by a Solvothermal Route with Better Electrochemical Properties towards Supercapacitor Applications. RSC Adv. 2015, 5, 17228–17235. [Google Scholar] [CrossRef]
- Betz, U.; Kharrazi Olsson, M.; Marthy, J.; Escolá, M.F.; Atamny, F. Thin Films Engineering of Indium Tin Oxide: Large Area Flat Panel Displays Application. Surf. Coat. Technol. 2006, 200, 5751–5759. [Google Scholar] [CrossRef]
- Garvey, T.R.; Farnum, B.H.; Lopez, R. Pulsed Laser Deposited Porous Nano-Carpets of Indium Tin Oxide and Their Use as Charge Collectors in Core-Shell Structures for Dye Sensitized Solar Cells. Nanoscale 2015, 7, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, J.W.; Yun, J.; Lee, K.; Ryu, J.; Lee, J.; Hwang, D.; Kim, S.K.; Jang, J. Outstanding Performance of Hole-Blocking Layer-Free Perovskite Solar Cell Using Hierarchically Porous Fluorine-Doped Tin Oxide Substrate. Adv. Energy Mater. 2017, 7, 1700749. [Google Scholar] [CrossRef]
- Sivakumar, R.; Gopalakrishnan, R.; Jayachandran, M.; Sanjeeviraja, C. Investigation of X-Ray Photoelectron Spectroscopic (XPS), Cyclic Voltammetric Analyses of WO3 Films and Their Electrochromic Response in FTO/WO3/Electrolyte/FTO Cells. Smart Mater. Struct. 2006, 15, 877–888. [Google Scholar] [CrossRef]
- Rana, C.; Bera, S.R.; Saha, S. Growth of SnS Nanoparticles and Its Ability as Ethanol Gas Sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 2016–2029. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Review—Recent Advances in Tin Oxide Nanomaterials as Electrochemical/Chemiresistive Sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Shi, Z.; Ju, Y.; Zhang, Z.; Gu, M. Fabrication and Enhanced H2O2-Sensing Properties of the Uniform Porous FTO Glasses with Tunable Pore Sizes and Densities. Appl. Surf. Sci. 2019, 465, 357–361. [Google Scholar] [CrossRef]
- Vazquez-Santos, M.B.; Tartaj, P.; Morales, E.; Amarilla, J.M. TiO2 Nanostructures as Anode Materials for Li/Na-Ion Batteries. Chem. Rec. 2018, 18, 1178–1191. [Google Scholar] [CrossRef]
- Yang, L.X.; Luo, S.L.; Cai, Q.Y.; Yao, S.Z. A Review on TiO2 Nanotube Arrays: Fabrication, Properties, and Sensing Applications. Chin. Sci. Bull. 2010, 55, 331–338. [Google Scholar] [CrossRef]
- Ilyas, A.M.; Gondal, M.A.; Yamani, Z.H.; Baig, U. Facile Synthesis of Titanium Dioxide-Cadmium Sulfide Nanocomposite Using Pulsed Laser Ablation in Liquid and Its Performance in Photovoltaic and Photocatalytic Applications. Int. J. Energy Res. 2017, 41, 1422–1435. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Nengzi, L.C.; Li, B.; Gou, J.; Cheng, X. Synthesis of SnS/TiO2 Nano-Tube Arrays Photoelectrode and Its High Photoelectrocatalytic Performance for Elimination of 2,4,6-Trichlorophenol. Sep. Purif. Technol. 2019, 228, 115742. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Lei, J.; Liu, Y.; Zhang, J. Fabrication of TiO2/Co-g-C3N4 Heterojunction Catalyst and Its Photocatalytic Performance. Catal. Commun. 2017, 89, 125–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, X.; Han, Y.; Zhang, X.; Shen, F.; Deng, S.; Xiao, H.; Yang, X.; Yang, G.; Peng, H. Photoelectrocatalytic Degradation of Recalcitrant Organic Pollutants Using TiO2 Film Electrodes: An Overview. Chemosphere 2012, 88, 145–154. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, F.; Cai, F.; Cheng, C.; Zhao, Y. Photoelectrochemical and Charge Transfer Properties of SnS/TiO2 Heterostructure Nanotube Arrays. Electron. Mater. Lett. 2013, 9, 287–291. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H.; Fan, B.; Hu, G. Photovoltaic Behavior of Nanocrystalline SnS/TiO2. J. Phys. Chem. C 2010, 114, 3256–3259. [Google Scholar] [CrossRef]
- Nengzi, L.C.; Yang, H.; Hu, J.Z.; Zhang, W.M.; Jiang, D.A. Fabrication of SnS/TiO2 NRs/NSs Photoelectrode as Photoactivator of Peroxymonosulfate for Organic Pollutants Elimination. Sep. Purif. Technol. 2020, 249, 117172. [Google Scholar] [CrossRef]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-Baena, J.P.; Gao, P.; Scopelliti, R.; Mosconi, E.; Dahmen, K.H.; et al. A Molecularly Engineered Hole-Transporting Material for Efficient Perovskite Solar Cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Cheraghizade, M.; Jamali-Sheini, F.; Shabani, P. Charge Transportation Mechanisms in TiO2/SnS/Ag Solar Cells. Mater. Res. Bull. 2020, 124, 110727. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.; Shih, W.P.; Dai, C.L. Wet Etching Rates of InGaZnO for the Fabrication of Transparent Thin-Film Transistors on Plastic Substrates. Thin Solid Film. 2010, 518, 3992–3998. [Google Scholar] [CrossRef]
- Volkert, C.A.; Minor, A.M. Focused Ion Beam Microscopy and Micromachining. MRS Bull. 2007, 32, 389–399. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.-P.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (N−i−p) and Inverted (P−i−n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Bronusiene, A.; Popov, A.; Barauskiene, I.; Ancutiene, I. Effect of Ascorbic Acid on the Properties of Tin Sulfide Films for Supercapacitor Application. Surf. Interfaces 2021, 25, 101275. [Google Scholar] [CrossRef]
- Vikraman, D.; Thiagarajan, S.; Karuppasamy, K.; Sanmugam, A.; Choi, J.H.; Prasanna, K.; Maiyalagan, T.; Thaiyan, M.; Kim, H.S. Shape- and Size-Tunable Synthesis of Tin Sulfide Thin Films for Energy Applications by Electrodeposition. Appl. Surf. Sci. 2019, 479, 167–176. [Google Scholar] [CrossRef]
- Bae, J.W.; Koo, B.R.; An, H.R.; Ahn, H.J. Surface Modification of Fluorine-Doped Tin Oxide Films Using Electrochemical Etching for Dye-Sensitized Solar Cells. Ceram. Int. 2015, 41, 14668–14673. [Google Scholar] [CrossRef]
- Triana, S.L.; Kusumandari; Suryana, R. Effect of Wet Etching Process on the Morphology and Transmittance of Fluorine Doped Tin Oxide (FTO). J. Phys. Conf. Ser. 2016, 776, 012005. [Google Scholar] [CrossRef]
- Lee, K.T.; Lu, S.Y. Porous FTO Thin Layers Created with a Facile One-Step Sn 4+-Based Anodic Deposition Process and Their Potential Applications in Ion Sensing. J. Mater. Chem. 2012, 22, 16259–16268. [Google Scholar] [CrossRef]
- Miao, F.; Chu, F.; Sun, B.; Tao, B.; Zhang, P.; Zang, Y.; Chu, P.K. Dye-Sensitized Solar Cells Based on Au/SnS/TiO2 sensitized by Natural Dye. J. Mater. Res. Technol. 2022, 21, 704–711. [Google Scholar] [CrossRef]
- Jarernboon, W.; Pimanpang, S.; Maensiri, S.; Swatsitang, E.; Amornkitbamrung, V. Effects of Multiwall Carbon Nanotubes in Reducing Microcrack Formation on Electrophoretically Deposited TiO2 Film. J. Alloys Compd. 2009, 476, 840–846. [Google Scholar] [CrossRef]
- Zhang, Y.; Weidenkaff, A.; Reller, A. Mesoporous Structure and Phase Transition of Nanocrystalline TiO2. Mater. Lett. 2002, 54, 375–381. [Google Scholar] [CrossRef]
- Parida, B.; Singh, A.; Oh, M.; Jeon, M.; Kang, J.W.; Kim, H. Effect of Compact TiO2 Layer on Structural, Optical, and Performance Characteristics of Mesoporous Perovskite Solar Cells. Mater. Today Commun. 2019, 18, 176–183. [Google Scholar] [CrossRef]
- Li, Z.; Haidry, A.A.; Plecenik, T.; Vidis, M.; Grancic, B.; Roch, T.; Gregor, M.; Durina, P.; Yao, Z.J.; Plecenik, A. Influence of Nanoscale TiO2 Film Thickness on Gas Sensing Properties of Capacitor-like Pt/TiO2/Pt Sensing Structure. Appl. Surf. Sci. 2020, 499, 143909. [Google Scholar] [CrossRef]
- Klugmann-Radziemska, E. Degradation of Electrical Performance of a Crystalline Photovoltaic Module Due to Dust Deposition in Northern Poland. Renew. Energy 2015, 78, 418–426. [Google Scholar] [CrossRef]
- Kumar, A.; Saxena, V.K.; Thangavel, R.; Nandi, B.K. A Dual Effect of Surface Roughness and Photocatalysis of Crystalline TiO2-Thin Film for Self-Cleaning Application on a Photovoltaic Covering Glass. Mater. Chem. Phys. 2022, 289, 126427. [Google Scholar] [CrossRef]
- Hong, S.; Park, B.; Balamurugan, C.; Lee, J.; Kwon, S. Impact of Solvents on Doctor Blade Coatings and Bathocuproine Cathode Interlayer for Large-Area Organic Solar Cell Modules. Heliyon 2023, 9, e18209. [Google Scholar] [CrossRef]
- Kamarulzaman, N.H.; Salleh, H.; Dagang, A.N.; Mohd Ghazali, M.S.; Ishak, N.; Ahmad, Z. Optimization of Titanium Dioxide Layer Fabrication Using Doctor Blade Method in Improving Efficiency of Hybrid Solar Cells. J. Phys. Conf. Ser. 2020, 1535, 012025. [Google Scholar] [CrossRef]
- Plecenik, A.; Haidry, A.A.; Plecenik, T.; Durina, P.; Truchly, M.; Mosko, M.; Grancic, B.; Gregor, M.; Roch, T.; Satrapinskyy, L.; et al. Metal Oxide Gas Sensors on the Nanoscale. In Proceedings of the Micro- and Nanotechnology Sensors, Systems, and Applications VI; SPIE: Bellingham, WA, USA, 2014; Volume 9083, p. 90830Y. [Google Scholar]
- Usher, C.R.; Baltrusaitis, J.; Grassian, V.H. Spatially Resolved Product Formation in the Reaction of Formic Acid with Calcium Carbonate (1014): The Role of Step Density and Adsorbed Water-Assisted Ion Mobility. Langmuir 2007, 23, 7039–7045. [Google Scholar] [CrossRef]
- Suprayogi, T.; Masrul, M.Z.; Diantoro, M.; Taufiq, A.; Fuad, A.; Hidayat, A. The Effect of Annealing Temperature of ZnO Compact Layer and TiO2 Mesoporous on Photo-Supercapacitor Performance. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012006. [Google Scholar] [CrossRef]
- Lance, R.; Tate, J. Optical Analysis of Titania: Band Gaps of Brookite, Rutile and Anatase. Bachelor’s Thesis, Oregon State University, Corvallis, OR, USA, 2018. [Google Scholar]
- Möls, K.; Aarik, L.; Mändar, H.; Kasikov, A.; Niilisk, A.; Rammula, R.; Aarik, J. Influence of Phase Composition on Optical Properties of TiO2: Dependence of Refractive Index and Band Gap on Formation of TiO2-II Phase in Thin Films. Opt. Mater. 2019, 96, 109335. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Selvaratnam, B.; Baride, A.; Koodali, R.; Ravirajan, P.; Velauthapillai, D.; Shivatharsiny, Y. Sns2/Tio2 Nanocomposites for Hydrogen Production and Photodegradation under Extended Solar Irradiation. Catalysts 2021, 11, 589. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, Z.-D.; Wang, X.-T.; Zhao, X.-D.; Ning, X.-B.; Lei, J.; Li, X.-R.; Hou, B.-R. Synthesis and Photocathodic Protection Properties of Nanostructured SnS/TiO2 Composites. J. Electrochem. Soc. 2018, 165, H601–H606. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Zhang, P.; Jin, H.; Ding, Y. Natural Assembly of a Ternary Ag-SnS-TiO2 Photocatalyst and Its Photocatalytic Performance under Simulated Sunlight. RSC Adv. 2018, 8, 13408–13416. [Google Scholar] [CrossRef]
- Bronusiene, A.; Popov, A.; Ivanauskas, R.; Ancutiene, I. Preparation and Characterization of Tin Sulfide Films with or without Sodium Chloride. Chem. Phys. 2020, 535, 110766. [Google Scholar] [CrossRef]
- Yun, H.S.; Park, B.W.; Choi, Y.C.; Im, J.; Shin, T.J.; Seok, S.I. Efficient Nanostructured TiO2/SnS Heterojunction Solar Cells. Adv. Energy Mater. 2019, 9, 1901343. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y. Synthesis of CPVC-Modified SnS2/TiO2 Composite with Improved Visible Light-Driven Photocatalysis. Mater. Res. Bull. 2021, 135, 111125. [Google Scholar] [CrossRef]



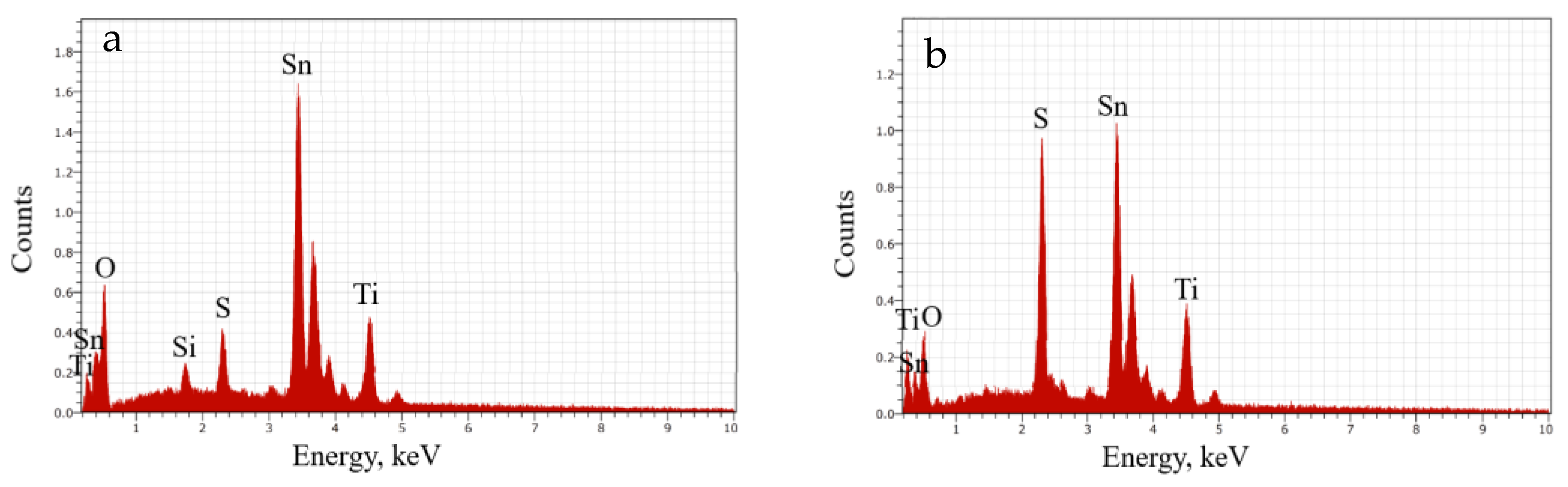

| Elements | Thin Film (a) | Thin Film (b) |
|---|---|---|
| Ti (at.%) | 9.28 | 11.93 |
| O (at.%) | 67.96 | 54.07 |
| Sn (at.%) | 16.01 | 15.11 |
| S (at.%) | 2.61 | 12.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronusiene, A.; Kleinauskas, R.; Ancutiene, I. Facile Synthesis and Characterization of TiO2/SnS Nanocomposites by Eco-Friendly Methods. Coatings 2024, 14, 88. https://doi.org/10.3390/coatings14010088
Bronusiene A, Kleinauskas R, Ancutiene I. Facile Synthesis and Characterization of TiO2/SnS Nanocomposites by Eco-Friendly Methods. Coatings. 2024; 14(1):88. https://doi.org/10.3390/coatings14010088
Chicago/Turabian StyleBronusiene, Asta, Ricardas Kleinauskas, and Ingrida Ancutiene. 2024. "Facile Synthesis and Characterization of TiO2/SnS Nanocomposites by Eco-Friendly Methods" Coatings 14, no. 1: 88. https://doi.org/10.3390/coatings14010088
APA StyleBronusiene, A., Kleinauskas, R., & Ancutiene, I. (2024). Facile Synthesis and Characterization of TiO2/SnS Nanocomposites by Eco-Friendly Methods. Coatings, 14(1), 88. https://doi.org/10.3390/coatings14010088






