Abstract
With the increasing shortage of petroleum resources and the growing seriousness of environmental pollution, the exploitation and application of bio-based coatings derived from renewable resources have become increasingly important for the woodworking industry. Wood wax oil (WWO) is a new type of bio-based natural coating material that offers an eco-friendly solution for wood protection. This paper focused on the utilization of tung oil and beeswax as the primary raw materials for the preparation of wood wax oil. The WWO was based on the oxidation polymerization of tung oil, which served as the foundation for the preparation process. The effects of the photoinitiator TPO-L on the curing performance of the WWO were investigated, and the curing mechanism of the WWO system induced by photoinitiators was analyzed and characterized by infrared spectroscopy. Through ultraviolet irradiation experiments and coating quality tests, the effects of incremental photoinitiators on the properties of the surface drying time, gloss, color, hydrophobicity, and solution resistance of the treated ash wood were studied. The results indicated that the addition of photoinitiators was beneficial for the rapid polymerization of wood wax oil. A UV light intensity of 30 w was found to be sufficient to initiate the curing process. Specifically, when using TPO-L as the initiator at a concentration of 3 wt%, the surface could be surface-dried within 10 min under UV exposure. Under these curing conditions, wood wax oil coatings based on tung oil with comprehensive curing properties can be obtained. Additionally, adding 6% beeswax to the tung oil can effectively enhance the hydrophobicity of pure tung-oil-based wood protective coatings.
1. Introduction
Due to the growing seriousness of environmental pollution and the excessive consumption of resources, the exploitation and application of coatings derived from renewable resources have become increasingly important for the woodworking industry [1]. More research is now focusing on eco-friendly and sustainable wood coatings, particularly those that are biomass-based. Tung oil, as a type of drying oil, is easily obtained from Vernicia fordii seeds [2]. Tung oil possesses several advantages such as biocompatibility, biodegradability, and good corrosion resistance. It finds wide applications in electronic appliances, architectural decoration, and other fields and has an especially important position in the light-curing wood coating field [3,4].
Tung oil is a highly unsaturated plant oil, the main component of which is alpha-eleostearic acid containing octadecyl-conjugated triene and other structures, which can form a solidified film through auto-oxidation at room temperature [5]. Triglycerides are an important source of biopolymers [6]; utilizing Diels–Alder, Friedel–Crafts, esterification, oxidative polymerization, amidation, photochemistry, and other principles, an active reaction can be carried out to endow tung oil with functional groups [7]. The prepolymers are synthesized and then applied to polyester resin, epoxy resin coatings, and other systems [8,9,10]. Bin Liang et al. used tung oil as a raw material to prepare tung-maleic anhydride (TMA) with methyl eleostearate and maleic anhydride. After hydrolysis, it reacted with epichlorohydrin to synthesize epoxidized tung maleic anhydride. After modification with pentaerythritol triacrylate (PETA), a prepolymer with good curing efficiency was synthesized [11]. Based on the former prepolymer, Yugang Huang et al. prepared an acrylate-functionalized tung-oil-based resin with glycidyl methacrylate (GMA) [12]. They converted vegetable oil into a valuable monomer via a pre-chemical modification and then synthesized plant-oil-based polymer materials with high added value with excellent performance; however, the reaction conditions and process were relatively complex, which cannot give full play to the cost advantage of vegetable oil [13].
The protective layer formed by tung oil on the surface of wood is very thin and cannot meet all the requirements of wood protection for wood coatings [14]. As a natural hydrophobic agent, wax has good film formation and water resistance and is widely used in the home and in automobile, construction, and even food science fields [15]. The hydrophobic modification of poplar wood with a mixed emulsion of paraffin wax and carnauba wax can delay the process of water infiltration into wood [16]. Paraffin emulsions combined with titania (TiO2) sol could improve the weathering resistance of thermo-modified Scots pine (Pinus sylvestris L.) [17]. Polypropylene wax is applied to wood coatings, and its surface has excellent thermal stability and adhesion [18]. In addition to synthetic wax, there are many natural waxes with environmental protection properties, such as beeswax, Chinese wax, palm wax, etc., the main components of which are fatty acids, long-chain fatty alcohols, and long-chain alkane esters [19,20,21]. The decoration technology of natural beeswax hot waxing has a long history in China [22]; it has a low melting point and high flexibility, contains gallate myricyl ester and other excellent antioxidant properties, and has certain anti-corrosion and antioxidant properties [23]. The dimensional stability and anti-fungal properties of bamboo can be effectively improved via the heat treatment of wood wax oil at 180 °C, which is mainly composed of tung oil, linseed oil, and beeswax [24]. Therefore, a natural and sustainable wood protection coating was prepared by using tung oil and beeswax. The raw materials of wood wax oil are bio-based and renewable. The reaction conditions and process are relatively simple and low-energy. WWO is very important in providing surface protection to wood and as a decorative coating to improve wood’s appearance, protect it from moisture, and prolong its service life.
For pure biomass coatings, there is a certain gap between their curing film formation speed and that of petroleum-based coatings, but their environmental protection properties are the focus of research, and providing a feasible method for their rapid curing is one of the purposes of this paper. The addition of drying agents, including metal drying agents and rare-earth drying agents, can effectively promote the decomposition of peroxides and speed up the curing speed of tung oil, but metals such as cobalt, manganese, and lead, as well as other metal complexes, are harmful to the environment and human body [25,26]. The current proven approach is carried out with the help of ultraviolet curing technology, which is one of the most efficient, clean, and sustainable technologies in coating and composite crosslinking applications [27,28,29]. UV light induces photoinitiators to change from a ground state to a free radical or cationic transition state and promotes the conversion of monomers to polymers, completing the crosslinking curing behavior [30]. Its light absorption properties directly affect the structure of crosslinked polymers and the properties of cured coatings [31,32]. UV-curable coatings are an important development direction for wood coatings.
In summary, to realize the wood protection effect of wood wax oil more quickly and reduce the processing time, the induction effect of photoinitiators was studied to promote the direct and efficient application of tung oil in the field of wood coatings. In this paper, the effect of photoinitiators on the curing behavior and the photopolymerization mechanism of tung oil is studied using the self-made WWO as a surface-coating material of ash wood. Surface performance and related characterization, such as the surface gloss, color, hydrophobicity, and solvent resistance, were tested to discuss the influence of the initiator amount on the curing property of the coating comprehensively.
2. Materials and Methods
2.1. Materials
Tung oil (TO), containing 81.5% alpha-eleostearic acid, was purchased from Wuhan Linde Chemical Co., Wuhan, China, LTD. Beeswax (BW) was obtained from GuiSen Bee Farm in YiChun, China, and the center part of wax block samples was melted in distilled water to remove impurities before use. The photoinitiator TPO-L (ethyl(2,4,6-trimethylbenzoyl) phenylphosphinate), an analytical-grade reagent, was purchased from Ron’s reagent. The above reagents can be used without further purification. White ash wood (Fraxinus spp.), a type of ring-porous hardwood that is commonly used as a material for home decoration and buildings [33], was sourced from a furniture factory in Harbin, Heilongjiang Province. According to coating and performance testing requirements, three groups of repeated experiments were performed. The uncracked and defect-free areas of the white ash wood were cut into 50 mm (L) × 50 mm (R) × 5 mm (T) wood blocks along the fiber direction after naturally drying in the atmosphere. Some structures of the above materials are shown in Figure 1.
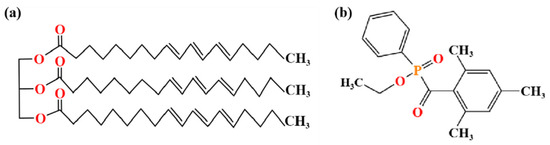
Figure 1.
Structure diagrams of tung oil (a) and photoinitiators TPO-L (b).
2.2. Preparation of Polymerized Tung Oil
We added quantitative tung oil into a 500 mL three-port round-bottom flask and connected a condensing device and thermometer to the flask. The device was placed in a constant-temperature oil bath at 105 °C with a stirring rate of 300 r/min to dehydrate the tung oil for 15 min. Subsequently, we set the desired temperature and stirring rate separately to 180 °C and 300 r/min and maintained this constant temperature for 90 min to allow polymerization to occur. After that, the heating and stirring device was turned off and the polymerized tung oil cooled to room temperature. Then, we obtained the polymerized tung oil.
2.3. Synthesis of Wood Wax Oil
A certain amount of beeswax was added to the pre-polymerized tung oil, and the temperature was set at a constant 75 °C. The stirring rate was set at 400 rpm and the mixing time was 15 min until the mixture was uniform and free of particles. The resulting mixture was a light yellow and transparent wood wax oil (WWO). When the WWO was cooled to room temperature, it appeared like a translucent gel. Photoinitiator TPO-L was added at concentrations of 1 wt%, 2 wt%, 3 wt%, 4 wt%, and 5 wt% to the WWO, separately labeled as N1 to N5, and stored in a shady place. The group without photoinitiator served as the control group (N0) to investigate the effects of different photoinitiator concentrations on the curing performance of the WWO. The specific compositions of the wood wax oil are detailed in Table 1.

Table 1.
Compositions of wood wax oil samples (mass ratio: wt%).
2.4. Preparation of Wood Wax Oil Coatings
The wood block surfaces were polished using sandpaper with three different grades (180, 240, and 600 mesh). Any dust on the surface was removed to ensure it was smooth and flat. Figure 2 illustrates the simplified process of the partial experiment. The abovementioned wood wax oil was used to prepare WWO coatings on wood. The WWO was evenly brushed following the direction of the wood fibers onto the surface with a coating amount of 25 ± 0.5 g·cm−2. The wood surface was wiped with a cotton cloth to allow the WWO to infiltrate into wood pores. Subsequently, the wood blocks were placed in a UV curing machine and the surface drying time was recorded. The selected UV wavelength was 320~400 nm, with a radiant power of 30 w. The control group was cured and dried in a room-temperature environment with good ventilation, and the drying time and hard time were also recorded. The wood samples coated with group N0~N5 were recorded as W0~W5.
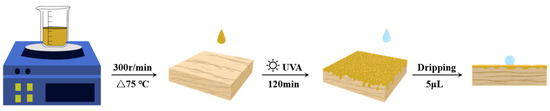
Figure 2.
Simplified diagram of experimental process.
2.5. Curing Performance Characterization
2.5.1. Surface Drying Time Test
To investigate the curing behavior of N0~N5, the wood wax oil was cured in the air at room temperature and under ultraviolet irradiation for a certain time. According to the Chinese national standard (GB/T 1728-89) [34], the wood wax oil surface drying time was determined as specified. According to the standard, we used the finger contact method of method B to measure the surface drying time. The coated wood surfaces were lightly touched with the finger every two minutes. If there was no WWO adhering to the finger, it could be considered that WWO was solidified on the surface of the wood. And then, we recorded the surface drying time.
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR) Test
The surface chemical changes of WWO-coated wood samples were analyzed by Fourier transform infrared spectroscopy (FTIR) employing a Nicolet is50 FTIR spectrometer (Thermo Fisher, Waltham, MA, USA). The spectra were obtained over the wavenumber range of 500–4000 cm−1 with a resolution of 4 cm−1 and averaged over 32 scans. The differences between active functional groups of N0~N5-coated ash wood were compared and analyzed.
2.5.3. Gloss Test
According to the Chinese national standard (GB/T 4893.6-2013) [35], the WCG-60 gloss meter (Pushen Chemical Machinery Co., Ltd., Shanghai, China) was used to determine the surface gloss of untreated wood and WWO-coated wood samples. The gloss meter was first calibrated by adjusting the gloss value while placed on a calibrated glass surface. Measurements were taken at a 60° incidence angle and along the grain, five various points were selected for each sample, and the arithmetic mean values were recorded.
2.5.4. Surface Color Test
The surface color of WWO-coated (UV: 120 min) and uncoated wood samples were tested by a CM-2300d spectrophotometer (Konica Minolta, Tokyo, Japan). The surface color test was based on the CIELAB color system. The lightness index L* (from black, 0, to white, 100), green–red-axis chromaticity index a* (from the negative value of green to the positive value of red), and blue–yellow-axis chromaticity index b* (from the negative value of blue to the positive value of yellow) were measured. The differences in surface color between the samples were expressed as ΔE. According to the standard ASTM D2244-02 [36], ΔE is calculated using L*, a*, and b* which is shown in Equation (1).
where Ln*, an*, and bn* are the measured values of the samples after irradiating with UV light for 120 min; L0*, a0*, and b0* are the measured values of the samples before coating.
△E = [(Ln* − L0*) + (an* − a0*) + (bn* − b0*)]1/2
2.5.5. Contact Angle Test
Under the conditions of relative humidity at 50% and temperature at 25 °C, the static contact angles of the distilled water on the films of untreated wood samples and WWO-coated wood samples were measured using a contact angle goniometer (CA-100B, Yingnuo Instrument Ltd., Shanghai, China). The contact angle was measured by the drop method and the volume of the deionized water droplet was 5 μL. The contact angle was immediately measured when the in-needle droplet contacted the sample surface. The measurements were taken 60 s after deposition using software to record the pictures. Three measurements per sample were taken at random locations along the grain.
2.5.6. Solvent Resistance Test
The surface solvent resistance performances of the cured coatings were tested according to the Chinese national standard (GB/T 17657-2013) [37]. The specimens were placed horizontally in a normal-temperature environment. We then cleaned the surfaces and dropped three drops of a normal-temperature sodium chloride solution, acid solution, alkaline solution, and anhydrous ethanol on the cured surfaces. And then, a sheet of glass was used to cover them separately; after remaining in contact for 16 h, the surfaces of the specimens were rinsed with deionized water. One hour later, the contamination and deformation of the specimens’ surfaces were observed. The evaluation was divided into five grades, with grade I meaning the surface has serious discoloration and grade V meaning the surface is not contaminated.
2.5.7. Surface Microscopy Characterization
The surface microscopy of uncoated wood and WWO-coated wood was observed by scanning electron microscopy (SEM, QUANTAN200, FEI, Eindhoven, The Netherlands). Tangential sections of the samples irradiated with UV light for 120 min were cut and exposed with pathological blades, and sample slices were fixed on a sample holder with conductive glue. Before testing, the coatings were sputter-coated with gold in a vacuum environment for 60 s. The voltage used in the microscope during image capture was 5.0 kV and the working distance was 10 mm.
3. Results and Discussion
3.1. Curing Behavior Analysis
3.1.1. Surface Drying Time
Figure 3 shows the surface drying time of WWO-coated wood with different amounts of photoinitiators. The W0 surface required more than 180 min to dry, which is faster than pure tung oil. The surface drying time decreased from 36 min to 10 min as the amount of photoinitiators increased from 1 wt% to 3 wt%. When the TPO-L was added at a concentration of more than 3 wt%, the curing rates of the coatings began to decrease, which meant that the wood coating needed more time to cure. The changing trend of surface drying time is similar to values reported in the literature in which different photoinitiators were used to research the UV curing activity of pure tung oil [38]. UV curing involves radical polymerization of the tung oil molecule which is converted from a wet to solid state.
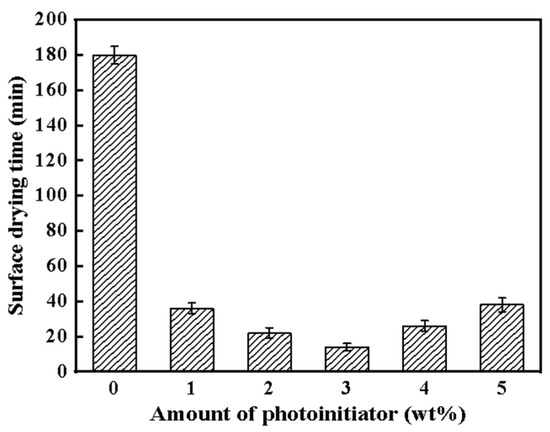
Figure 3.
Surface drying time of different amounts of photoinitiators for the samples in Table 1.
The crosslinking density of wood wax oil increased rapidly due to excessive amounts of photoinitiators, and molecular weight significantly increased in a short time. The synergistic effect between the tung oil film formed on the surface and the condensed beeswax impeded oxygen and ultraviolet radiation from infiltrating into the interior of the coating. Therefore, it reduced the rate of oxidation polymerization in the deep layer of the WWO system. In addition, excessive photoinitiators may produce excess free radicals, resulting in the quenching of active radicals which means termination of polymerization. Therefore, when the amount of photoinitiator added was 4 wt%, the promotional effect on curing was not obvious.
3.1.2. Fourier Transform Infrared Spectra (FTIR) Analysis
The FTIR spectra of wood wax oil-coated wood (W0~W5) with TPO-L (N1~N5) and without TPO-L (N0) are shown in Figure 4. The broad band of untreated white ash wood at 3332 cm−1 corresponds to the stretching vibration of hydroxyl groups (–OH) in wood, and this band did not change obviously in the FTIR spectrum of W0. It was not hard to find that the number of hydroxyl groups in W0~W5 wood was relatively reduced and the –C–O– band of saturated ester was enhanced at 1157 cm−1. It can be reasonably inferred that alpha-eleostearic acid and other unsaturated fatty acids in tung oil undergo esterification, resulting in a decrease in the number of hydroxyl groups in wood, and the unsaturated components of tung oil change under the action of photoinitiator.
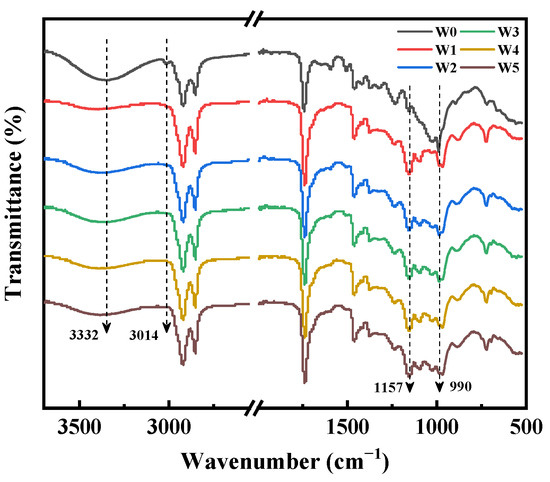
Figure 4.
FTIR spectra of W0~W5.
The absorption peaks of =C–H disappeared at 3014 cm−1 and the tensile vibration peaks of =C–H at 990 cm−1 became weakened in group W1~W5. This means that conjugated double bonds participated in polymerization during the curing of wood wax oil. The photoinitiator successfully cracked to produce free radicals after light irradiation, which led to crosslinking curing of the tung oil monomer. The peak at 1732 cm−1 corresponds to the stretching vibration of unconjugated carbonyl groups of esters [39]. With an increase in photoinitiator concentration, the main functional groups of beeswax did not change significantly, which meant that beeswax and wood only underwent a physical combination, rather than a chemical combination.
The above analysis shows that photoinitiators were activated during the curing reaction and were then copolymerized with a conjugated double bond from a tung oil molecule. The interfacial bonding between beeswax and wood mainly involved weak physical interactions.
3.2. The Effect of Photoinitiator Addition on Coating Properties
3.2.1. Hydrophobicity
The surface of uncoated wood is hydrophilic, which leads to a low water contact angle and the water drop was completely absorbed by capillary forces within 5 s. Figure 5a shows the initial water contact angle of W0 was 105° and decreased to 82° after 60 s. The wood surface changed from hydrophilic to hydrophobic after coating with wood wax oil. Furthermore, the initial water contact angle of W3 was 134° and slightly changed to 133° after 60 s. In the control group, no photoinitiator was added to wood wax oil; therefore, the coating was discontinuous and hydrophilic due to incomplete curing during the same reaction time. Figure 5b shows the water contact angle of coated wood at 0 s and 60 s for all groups. Compared to the other experimental groups, the water contact angle of W1 was lowest. The proportion of early wood in W1 was more and the structure was compacter. Therefore, the penetration rate of oil was slow. The TPO-L content of 1 wt% was not enough to promote the curing of all WWO so the protective coating formed was too thin to obtain a lower contact angle.
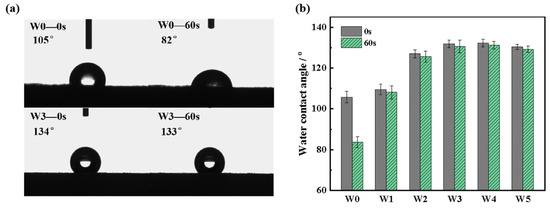
Figure 5.
The water contact angles of wood wax oil-coated wood: (a) W0 and W3; (b) W0~W5.
The hydrophobicity of wood was significantly enhanced by adding photoinitiators to the WWO coating. The photoinitiators were induced by UV light and cleaved into active intermediates, which accelerated the ionic reactivity of the WWO system and was conducive to free radical polymerization of tung oil double bonds. At the same time, it accelerated the solidification of beeswax and increased the intermolecular force between tung oil and beeswax. The content of long-chain fatty acid esters and alcohols in beeswax is higher, and the synergistic effect with tung oil also plays a role in improving the hydrophobicity of wood [40]. It was beneficial for the wood wax oil to cover the grooves on the wood and form a coating that covered the hydrophilic functional groups of wood.
3.2.2. Gloss
Figure 6a shows the surface gloss of WWO-treated wood with different photoinitiator concentrations. In the beginning, the change degree of gloss was obvious. As the coating solidified, the gloss of groups W1 to W5 became stable gradually after UV irradiation for 40 min. It is worth noting that the stable gloss of the wood wax oil coating containing photoinitiator was lower than control group W0. According to the European standard (EN ISO 2813) [41], the stable gloss decline was not substantial. But it shows that the just-coated gloss of WWO coating was affected by the amount of photoinitiator added. For the control group, although the ultraviolet light exceeded 120 min, the surface still kept wet, so the gloss was continuously reduced.
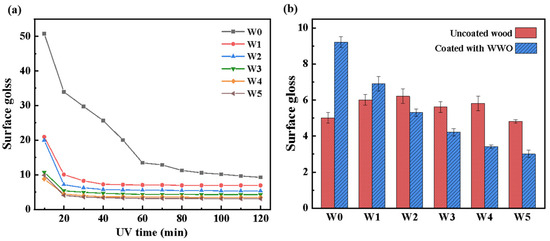
Figure 6.
The surface gloss of W0~W5: (a) gloss evolution with the UV time; (b) the surface gloss of uncoated wood and wood coated with WWO.
As shown in Figure 6b, the surface gloss results of groups W0 and W1 were higher than those of uncoated wood. However, the stable gloss of WWO coating containing photoinitiator is lower than that of uncoated wood. The reason may be that an excessive concentration of photoinitiator will form excessive free radicals locally, causing the recombination of free radicals [42], resulting in incomplete curing of the wood wax oil coating. And beeswax formed a relatively rough coating on the wood surface, which caused diffuse reflection of incident light between the wax particles Previous research has shown that the gloss of photocurable coatings is related to UV irradiation time, and gloss is affected by many factors. The photoinitiator will cause the coating to turn yellow, and the oxygen inhibition will also cause a decrease in gloss.
3.2.3. Color Changes of Wood Surface
After UV curing for 120 min, the color changes of ash wood in the experimental group and the control group are shown in Figure 7. It can be seen that the wood wax coating changes the color of the white ash wood. Compared with the color index of W0, the lightness index (L*) of W1~W5 wood samples decreased; this was consistent with the results of the gloss test. The green–red-axis color index (a*) and blue–yellow-axis color index (b*) increased, indicating that the color of the wood surface deepened and changed to red and yellow after wood wax oil treatment. In general, the values of index △E* of experimental groups were all greater than W0 (△E* = 6.042). Among them, the changes in color parameters of W3~W5 were similar but higher than those of W1 and W2. Photoinitiators were very sensitive to UV light, and photoinitiator additions exceeding 3 wt% led to more obvious changes in the wood surface. According to the six-degree measurement scale [43], the total color difference ΔE* values ranged from degree five to six. The discoloration varied from just visually detectable changes up to a completely new color. To some extent, the coating influence was positive and the deepened color was closer to precious wood.
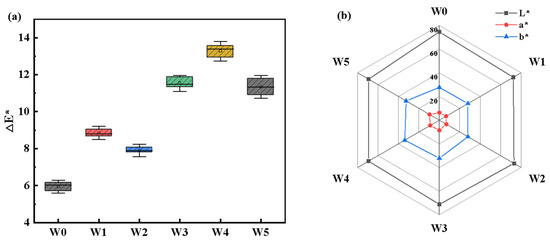
Figure 7.
Color changes of coated wood with N0~N5 during UV-120 min: (a) Global color changes (ΔE*); (b) Lightness changes (ΔL*), redness changes (Δa*), and yellowness changes (Δb*).
3.2.4. Solvent Resistance
It can be seen from Table 2 that the WWO-coated wood surfaces of W0~W5 exhibited varying resistance to different solvents. All the curing surfaces were grade V after contact with the sodium chloride solution, and there was basically no change. The curing surface was grade IV after contact with acetic acid, and there was a slight change. There were only slight changes noted after exposure to acetic acid. The surfaces coated with WWO were seriously discolored under the influence of alkali liquor. The changes in the W1 and W2 groups were grade I, which were more obvious than grade II.

Table 2.
Solvent resistance class of photocurable coatings.
These results indicate that the crosslinking density of the UV-cured wood wax oil coating increases, leading to a relatively dense three-dimensional network structure. Consequently, the solvent resistance of the cured coating is significantly improved, effectively slowing down the damage caused by salt, acid, ethanol, or other solutions to white ash wood. After contact with the alkali solution, the surface changes, as the tung oil acid still contained in the WWO is sensitive to alkaline substances, resulting in slightly weaker alkali resistance than other solutions. Considering the problem of poor durability of tung oil in outdoor applications, it is necessary to further study the enhancement of wood wax oil coatings [44].
3.2.5. Scanning Electron Microscopy (SEM) Analysis
The SEM images of the tangential section of W3 in Figure 8b,d are compared with untreated wood in Figure 8a,c. The characteristic tangential structure of uncoated wood with open cell walls and pits can be observed. After coating with N3, these microstructures were covered by a rough coating formed by wood wax oil. WWO was found in wood rays, fibers, and vessels and filled in the wood cell cavities. This resulted in a protective layer covering the wood surface on a macro level [45]. The photocurable coating exhibited a micron-scale roughness; according to the lotus effect, a rough micro/nanolayered structure can make the surface non-wettable [46]. WWO-coated ash wood had better hydrophobicity, which was also proved by the contact angle test results. Additionally, the induction of photoinitiators resulted in faster tung oil polymerization within the same UV exposure time. The presence of wrinkled oil film and beeswax particles contributed to the formation of a rough surface, which in turn led to reduced gloss due to diffuse reflection between the concave and convex layers of the wax.
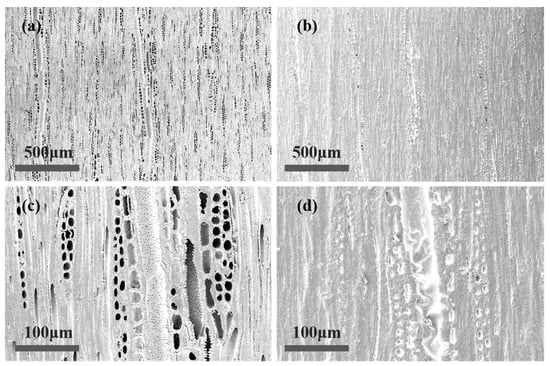
Figure 8.
SEM micrographs of white ash wood: (a) uncoated tangential section (100×), (b) W3′s tangential section (100×), (c) uncoated tangential section (500×), (d) W3′s tangential section (500×).
4. Conclusions
In this fundamental study, we prepared a UV-curable wood-protective coating using natural materials. The practical impact of different amounts of photoinitiator TPO-L on the photochemical reaction of wood wax oil was investigated through Fourier transform infrared spectroscopy. The surface drying time was directly affected by the additive amount. Specifically, when it was 3 wt%, the surface drying time was found to be the shortest. Wood wax oil can fill in the wood cell cavity, leading to an improvement in wood’s hydrophobicity. The resulting cured coatings exhibited moderate chromaticity and demonstrated good solvent resistance. The stability of the surface gloss was found to be directly linked to the addition of the initiator, with excessive photoinitiators leading to a reduction in gloss.
Wood wax oil, as a bio-based environmental protection material, holds significant potential for use in environmentally friendly wood coatings, especially when combined with ultraviolet curing technology. The synergistic effect of other natural waxes in WWO can be studied in the future. This work can serve as a starting point for future exploration and application in the preparation of improved coatings based on drying oils and natural wax.
Author Contributions
Conceptualization, D.Z.; methodology, D.Z. software, D.Z.; validation, D.Z. and K.S.; formal analysis, D.Z.; investigation, D.Z.; resources, D.Z.; data curation, D.Z.; writing—original draft preparation, D.Z.; writing—review and editing, D.Z.; visualization, D.Z.; supervision, K.S.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31770592).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, P.D.; Matsunaga, H.; Preston, A.F.; Kewish, C.M. Wood Protection for Carbon Sequestration—A Review of Existing Approaches and Future Directions. Curr. For. Rep. 2022, 8, 181–198. [Google Scholar] [CrossRef]
- Ribeiro, B.O.; Valério, V.S.; Gandini, A.; Lacerda, T.M. Copolymers of Xylan-Derived Furfuryl Alcohol and Natural Oligomeric Tung Oil Derivatives. Int. J. Biol. Macromol. 2020, 164, 2497–2511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, Z.; Liu, X.; Kang, D.; Dong, W.; Lin, Q.; Zhang, A. Research Progress of Tung Oil/UV Photocomposite Curing Material. J. Renew. Mater. 2022, 11, 1661–1686. [Google Scholar] [CrossRef]
- Yu, X.; Hu, Y.; Lei, W.; Liu, C.; Zhou, Y. Development of Catalyst-Free Self-Healing Biobased UV-Curable Coatings via Maleate Monoester Transesterification. Coatings 2023, 13, 110. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Li, W. The Performance of Biodegradable Tung Oil Coatings. Prog. Org. Coat. 2015, 85, 216–220. [Google Scholar] [CrossRef]
- Yi, W. Drying Oils Treated by Irradiation as Coatings for Biobased Films. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2008. [Google Scholar]
- Seniha Güner, F.; Yağcı, Y.; Tuncer Erciyes, A. Polymers from Triglyceride Oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Castor Oil-Based Hyperbranched Polyurethanes as Advanced Surface Coating Materials. Prog. Org. Coat. 2013, 76, 157–164. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.; Mustata, F.; Rosu, D.; Rosca, I.; Rusu, T. Epoxy Coatings Based on Modified Vegetable Oils for Wood Surface Protection against Fungal Degradation. ACS Appl. Mater. Interfaces 2020, 12, 14443–14458. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent Advances in Vegetable Oils Based Environment Friendly Coatings: A Review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile Synthesis and Characterization of Novel Multi-Functional Bio-Based Acrylate Prepolymers Derived from Tung Oil and Its Application in UV-Curable Coatings. Ind. Crops Prod. 2019, 138, 111585. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Vuluga, D.; Lecamp, L.; Burel, F. A Rapid, Eco- and Environmental Friendly Alternative to Oil Oxidation for the Preparation of Fatty Coatings Using Photoinitiated Thiol-Ene Chemistry. Prog. Org. Coat. 2016, 101, 216–224. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chatterjee, K. Polymers and Composites Derived from Castor Oil as Sustainable Materials and Degradable Biomaterials: Current Status and Emerging Trends. Biomacromolecules 2020, 21, 4639–4662. [Google Scholar] [CrossRef]
- He, L.; Zhang, T.; Zhao, X.; Zhao, Y.; Xu, K.; He, Z.; Yi, S. Synergistic Effect of Tung Oil and Heat Treatment on Surface Characteristics and Dimensional Stability of Wood. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131233. [Google Scholar] [CrossRef]
- Fei, T.; Wang, T. A Review of Recent Development of Sustainable Waxes Derived from Vegetable Oils. Curr. Opin. Food Sci. 2017, 16, 7–14. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Zhang, S.; Cao, J.; Wang, W. Forming Textured Hydrophobic Surface Coatings via Mixed Wax Emulsion Impregnation and Drying of Poplar Wood. Wood Sci. Technol. 2020, 54, 421–439. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, S.; Cao, J.; Jiang, J.; Wang, W. Improving Anti-Weathering Performance of Thermally Modified Wood by TiO2 Sol or/and Paraffin Emulsion. Constr. Build. Mater. 2018, 169, 372–378. [Google Scholar] [CrossRef]
- Wang, X.; Song, K. Improvement of Surface Coating and Interfacial Properties of Hot-Waxed Wood Using Maleic Anhydride Grafted Polypropylene Wax. Forests 2022, 13, 1205. [Google Scholar] [CrossRef]
- Liu, X.Y.; Timar, M.C.; Varodi, A.M. A Comparative Study on the Artificial UV and Natural Ageing of Beeswax and Chinese Wax and Influence of Wax Finishing on the Ageing of Chinese Ash (Fraxinus mandshurica) Wood Surfaces. J. Photochem. Photobiol. B-Biol. 2019, 201, 111607. [Google Scholar] [CrossRef] [PubMed]
- Lesar, B.; Pavlič, M.; Petrič, M.; Škapin, A.S.; Humar, M. Wax Treatment of Wood Slows Photodegradation. Polym. Degrad. Stab. 2011, 96, 1271–1278. [Google Scholar] [CrossRef]
- Tulloch, A.P. The Composition of Beeswax and Other Waxes Secreted by Insects. Lipids 1970, 5, 247–258. [Google Scholar] [CrossRef]
- Niu, K.; Song, K. Hot Waxing Treatment Improves the Aging Resistance of Wood Surface under UV Radiation and Water. Prog. Org. Coat. 2021, 161, 106468. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Zhao, Z.; Guo, H.; Wang, Z.; Jin, C. Improved Properties of Bamboo by Thermal Treatment with Wood Wax Oil. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128807. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, N.; Xu, R.; Huang, B.; Yang, Y.; Liao, Y. Preparation of High-efficient Tung Oil Drier and Its Drying Performance. Chem. Ind. For. Prod. 2019, 39, 101–107. [Google Scholar]
- Soucek, M.D.; Khattab, T.; Wu, J. Review of Autoxidation and Driers. Prog. Org. Coat. 2012, 73, 435–454. [Google Scholar] [CrossRef]
- Xi, X.; Yuan, W. UV-Curable Fluorocarbon Polyurethane Coatings for Marble Kitchen Countertops. Coatings 2023, 13, 1394. [Google Scholar] [CrossRef]
- Chen, G.; Guan, X.; Xu, R.; Tian, J.; He, M.; Shen, W.; Yang, J. Synthesis and Characterization of UV-Curable Castor Oil-Based Polyfunctional Polyurethane Acrylate via Photo-Click Chemistry and Isocyanate Polyurethane Reaction. Prog. Org. Coat. 2016, 93, 11–16. [Google Scholar] [CrossRef]
- Rawat, R.S.; Chouhan, N.; Talwar, M.; Diwan, R.K.; Tyagi, A.K. UV Coatings for Wooden Surfaces. Prog. Org. Coat. 2019, 135, 490–495. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Xie, C.; Tian, X.; Song, L.; Liu, L.; Wang, Z.; Yu, Q. Naphthyl-Based Acylphosphine Oxide Photoinitiators with High Effiency and Low Migration. Prog. Org. Coat. 2020, 142, 105603. [Google Scholar] [CrossRef]
- Kim, G.-T.; Go, H.-B.; Yu, J.-H.; Yang, S.-Y.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Cytotoxicity, Colour Stability and Dimensional Accuracy of 3D Printing Resin with Three Different Photoinitiators. Polymers 2022, 14, 979. [Google Scholar] [CrossRef]
- Sanai, Y.; Ninomiya, T.; Arimitsu, K. Improvements in the Physical Properties of UV-Curable Coating by Utilizing Type II Photoinitiator. Prog. Org. Coat. 2021, 151, 106038. [Google Scholar] [CrossRef]
- Eun, S.; Chun, W. The Use of Ring-Porous East Asian Ash (Fraxinus Japonica (Thunb.) Steud.) and Oak (Quercus spp.) Cross-Sections as Eco-Friendly Resonance-Absorbing Materials for Building. Wood Mate. Sci. Eng. 2021, 17, 1002–1009. [Google Scholar] [CrossRef]
- GB/T 1728-2020; Etermination of Drying Time of Coating and Putty Films. Standardization Administration of China, SAC: Beijing, China, 2020.
- GB/T 4893.6-2013; Test of Surface Coatings of Furniture—Part 6: Determination of Gloss Value. Standardization Administration of China, SAC: Beijing, China, 2013.
- ASTM D2244-02,2003; Standard Practice for Calculation of Color Tolerances and Color Differences from Instrumentally Measured Color Coor-Dinates. ASTM International: West Conshohocken, PA, USA, 2003.
- GB/T 17657-2013; Test Methods of Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels. Standardization Administration of China, SAC: Beijing, China, 2013.
- Huang, J.; Yuan, T.; Ye, X.; Man, L.; Zhou, C.; Hu, Y.; Zhang, C.; Yang, Z. Study on the UV Curing Behavior of Tung Oil: Mechanism, Curing Activity, and Film-Forming Property. Ind. Crops Prod. 2018, 112, 61–69. [Google Scholar] [CrossRef]
- Niu, K.; Song, K. Surface Coating and Interfacial Properties of Hot-Waxed Wood Using Modified Polyethylene Wax. Prog. Org. Coat. 2021, 150, 105947. [Google Scholar] [CrossRef]
- Janesch, J.; Arminger, B.; Gindl-Altmutter, W.; Hansmann, C. Superhydrophobic Coatings on Wood Made of Plant Oil and Natural Wax. Prog. Org. Coat. 2020, 148, 105891. [Google Scholar] [CrossRef]
- EN ISO 2813,2014; Paints and Varnishes—Determination of Specular Gloss of Non-Metallic Paint Films at 20°, 60° and 85°. British Standards Institution (BSI): Brussels, Belgium, 2014.
- Jančovičová, V.; Mikula, M.; Havlínová, B.; Jakubíková, Z. Influence of UV-Curing Conditions on Polymerization Kinetics and Gloss of Urethane Acrylate Coatings. Prog. Org. Coat. 2013, 76, 432–438. [Google Scholar] [CrossRef]
- Allegretti, O.; Travan, L.; Cividini, R. Drying Techniques to Obtain White Beech. In Proceedings of the Wood EDG Conference, Bled, Slovenia, 23 April 2009; Available online: http://timberdry.net/downloads/EDG-SeminarBled/Presentation/EDG (accessed on 10 December 2023).
- Kudela, J.; Gondas, L. Stability testing of coating materials proposed for renovation of surface finish windows. Acta Fac. Xylologiae Zvolen 2022, 64, 25–37. [Google Scholar] [CrossRef]
- Song, X. Preparation of Fragrance Releasing Wood Wax Oil and Their Effect on Finished Wood. Degree of Master, Northeast Forestry University, Harbin, China, 2022. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Kumar Sadasivuni, K.; Xing, R.; Liu, S. Self—Cleaning Superhydrophobic Coatings: Potential Industrial Applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).