Impact of Molecular Weight on Anti-Bioadhesion Efficiency of PDMS-Based Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PDMS Functionalization
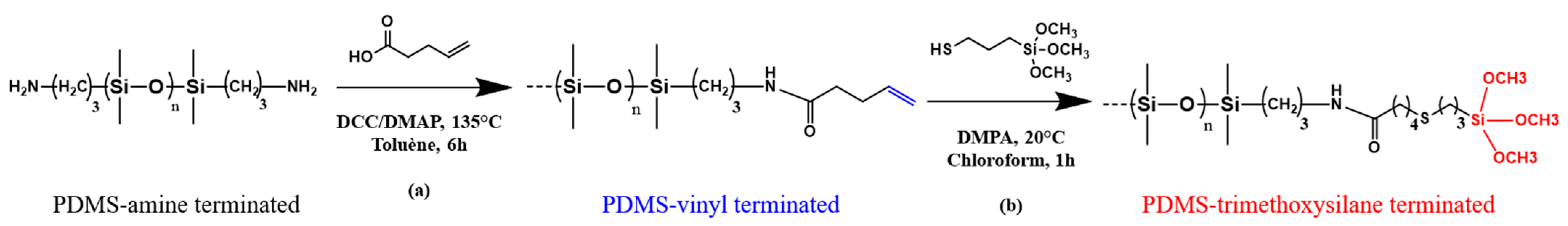
2.2.1. PDMS-NH2 Functionalization via Steglich Esterification: PDMS-V2.5K
2.2.2. PDMS-V-MW Functionalization via Thiol-Ene Reaction: PDMS-TMS-MW
2.3. Coating Preparation
2.4. Characterization
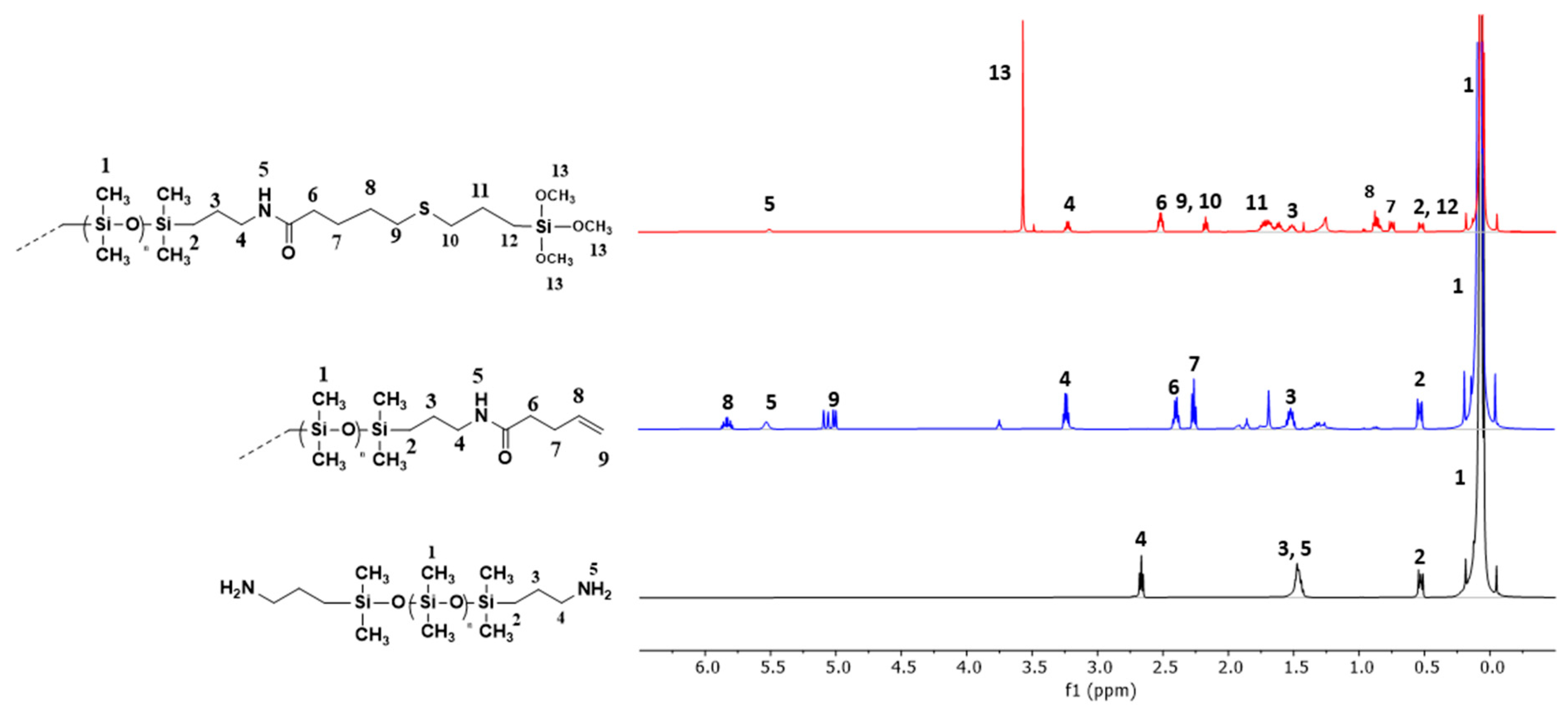
2.4.1. Proton Nuclear Magnetic Resonance Spectroscopy
2.4.2. Gel Permeation Chromatography
2.4.3. Soxhlet Extraction
2.5. Surface Characterization
2.6. Marine Field Test
3. Results and Discussion
3.1. Modification of PDMS Polymers
3.2. Preparation of PDMS Coating
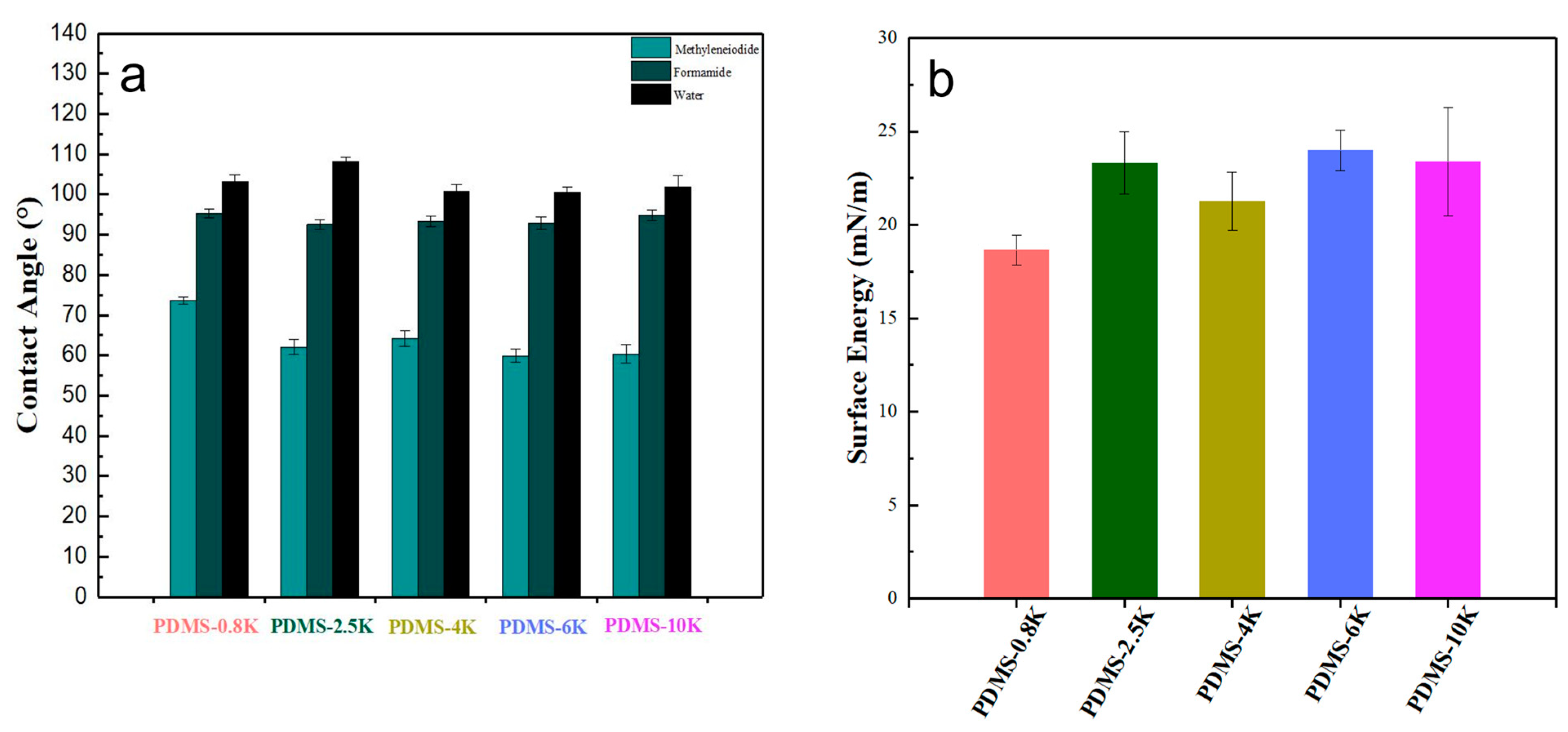
3.3. Surface Properties of PDMS Coatings
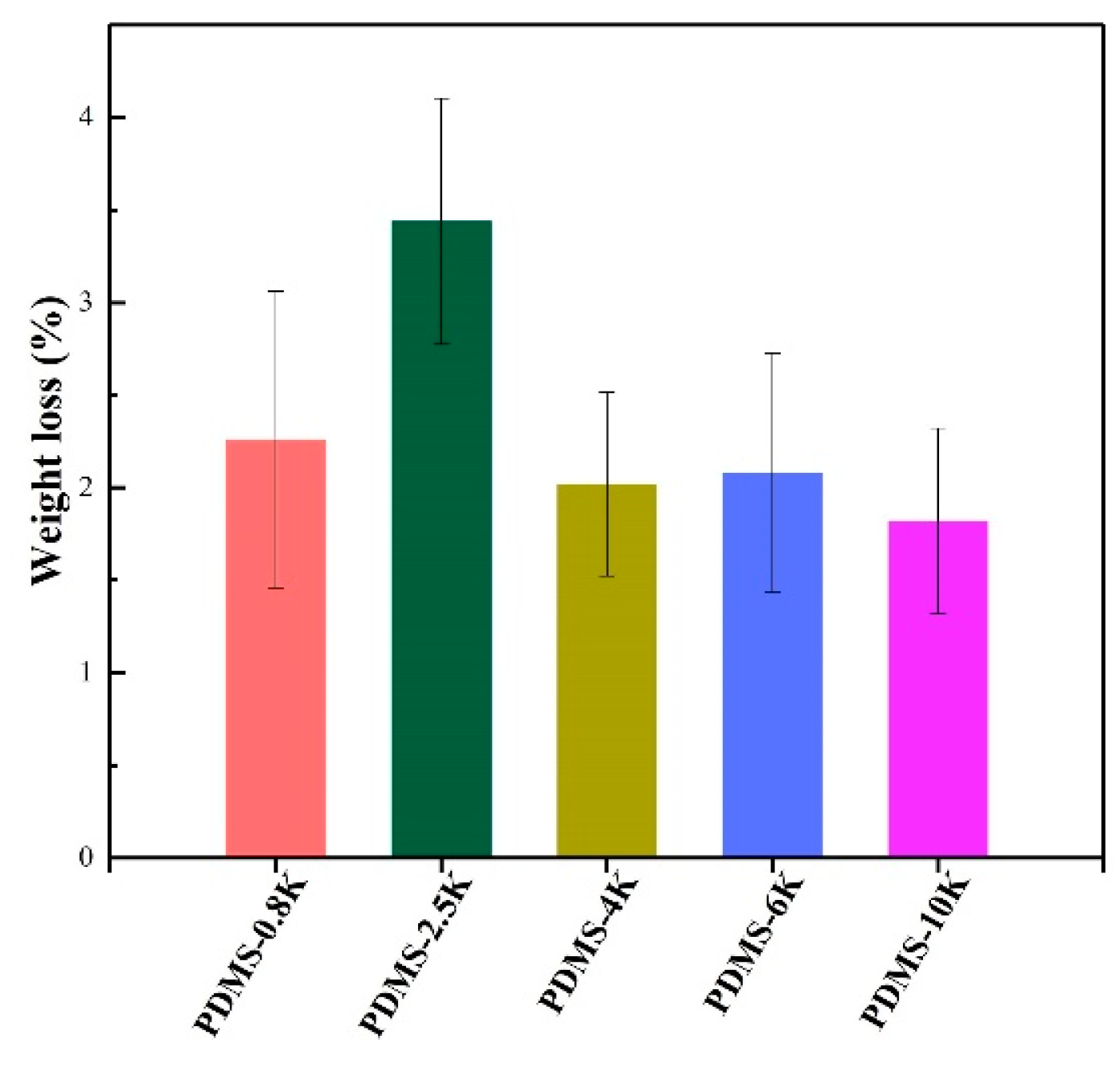
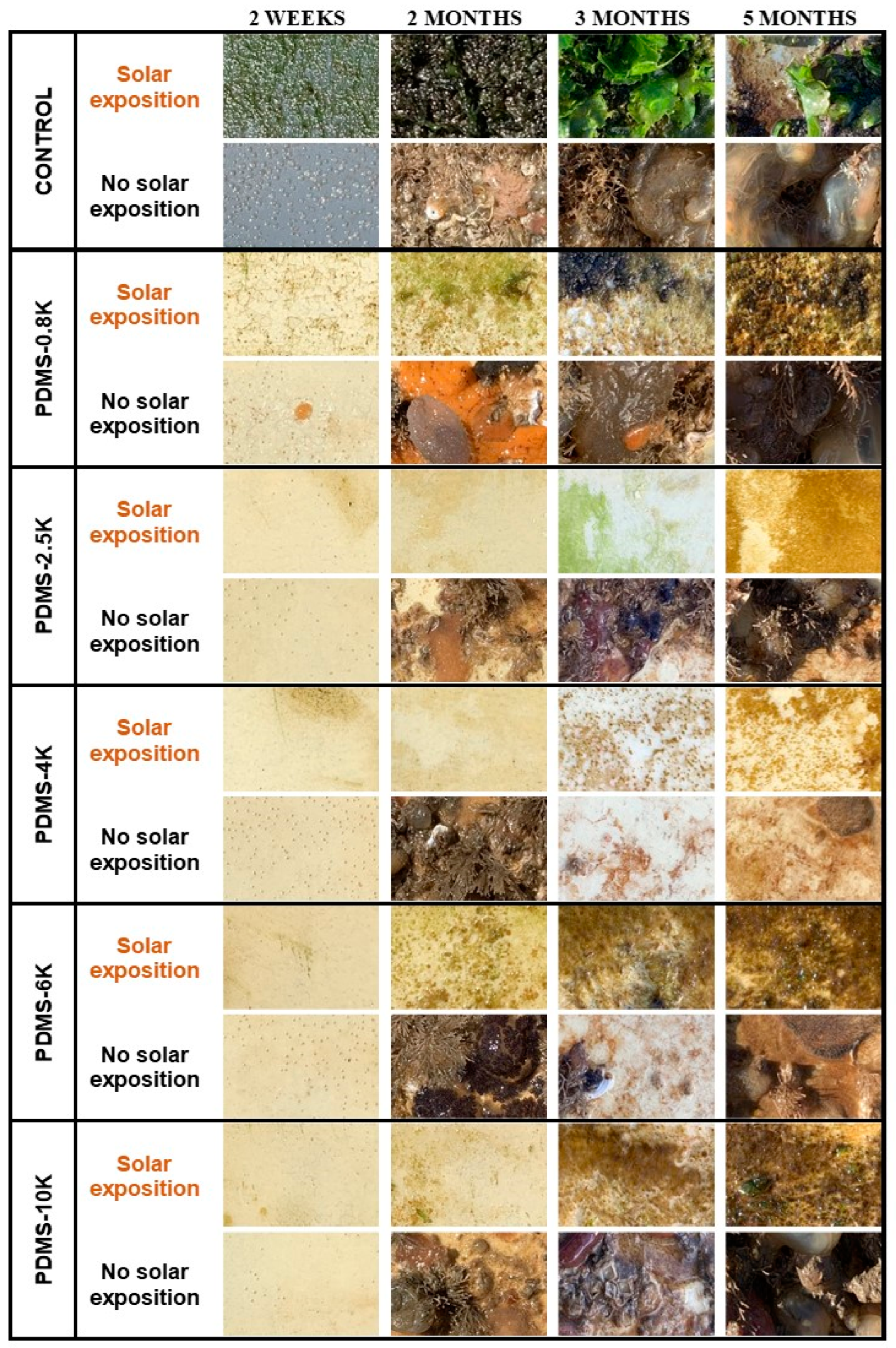
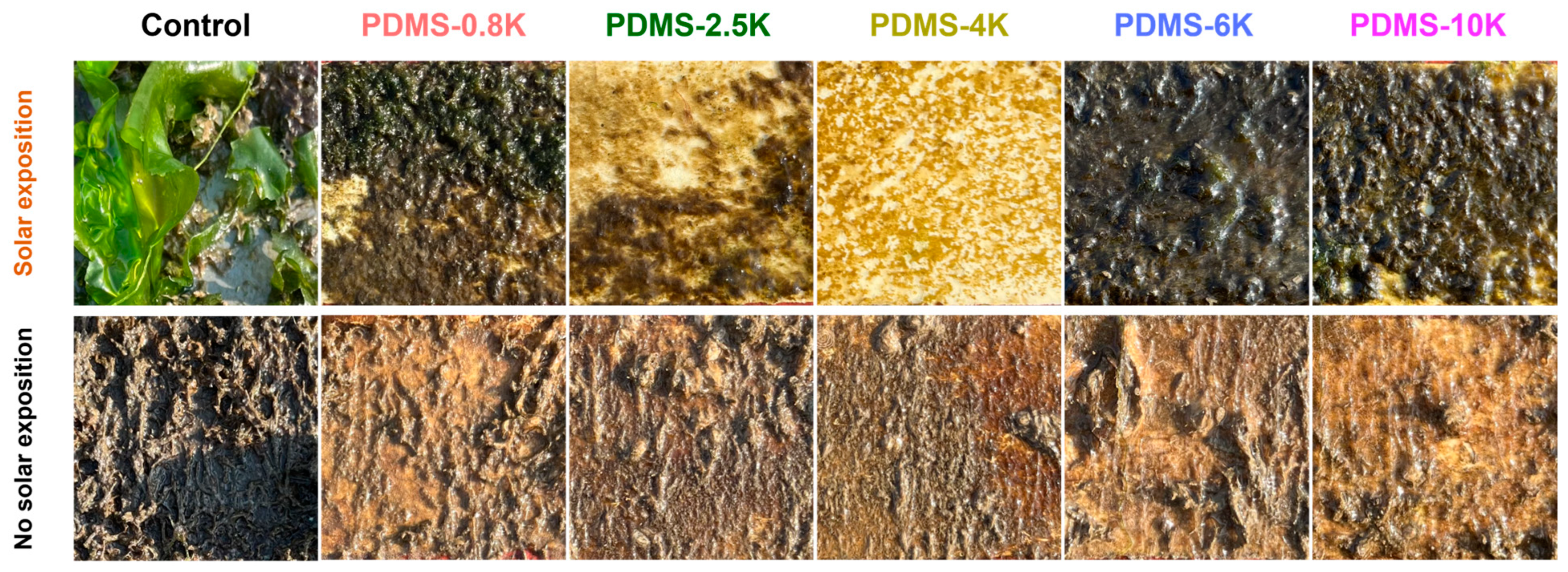
3.4. Marine Field Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling Technology—Past, Present and Future Steps towards Efficient and Environmentally Friendly Antifouling Coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.E. Surface Behaviour of Biomaterials: The Theta Surface for Biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, M.K.; Finlay, J.A.; Chung, J.Y.; Callow, M.E.; Callow, J.A. The Influence of Elastic Modulus and Thickness on the Release of the Soft-Fouling Green Alga Ulva linza (Syn. Enteromorpha Linza) from Poly(Dimethylsiloxane) (PDMS) Model Networks. Biofouling 2005, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chisholm, B.J.; Bahr, J. Adhesion Study of Silicone Coatings: The Interaction of Thickness, Modulus and Shear Rate on Adhesion Force. Biofouling 2007, 23, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pouget, E.; Tonnar, J.; Lucas, P.; Lacroix-Desmazes, P.; Ganachaud, F.; Boutevin, B. Well-Architectured Poly(Dimethylsiloxane)-Containing Copolymers Obtained by Radical Chemistry. Chem. Rev. 2010, 110, 1233–1277. [Google Scholar] [CrossRef]
- Lampe, W.R.; Moore, A.A.; Hartley, K.R. Marine Foulant Release Coating. U.S. Patent 4861670A, 29 August 1989. [Google Scholar]
- Wynne, K.; Swain, G.; Fox, R.; Bullock, S.; Uilk, J. Two Silicone Nontoxic Fouling Release Coatings: Hydrosilation Cured PDMS and CaCO3 Filled, Ethoxysiloxane Cured RTV11. Biofouling 2000, 16, 277–288. [Google Scholar] [CrossRef]
- Edward, R. Ship’s Hull Coated with Anti-Fouling Silicone Resin and Method of Coating. U.S. Patent 2986474A, 30 May 1961. [Google Scholar]
- Truby, K.; Wood, C.; Stein, J.; Cella, J.; Carpenter, J.; Kavanagh, C.; Swain, G.; Wiebe, D.; Lapota, D.; Meyer, A.; et al. Evaluation of the Performance Enhancement of Silicone Biofouling-Release Coatings by Oil Incorporation. Biofouling 2000, 15, 141–150. [Google Scholar] [CrossRef]
- Qiu, C.; Xiong, W.; Zhang, H.; Zhang, R.; Parkin, I.P.; Wang, S.; Li, L.; Chen, J.; Chen, Z.; Tapa, A.R.; et al. Superhydrophobicity Transfer Effect in Superwetting Coatings for Strengthening Anti-Pollution Flashover Performance. Prog. Org. Coat. 2024, 186, 107955. [Google Scholar] [CrossRef]
- Stein, J.; Truby, K.; Wood, C.D.; Stein, J.; Gardner, M.; Swain, G.; Kavanagh, C.; Kovach, B.; Schultz, M.; Wiebe, D.; et al. Silicone Foul Release Coatings: Effect of the Interaction of Oil and Coating Functionalities on the Magnitude of Macrofouling Attachment Strengths. Biofouling 2003, 19, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.L.; Banta, W.C.; Loeb, G.I. Aquatic Biofouling Larvae Respond to Differences in the Mechanical Properties of the Surface on Which They Settle. Biofouling 2002, 18, 269–273. [Google Scholar] [CrossRef]
- Gillet, G.; Azemar, F.; Faÿ, F.; Réhel, K.; Linossier, I. Non-Leachable Hydrophilic Additives for Amphiphilic Coatings. Polymers 2018, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of Contact Deformations on the Adhesion of Particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Drebezghova, V.; Hakil, F.; Grimaud, R.; Gojzewski, H.; Vancso, G.J.; Nardin, C. Initial Bacterial Retention on Polydimethylsiloxane of Various Stiffnesses: The Relevance of Modulus (Mis)Match. Colloids Surf. B Biointerfaces 2022, 217, 112709. [Google Scholar] [CrossRef]
- Murthy, R.; Cox, C.D.; Hahn, M.S.; Grunlan, M.A. Protein-Resistant Silicones: Incorporation of Poly(Ethylene Oxide) via Siloxane Tethers. Biomacromolecules 2007, 8, 3244–3252. [Google Scholar] [CrossRef]
- de Dantas, L.C.M.; da Silva-Neto, J.P.; Dantas, T.S.; Naves, L.Z.; das Neves, F.D.; da Mota, A.S. Bacterial Adhesion and Surface Roughness for Different Clinical Techniques for Acrylic Polymethyl Methacrylate. Int. J. Dent. 2016, 2016, 8685796. [Google Scholar] [CrossRef]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The Influence of Nano-Scale Surface Roughness on Bacterial Adhesion to Ultrafine-Grained Titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef]
- Drebezghova, V.; Gojzewski, H.; Allal, A.; Hempenius, M.A.; Nardin, C.; Vancso, G.J. Network Mesh Nanostructures in Cross-Linked Poly(Dimethylsiloxane) Visualized by AFM. Macromol. Chem. Phys. 2020, 221, 2000170. [Google Scholar] [CrossRef]
- Guennec, A.; Brelle, L.; Balnois, E.; Linossier, I.; Renard, E.; Langlois, V.; Faÿ, F.; Chen, G.Q.; Simon-Colin, C.; Vallée-Réhel, K. Antifouling Properties of Amphiphilic Poly(3-Hydroxyalkanoate): An Environmentally-Friendly Coating. Biofouling 2021, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ren, D. Stiffness of Cross-Linked Poly(Dimethylsiloxane) Affects Bacterial Adhesion and Antibiotic Susceptibility of Attached Cells. Langmuir 2014, 30, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Straub, H.; Bigger, C.M.; Valentin, J.; Abt, D.; Qin, X.-H.; Eberl, L.; Maniura-Weber, K.; Ren, Q. Bacterial Adhesion on Soft Materials: Passive Physicochemical Interactions or Active Bacterial Mechanosensing? Adv. Healthc. Mater. 2019, 8, 1801323. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, K.; Hao, R.; Zhang, Z.; Feng, Z.; Shi, Z.; Yuan, W.; Jing, Z.; Zhang, L. Disregarded Free Chains Affect Bacterial Adhesion on Cross-Linked Polydimethylsiloxane Surfaces. ACS Appl. Mater. Interfaces 2023, 15, 36936–36944. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, R.R.; Kato, N.; Awaji, H.; Matsuyama, H. Development of Polydimethylsiloxane Composite Membrane for Organic Solvent Separation. Sep. Purif. Technol. 2022, 285, 120369. [Google Scholar] [CrossRef]
- Gevaux, L.; Lejars, M.; Margaillan, A.; Bressy, C. Water Erodible Coatings Based on a Hydrolyzable PDMS/Polyester Network. Mater. Today Commun. 2018, 17, 517–526. [Google Scholar] [CrossRef]
- Dobretsov, S.; Coutinho, R.; Rittschof, D.; Salta, M.; Ragazzola, F.; Hellio, C. The Oceans Are Changing: Impact of Ocean Warming and Acidification on Biofouling Communities. Biofouling 2019, 35, 585–595. [Google Scholar] [CrossRef]
- Vinagre, P.A.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. J. Mar. Sci. Eng. 2020, 8, 495. [Google Scholar] [CrossRef]
- Brady, R.F.; Singer, I.L. Mechanical Factors Favoring Release from Fouling Release Coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef]
- Liu, L.; Ercan, B.; Sun, L.; Ziemer, K.S.; Webster, T.J. Understanding the Role of Polymer Surface Nanoscale Topography on Inhibiting Bacteria Adhesion and Growth. ACS Biomater. Sci. Eng. 2016, 2, 122–130. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial Anti-Adhesion Surface Design: Surface Patterning, Roughness and Wettability: A Review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Valentin, J.D.P.; Qin, X.-H.; Fessele, C.; Straub, H.; van der Mei, H.C.; Buhmann, M.T.; Maniura-Weber, K.; Ren, Q. Substrate Viscosity Plays an Important Role in Bacterial Adhesion under Fluid Flow. J. Colloid Interface Sci. 2019, 552, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Azemar, F.; Faÿ, F.; Réhel, K.; Linossier, I. Ecofriendly Silicon-Poly(Lactic Acid) Hybrid Antifouling Coatings. Prog. Org. Coat. 2020, 148, 105841. [Google Scholar] [CrossRef]


| Commercial Polymers | Theoretical Molar Masses g·mol−1 | Modified Polymers | Molar Masses by 1H NMR (g·mol−1) a | Molar Masses by SEC (g·mol−1) b | ÐM |
|---|---|---|---|---|---|
| PDMS-V0.8K | 800 | PDMS-TMS0.8K | 858 | 1341 | 1.83 |
| PDMS-N2.5K | 2500 | PDMS-TMS2.5K | 2775 | 4404 | 2.18 |
| PDMS-TMS4K | 4010 | - | 3774 | 4775 | 2.06 |
| PDMS-V6K | 6000 | PDMS-TMS6K | 5164 | 7173 | 3.14 |
| PDMS-V10K | 9400 | PDMS-TMS10K | 9021 | 11,720 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangoura, M.A.; Mimeau, D.; Balnois, E.; Réhel, K.; Azemar, F.; Linossier, I. Impact of Molecular Weight on Anti-Bioadhesion Efficiency of PDMS-Based Coatings. Coatings 2024, 14, 149. https://doi.org/10.3390/coatings14010149
Bangoura MA, Mimeau D, Balnois E, Réhel K, Azemar F, Linossier I. Impact of Molecular Weight on Anti-Bioadhesion Efficiency of PDMS-Based Coatings. Coatings. 2024; 14(1):149. https://doi.org/10.3390/coatings14010149
Chicago/Turabian StyleBangoura, Mama Aïssata, David Mimeau, Eric Balnois, Karine Réhel, Fabrice Azemar, and Isabelle Linossier. 2024. "Impact of Molecular Weight on Anti-Bioadhesion Efficiency of PDMS-Based Coatings" Coatings 14, no. 1: 149. https://doi.org/10.3390/coatings14010149
APA StyleBangoura, M. A., Mimeau, D., Balnois, E., Réhel, K., Azemar, F., & Linossier, I. (2024). Impact of Molecular Weight on Anti-Bioadhesion Efficiency of PDMS-Based Coatings. Coatings, 14(1), 149. https://doi.org/10.3390/coatings14010149







