Abstract
Titanium alloys are acclaimed for their remarkable biocompatibility, high specific strength, excellent corrosion resistance, and stable performance in high and low temperatures. These characteristics render them invaluable in a multitude of sectors, including biomedicine, shipbuilding, aerospace, and daily life. According to the different phases, the alloys can be broadly categorized into α-titanium and β-titanium, and these alloys demonstrate unique properties shaped by their respective phases. The hexagonal close-packed structure of α-titanium alloys is notably associated with superior high-temperature creep resistance but limited plasticity. Conversely, the body-centered cubic structure of β-titanium alloys contributes to enhanced slip and greater plasticity. To optimize these alloys for specific industrial applications, alloy strengthening is often necessary to meet diverse environmental and operational demands. The impact of various processing techniques on the microstructure and metal characteristics of titanium alloys is reviewed and discussed in this research. This article systematically analyzes the effects of machining, shot peening, and surface heat treatment methods, including surface quenching, carburizing, and nitriding, on the structure and characteristics of titanium alloys. This research is arranged and categorized into three categories based on the methods of processing and treatment: general heat treatment, thermochemical treatment, and machining. The results of a large number of studies show that surface treatment can significantly improve the hardness and friction mechanical properties of titanium alloys. At present, a single treatment method is often insufficient. Therefore, composite treatment methods combining multiple treatment techniques are expected to be more widely used in the future. The authors provide an overview of titanium alloy modification methods in recent years with the aim of assisting and promoting further research in the very important and promising direction of multi-technology composite treatment.
1. Introduction
A titanium alloy is recognized as a high-strength lightweight structural material with excellent biocompatibility, high specific strength, robust corrosion resistance, and good performance under high–low-temperature conditions. Titanium alloys are widely used in various fields, for instance, biomedicine, shipbuilding, aerospace, and in daily life scenarios [1,2,3,4,5]. Generally, based on their microstructure, Ti alloys are typically categorized into α, α+compound, near-α, α+β, metastable β, and β alloys [6,7,8,9,10]. A near-α titanium alloy, Ti6242S, is notably employed in the hot zones of jet engines, effectively operating up to 550 °C for its remarkable high-temperature mechanical strength [11]. As a typical dual-phase alloy, Ti6Al4V is widely used in the aerospace, automotive manufacturing, and biomedical fields for its excellent comprehensive performance [12]. Additionally, the β titanium alloy Ti-13V-13Cr-3Al (wt. %) is extensively utilized in the SR-71 Blackbird aircraft because of its high specific strength and good high-temperature stability [6].
However, considering the need for high wear resistance, high quality, and long service life of titanium alloys in the industrial field, traditional manufacturing processes are increasingly inadequate to fulfill these stringent requirements. Accordingly, surface strengthening technology is proposed to improve the mechanical properties of titanium alloys and minimize their surface roughness in order to achieve excellent service characteristics. Huang et al. [13] demonstrated that the elongation of the TA15 alloy was increased to 46.7% after 1073 K heat treatment. The observed fracture morphology showed that the size and depth of dimples increased with heat treatment time, improving the toughness of TA15. Yan et al. [14] found that the fatigue strength limit increased by 37.6% after shot peening, which was attributed to the formation of ~260 μm thick gradient-hardened layers on the surface. Li et al. [15] performed ion implantation on titanium alloy surfaces and found that nitrogen ion implantation can notably improve the surface hardness of the titanium alloy.
Surface properties exhibit high sensitivity to treatment, and distinct surface treatment techniques yield titanium alloys with varying microstructural and mechanical properties, which, in turn, influence their appropriateness for particular industrial uses. Numerous advanced surface treatment technologies have been used to modify the surface of titanium alloys in tandem with the dramatic rise in demand for titanium. The majority of current research on titanium alloy surface treatment involves either a single treatment or a composite treatment including two treatment techniques. Current research summaries on different novel therapeutic approaches are rare. There is a relative lack of summarized research on various new treatment methods. This work covers the research development on alloy composition and surface treatment from the viewpoints of the preparation method, microstructure, mechanical characteristics, and strengthening mechanism of titanium alloys in an effort to improve the findings of previous studies in this area.
2. Titanium and Titanium Alloys
2.1. Basic Properties of Titanium
Titanium, ranking as the 10th most abundant element in the Earth’s crust, predominantly exists in the form of titanium-rich ores: ilmenite and rutile. Renowned for its lightweight nature, formidable strength, ease of processing, and exceptional specific strength, titanium and its alloys have become staples in various industrial applications. A comparison of the density and specific strength of titanium with other metals is shown in Table 1.

Table 1.
Comparison of density and specific strength of titanium and other metals.
2.2. The Main Structure of Titanium Alloy and Its Properties
As an allotropic element, crystallographically titanium exists in two structures: hexagonal close-packed structure (hcp) α-phase at room temperature; and body-centered cubic (bcc) structure β-phase, prevalent under high-temperature conditions. The transition temperature between these two phases is approximately 882 °C. The crystal structure of titanium alloy is shown in Figure 1 [16]. In addition to elevated temperatures to force the phase transition, the addition of different alloys to change the alloy phase transition temperature and component content is the main method of obtaining different organizations of titanium alloys.

Figure 1.
Crystal structure of titanium alloy: (a) α-phase crystal structure, (b) β-phase crystal structure [16].
The alloying of titanium alloys mainly affects the properties of the titanium matrix by changing the lattice structure of titanium atoms, adjusting the lattice constants and electronic structure, etc. These changes give rise to a variety of alloy types and microstructures. Alloying serves as the principal means to improve the organization and enhance the mechanical properties of titanium alloys, enabling them to maintain the stability of the original crystal structure. With to different alloys, the stability preferences can be divided into three categories of titanium alloying elements: (1) α-stabilizers, mainly replacement elements Al and interstitial elements O, N, C, etc. (2) β-stabilizers, containing eutectic elements Mo, V, Nb, Ta, and the eutectic elements Fe, W, Cr, Si, Co, Mn, H, etc. (3) Neutral elements, notably Zr and Sn. After alloying, depending on the conditions of room temperature, the microstructure is broadly divided into: α-phase alloys, two-phase α+β alloys, and β-phase alloys.
According to the stabilizer content, α-type titanium alloys can be divided into α alloys with a single α-phase and near-α alloys with 1%–2% β-stabilizers [7]. The microstructure characteristics of α-type titanium alloys are shown in Figure 2. The low-symmetric hexagonal close-packed row (HCP) crystal structure makes α-type titanium alloys show obvious plastic anisotropy at both room and working temperatures. Additionally, the close-packed row tends to be transformed into quasi-disintegration surfaces when subjected to tension, which is prone to premature fracture initiation [17]. This tendency significantly reduces the fatigue resistance of the alloy and adversely affects its properties.
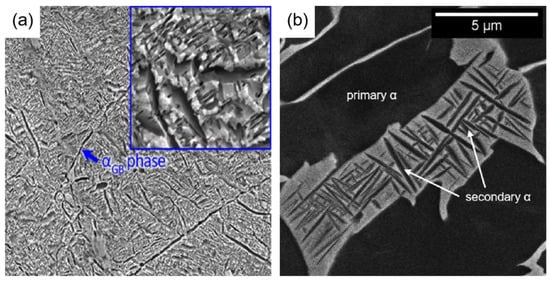
Figure 2.
Microstructure of α-type titanium alloy: (a) α-phase boundary [18]; (b) primary α-phase and secondary α-phase [10].
Unlike at room temperature, α-type titanium alloys excel in high and low temperatures. For example, the α alloy Ti-5Al-2.5-Sn ELI shows good strength and toughness under 20 K low-temperature conditions due to its low interstitial element content [19]. So, it is an ideal choice for applications like low-temperature containers and impellers in rocket engine turbine oil pumps. In addition, the densely arranged hexagonal crystal structure gives it good thermal stability and high-temperature creep properties, which makes it have good material properties under high-temperature conditions. Taking the near-α alloy Ti-6Al-2Sn-4Zr-2Mo (Ti-6242) as an example, its mechanical properties are better than those of the two-phase alloy Ti-6Al-4V (TC4) under high-temperature conditions, positioning it as an optimal material for aerospace field [9].
β-type titanium alloys generally have the advantages of high strength, low modulus of elasticity and good toughness [16,20]. Their body-centered cubic structure gives them more slip systems, thereby granting β-type titanium alloys a more pronounced plastic deformation capacity compared to α-type titanium alloys. The lower densification and higher self-diffusivity under high-temperature conditions result in them being relatively poorly thermally stable, which limits their suitability for high-temperature applications. According to the different concentrations of β-phase stabilizing elements, β-type titanium alloys can be further subdivided into nearly β alloys, sub-stable β alloys, and stable β alloys.
Near-β alloys and substable β alloys are representative high-strength β alloys with a relatively low content of β-phase stabilizing elements and good mechanical properties, which are widely used in large load-bearing components in aerospace [21]. Among them, near-β alloys, which contain the least amount of β-phase stabilizing elements, boast exceptional qualities such as high specific strength and corrosion resistance. This makes them highly suitable for critical structural parts in aerospace and other industries, such as high-strength new experimental alloys Ti-7Mo-3Nb-3Cr-3Al, Ti-4Al-7Mo-3Cr-3Vl, etc. [6]. The microstructure of near-β alloys is shown in Figure 3 [20]. Near-β alloys have strict requirements for thermo-mechanical processing, and improper treatment processes are prone to form abnormally coarse grains, affecting the quality of the structural components [22].

Figure 3.
Microstructure of near-β alloy Ti-17 (Ti-5Al-2Sn-2Zr-4Mo-4Cr) [20].
Substable β alloys with a relatively high content of β-phase stabilizing elements are widely used in the aerospace industry and biomedical fields because of their excellent overall properties compared to those of near-β alloys. For example, the substable β alloy Ti-35Nb-7Zr-6Ta-2F-0.5Si alloy developed by Kopova et al. [23] has good biocompatibility, and its higher strength and lower elastic modulus than those of Ti-6Al-4V make it ideal for biomedical applications. The high-moldability and high-strength properties make substable β alloys such asTi-6Cr-5Mo-5V-4Al (Ti-6554) show good potential for application in making large parts such as landing gears of airplanes [24]. However, they are more expensive than other alloys; so, their development in some fields is constrained. The microstructure transformation of substable β alloys is shown in Figure 4 [25].
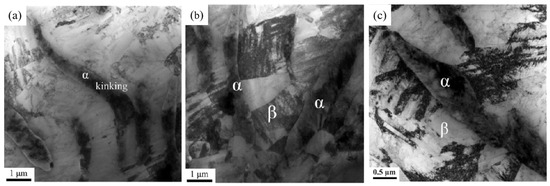
Figure 4.
Microstructural transformation of substable β alloys at 785 °C. (a) Phase kinking; (b) interlaced lamellar α-phase restricting the growth of β subcrystals; (c) phase resistance to dislocation motion [25].
Stabilized β alloys represent the apex of β-type titanium alloys, enriched with the highest concentration of β-phase stabilizing elements. This unique composition endows them with distinct performance characteristics, diverging significantly from other β-type titanium alloys. Xin et al. [26] found that various heat treatment techniques applied to stabilized β-type titanium alloys can profoundly influence their properties. Notably, the presence of grain boundaries has been identified as a key factor in enhancing the high-temperature creep resistance of these alloys. This enhancement renders stabilized β-type titanium alloys particularly suitable for high-temperature applications. The current typical heat-strengthened β titanium alloys are Ti-35V-15Cr-0.3Si-0.1C, Ti-40, etc. The organization image of stabilized β titanium alloy is shown in Figure 5 [27].
Figure 5.
Microstructures of as-received Ti3515 alloy [27].
In addition to the two phases mentioned above, the transition phase occurring during the transformation from α-phase to β-phase is generally referred to as the α+β-phase. An alloy exhibiting this transitional phase is referred to as a α+β-type titanium alloy. As the most commonly used type of alloy in the industrial field, two-phase alloys have excellent comprehensive performance and are widely used in aerospace, medical, marine and other fields. For example, Ti-6Al-4V is employed in manufacturing engine compressor blades [28]. The excellent performance of two-phase alloys mainly depends on the specificity of their internal organization, such as α-phase, β-phase two-phase organization, so that it combines the advantages of both α-type titanium alloys and β-type titanium alloys and exhibits higher strength. A typical two-phase organization is shown in Figure 6 [29].
Figure 6.
Typical two-phase organization chart [29]. (a–d): Non-IPM, IPM200, IPM300, IPM400 metallurgical microstructure of the top.
Diverse heat treatment methodologies can induce significant alterations in the microstructure of titanium alloys, consequently affecting their material properties. Alessandro et al. [10] obtained the following conclusions by comparing the changes in the organization and mechanical properties of Ti-6Al-2Sn-4Zr-6Mo alloys after heat treatments at different temperatures: At 600 °C, the α’ phase undergoes an a + b transformation, resulting in a marked increase in material strength to 1383 MPa. At 875 °C, the appearance of the bilayer organization improves the ductility of the material, but the extensively roughened microstructure results in a significant decrease in the hardness (339 HV). At 950 °C, the strength (1012 MPa) and ductility (17%) decrease slightly, accompanied by a minor increase in hardness (352 HV). At the same time, the complete recrystallization of the microstructure of the isotropy was improved. The microscopic images of the material at different temperatures are depicted in Figure 7 [10] and Figure 8 [30].
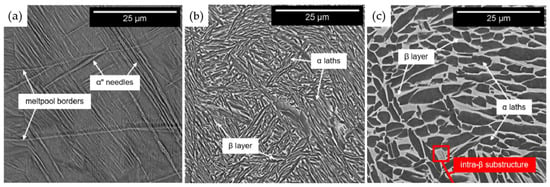
Figure 7.
Microstructure of duplex alloys after treatment at different temperatures (a) 600 °C; (b) 875 °C; (c) 950 °C [10].
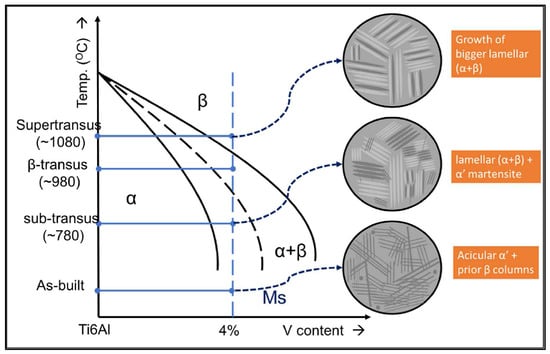
Figure 8.
Summary of microstructure growth with heat treatment temperature [30].
2.3. Classification of Titanium Alloys
According to the different uses of titanium alloys, they can be categorized into corrosion-resistant alloys, heat-resistant alloys, low-temperature alloys and high-strength alloys, as well as alloys with special functions.
2.3.1. Corrosion-Resistant Alloys
Compared to other metallic materials, titanium exhibits higher activity at room temperature and reacts very easily in atmospheric and aqueous solutions to produce dense oxides. This formation of TiO2 oxide film is the cornerstone of the exceptional corrosion resistance observed in titanium alloys [31]. Protected by the surface layer of oxide, titanium alloy products are well-suited for use in highly corrosive environments, such as marine engineering and oil extraction, maintaining their integrity under challenging work conditions. Since their inception, corrosion-resistant titanium alloys have been developed continuously. Currently, there are four relatively mature corrosion-resistant alloy families, namely titanium–molybdenum alloys, titanium–palladium alloys, titanium–nickel alloys, and titanium–tantalum alloys.
Accordingly, it is easy to see that the incorporation of different alloying elements is one of the main methods to improve the corrosion resistance of alloys. The results of Masahiko Morinaga’s research have shown that the introduction of elements with high bonding sequence values (Bo) (e.g., Ta, Nb, etc.) effectively strengthens the chemical bonding between the matrix and the alloying elements. This enhancement leads to a reduction in the critical anodic current density on the polarization curve, consequently elevating the corrosion resistance properties of the alloys [32]. Bosung et al. [33] found that the introduction of elements such as Cr and Mo into titanium alloys can induce the passivation film to form Ti-Mo and Ti-Cr double hydroxides. This process accelerates the spontaneous passivation of the alloys, making the oxide film more stable and thus showing high corrosion resistance in highly concentrated reducing acids. Currently, titanium alloys with Pt or Pd are recognized as the most corrosion-resistant alloys. However, their widespread application is substantially limited by cost constraints and other practical factors.
The variability of the microstructure also has an important effect on the corrosion resistance of materials. Dong et al. [34] have shown that at a constant strain rate, the lamellar organization has higher stress corrosion sensitivity than the equiaxial organization due to a higher content of the α-phase. In addition, it has been shown that the lamellar organization can enhance the stress corrosion sensitivity of the material by changing the crack extension pathway. The effects of grain size and the number of grain boundaries are also critical factors in determining the corrosion resistance. When the alloy’s grain size is reduced to microns or smaller by processing means, the material properties are significantly optimized with increased strength, hardness, and densification, coupled with a rise in the number of grain boundaries [35,36]. It is beneficial to increase the contact area of the alloy surface with the external environment, form a dense and uniform oxide layer, and improve corrosion resistance. Compared with alloying, such means (e.g., violent plastic deformation) can effectively reduce costs, and eliminate the original defects within the alloy material, thereby improving the mechanical properties of the material.
Surface treatment is also an effective means of improving the corrosion resistance of alloys, with the coating method being often considered to be the preferred method for this purpose [37]. In addition, processes such as heat treatment can significantly affect the corrosion resistance of materials by changing the properties of surface alloy materials. Taking nitriding treatment as an example, plasma nitriding (PN) is known to improve the surface hardness and corrosion resistance through the formation of a titanium nitride layer [38,39,40,41]. Li et al. [31] compared the corrosion current densities of the electron-beam melting (EBM) Ti-6Al-4V alloy in seawater before and after PN treatment. They found that the corrosion current density of the alloy after PN (PN-EMM) was significantly lower than that of the original sample, which indicated that the corrosion resistance of the treated material was effectively improved. The kinetic potential polarization curves of EBM and PN-EBM samples in natural seawater are illustrated in Figure 9 [31].
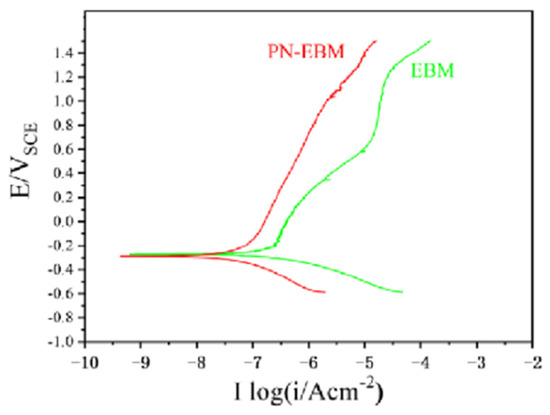
Figure 9.
Dynamic potential polarization curves of EBM and PN-EBM samples [31].
2.3.2. Heat-Resistant Alloys
High-temperature alloys are widely used in the aerospace field because of their exceptional specific strength and good high-temperature mechanical properties. Currently, the service temperature of high-temperature alloys is mostly in the range of 550–650 °C. Predominantly, these alloys are near-α-type titanium alloys, such as Ti60, Ti-1100, etc. The continuous development of aviation technology has put forward higher requirements for the operating temperature and other critical properties of these high-temperature alloys. However, the increase in service temperature will result in a consequent decrease in the antioxidant properties of the alloys, which poses a significant limitation on their application in high-temperature components [42,43,44].
The addition of alloying elements and the use of ordered phase hardening are effective methods to improve the oxidation resistance and high-temperature performance of alloys. Xu et al. [45] found that the addition of high W content can increase the oxidation activation energy of the alloy, so that the alloy generates a more homogeneous and denser oxidation layer, which significantly improves the oxidation resistance of the alloy. The addition of W also plays an important role in refining the α-sheet layer and ordered α2-Ti3Al phase. This results in improved room-temperature yield strength and high-temperature yield strength, and better plasticity after extended thermal exposure. SEM images of the W-free alloy and the alloy with 4.0 W (wt.%) addition (W40) after 100 h exposure at 650 °C are shown in Figure 10 [45].
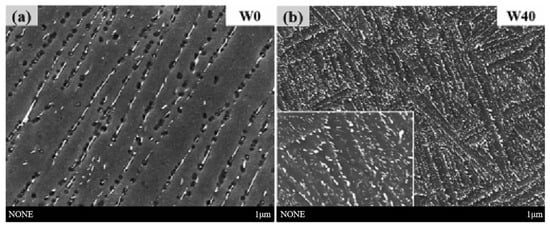
Figure 10.
SEM images of the (a) W0 and (b) W40 alloys after thermal exposure [45].
Most high-temperature alloys frequently incorporate aluminum to reinforce the α-phase. However, aluminum-containing titanium alloys tend to experience coarsening due to the precipitation of the ordered α2-Ti-3Al phase after prolonged exposure to high temperatures. This would be detrimental to the alloy plasticity and make the alloy more susceptible to cold residence fatigue [46,47]. Increasing the Al solubility in the alloy can inhibit the growth of the ordered α2-Ti3Al phase, thereby enhancing the thermal strength and thermal stability of the material. Cao et al. [48] compared the interactions of Al with other alloy atoms in α-Ti, and found that the traps of Al atoms, such as Mo, Ru, etc., can increase the solubility of Al and inhibit the growth of the intermetallic compound of Ti3Al in α-Ti. This improves the thermodynamic properties of aluminum-containing high-temperature alloys. Rare earth elements can affect the thermomechanical properties of alloys by changing the metallographic organization of the alloys. Li et al. [49] found that the introduction of Re increased the β-phase content of the alloys significantly, and the tensile strength of the alloys was improved. This element also played a role in refining the grain size and the thickness of the sheet layer and effectively inhibited the Al, Ta, and other elements’ diffusion. While these alloys exhibit higher thermal strength post-Re addition, their plasticity is somewhat compromised. The comparison of the organization images of Ti-Al-Ta-Nb alloy before and after the addition of Re is shown in Figure 11 [49].
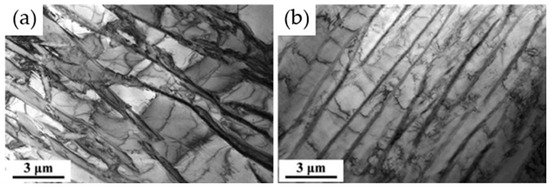
Figure 11.
Ti-Al-Ta-Nb TEM image. (a) Original alloy. (b) Alloy after introduction of Re [49].
Compared with single-element alloying, multi-element co-alloying has proven to be more effective in improving the high-temperature creep performance of alloys. For example, Wen et al. [50] found that when Al-Ta coexisted on the surface of the alloy, the superposition of hindering oxygen adsorption and oxygen diffusion effects could be realized. Thus, the antioxidant performance of the material could be optimized. Table 2 shows typical high-temperature alloys used in various countries [4].

Table 2.
Typical high-temperature alloys in various countries [4].
Currently, the physical mechanism of the effect of alloying elements on the thermal strength and thermal stability of high-temperature alloys remains an area of ongoing investigation [48]. It is believed that this will be one of the breakthrough directions to improve the performance of high-temperature alloys in the future.
2.3.3. Low-Temperature Alloys
Compared with other metal materials such as aluminum alloys and stainless steel, titanium alloys show excellent corrosion resistance, toughness, and high specific strength in cryogenic environments. They are commonly used in the manufacturing of cryogenic equipment, such as hydrogen storage tanks, hydrogen pump impellers, and structural parts for the aerospace industry. Currently, low-temperature alloys used both domestically and internationally are dominated by α-type titanium alloys, such as TA7 and TC4 ELI.
Normally, the alloy’s hardness increases with decreasing temperature, while its elongation and fracture toughness tend to decrease. This makes the alloys exhibit significant brittleness at low temperatures. In addition to temperature, the low-temperature toughness and plasticity of alloys are also affected by the gap element content, alloy composition, and microstructure. Researchers both domestically and internationally have managed to enhance these properties by reducing the content of interstitial elements like carbon (C), hydrogen (H), and oxygen (O) in the alloy. For instance, TA7 ELI alloy can even be used at −253 °C. Compared with low-Al-content alloys, high-Al-content alloys are prone to chemical interactions with surrounding atoms due to the presence of Al, which hinders the dislocation movement and thereby reduces the alloy’s plasticity [51].
Microstructure is another important factor affecting the low-temperature plasticity of the material. The lamellar organization under low-temperature conditions shows better low-temperature plasticity than the equiaxial organization because of the higher number of serrations on the deformation curve and the greater susceptibility to twinning [51].
Currently, the prevalent cryogenic titanium alloys are mostly near-α alloys and two-phase alloys with a minor β-phase component. Generally, these alloys exhibit poor plasticity. Moreover, α titanium alloys cannot be strengthened through heat treatment; so, the use of cryogenic alloys has been greatly restricted, relegating their use to low-stress components [19]. With the rapid development of science and technology, the plasticity and strength of alloys at low temperatures will certainly put forward higher requirements. Therefore, the development of low-cost, high-strength, and high-plasticity alloys will likely become a principal focus in the field of high-temperature alloys in the foreseeable future. Research into the low-temperature performance of β-phase alloys may emerge as a key direction in the evolution of low-temperature alloy research.
2.3.4. High-Strength Alloys
High-strength titanium alloys, characterized by a tensile strength greater than 1100 MPa at room temperature, primarily consist of near-β titanium alloys and sub-stable β titanium alloys, such as Ti5Si3 and TB17. High-strength titanium alloys usually have a high specific strength, good toughness, excellent corrosion resistance, and other characteristics. Consequently, they find extensive applications in the aerospace and defense industries.
The strength and toughness of titanium alloys are usually affected by grain size, metallographic structure, and alloying elements. Lu et al. [52] found that α lamellae within the grain can strengthen the β matrix and prolong the crack extension path, thus optimizing the alloy’s strength and toughness. Mantri et al. [53] successfully optimized the strength and toughness of titanium alloys by aging the β-21S titanium alloy (Ti-15Mo-3Nb-2.7Al-0.2Si, wt.%) by adopting an aging treatment to precipitate lamellar αs phase, which effectively improved the strength of this alloy.
Heat treatment techniques, such as solid solution aging, are effective means to increase the strength of β alloys. Comparative observations of Figure 12 [54] show that the width of the α-phase grows rapidly as the heat treatment temperature is increased, and the number of strength-enhancing α’ phases precipitated in the alloy increases significantly. This phase not only becomes more refined but also contributes to an increase in the alloy’s strength as the process continues. The incorporation of alloying elements is the most commonly used method to improve the strength of alloys. For instance, the introduction of Al elements can encourage the precipitation of the α-phase, contributing to solid solution strengthening and amplifying the solid solution aging strengthening effect. High-strength alloys are widely used in a variety of applications. The chemical composition of common high-strength titanium alloys is shown in Table 3 [55].
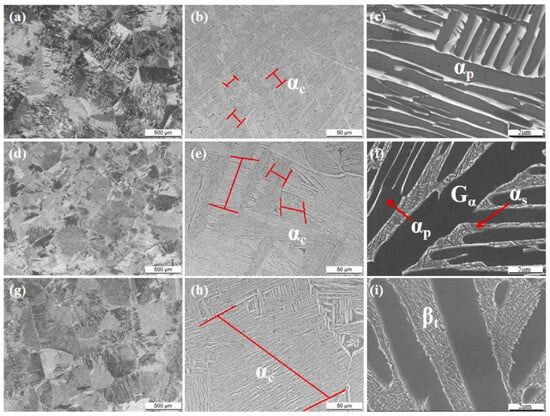
Figure 12.
Microstructures of the alloy annealed at different temperatures: (a–c) 760 °C, (d–f) 800 °C, (g–i) 840 °C [54].

Table 3.
Chemical composition of ordinary high-strength titanium alloys [55].
2.3.5. Special Function Alloys
Beyond the aforementioned high-performing alloys, titanium can be alloyed with various other metals to create materials with specialized functionalities. For example, the nickel–titanium alloy generated by the combination of titanium and nickel has a shape memory function. Ti-Nb alloy is a superconducting material commonly used in the industrial and medical fields. Additionally, Ti-Cr-based alloy is used as a solid-state hydrogen storage material, demonstrating the versatility of titanium when alloyed with different elements.
Shape memory function refers to the ability of a severely deformed alloy to recover its intended shape when heated above its transformation temperature. According to the different alloy compositions, they can be categorized into three types: copper-based, iron-based, and nickel–titanium-based alloys. Among them, Ni-Ti-based alloys are widely used as implants and surgical tools for medical applications due to their bidirectional shape memory, superplastic effect, and good biocompatibility [56]. In addition, Ni-Ti alloys are also commonly used in the industrial field. And in the early stage, the United States pioneered the use of NiTiFe alloy pipe joints in the industrial domain, notably on the F14 fighter jet. In recent years, the application of NiTiFe alloys has been further expanded as the research on the basic properties of shape memory alloys has been deepened in various countries.
The β-type Ti-Nb alloy’s capacity to withstand stress during magnetic field establishment renders it a viable superconducting material for industrial applications. Niobium–titanium alloys offer several significant advantages over other materials, including high strength, good plasticity, superior mechanical properties, and lower cost. These benefits have led to their widespread use across various fields, making them the most extensively utilized material among low-temperature superconducting materials [57]. They can be seen in particle gas pedals, nuclear magnetic resonance, military minesweeping, superconducting power transmission and energy storage, and magnetic levitation trains. In recent years, with the continuous research on superconducting materials in various countries, the demand for niobium–titanium materials has been rising, and the annual consumption of niobium–titanium superconducting wires for only one item of nuclear magnetic resonance in medical research has reached more than 1000 tons. In the future, the field of superconducting materials is poised for rapid advancement. As an important component of superconducting materials, the niobium–titanium alloy is expected to find even broader and deeper applications.
In addition, researchers have found that certain titanium alloys can inhale hydrogen under specific conditions, and release it following a designated process, demonstrating that titanium alloys have the function of hydrogen storage. In recent years, the research and application of new energy sources such as hydrogen has become the main way to solve the current energy crisis, which puts forward higher requirements on the capacity, safety and cost of hydrogen storage materials. Ti-Cr-based alloys have been emphasized by researchers because of their low price and large hydrogen storage capacity. A study by Lv et al. [58] explored the impact of Mn on the hydrogen storage performance of Ti-Cr-based alloys. They discovered that introducing Mn not only effectively reduces the oxidation of Ti and Cr but also minimizes the loss of the hydrogen-absorbing C14-type phase in TiCr2 alloy. This modification results in superior hydrogen storage properties under low-temperature conditions.
In addition, the addition of multiple alloys, using the lattice distortion caused by differences in atomic size, enables hydrogen atoms to occupy more interstitial positions in the alloy, thereby effectively improving its hydrogen storage capacity. Multi-major element alloys are relatively inexpensive to produce, exhibit a large hydrogen storage capacity, and absorb hydrogen quickly, which is one of the main directions for the development of hydrogen storage materials in the future.
3. Titanium Alloy Surface Treatment
Titanium and its alloys are celebrated for their excellent material properties, contributing to their extreme versatility. However, in most industrial cases, they cannot directly meet the requirements of the working environment. Therefore, it is usually necessary to improve the mechanical, force, and chemical properties of the material to suit varying operational conditions. Metal properties are usually directly related to the friction and corrosion resistance of the surface material and many other physical and chemical surface properties [59]. The performance of titanium alloys depends greatly on the quality of the surface material, which is affected by the wear and corrosion of the surface metal. Zhang et al. [60] demonstrated that, according to the variations in the working environment, selecting the processing technique and parameters wisely is essential to achieving a good functional performance of the surface. The qualities of the material are positively impacted by good surface morphology. To increase a part’s fatigue resistance, surface strengthening is often applied as the final step in the manufacturing process [61].
All established metal surface treatment methods have been adapted for titanium alloys, which can be categorized into mechanical, thermal, and chemical treatments depending on the nature of the process. Essentially, these treatments aim to either improve the surface properties of the alloy or form a protective layer on it, mitigating the effects of hydrogen, oxygen, or corrosive agents on the substrate. Selecting an appropriate treatment method according to the specific working environment can significantly enhance the alloy’s performance while preserving its inherent material and mechanical properties. This section will delve into the mechanical treatment methods for titanium and its alloys, as well as discuss various heat treatment techniques.
3.1. Mechanical Processing
Mechanical processing, including techniques such as mechanical shot peening and mechanical grinding treatment, is a common means of mechanical treatment. The underlying principle shared by these techniques is the application of kinetic energy to a solid medium, which then interacts with the surface of the workpiece. This interaction utilizes the imparted force to modify the surface contour of the workpiece. The primary objectives of these methods are to strengthen the material’s surface or to achieve desired surface morphology characteristics, such as specific patterns or surface roughness levels.
3.1.1. Mechanical Processes
Mechanical processing is one of the common surface treatment technologies for titanium alloys. However, given their status as materials typically challenging to machine, enhancing the surface quality and reducing tool wear during the machining of titanium alloys present considerable challenges [62]. Du et al. [62] propose a novel hybrid machining process to improve the machinability and mechanical properties of alloys. This process is shown in Figure 13 [62]. In this hybrid machining process, laser-assisted diamond turning is used to improve machinability by softening material surfaces. Additionally, slow-tool-servo cutting is employed to create various microstructure arrays.
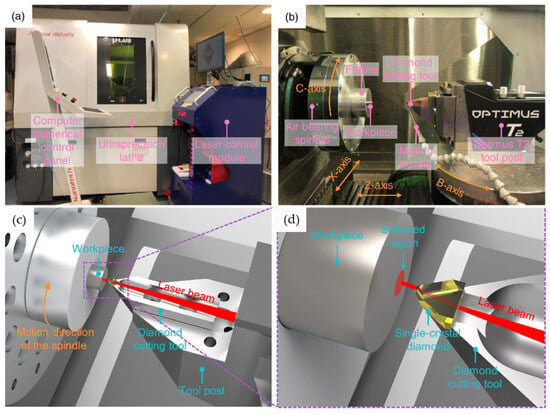
Figure 13.
(a,b) Experiment setup of machining high-quality microstructure arrays; (c,d) working principle of the in situ laser-assisted diamond turning [62].
In the machining process, the high temperature caused by friction can increase the titanium’s chemical activity, so that it reacts with the oxygen in the air to generate high-hardness oxides. These reactions often produce high-hardness oxides, complicating the subsequent processing steps. Moreover, the elevated chemical activity can cause portions of the titanium to cold weld onto the cutting tool. Therefore, titanium machining, due to different hardness requirements, is not easy to process with the titanium in the bonding diffusion of the tool material. Ahmed et al. [63] investigated the machinability of titanium alloy (Ti-6Al-4V) through electric discharge machining and identified the most appropriate tool material for better machining performance. This shows that superhard materials are the first choice for turning tools. Research [64] further supports that superhard cutting tool materials can effectively mitigate bonding issues, facilitating the finishing and high-speed machining of titanium alloys. For example, polycrystalline cubic boron nitride tools can effectively cut titanium alloy materials under the conditions of high cutting speed, low feed, and low back draft, maintaining a stable cutting force and low machining surface roughness. Additionally, polycrystalline diamond tools can still maintain good tool life and machining surface quality when machining a titanium alloy at a speed of more than 200 m/min.
Furtherly, Cui et al. [65] evaluated the grindability of the titanium alloy by considering the grinding temperature, grinding force, surface roughness, and defect ratio. The experimental results indicated that the values of surface roughness and grinding force obviously decreased under the assistance of the cryogenic nano-lubricant minimum quantity lubrication. Moreover, they observed a substantial 84.5% reduction in the defect ratio of the workpiece surface compared to cryogenic air cooling. Fábio et al. [66] compared the machinability of the casting Ti-6Al-4V with the selective laser-melted Ti-6Al-4V in terms of cutting forces, surface roughness, burr formation analysis, and microchips morphology, and found that the selective laser-melted Ti-6Al-4V had higher machinability with lower cutting forces, lower surface roughness, and less burr formation.
Apart from grinding and milling, turning is another machining process to remove the workpiece material and create the desired shape. Dandekar et al. [67] combined laser-assisted machining with the cryogenic cooling of the cutting tool to improve the machinability of a titanium alloy (Ti-6Al-4V) during the turning process. But the nanometric surface roughness was not achieved due to the limitations of the lathe and cutting tool. Addressing a similar challenge, Li et al. [68] utilized the femtosecond laser to texture the submillimeter-scaled structures on the rake faces of the uncoated cemented carbide cutting tool. Their findings showed that the textured cutting tool enhanced the machinability of Ti-6Al-4V because the cutting forces were reduced. Ni et al. [69] investigated the influences of anisotropic mechanical properties and microstructure features of additively manufactured Ti-6Al-4V alloys on machining performance in terms of cutting force and surface roughness. Yip et al. [70] studied the single-point diamond turning of titanium alloy Ti-6Al-4V under the assistance of the magnetic field. They found that the surface quality had been improved and marks on the workpiece surface had been obviously reduced. Considering that the mechanical properties of titanium alloys were dramatically influenced by their microstructures, Zhao et al. [71] applied a commercial electropulsing treatment to Ti-6Al-4V. The results showed that the single-point diamond turning improved the machinability by adding the magnetic field, electropulsing treatment, or using the hybrid cooling strategy.
In addition to traditional tool machining, Electrical Discharge Machining (EDM), as a new precision machining method, is commonly used for the treatment of difficult-to-machine materials such as titanium alloys. This method achieves material removal by applying a regulated electrical pulse between the tool electrode and the workpiece specimen [72]. The EDM setup is shown in Figure 14 [73].
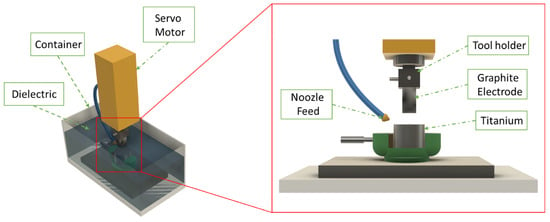
Figure 14.
Graphical representation of EDM setup [73].
The surface quality and machining efficiency of EDM are mainly affected by the electrode material and pulse on conductive current. Panagiotis et al. [73] found that the effect of pulse on conductive current (IP) and pulse on time (Ton) on the material removal rate is significant by comparing the effect of pulse on conductive current (IP) and pulse on time (Ton) on the material removal rate. Ahmed et al. [74] found the effect of pulse on conductive current (IP) and pulse on time (Ton) on the material removal rate of α-β titanium alloys by exploring the effect of tool electrode on the machinability of EDM. The evaluation was based on material removal rate and surface morphology. It was finally determined that the copper electrode enhances the material removal mechanism, and the tungsten carbide electrode produces small craters after machining due to its high melting point. And the surface roughness after processing is superior to the other electrode materials. Brass electrodes have a lower melting point, produce larger craters on the surface after machining, and have higher surface roughness and electrode wear.
EDM can be used to fabricate complex shapes and geometries with high dimensional accuracy compared to conventional manufacturing processes [73]. And EDM is relatively inexpensive compared to other mechanical treatments. However, EDM can produce machined surfaces with high roughness, high white layer thickness and large heat-affected zones. Therefore, the selection of a suitable pulse generator is critical to improve the quality of EDM machining.
3.1.2. Machine Shot Peening
Shot peening (SP) is a robust technique for surface layer strengthening, effectively enhancing the surface life and durability of the components while preserving their original internal chemical structure. Its operating principle involves utilizing compressed air, pressurized water, ultrasonic energy, or centrifugal force to propel spherical particles at high velocities against the material’s surface. The impact of the projectile produces compressive residual stress on the surface of the component [75]. The introduction of compressive stresses effectively prevents crack initiation and extension and improves the fatigue resistance of the material. In addition, shot peening can also effectively refine the grain structure, thus playing a role in improving the fatigue strength and resistance to stress corrosion cracking [76]. Figure 15 shows a schematic diagram of the SP process and the plastic deformation introduced [77].
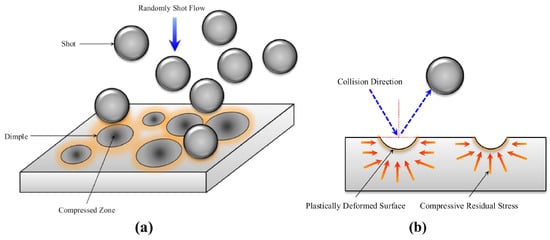
Figure 15.
Schematic illustration of (a) the SP process and (b) the plastic deformation made by SP [77].
The plastic deformation capacity of metallic materials depends on the dislocation mobility and plastic strain rate. Constraining the movement of dislocations can lead to an increase in the material’s strength [78]. The degree of plastic deformation during SP is directly related to the amount of total impact energy. This suggests three major factors that determine the effectiveness of SP: blast size, blast velocity, and number of blasts. Each of these factors plays a pivotal role in determining the level of impact energy and, consequently, the degree of plastic deformation achieved during the SP process.
A comparative study of conventional SP (CSP) and micro-SP (MSP) by Su et al. [79] found that micro-shot peening can better inhibit crack initiation than the impact of high-speed, large-size particles. This efficacy results in surfaces exhibiting a superior finish and enhanced fatigue properties. However, the smaller size used in MSP also equates to lower kinetic energy, leading to a shallower impact layer depth. Consequently, MSP is generally not preferred for alloys that require stringent corrosion and wear resistance. Wang et al. [80] investigated the effect of different shot sizes on the microstructure via coupled intrinsic modeling and found that larger shot sizes increased the depth of the dislocation density layer. The alloy was made to possess higher compressive residual stresses and exhibit greater corrosion resistance.
Research indicates that employing a dual shot peening approach can enhance the fatigue strength of an alloy more effectively than a single shot peening method. Shi et al. [81] determined the fatigue strength of the TC17 titanium alloy using a combination of classical shot peening and vibratory shot peening. Their findings demonstrated that this combined technique resulted in a greater increase in fatigue strength compared to the application of classical shot peening alone.
Shot peening has the advantage of being a simple process without heat exposure, and adaptable to complex and intrinsic conditions [82]. However, the determination of its process parameters remains a challenging task. The use of the small-size shot peen may not affect the deeper alloys, thus limiting the improvements in corrosion resistance. Conversely, the bombardment of large-size particles tends to increase the surface roughness and friction coefficients, which leads to pitting corrosion and the creation of stress concentration defects. Therefore, optimizing the coefficient of the shot peening process and developing better means for the shot peening process are an effective way to improve the shot peening technology.
3.1.3. Surface Mechanical Grinding
Surface mechanical grinding (SMAT) is a new surface nanosizing technology based on the high-frequency impact of balls. It operates on the principle of high-frequency ball impacts, inducing intense local plastic deformation on the surface. This process achieves grain size refinement within the impacted layer, alongside the introduction of dislocation density and residual stresses. Notably, SMAT enhances the material properties without altering the material’s chemical composition [83].
The surface generated by the SMAT technique is mainly affected by the vibration frequency and the treatment time. Yao et al. [84] found that the thickness of the surface nanocrystalline layer increased with increasing treatment time by comparing the thickness of the crystalline layer after STMA treatment of different durations. Additionally, Aoudia et al. [83] investigated the impact of SMAT on the mechanical properties of coatings and their corrosion resistance. They discovered that the SMAT treatment effectively increased the hardness of the affected zone and improved the wear rate of the coating. SEM images of the cross-section microstructure of titanium alloy at different processing speeds are shown in Figure 16 [85]. Furthermore, they noted a positive correlation between the wear rate of the material and the amplitude of ultrasonic electrode vibration. Figure 17 [85] shows the 3D morphology of the wear trajectories of the specimens after different processing treatments. Chamgordani et al. [86] demonstrated that an ultrafine grain layer treated via a surface mechanical attrition treatment was generated on the surface of commercial pure titanium, resulting in a 60% reduction in the friction coefficient.
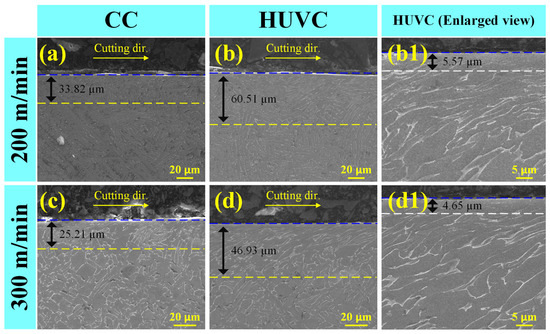
Figure 16.
SEM images of cross-sectional microstructure of (a,c) CC and (b,b1,d,d1) HUVC samples with cutting speeds of 200 m/min (a,b,b1) and 300 m/min (c,d,d1) [85].
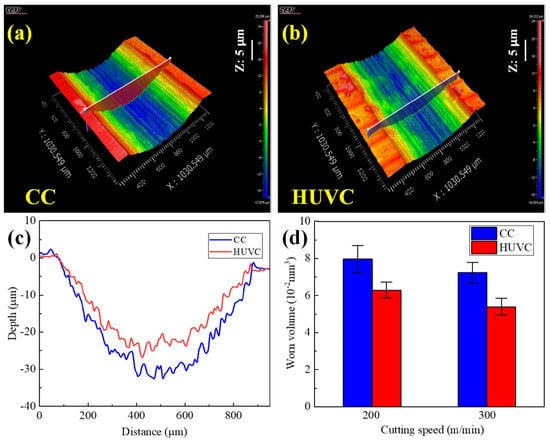
Figure 17.
Three-dimensional topography of wear tracks of (a) CC- and (b) HUVC-machined samples; (c) cross-sectional profile curves at cutting speed of 200 m/min; (d) summary of worn volume [85].
SMAT treatment technology is inexpensive and flexible, significantly improving the surface properties of materials. It has substantial potential value addition, particularly when integrated with other surface treatment technologies such as coatings. The working principle is similar to that of mechanical shot peening, but there are still some differences. For instance, SMAT involves random directional impact that facilitates grain refinement. Additionally, the shots used in SMAT are typically larger and smoother. In addition, compared with ordinary machining and mechanical shot peening, SMAT can achieve high-speed shear deformation and large strain gradients on large-scale materials without size constraints, and achieve the purpose of surface auto-nanosizing [87].
The methods discussed above represent some of the more commonly employed mechanical treatments for titanium alloys. These techniques are characterized by their simplicity in operation and the ability to be applied to large-sized metals. They enhance the overall chemical and mechanical properties of the material. Moreover, certain methods among these can even boost the material’s strength and further improve its comprehensive mechanical properties. This enhancement is achieved through surface nanonization, which is accomplished while preserving the original toughness of the material.
3.2. Surface Heat Treatment
As a traditional treatment process, heat treatment is widely utilized to prevent the formation of cracks and voids, as well as to overcome the generation of unstable phases and internal stresses [30]. By heating and cooling the material, the metal undergoes phase transformation or recrystallization to achieve changes in the internal organization and structure so that the alloy achieves the specific desired material properties. The properties of titanium alloys are strongly related to the properties of their surface metals; so, it is necessary to perform targeted surface heat treatments on titanium alloys according to the demands of the working environment.
According to researchers, titanium alloys can be broadly classified into two categories based on the introduction of chemical elements: general heat treatment and thermochemical treatment [30]. The first method can take advantage of the hot and cold deformation of the material to change the metal organization structure, optimize the stress distribution within the material, and obtain better wear resistance and fatigue resistance. Thermochemical treatment, on the other hand, is used to achieve surface modification by injecting different alloying elements, resulting in the formation of alloys with higher hardness. At present, the prevalent thermochemical methods include boron infiltration, carburization, nitriding, and carbonitriding.
3.2.1. Normal Surface Heat Treatment
Surface heat treatment of titanium alloys facilitates alterations in the microstructure and organization of the surface material, consequently influencing the material’s properties. Surface heat treatment operations have also been proven to be an effective way to extend the service life of titanium alloy materials and improve the wear resistance of titanium alloy components. This section focuses on surface quenching and annealing processes as applied to titanium alloys.
- Surface hardening
Quenching is known to significantly enhance material properties through the alteration of phase composition and redistribution of elements within the alloy. It has been shown that quenching above and below the solid-phase temperature results in a uniformly distributed single-phase organization. This process effectively eliminates defects associated with the segregation of the constituent elements and mitigates selective phase corrosion. As a result, the wear resistance of the material is substantially improved [88].
Dang et al. [89] developed a new forming process of fast gas forming with in-die quenching for titanium alloys. The formed component could be significantly strengthened through the formation of abundant fine martensitic microstructure during in-die quenching. For example, the yield strength and tensile strength of the Ti-3Al-2.5V alloy component increased by 26.5% and 15.2%, respectively, while the elongation still reached up to 16.2%. Traditional quenching methods, which typically involve treating the entire workpiece, can lead to reduced plasticity and potential brittle fracture, thus adversely affecting the mechanical toughness of the substrate [90]. This challenge led to the development of surface quenching, which targets only the surface layer of the material, forming a supersaturated layer with uniform element distribution and enhanced corrosion resistance. Since surface quenching primarily affects the surface, the internal microstructure and properties of the metal matrix remain largely unchanged, preserving the original mechanical characteristics of the interior metal. At present, laser quenching and induction quenching are the two main ways of titanium alloy quenching.
Laser quenching is currently the most prevalent method for quenching titanium alloys. This process involves directing a high-density energy beam onto the metal surface, where the photothermal effect instantaneously melts and then rapidly solidifies the surface metal. This results in the formation of ultrafine, acicular martensite with a uniform distribution and well-developed vertical grain boundaries, significantly enhancing the material’s wear and corrosion resistance. Laser quenching has the advantages of high processing efficiency, and controllable quenching depth. It also minimizes stress introduction and is less likely to induce porosity defects. Additionally, the rapid cooling effect associated with laser treatment ensures minimal changes in surface roughness and contour.
As a rapid thermal treatment technology, high-frequency induction quenching has been widely used in the industrial field due to its advantage of process controllability, fast heating rate, cleanliness, low energy consumption, and environmental friendliness. A temperature gradient can be formed via fast quenching. The surface temperature of the material reduces rapidly, in contrast to the more gradual temperature decline within the substrate. This differential cooling rate facilitates the formation of distinct microstructures across the material’s depth during high-frequency induction quenching, thereby engendering heterogeneous mechanical properties at the surface and the core. As reported by Jian et al. [91], a gradient microstructure was successfully fabricated through a high-frequency induction quenching treatment to improve the mechanical behavior of Ti-6Al-4V alloy. The results showed that the gradient microstructure of the alloy varied from a fine, as lamellae decomposed from a’-martensite at the surface layer, to a bimodal microstructure at the center. Moreover, the alloy with a gradient microstructure presents an optimal strength–ductility synergy. However, it is worth noting that the application of high-frequency induction quenching for surface enhancement in titanium alloys remains underexplored, signifying a potential avenue for further research in material science.
During a quenching treatment, the thermal gradients induce internal stresses and plastic strains. The mechanical behavior depends on the local temperature and microstructure. In an effort to demystify this phenomenon, Teixeira et al. [92] conducted a comprehensive numerical simulation, meticulously examining the Ti17 alloy. Their study was pioneering in considering the synergistic effects of thermal, mechanical, and microstructural evolutions during the quenching process. Simultaneously, emphasis was put on the influence of the β and α+β phases transformation on the internal stress evolutions during quenching. Furthermore, for the quantitative assessment of the parameters related to the morphological structure generated during laser treatment, Lytvynenko et al. [93] introduced a method of mathematical analysis. This method, coupled with the consideration of the stochastic and cyclic nature inherent in laser treatment, is employed to analyze the ordered undulations that manifest on the surface of titanium nanoparticles following laser shock wave treatment.
- Surface annealing
Annealing stands as a pivotal process in the realm of material fabrication, primarily for its efficacy in mitigating or entirely rectifying the non-uniform microstructures typically induced by uneven deformation during processing, as outlined in [94]. This thermal treatment is instrumental in alleviating residual stresses inherent within the material, thereby enhancing both the stability of the alloy’s microstructure and its overall toughness. Furthermore, annealing contributes significantly to the homogenization of the material’s microstructure, ensuring a more consistent and predictable response to subsequent processing or operational stresses. To illustrate this process, Figure 18, as referenced in [95], presents a detailed schematic that delineates both the preparation of specimens for annealing and their subsequent characterization.
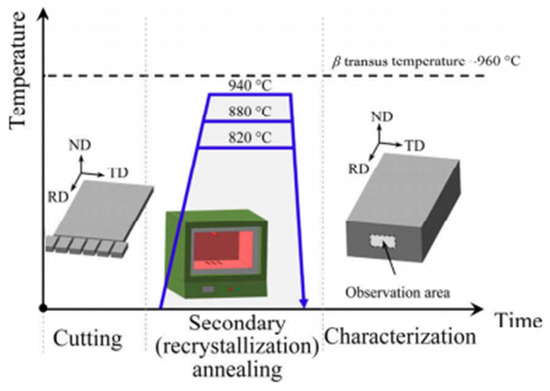
Figure 18.
Schematic diagram of specimen preparation and characterization of annealed specimens [95].
Annealing temperature and annealing time are the main factors affecting the usable properties of the treated material. In a seminal study conducted by He et al. [94], the impact of annealing on the microstructure of the TB8 alloy post cold rolling was meticulously investigated. Their findings underscored that the annealing temperature positively correlates with the grain growth time index. Furthermore, as the holding time extends, the activation energy required for grain growth exhibits an increasing trend. This phenomenon is vividly illustrated in Figure 19 [96], which compares the microstructural transformation of TA10 alloys under different heat treatment regimes. The micrographs show that the recrystallization drive increases with annealing time. On the other hand, a high annealing time will lead to the consumption of small grains by large grains. The β-phase dispersed between α-phases gradually decreases, while the volume fraction of the α-phase shows an increasing tendency [96]. This transition leads to a morphological change from an equiaxed to a Weissian organization. Such microstructural evolution results in a material that displays enhanced plasticity, albeit at a slight compromise in strength.
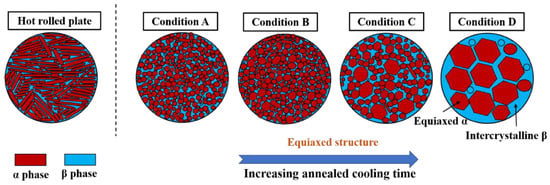
Figure 19.
Microstructural evolution of the TA10 sheet at different annealing times [96].
Annealing is also commonly used to improve the organization and mechanical properties of welded joints and so on. In this context, it serves as a crucial technique for improving both the microstructural organization and mechanical properties of the joints. A notable investigation by Kumar [97] delved into the effects of annealing on titanium alloy welded joints under various thermal conditions. This study illuminated the multifaceted impact of annealing on the microstructure of these joints.
Surface heat treatment is one of the pivotal techniques to regulate the microstructure of the alloy and change the mechanical properties. Compared with deformation processing, it has the advantages of energy saving and high efficiency, short cycle time and convenient operation. Therefore, this tailored approach to heat treatment not only conserves energy and reduces processing time but also unlocks the potential for customizing the mechanical profile of alloys to meet specific application requirements [98].
3.2.2. Thermochemical Treatment
Thermochemical treatments, as one of the most common surface methods, include carburizing, nitriding, and other elemental carburizing surface treatments. These methods fundamentally enhance the material properties of surface alloys by infusing elements such as carbon and nitrogen into the alloy matrix. This infusion results in the formation of a hardened layer, which is a composite of ceramic particles and a solid solution, as detailed in [99]. The quality of the hardened layer formed via this method is influenced by a number of factors such as the oxide layer, gas atmosphere, treatment time and temperature. The oxide layer is a significant impediment to the penetration of elements such as carbon and nitrogen. So, it is usually necessary to remove the surface oxide layer by grinding and cleaning before carburizing. Increasing the treatment temperature and time can also lead to the generation of a thicker saturated penetration layer. This enhanced penetration contributes to a marked improvement in the treated material’s wear resistance and hardness, thereby extending the material’s operational life and broadening its application spectrum in demanding environments.
- Carburization
Carbon is one of the elements with the greatest influence on the surface hardness of titanium alloys. And the injection of carbon into the surface layer of the alloy can effectively improve the hardness and wear-resistant properties of the surface metal. It is usually believed that the main strengthening effect on the surface of the material is the hard TiC particles in the carburized layer. Take the carburization treatment of Ti-6Al-4V as an example: a hardened layer composed of carbide particles is formed on the surface of the material after the carbonization treatment. Compared with the pre-treatment material, the hardness of the treated material was increased by about 128%, and the fatigue strength and wear resistance of the material were improved [100]. The thickness of the penetration layer generated by the carburizing treatment is thickened with the increase in the treatment temperature. And there is no obvious sharpness between the penetration layer and the subsurface. These methods include solid carburizing, where the alloy is exposed to a carbon-rich solid medium; liquid carburizing, involving immersion in a carbonaceous liquid; gas carburizing, which employs a carbon-rich gas atmosphere; and plasma carburizing, which utilizes ionized gases in a plasma state for the process.
- Solid carburization
Solid carburization, a prominent method in hydrogen-free carburization, stands out as an efficient technique to enhance the surface properties of titanium alloys, notably with minimal alteration to the matrix properties of the material. Prior to the carburizing process, it is imperative to remove any oxide layer present on the sample’s surface. Subsequently, the sample is enveloped in a carburizing agent and placed within a sealed chamber. The solid vacuum carburizing unit is shown in Figure 20 [99]. After solid carburizing, TiC reinforcing phase and Ti-C solid solution are introduced into the α-Ti phase of the alloy. The carbon content of α-Ti is increased and the hardness of the surface layer is improved. With the increase in sample depth, the volume fraction of α-Ti decreases gradually, and the carbon content of the inner metal layer decreases. So, the solid-nitriding-treated samples usually show a decrease in metal hardness with the increase in the samples’ layer depth. This phenomenon is corroborated by the experimental findings of Duan et al. [99] on the Ti-6Al-4V alloy, which demonstrate a significant decrease in the volume fraction of the α-Ti phase in the diffusion layer as the sample depth increases. In addition, the hardness test results showed that the microhardness of both carburized surfaces significantly increased by about 100% compared to the untreated material.
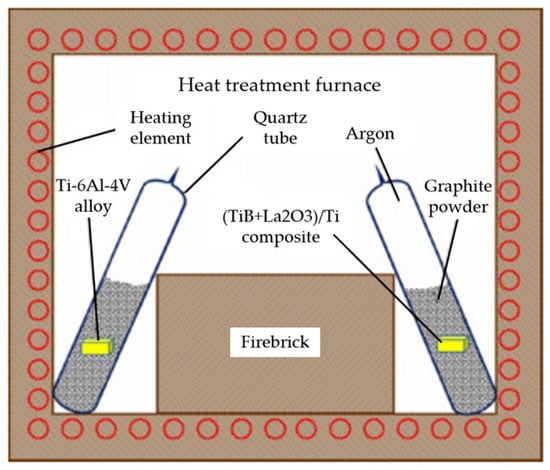
Figure 20.
Schematic diagram of solid vacuum carburization process [99].
The diffusion coefficient of carbon in titanium is very low, and the titanium carbide layer generated on the surface will hinder the diffusion of carbon. Therefore, more efficient carburizing methods and better carburizing processes have become the main direction for the development of solid carburizing. Zhao et al. [101] proposed a contact solid carburization method to fabricate TiC coatings on titanium alloy using a “carbon sponge” cast iron. The schematic illustration of the contact solid carburization is shown in Figure 21 [101].
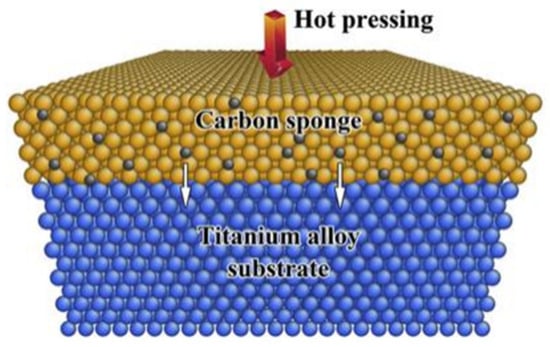
Figure 21.
Schematic illustration of the contact solid carburization [101].
Solid titanium alloy (depicted in blue) and “carbon sponge” (illustrated in yellow) contact in atomic scale at high temperatures to enable the directional diffusion of interstitial carbon atoms (represented in black) from “carbon sponge” to the substrate. Specifically, when titanium alloy (Ti6Al4V) and cast iron contact in atomic scales at high temperatures below the melting point of the cast iron (e.g., 1100 °C), the interstitial carbon atoms in the cast iron diffuse into the titanium alloy, forming a TiC layer. This process is marked by the negligible interdiffusion of metallic atoms, which notably facilitates the subsequent removal of iron, leaving behind a titanium alloy coated with TiC. The resultant coating is characterized by its composition of equiaxed TiC grains and its complete density, signifying a noteworthy advancement in the field of surface-coating technologies for titanium alloys.
Solid carburizing, recognized for its operational simplicity and cost-effectiveness, plays a pivotal role in augmenting the hardness and wear resistance of alloys. However, this technique is currently limited by its relatively low carburizing efficiency. The resulting penetration layer often exhibits limitations such as being thin, porous, and prone to uncontrollable oxidation reactions. Moreover, the quality of this layer is significantly and adversely influenced by the process temperature, highlighting intrinsic drawbacks in its application under certain conditions.
Given these constraints, we posit that the development of innovative process technologies and treatments aimed at enhancing the quality of the solid carburized layer is imperative. Such advancements will not only address the existing limitations but also significantly broaden the application spectrum of solid carburization. Therefore, it stands to reason that refining and innovating in this domain will constitute a primary direction for future development in solid carburizing techniques.
- 2.
- Liquid carburization
Liquid carburizing, also known as salt bath carburizing, is a method of carburizing in a liquid medium. Molten salts can provide a liquid reaction media at high temperature for surface treatment of metals. Comparing to an aqueous solution, they have excellent thermal conductivity, rapid ion migration and diffusion at elevated temperature, resulting in a faster reaction rate at the solid/liquid (S/L) and metal/carbide interfaces [102]. In addition, inorganic carbon sources such as CO2 [103] and carbonates [104,105,106] can be employed as raw materials for the preparation of carbides. These sources can be converted into carbon via electrochemical reduction or oxidation [107,108] to prepare carbon films or metal carbides. Furthermore, due to the good wettability of molten salt to the metal substrate, it also offers possibility for preparing coatings on shaped structural parts. Zhao et al. [109] demonstrated a general salt-thermo-carburizing method to prepare TiC coatings, as shown in Figure 22 [109]. In molten CaCl2-CaC2, TiC coatings were efficiently prepared on the Ti substrate at a temperature below 900 °C. The spontaneous carbonization of Ti is attributed to the negative formation of Gibbs free energies [110]. The growth of TiC is determined by the diffusion of C in the TiC coating, which is driven by the high carbon potential at the molten salt/TiC interface. The growing kinetics of the coating follow a parabolic law, demonstrating a diffusion-controlled process [111].

Figure 22.
(a–c) The SEM images and structural composition of the initial sample and the sample after carburizing for 2 h at 900 °C, respectively. (d) The SEM-mappings, (e) linear scan of the elements, and (f) the carburizing mechanism process of the TiC coating [109].
This simplicity and effectiveness of the salt-thermo-carburizing method suggests a general way to prepare metallic carbide coatings on the relative metal substrate at a medium temperature. However, due to the limitations of the container, it is difficult to carburize larger sizes and slender parts via salt bath carburizing, rendering this method more suitable for small batch production rather than large-scale manufacturing.
- 3.
- Gas carburization
Gas carburization stands as a prevalent method for the carburization of titanium alloys, predominantly utilizing high-carbon-content gases such as methane and carbon monoxide (CO) as the carbon sources. This process typically involves constant-temperature heating. Concurrently, inert gases like nitrogen (N2) are introduced as protective agents to circumvent the potential oxidation of the material or to prevent hydrogen embrittlement, a common concern in high-temperature treatments. The elevated furnace temperatures facilitate the disassociation of carbon from the gas source, subsequently enabling the activated carbon atoms to react with the surface titanium atoms, leading to the formation of TiC (titanium carbide). A depiction of a typical gas nitriding setup is illustrated in Figure 23.
Figure 23.
Schematic diagram of gas carburization device.
This process not only ensures a controlled introduction of carbon into the titanium alloy but also maintains the integrity of the material by preventing adverse reactions that could compromise its mechanical properties. The resultant TiC layer is known for its enhanced hardness and wear resistance, making gas carburizing a vital technique in the surface treatment of titanium alloys.
The carburizing temperature is the factor that has the greatest influence on the quality of the layer. A lower temperature will lead to lower carbon atom activity, resulting in a thinner thickness of the generated layer. With increasing carburization temperature, there is a decrease in reaction activity but an increase in the availability and diffusion capability of active carbon atoms. This change facilitates deeper penetration of carbon into the titanium matrix, leading to the formation of a thicker carburized layer. Such temperature-dependent dynamics are crucial for tailoring the depth and quality of the carburized layer in titanium alloys. Wu et al. [112] compared the XRD physical and metallographic organization of TA2 alloy under different carburizing temperatures. It was also concluded that the thickness of the carburized layer increased with the increase in the carburizing temperature. And when the treatment temperature exceeds 882.5 °C, the organization of the titanium alloy changes, while the abnormal coarsening of martensitic organization provides more possibilities for carbon atoms to enter the interstices. The XRD physical phase diagrams and metallographic histograms of TA2 alloy carburized at different temperatures are shown in Figure 24 [112]. And the related microstructural characteristics are shown in Figure 25 [112].
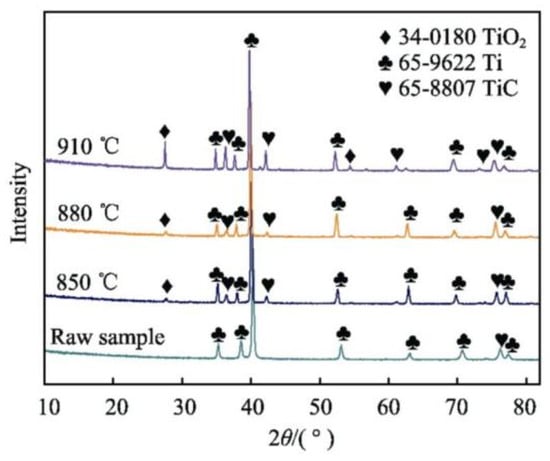
Figure 24.
Phase analysis of TA2 titanium alloy via XRD at different temperature [112].
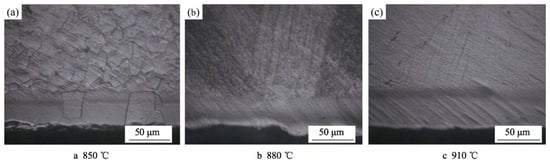
Figure 25.
Microstructure of TA2 titanium alloy carburized at different temperature, (a) 850 °C, (b) 880 °C, (c) 910 °C [112].
The second important factor affecting the quality of the penetrating layer is the treatment time. It is generally believed that the longer the treatment time, the greater the thickness of the carburized layer, and the more carbon atoms there are in the carburized layer. The hardness distribution of the carburized layer is positively correlated with the carbon concentration distribution [113]. Therefore, longer treatment times typically yield a carburized layer of superior quality. Amar et al. [114] observed that the carburized layer’s thickness and the tribological properties of TC4 alloys improve proportionally with increased treatment durations.
Upon examining the comparative graph of the tribological properties in Figure 26 [114], a distinct trend is evident. Alloys subjected to prolonged carburization times demonstrate significantly lower friction coefficients. This reduction in friction is directly linked to an increased resistance to mass loss over distance, indicating enhanced wear properties.
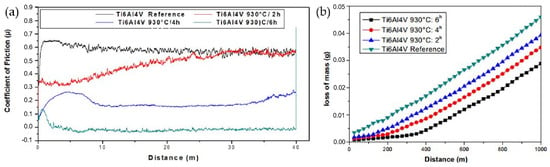
Figure 26.
Comparison chart of friction performance. (a) Coefficient of friction (untreated and carburizing 930 °C: 2 h, 4 h, 6 h). (b) Variation in mass loss (untreated state (reference) and carburizing 930 °C: 2 h, 4 h, 6 h) [114].
Gas carburizing can effectively improve the material hardness, coefficient of friction and wear resistance. Characterized by high processing temperatures, this method offers rapid carburization, with cycles approximately half the duration of those in solid carburizing. Its operational simplicity, coupled with minimal pollution, makes gas carburizing a preferable choice for continuous and large-scale production. Furthermore, the ability to control the thickness of the carburized layer by adjusting the carbon potential is a significant advantage of this technique, allowing for precise tailoring of surface properties to meet specific application requirements. But too high carburizing temperature will make the martensite grain abnormally coarse, resulting in a decline in the mechanical properties of the material.
Therefore, the authors believe that optimizing gas carburizing to ensure material properties while lowering the carburizing temperature represents a key direction for future advancements in this field. This approach not only maintains the desired surface characteristics but also aims to enhance the overall efficiency and sustainability of the carburizing process. Research has shown that intense plastic deformation and surface nano-means such as active ions can provide diffusion channels, effectively improve the diffusion of active ions, and reduce the temperature required for thermochemical treatment.
Integrating gas carburizing with additional surface strengthening techniques, such as shot peening, could potentially reduce the required carburizing temperature while simultaneously enhancing the mechanical properties of the carburized alloy and the quality of the carburized layer. This combined approach may offer a synergistic effect, optimizing both the process efficiency and the performance of the treated material. In addition, constant-temperature, low-pressure intermittent carburizing can repeat the saturation and diffusion cycle by repeatedly pumping in and out the carburizing gas. It effectively avoids hydrogen embrittlement and maintains a high carbon ion concentration to generate a high-quality carburized layer [115].
- 4.
- Ion carburizing
Ion carburizing is a process of surface carburization using the glow discharge effect between the cathode and anode in a low carburizing atmosphere, which is commonly used to improve the hardness, corrosion resistance and tribological properties of alloys. Compared with the traditional means of carburizing, ionic carburizing can obtain a higher quality and greater thickness of the layer in 3–6 h. This represents a substantial improvement in both processing efficiency and layer quality. Ionic carburizing is derived from a variety of technologies, including plasma electrolytic carburizing and double-layer glow plasma carburizing, broadening its application and efficacy in surface treatment processes.
The quality of ionic carburizing layers depends mainly on the carburizing temperature. Numerous studies have shown that the carburizing temperature plays a decisive role in the diffusion process of atoms. Under the same conditions, high temperatures are more likely to form a composite layer on the metal surface, and the composite layer is likely to hinder the penetration of carbon atoms into deeper specimens. Zhang et al. [116] compared the effects of different temperatures on the quality of TC4 carburized layers, and found that the carburized layers obtained at 950 °C were of higher quality compared with those obtained at 1000 °C and 900 °C, highlighting the significance of optimizing the temperature to achieve the best carburizing results.
The SEM cross-section of SLM-C-TC4 is shown in Figure 27 [117]. A black TiC layer is formed on the surface, followed by a gray transition layer. In contrast, the titanium substrate contains α-Ti and β-Ti, while the transition layer contains only β-Ti. This suggests that the diffusion of carbon in the titanium matrix promotes the transition of titanium from the α-phase to the β-phase. On the other hand, a thicker transition layer indicates a good bonding between the carburized layer and the titanium substrate [117].
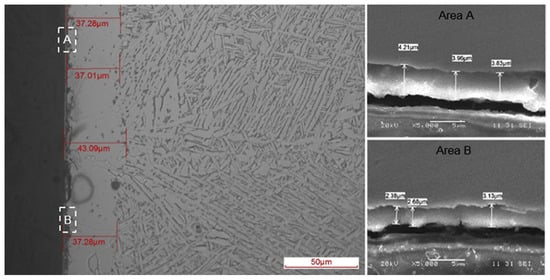
Figure 27.
Typical microstructures of SLM-C-TC4 specimen (left) and the enlarged views of areas A and B (right) [117].
Dong et al. [118] investigated the effect of carburizing on the bond strength of titanium alloy DLC coatings and found that the carburizing treatment can effectively reduce the grain size of DLC coatings and thus improve the densification of the coatings. The carburizing layer also inhibits grain boundary migration in the bulk of the DLC coatings, preventing grain coarsening and imparting higher thermal stability [118]. SEM micrographs and 3D images of the carburized DLC coatings are shown in Figure 28 [118]. The cross-sectional SEM micrographs and EDS line scan analysis of the carburized DLC coatings are shown in Figure 29 [118].

Figure 28.
SEM micrographs and 3D images of carburized DLC coatings [118]. (a). Coated surface under low magnification. (b). DLC coating under high magnification. (c). Surface profile of coatings deposited on the different pretreated substrates.
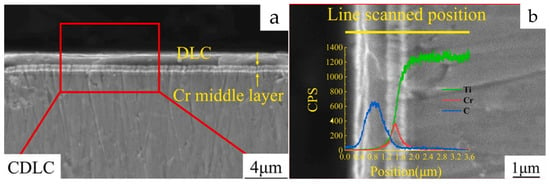
Figure 29.
Cross-sectional SEM micrographs and EDS line scan analysis of carburization-treated DLC layers [118]. (a). SEM micrograph of DLC coated sample after carburizing treatment. (b). EDS line scan analysis of DLC coated sample after carburizing treatment.
Compared with glow plasma carburization, dual-glow plasma carburization technology further improves the hardness and service life of the carburized alloy by forming two different layers of glow ion carburization. Zheng et al. [119] demonstrated that dual-glow plasma carburization can introduce supersaturated vacancies to form a gradient structure layer, which accelerates the transport of metallic elements to the outside. This process accelerates the outward transport of metallic elements, leading to a more rapid development of the gradient structure layer. Post carburization, there is a significant improvement in the surface layer hardness due to the vacancy concentration gradient.
Ion carburizing, known for its brief heat treatment duration, produces a high-quality penetration layer and is versatile enough to treat workpieces of varied shapes and complexities in a clean manner [120]. It can improve the hardness and wear resistance of the material surface without damaging the original properties of titanium. However, its drawbacks such as an unavoidable hollow cathode effect and difficulty in scale production remain significant obstacles to its broader adoption. It is worth mentioning that the emergence of new treatment methods such as physical vapor deposition and chemical vapor deposition provides a new choice and development direction for the ion carburization of titanium alloys.
Carburizing is an effective means to improve the surface properties of titanium alloy. Compared with nitrogen atoms, carbon has a weaker solubility in titanium, which makes carburizing titanium alloys more difficult than nitriding [121]. In addition, the nitrogen element has a greater effect on the surface hardness of titanium alloys than carbon. Consequently, both domestically and internationally, there is a notable prevalence of research focusing on the nitriding of titanium alloys.
- Nitriding
Nitrogen significantly influences the surface hardness of titanium alloys, with nitriding treatments notably enhancing their surface hardness and friction corrosion resistance. Currently, the more common nitriding treatments include solid nitriding, gas nitriding and plasma nitriding. These treatments typically require several hours to effectuate the desired surface modification. However, solid and gas nitriding often result in a loosely structured and porous layer, which may lead to hydrogen embrittlement and thus fail to meet quality standards for the workpieces. Another research report shows that plasma nitriding can complete the surface alloy nitriding within one hour, offering a marked improvement in both nitriding efficiency and layer quality compared to the other two methods [122]. In this section, three different nitriding methods for titanium alloys are presented.
- Salt bath nitridation
Salt bath nitridation is a surface modification technique that involves the decomposition of cyanide into cyanate in a molten salt medium at temperatures ranging from 500 to 600 °C. This process facilitates the diffusion of nitrogen from the salt into the surface of the workpiece, with nitrogen atoms infiltrating the surface in the form of an interstitial solid solution. Because of a relatively low working temperature, salt bath nitriding is categorized as a low-temperature surface treatment. The primary nitrogen source in salt bath nitriding originates from the cyanate ion in the nitriding salt. However, the high temperatures involved in the nitriding process can lead to the gradual decomposition of cyanate and the formation of cyanogen, which poses health risks. Consequently, the development of salt bath nitriding techniques has been oriented towards low-cyanogen, cyanide-free solutions, and faster processing rates to mitigate these concerns.
Typically, salt bath nitriding involves extended treatment durations. However, prolonged exposure to high temperatures during nitriding can lead to porosity in the nitride layer, adversely affecting the hardness and wear resistance of the alloy [123]. In order to improve the nitriding efficiency of salt bath nitriding, researchers have developed many directions, including the addition of rare earth elements to change the metallographic structure of the alloy. For example, Zhu et al. [124] compared the metallographic structures of nitride titanium alloys before and after the addition of rare earth elements. They discovered that rare earth atoms, due to their strong surface adsorption capabilities, can attract a significant amount of reactive nitrogen atoms. These atoms are adsorbed onto the workpiece’s surface at low energy, significantly increasing the nitrogen concentration in the substrate’s surface layer.
Salt bath nitriding has the advantages of low equipment cost, reduced nitriding temperatures, and minimal deformation of the workpiece. This method effectively enhances the roughness, hardness, and wear resistance of samples, thereby broadening their application spectrum. The hardness of the workpiece quenched in water after salt bath nitriding will be greatly improved, and the workpieces treated by this means can usually be used for making fasteners and automotive parts (e.g., nuts and bolts), etc. [125]. However, the highly toxic cyanogen produced by decomposition in the salt bath process is still an urgent problem to be solved.
- 2.
- Gas nitriding
Gas nitriding, a method for surface modification of titanium alloys, involves exposing these alloys to an atmosphere of nitrogen or a nitrogen–hydrogen mixture and heating them to form a hardened layer of Ti2N or TiN on the surface. The nitrogen source mainly comes from nitrogen or nitrogen–hydrogen and other gaseous media. According to the different heating methods, gas nitriding can be divided into vacuum nitriding and laser nitriding.
Vacuum nitriding is an innovative technique developed in recent years, which involves a series of steps to enhance the surface properties of titanium alloys. The process begins with placing the titanium alloy into the furnace, followed by purging impurity gases using an inert gas. The furnace is then evacuated, and this vacuum–gas washing cycle is repeated 2–3 times. The alloy is heated to 800 °C and maintained at this temperature for approximately an hour. This step is crucial for decomposing the titanium surface oxidation layer, thereby reducing the hindrance it poses to nitrogen atom infiltration into the substrate. Such a temperature setting also facilitates the formation of a fine lamellar structure on the alloy’s surface, aiding in grain refinement and reducing lamellar organization. Finally, high-purity nitrogen is introduced into the nitriding treatment for 30 min, so that nitrogen is fully diffused into the substrate, and the above operation is repeated until the nitriding treatment time is reached [126], which can generate a layer of dense nitriding layer. Intermittent vacuum nitriding treatment is particularly effective in breaking down residual oxide layers on the titanium alloy’s surface, enhancing the number of reactive nitrogen atoms, and improving both nitrogen adsorption rates and reaction speeds on the surface. A higher nitrogen potential further facilitates nitrogen’s inward diffusion, resulting in a thicker nitriding layer.
Vacuum nitriding, characterized by its simplicity, cost effectiveness, and flexibility in accommodating various workpiece geometries, has certain limitations. Typically, the treatment duration is lengthy, and as the nitriding time extends, the thickness of the nitride layer increases. However, this newly formed nitriding layer can hinder further nitrogen diffusion, leading to issues such as brittleness, thinness, and poor adhesion strength with the substrate. Managing the duration of the nitriding process is crucial; excessively long treatment times can cause the nitride layer to peel off due to prolonged high temperatures, while insufficient treatment time may not satisfy the material’s wear resistance requirements [127]. To overcome these shortcomings, laser nitriding has been gradually used for surface modification of titanium alloys. This technique, developed through continuous exploration, offers a solution to the challenges posed by traditional vacuum nitriding.
High-purity nitrogen then reacts with this molten pool to form a nitride layer, significantly improving the wear and abrasion corrosion resistance of the treated surface. A key advantage of this technique is its surface-specific treatment, which leaves the mechanical properties of the titanium alloy matrix largely unaffected. By restricting the process parameters during the treatment, it is possible to control the thickness and hardness of the titanium alloy nitriding layer. The working schematic of laser nitriding is shown in Figure 30 [128]. Figure 31 shows a schematic diagram of the wear mechanism for samples with different laser nitriding energies [128].

Figure 30.
Pulsed laser nitriding process flow diagram [128].
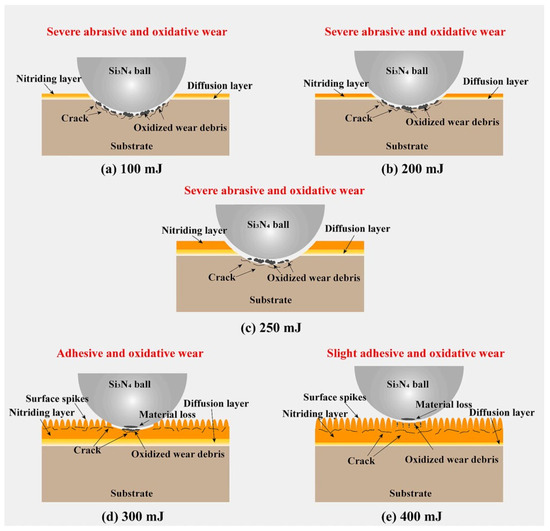
Figure 31.
Schematic of the wear mechanism of different laser nitriding energy samples [128].
With the change in depth, the nitrogen concentration shows a gradient from the surface of the percolated layer to the bottom of the molten pool. Meanwhile, the formed nitrides also show different metallographic structures. On the near surface of the percolated layer, TiN exists in the form of dendrites, and the secondary dendrites grow perpendicular to the primary dendrites, as shown in Figure 32a [129]. Originating from the bias effect of TiN dendrites on nitrogen, the near-surface region has the largest nitride concentration and the highest hardness. This inference is consistent with the results exhibited in Figure 32d. In the intermediate region, often referred to as the heat-affected zone, the microstructure primarily consists of parallel rows of martensite, exhibiting a predominantly needle-like organization, as illustrated in Figure 32b [129]. The TiN content in the heat-affected region is relatively lower than that in the near-surface region. Its hardness is relatively low compared to the nitride region, which is generally twice the hardness of the matrix. With increasing depth, nitrides precipitate in the interphase region in the form of particles (Figure 32c) [129]. In this region, the heat-affected zone is less pronounced, and the overall structure of the alloy remains largely unchanged.
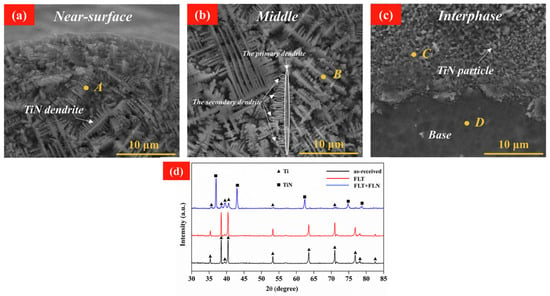
Figure 32.
(a–c) SEM images of different nitride zones. (d) The microhardness and nitrogen contents measured at points A, B, C, and D [129].
Compared with vacuum nitriding, the processing time of laser nitriding is greatly shortened. The thickness of the nitride layer can be increased via repeated laser scanning. Among the nitriding treatments discussed in this paper, laser nitriding achieves the highest surface hardness post treatment. However, defects, such as surface cracks, triggered by the heat buildup effect are still a challenge that needs to be solved urgently. Therefore, maintaining high hardness and limiting the generation of cracks are still important issues in optimizing the parameters of the laser nitriding process. It is worth mentioning that in recent years, scholars have proposed a new surface-strengthening strategy combining surface nitriding and surface weaving. This approach has been shown to produce a uniform, crack-free nitride layer of 40–60 μm on the weave’s surface [129]. Kang et al. [130] also reported that the composite treatment can significantly reduce the material wear rate and friction coefficient. And it can effectively control the thermal deformation and inhibit the generation of cracks, addressing one of the primary drawbacks of traditional laser nitriding.
Gas nitriding, as one of the most convenient and effective nitriding methods, has an extremely wide range of applications in the field of industry and scientific research. But prolonged high-temperature treatment will cause a large amount of energy loss, and may lead to the growth of secondary α-phase or the decomposition of the substable phase, which will affect the internal organization and structure [131]. Therefore, reducing the nitriding temperature and improving the nitriding efficiency is a major critical issue in the development of gas nitriding. Recent developments in low-temperature nitriding have led researchers to explore innovative strategies for improving nitrogen diffusion. These strategies include creating diffusion channels and utilizing surface metal phase transitions to provide auxiliary driving forces for nitrogen diffusion [131]. For example, Liu et al. [132] reduced the nitriding temperature of the Ti-6Al-4V alloy to 650 °C via surface nanosizing. Additionally, Jiang et al. [131] increased the effective thickness of the nitride layer by 15 μm from 500 °C nitriding treatment through post-laser nitriding, demonstrating the potential for significant improvements in gas nitriding efficiency.
- 3.
- Plasma nitridation
Plasma nitriding (PN) is an important surface modification technique that significantly increases surface hardness and improves the anti-corrosion ability by forming a titanium nitride layer [38,39,40,41]. When the nitrogen ions hit the surface of the workpiece at high speed, the kinetic energy is converted into thermal energy, which increases the temperature of the surface of the titanium alloy. In this process, part of the nitrogen ions seizes electrons to become nitrogen atoms and penetrate into the alloy surface [133]. Another part causes cathodic sputtering upon impact with the alloy surface. The sputtered titanium atoms react with nitrogen atoms to form nitrides, which subsequently deposit on the surface of the workpiece [134]. Under the effect of sputtering and diffusion of ions, nitrogen continuously diffuses to the inside of titanium and forms a new nitride layer. The schematic diagram of the plasma nitriding device is shown in Figure 33 [135].
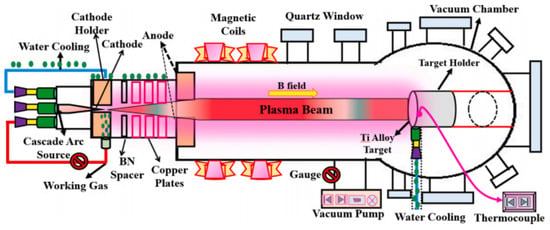
Figure 33.
A schematic representation of the plasma nitriding setup [135].
From the working principle of ion nitriding, the rate of ion nitriding has a greater relationship with the nitrogen content in the gas medium and the voltage or temperature of the glow discharge. A higher nitrogen content in the gas medium accelerates the nitriding rate, but it can also lead to rapid formation of surface nitrides, which may hinder the inward diffusion of nitrogen and the growth of the nitriding layer, thereby impacting the wear resistance of the material. It has been found that gas nitriding of Ti6Al4V alloy is conducted at temperatures near or exceeding 1000 °C, whereas plasma nitriding is optimally performed within the 700 to 900 °C range. This enables the formation of a protective surface layer of TiN compound while preserving the mechanical properties of the workpiece [136]. The nitride layer is composed of a compound layer (TiN and Ti2N) on the surface and a subsequent nitrogen diffusion zone (α-Ti(N)) [41,137,138]. In previous work, researchers have demonstrated that a protective Ti–N layer could be formed on Ti6Al4V surface via hollow cathode plasma source nitriding [139]. And a special compound layer structure with nanocrystalline/amorphous TiN formed during this process.
Ionic nitriding can significantly enhance the surface hardness and wear resistance of titanium alloys, and it can form a fine grain layer by changing the microstructure of the alloy, thus enabling it to exhibit better mechanical properties [140]. However, prolonged high-temperature nitriding is prone to lead to the growth and precipitation of undesirable grains, which is not conducive to the enhancement of the material properties of titanium alloys [141]. The study of She et al. [142] also showed that the hardness and brittleness of the nitride layer increase with the increase in the temperature, which has a significant effect on the abrasion resistance. The low-temperature environment is not conducive to the growth of nitriding layer. Therefore, the nitriding layer may not meet the requirements of surface modification. So, the selection of the appropriate treatment temperature and treatment time is an important part to ensure the quality of the nitriding layer.
Ion nitriding’s characteristics are its high energy efficiency, fast speed, small deformation of workpiece, low pollution levels, and suitability for a complex surface workpiece. However, it has a high cost, energy consumption and other shortcomings, which limit the application of this technology. And traditional DC plasma nitriding often results in surface damage, edge effect and hollow cathode effect, which can negatively impact the quality of the nitride layer [143]. In order to overcome these defects, researchers have made many efforts. Active screen plasma nitriding technology is one of the active screen plasma nitriding technologies. This innovative approach utilizes a metal mesh cage to transfer the cathodic potential, shifting the formation of plasma from the component’s surface to the cage. Consequently, it effectively mitigates many of the inherent problems associated with DC plasma nitriding. The experimental setup of this technology is shown in Figure 34 [144].
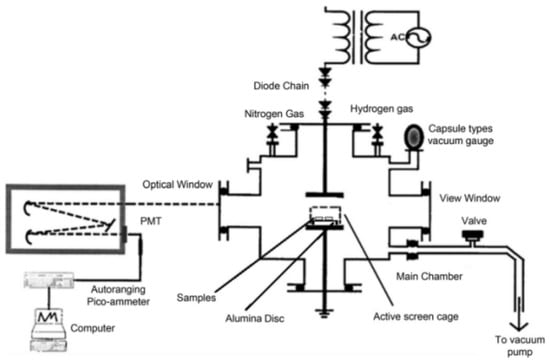
Figure 34.
Experimental diagram of active screen plasma nitriding device [144].
To address these issues, significant efforts have been made, leading to the development of active screen plasma nitriding technology. This innovative approach utilizes a metal mesh cage to transfer the cathodic potential, shifting the formation of plasma from the component’s surface to the cage. Consequently, it effectively mitigates many of the inherent problems associated with DC plasma nitriding. The experimental setup of this technology is illustrated in Figure 34 [144], demonstrating its unique approach to improving the nitriding process.
Although this method of active screen plasma nitriding solves many of the inherent shortcomings of traditional plasma nitriding, it also introduces a new problem: the process’s high nitriding temperature of around 800 °C. When applied to titanium alloys, this elevated temperature may lead to the growth of large grains, potentially compromising the mechanical properties of the titanium alloy matrix. Therefore, when employing this method, additional measures are often necessary to mitigate the required temperature of titanium nitriding. Some studies have shown that shot peening can obtain a nanocrystalline structure with a surface gradient. The high-energy grain boundaries present in the surface nanocrystalline layer can provide channels for the diffusion of nitrogen, which is conducive to saving the nitriding time and lowering the nitriding temperature [143].
For example, Yao et al. [145] carried out nitriding treatment of pure titanium after shot peening and found that the nitriding temperature was reduced to 550 °C. Similarly, Wen et al. [146] reduced the optimum plasma nitriding temperature to 500 °C for TC4 titanium alloy via ASPN after SP. Zhang et al. [143] further confirmed the effectiveness of this approach by comparing nitride TA17 samples, both pre-treated with shot peening and without pre-treatment. They found that the surface nanocrystals generated by shot peening significantly enhanced the nitriding kinetics and promoted the formation of nitrides. Observing the surface morphology and SEM cross-sectional images of the original TA17 and the SP-treated TA17 samples in Figure 35 [143], it can be found that the nitriding samples treated with shot peening have more titanium nitride particles generated in the surface layer, forming a reticulated structure. Additionally, the composite layer generated is also thicker. Combined with the previous research, it can be found that SP-ASPN nitriding technology not only effectively reduces the nitriding temperature but also yields a nitriding layer of superior quality and thickness.
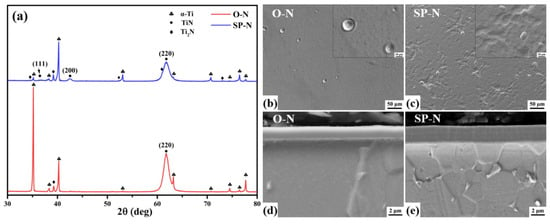
Figure 35.
The O-N and SP-N samples nitrided at 500 °C for 20 h via ASPN: (a) XRD patterns, (b,c) surface morphologies, (d,e) cross-sectional SEM images [143].
In summary, the combination of shot peening and ASPN nitriding technology has demonstrated significant advantages in the surface treatment of titanium alloys. This approach not only effectively reduces the required temperature for nitriding but also enhances the quality and thickness of the nitride layer.
- Oxidized coating
Oxygen is another important element that significantly affects the quality of titanium alloys, and this paper focuses on plasma electrolytic oxidation (PEO) technology. Compared with the nitriding and carbonizing treatments, this coating method is a more cost-effective and environmentally friendly surface treatment technique. Taking flame-retardant alloys as an example, the preparation of flame-retardant coatings on the surface of parts can effectively maintain the original mechanical properties of the metal and improve the flame-retardant properties of the parts, avoiding the introduction of low-strength and high-cost alloys through alloying [147].
Plasma electrolytic oxidation (PEO), as a promising high-pressure plasma-assisted electrochemical method, can produce oxide ceramic-like coatings on metal substrates submerged in electrolyte [148]. Their coatings are grown in situ and consist of a two-layer structure: the inner layer is dense, while the outer layer is porous [149,150,151,152]. Currently, PEO has been shown to be an effective surface treatment for additive manufacturing of Ti6Al4V alloys to obtain coatings with high hardness and high abrasion and corrosion resistance [153]. However, inherent defects such as porosity and cracks limit the development of this coating in the medical field [154].
Composite surface modification technology is an effective measure to compensate for the defects of a single type of technology. Arash et al. [148] showed that pre-treatment or post-treatment methods, such as laser surface melting (LSM) or laser surface texturing (LST), can effectively improve the densification of PEO coatings, which leads to a reduction in the porosity and number of the pores and an improvement of the coating quality.
With ongoing advancements in technology and metal surface treatment, the application of titanium alloys in biomedical, shipbuilding, and aerospace industries is expected to experience further expansion [155,156,157]. Simultaneously, these advanced technologies are likely to be adapted for the fabrication of a broader spectrum of alloys, such as aluminum alloys and high-temperature-resistant alloys [158,159]. The advancements discussed herein not only highlight the evolution of surface treatment techniques but also pave the way for future research in optimizing the mechanical and chemical properties of metal alloys. Our comprehensive review underscores the importance of continued innovation and interdisciplinary research in material science to develop more efficient, cost-effective, and environmentally friendly surface treatment methods.
4. Conclusions
In conclusion, this review comprehensively examines the characteristics of titanium alloys with diverse microstructures and investigates the impact of various surface processing methods, including machining, conventional heat treatment, and thermochemical treatment, on the surface quality and material properties of Ti and its alloys at the microstructural level. The findings underscore the superiority of composite surface modification technologies in enhancing the material properties of metals compared to single treatment methods.
Addressing the challenges posed by the difficult machinability of titanium alloys, Electrical Discharge Machining (EDM) emerges as an effective technique for Ti alloy cutting. Additionally, shot peening and surface mechanical grinding play crucial roles in enhancing surface metal densification through nanosizing, thereby contributing to improved material properties. Innovative approaches such as dual shot peening and the combination of vibratory and conventional shot peening exhibit superior efficacy in increasing metal fatigue strength.
Common heat treatment methods like quenching and annealing are identified as effective means to alter the metallurgical structure of alloys, thereby enhancing the performance of the metal’s surface layer. Thermochemical treatment, involving the introduction of titanium-sensitive elements, leads to the formation of a protective layer on titanium alloy surfaces, mitigating external influences and contact with the alloy matrix. Carburizing elements, when introduced before heat treatment, nanostructure the surface, facilitating element diffusion and reducing the carburization temperature requirements, ultimately improving heat treatment efficiency and forming higher quality carburized alloys.
The authors assert that composite surface modification technology serves as a potent strategy for elevating the surface hardness and friction corrosion resistance of titanium alloys. This review aims to aid researchers seeking to enhance the surface properties of titanium and its alloys by delving into the mechanisms through which diverse processing methods bolster the surface characteristics of these alloys. Given the widespread use of titanium, continuous development and refinement of surface modification techniques remain imperative, leaving room for further exploration and summarization of various technical approaches.
Author Contributions
Y.Z.: Conceptualization, Investigation, Resources, Supervision, Project Administration, Writing—Review and Editing; K.G.: Investigation, Methodology, Validation, Data curation, Writing—Original Draft Preparation; P.C.: Methodology, Validation, Supervision; J.Y. and F.D.: Investigation, Data Curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Project Founded by the Education Department of Hunan Province (23B0499), and the National Natural Science Foundation of China (No. 52005517).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, C.; Li, M.; Liu, Y. Severe plastic deformation induced precipitation of the ordered α2-Ti3Al phase in Ti-5Al-2Sn-2Zr-4Mo-4Cr. J. Alloys Compd. 2021, 854, 157277. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, X.F. Improvement of mechanical properties and microstructural refining of cast titanium alloys by coupling of electropulsing and temporary alloying element hydrogen. Mater. Sci. Eng. A 2022, 858, 144176. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, L.; Fan, Q.B.; Lei, W.; Chen, K.; Zhu, X.J.; Mu, X.N.; Yao, J.H. Effect of Fe content on microstructure and hardness of Ti-4.5Mo–5Al-1.8Zr-2.5Cr-1.1Sn titanium alloy based on high-throughput diffusion couple. Mater. Sci. Eng. A 2022, 842, 143089. [Google Scholar] [CrossRef]
- Zhao, E.T.; Sun, S.C.; Zhang, Y. Recent advances in silicon containing high temperature titanium alloys. J. Mater. Res. Technol. 2021, 14, 3029–3042. [Google Scholar] [CrossRef]
- Tan, Y.M.; Chen, R.R.; Fang, H.Z.; Liu, Y.L.; Cui, H.Z.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Enhanced strength and ductility in Ti46Al4Nb1Mo alloys via boron addition. J. Mater. Sci. Technol. 2022, 102, 16–23. [Google Scholar] [CrossRef]
- Kolli, P.R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Singh, S.S.; Harpreet, S.; Hady, A.M.G. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2020, 121, 111661. [Google Scholar]
- Masoud, S.; Erfan, G.R.; Saeid, A.; Seeram, R.; Nazatul, S.L. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2021, 5, 371–395. [Google Scholar]
- Fan, H.; Yang, S. Effects of direct aging on near-alpha Ti–6Al–2Sn–4Zr–2Mo (Ti-6242) titanium alloy fabricated by selective laser melting (SLM). Mater. Sci. Eng. A 2020, 788, 139533. [Google Scholar] [CrossRef]
- Alessandro, C.; Alberta, A.; Paolo, F.; Mariangela, L. Towards customized heat treatments and mechanical properties in the LPBF-processed Ti-6Al-2Sn-4Zr-6Mo alloy. Mater. Des. 2022, 215, 110512. [Google Scholar]
- Optasanu, V.; Berger, P.; Vincent, B.; Lucas, D.M.C.M.; Herbst, F.; Montesin, T. Strong correlation between high temperature oxidation resistance and nitrogen mass gain during near alpha titanium alloys exposure in air. Corros. Sci. 2023, 224, 111547. [Google Scholar] [CrossRef]
- Si, Y.H.; Li, M.S.; Liu, H.Y.; Jiang, X.Z.; Yu, H.Y.; Sun, D.B. Evaluation of tribocorrosion performance of Ti6Al4V alloy in simulated inflammatory and hyperglycemic microenvironments. Wear 2023, 532–533, 205077. [Google Scholar] [CrossRef]
- Huang, S.; Sun, B.B.; Guo, S.Q. Microstructure and property evaluation of TA15 titanium alloy fabricated by selective laser melting after heat treatment. Opt. Laser Technol. 2021, 144, 107422. [Google Scholar] [CrossRef]
- Yan, Z.; Liang, Y.L.; Zhang, Z.J.; Xu, J.; Yi, Y.L. Effect of shot peening on high-frequency fatigue properties of TC11 titanium alloy. Chin. J. Rare Met. 2014, 38, 554–560. (In Chinese) [Google Scholar]
- Li, Z.Y.; Cai, Z.B.; Wu, Y.Q.; Zhu, M.H. Effect of nitrogen ion implantation dose on torsional fretting wear behavior of titanium and its alloy. Trans. Nonferr. Met. Soc. 2017, 27, 324–355. [Google Scholar] [CrossRef]
- Nihal, Y.; Kubilay, A. A review on heat treatment efficiency in metastable β titanium alloys: The role of treatment process and parameters. J. Mater. Res. Technol. 2020, 9, 15360–15380. [Google Scholar]
- Xu, S.; Zhang, H.M.; Xiao, N.M.; Qiu, R.S.; Cui, Z.S.; Fu, M.W. Mechanisms of macrozone elimination and grain refinement of near α Ti alloy via the spheroidization of the Widmannstätten structure. Acta Mater. 2023, 260, 119339. [Google Scholar] [CrossRef]
- Sun, S.C.; Fang, H.Z.; Li, Y.L.; Chen, R.R.; Zhu, B.H.; Guo, J.J. Formation mechanism of titanium solid solution and its influence on equiaxed behavior of α phase of Ti–5Al–5Mo–5Cr–2Zr-xNb alloys. J. Mater. Res. Technol. 2023, 26, 434–444. [Google Scholar] [CrossRef]
- Xu, A.J.; Wan, H.F.; Liang, C.Z.; Niu, Y.T.; Tao, Q.; Tang, Z.J. Current status and development trend of low-temperature titanium alloy materials. J. Netshape Form. Eng. 2020, 12, 145–156. (In Chinese) [Google Scholar]
- Huang, L.; Li, C.M.; Li, C.L.; Hui, S.X.; Yu, Y.; Zhao, M.J.; Guo, S.Q.; Li, J.J. Research progress on microstructure evolution and hot processing maps of high strength β titanium alloys during hot deformation. Trans. Nonferr. Met. Soc. 2022, 32, 3835–3859. [Google Scholar] [CrossRef]
- Shi, S.X.; Liu, X.S.; Zhang, X.V.; Zhou, K.C. Comparison of flow behaviors of near beta Ti-55511 alloy during hot compression based on SCA and BPANN models. Trans. Nonferr. Met. Soc. 2021, 31, 1665–1679. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Zhang, S.; Liu, X.J.; Wu, X.X.; Zhang, S.Q.; Zhou, G. High Temperature Deformation Behavior of Near-β Titanium Alloy Ti-3Al-6Cr-5V-5Mo at α+β and β Phase Fields. Crystals 2023, 13, 371. [Google Scholar] [CrossRef]
- Kopova, I.; Stráský, J.; Harcuba, P.; Landa, M.; Janeček, M.; Bačákova, L. Newly developed Ti–Nb–Zr–Ta–Si–Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C 2016, 60, 230–238. [Google Scholar] [CrossRef]
- Li, C.M.; Huang, L.; Zhao, M.J.; Guo, S.Q.; Li, J.J. Hot deformation behavior and mechanism of a new metastable β titanium alloy Ti–6Cr–5Mo–5V–4Al in single phase region. Mater. Sci. Eng. A 2021, 814, 141231. [Google Scholar] [CrossRef]
- Li, C.H.; Cui, Y.M.; Zheng, W.W.; Song, L.; Wu, Y.L.; Wu, Y.D.; Yu, C.Y.; Hui, X.D. Unveiling the dynamic softening mechanism via micromechanical behavior for a near-β titanium alloy deformed at a high strain rate. J. Mater. Res. Technol. 2023, 26, 9392–9405. [Google Scholar] [CrossRef]
- Xin, S.W.; Zhao, Y.Q.; Lu, Y.F.; Li, Q.; Yang, H.Y. Role of grain boundaries in the high-temperature performance of a highly stabilized beta titanium alloy II: Creep behavior. Mater. Sci. Eng. A 2013, 559, 7–13. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zeng, W.D.; Wang, Y.B.; Zhou, D.D.; Gao, X.X. High strain rate compression behavior of a heavily stabilized beta titanium alloy: Kink deformation and adiabatic shearing. J. Alloys Compd. 2017, 708, 84–92. [Google Scholar] [CrossRef]
- Xu, Q.B.; Liu, S.Y. Titanium and titanium alloy grades and applications in foreign aerospace industry. Lead. Edge Technol. 2022, 16, 96–99. (In Chinese) [Google Scholar]
- Chen, C.; Feng, T.T.; Zhang, Y.W.; Ren, B.Q.; Hao, W.; Zhao, X.H. Improvement of microstructure and mechanical properties of TC4 titanium alloy GTAW based wire arc additive manufacturing by using interpass milling. J. Mater. Res. Technol. 2023, 27, 1428–1445. [Google Scholar] [CrossRef]
- Iliana, J.F.; Mohd, F.F.; Ahmad, B.M.; Bakar, S.A.; Afiqah, M.R.N.; Norhamidi, M.; Fadhlina, M.I.; Hani, J.N.; Seah, T.K. Influence of heat treatment parameters on microstructure and mechanical performance of titanium alloy in LPBF: A brief review. J. Mater. Res. Technol. 2023, 24, 4091–4110. [Google Scholar]
- Li, Y.; Zhou, Z.L.; Yi, X.N.; Yan, J.W.; Xiu, J.J.; Fang, D.Z.; Shao, M.H.; Ren, P.; He, Y.Y.; Qiu, J.X. Improved seawater corrosion resistance of electron beam melting Ti6Al4V titanium alloy by plasma nitriding. Vacuum 2023, 216, 112463. [Google Scholar] [CrossRef]
- Morinaga, M. The Molecular Orbital Approach to Titanium Alloy Design. Key Eng. Mater. 2018, 770, 217–223. [Google Scholar] [CrossRef]
- Bosung, S.; Hyung-Ki, P.; Chang-Soo, P.; Kwangsuk, P. Effect of alloying elements on corrosion properties of high corrosion resistant titanium alloys in high concentrated sulfuric acid. Mater. Today Commun. 2023, 34, 105113. [Google Scholar]
- Dong, Y.C.; Huang, S.; Wang, Y.Y.; Zhang, B.; Alexandrov, I.V.; Chang, H.; Dan, Z.H.; Ma, L.; Zhou, L. Stress corrosion cracking of TC4 ELI alloy with different microstructure in 3.5% NaCl solution. Mater. Charact. 2022, 194, 112357. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yuasa, M.; Rifai, M.; Fujiwara, H. Corrosion Behavior of Severely Deformed Pure and Single-Phase Materials. Mater. Trans. 2019, 60, 1243–1255. [Google Scholar] [CrossRef]
- Chuvil’deev, V.; Kopylov, V.; Nokhrin, A.; Tryaev, P.; Kozlova, N.; Tabachkova, N.; Lopatin, Y.; Ershova, A.; Mikhaylov, A.; Gryaznov, M.; et al. Study of mechanical properties and corrosive resistance of ultrafine-grained α-titanium alloy Ti-5Al-2V. J. Alloys Compd. 2017, 723, 354–367. [Google Scholar] [CrossRef]
- Abhinay, T.; Savaş, K.; Ashish, K. Recent Trends in the Characterization and Application Progress of Nano-Modified Coatings in Corrosion Mitigation of Metals and Alloys. Appl. Sci. 2023, 13, 730–763. [Google Scholar]
- Zhang, F.; Yan, S.; Yan, M. In-Situ fabrication of novel (Ti, Cr)-N/aluminide multilayer coatings by plasma nitriding Ti-Cr coated Al alloy. Ceram. Int. 2018, 44, 7259–7266. [Google Scholar] [CrossRef]
- Naeem, M.; Shabana, A.; Shafiq, M.; Raza, H.A.; Javed, I.; Díaz-Guillén, J.; Sousa, R.; Jelani, M.; Abrar, M. Wear and corrosion studies of duplex surface-treated AISI-304 steel by a combination of cathodic cage plasma nitriding and PVD-TiN coating. Ceram. Int. 2022, 48, 21473–21482. [Google Scholar] [CrossRef]
- Keisuke, F.; Masataka, I.; Yoichi, I.; Shoichi, K. Rapid Nitriding of Titanium Alloy with Fine Grains at Room Temperature. Adv. Mater. 2021, 33, 2008098. [Google Scholar]
- Ahmadi, M.; Hosseini, S.; Hadavi, S. Comparison of auxiliary cathode and conventional plasma nitriding of gamma-TiAl alloy. Vacuum 2016, 131, 89–96. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhu, J.Y.; Chen, C.Z.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Jiang, B.B.; Wen, D.H.; Wang, Q.; Che, J.D.; Dong, C.; Peter, K.L.; Xu, F.; Sun, L.X. Design of near-α Ti alloys via a cluster formula approach and their high-temperature oxidation resistance. J. Mater. Sci. Technol. 2019, 35, 1008–1016. [Google Scholar] [CrossRef]
- Ebach-Stahl, A.; Eilers, C.; Laska, N.; Braun, R. Cyclic oxidation behaviour of the titanium alloys Ti-6242 and Ti-17 with Ti–Al–Cr–Y coatings at 600 and 700 °C in air. Surf. Coat. Technol. 2013, 223, 24–31. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Fu, Y.; Li, J.; Xiao, W.L.; Zhao, X.Q.; Ma, C.L. Effects of tungsten addition on the microstructural stability and properties of Ti-6.5Al-2Sn-4Hf-2Nb-based high temperature titanium alloys. J. Mater. Sci. Technol. 2021, 93, 147–156. [Google Scholar] [CrossRef]
- Ozturk, D.; Pilchak, A.; Ghosh, S. Experimentally validated dwell and cyclic fatigue crack nucleation model for α–titanium alloys. Scr. Mater. 2016, 127, 15–18. [Google Scholar] [CrossRef]
- Hémery, S.; Villechaise, P. On the influence of ageing on the onset of plastic slip in Ti-6Al-4V at room temperature: Insight on dwell fatigue behavior. Scr. Mater. 2016, 130, 157–160. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, S.Z.; Liu, J.R.; Li, S.J.; Sun, T.; Li, J.P.; Gao, Y.; Yang, R.; Hu, Q.M. Interaction between Al and other alloying atoms in α-Ti for designing high temperature titanium alloy. Comp. Mater. Sci. 2021, 197, 110620. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.Q.; Xiao, W.L.; Ma, C.L.; Huang, X. Development of Ti-Al-Ta-Nb-(Re) near-α high temperature titanium alloy: Microstructure, thermal stability and mechanical properties. J. Mater. Sci. Technol. 2022, 109, 1–11. [Google Scholar] [CrossRef]
- Wen, P.C.; Yuan, L.J.; Tao, R.; Li, J.; Li, D. First-principles investigation of interaction between surface oxygen and other alloy atoms in α-Ti (0001) for designing high-temperature titanium alloy. Appl. Surf. Sci. 2022, 604, 154535. [Google Scholar] [CrossRef]
- Huang, C.W.; Ge, P.; Zhao, Y.Q.; Xin, S.W.; Zhou, W.; Li, Q.; Zeng, W.D. Research progress of low-temperature titanium alloy. Rare Met. Mat. Eng. 2016, 45, 254–260. [Google Scholar]
- Lu, J.W.; Zhao, Y.Q.; Ge, P.; Niu, H.Z.; Zhang, Y.S.; Zhang, W.; Zhang, P.X. Microstructure and mechanical properties of new high strength beta-titanium alloy Ti-1300. Mater. Sci. Eng. A 2015, 621, 182–189. [Google Scholar] [CrossRef]
- Mantri, S.; Choudhuri, D.; Alam, T.; Viswanathan, G.; Sosa, J.; Fraser, H.; Banerjee, R. Tuning the scale of α precipitates in β-titanium alloys for achieving high strength. Scr. Mater. 2018, 154, 139–144. [Google Scholar] [CrossRef]
- Wang, J.X.; Ye, X.W.; Li, Y.H.; Wan, M.P.; Huang, C.W.; Huang, F.; Lei, M.; Liu, D.; Ma, R.; Ren, X.L. Effect of annealing temperature on mechanical properties of TC21 titanium alloy with multilevel lamellar microstructure. Mater. Sci. Eng. A 2023, 869, 144788. [Google Scholar] [CrossRef]
- Li, C.C.; Xin, C.; Wang, Q.; Ren, J.Q.; Zhao, B.; Wu, J.P.; Pan, X.L.; Lu, X.F. A novel low-cost high-strength β titanium alloy: Microstructure evolution and mechanical behavior. J. Alloys Compd. 2023, 959, 170497. [Google Scholar] [CrossRef]
- Karolina, D.; Tomasz, G.; Mateusz, D.; Bronisław, P.; Agnieszka, S.; Zdzisław, L. Functionalization of the Implant Surface Made of NiTi Shape Memory Alloy. Materials 2023, 16, 1609–1620. [Google Scholar]
- Wang, S.Y. Investigation of chemical analysis methods of niobium-titanium alloys for superconductivity. Ind. Technol. Innov. 2022, 4, 33–35. (In Chinese) [Google Scholar]
- Lv, P.; Zhong, C.L.; Huang, D.F.; Zhou, X.S.; Liu, Z.C.; Huang, D.J. Effect of introducing manganese as additive on microstructure, hydrogen storage properties and rate limiting step of Ti–Cr alloy. Int. J. Hydrogen Energy 2022, 47, 459–469. [Google Scholar] [CrossRef]
- Ilias, G.S.; Papazoglou, E.L.; Panagiotis, K.O.; Karkalos, N.E.; Markopoulos, A.P. Surface antibacterial properties enhanced through engineered textures and surface roughness: A review. Colloid. Surf. B 2023, 231, 113584. [Google Scholar]
- Zhang, Y.B.; Bai, Q.S.; Wang, P. 3D surface topography analysis and functionality-related performance of the machined surface in slot micro-milling titanium alloy Ti6Al4V. Int. J. Adv. Manuf. Technol. 2023, 127, 1609–1629. [Google Scholar] [CrossRef]
- Yu, W.W.; Wu, J.; Li, Y.G.; An, Q.L.; Ming, W.W.; Chen, D.; Wang, H.W.; Chen, M. Investigations on surface modification of nickel-based superalloy subjected to ultrasonic surface rolling process. Int. J. Adv. Manuf. Technol. 2023, 129, 1473–1488. [Google Scholar] [CrossRef]
- Du, H.H.; Chen, H.W.; Zhu, Z.W.; Wang, Z.K.; Suet, T. Novel hybrid machining process of titanium alloy for texturing high-quality microstructure array surfaces. Surf. Coat. Technol. 2023, 462, 129494. [Google Scholar] [CrossRef]
- Ahmed, N.; Ishfaq, K.; Moiduddin, K.; Ali, R.; Al-Shammary, N. Machinability of titanium alloy through electric discharge machining. Mater. Manuf. Process. 2019, 34, 93–102. [Google Scholar] [CrossRef]
- Rebecka, L.; Filip, L.; Henrik, P.; Rachid, M.; Eric, S.; Volodymyr, B. Performance and wear mechanisms of PCD and pcBN cutting tools during machining titanium alloy Ti6Al4V. Wear 2020, 454–455, 203329. [Google Scholar]
- Cui, X.; Li, C.; Zhang, Y.; Said, Z.; Debnath, S.; Sharma, S.; Ali, H.M.; Yang, M.; Gao, T.; Li, R.Z. Grindability of titanium alloy using cryogenic nanolubricant minimum quantity lubrication. J. Manuf. Process. 2022, 80, 273–286. [Google Scholar] [CrossRef]
- Fábio, C.O.D.; Carla, A.A.; Luiz, A.M.J.; Gopal, S.K. The influence of additive manufacturing on the micromilling machinability of Ti6Al4V: A comparison of SLM and commercial workpieces. J. Manuf. Process. 2020, 60, 299–307. [Google Scholar]
- Dandekar, R.C.; Shin, C.Y.; Barnes, J. Machinability improvement of titanium alloy (Ti–6Al–4V) via LAM and hybrid machining. Int. J. Mach. Tool Manuf. 2010, 50, 174–182. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.J.; Kong, D.D.; Tan, S.L. Experimental investigation with respect to the performance of deep submillimeter-scaled textured tools in dry turning titanium alloy Ti-6Al-4V. Appl. Surf. Sci. 2017, 403, 187–199. [Google Scholar] [CrossRef]
- Ni, C.B.; Zhu, L.D.; Zheng, Z.P.; Zhang, J.Y.; Yang, Y.; Yang, J.; Bai, Y.C.; Weng, C.; Lu, W.F.; Wang, H. Effect of material anisotropy on ultra-precision machining of Ti-6Al-4V alloy fabricated by selective laser melting. J. Alloys Compd. 2020, 848, 156457. [Google Scholar] [CrossRef]
- Yip, S.; To, S. Reduction of tool tip vibration in single-point diamond turning using an eddy current damping effect. Int. J. Adv. Manuf. Technol. 2019, 103, 1799–1809. [Google Scholar] [CrossRef]
- Zhao, Z.J.; To, S.; Sun, Z.W.; Ji, R.J.; Yu, K.M. Microstructural effects of Ti6Al4V alloys modified by electropulsing treatment on ultraprecision diamond turning. J. Manuf. Process. 2019, 39, 58–68. [Google Scholar] [CrossRef]
- Vijaykumar, S.J. Multi-characteristics optimization in EDM of NiTi alloy, NiCu alloy and and BeCu alloy using Taguchi’s approach and utility concept. Alex. Eng. J. 2017, 57, 2807–2817. [Google Scholar]
- Panagiotis, K.O.; Papazoglou, E.L.; Beata, L.; Krzysztof, Z.; Markopoulos, A.P. Surface and Subsurface Quality of Titanium Grade 23 Machined by Electro Discharge Machining. Materials 2021, 15, 164–185. [Google Scholar]
- Khoshaim, A.B.; Muthuramalingam, T.; Moustafa, E.B.; Elsheikh, A. Influences of tool electrodes on machinability of titanium α-β alloy with ISO energy pulse generator in EDM process. Alex. Eng. J. 2023, 63, 465–474. [Google Scholar] [CrossRef]
- Unal, O.; Maleki, E.; Karademir, I.; Husem, F.; Efe, Y.; Das, T. Effects of conventional shot peening, severe shot peening, re-shot peening and precised grinding operations on fatigue performance of AISI 1050 railway axle steel. Int. J. Fatigue 2022, 155, 106613. [Google Scholar] [CrossRef]
- Dunja, R.; Roman, Š.; Sebastjan, Ž. Effect of Shot Peening on the Strength and Corrosion Properties of 6082-T651 Aluminium Alloy. Materials 2023, 16, 4976–4990. [Google Scholar]
- Maleki, E.; Unal, O.; Amanov, A. Novel experimental methods for the determination of the boundaries between conventional, severe and over shot peening processes. Surf. Interfaces 2018, 13, 233–254. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Balint, S.D.; Dunne, P.F. Investigation of slip transfer across HCP grain boundaries with application to cold dwell facet fatigue. Acta Mater. 2017, 127, 43–53. [Google Scholar] [CrossRef]
- Su, K.X.; Zhang, J.W.; Li, H.; Ji, D.D.; Hu, L.K. Anti-fatigue strengthening mechanism of conventional shot peening and micro-shot peening on bare and micro-arc oxidation coated 6082-T6 aluminum alloy. Mater. Lett. 2023, 331, 133442. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xie, L.L.; Zhang, Q.; Abd, A.R.; Chen, W.L.; Zhou, L.J. Surface layer strengthening mechanism of 2060 aluminum–lithium alloy after shot-peening. J. Mater. Res. Technol. 2023, 23, 4615–4633. [Google Scholar] [CrossRef]
- Shi, H.L.; Liu, D.X.; Pan, Y.F.; Zhao, W.D.; Zhang, X.H.; Ma, A.M.; Liu, B.; Hu, Y.H.; Wang, W. Effect of shot peening and vibration finishing on the fatigue behavior of TC17 titanium alloy at room and high temperature. Int. J. Fatigue 2021, 151, 106391. [Google Scholar] [CrossRef]
- Efe, Y.; Karademir, I.; Husem, F.; Maleki, E.; Unal, O. Surface Severe Plastically Deformed Nanostructured AA7075 Alloy: Assessment on Tribological and Axial Fatigue Behaviors. J. Mater. Eng. Perform. 2020, 29, 1774–1783. [Google Scholar] [CrossRef]
- Aoudia, K.; Retraint, D.; Verdy, C.; Langlade, C.; Creus, J.; Sanchette, F. Enhancement of Mechanical Properties and Corrosion Resistance of HVOF-Sprayed NiCrBSi Coatings Through Mechanical Attrition Treatment (SMAT). J. Therm. Spray Technol. 2020, 29, 2065–2079. [Google Scholar] [CrossRef]
- Yao, Q.T.; Tong, W.P.; Li, M.Y.; Zhang, G.L. Neutral Molten Salt-Bath Carburizing of Ti6Al4V Alloy with Nanocrystalline Surface Layer at Low Temperature Assisted by Surface Mechanical Attrition Treatment. Key Eng. Mater. 2017, 727, 1001–1008. [Google Scholar] [CrossRef]
- Peng, Z.L.; Zhang, X.Y.; Liu, L.B.; Xu, G.T.; Wang, G.; Zhao, M.H. Effect of high-speed ultrasonic vibration cutting on the microstructure, surface integrity, and wear behavior of titanium alloy. J. Mater. Res. Technol. 2023, 24, 3870–3888. [Google Scholar] [CrossRef]
- Chamgordani, A.S.; Miresmaeili, R.; Aliofkhazraei, M. Improvement in tribological behavior of commercial pure titanium (CP-Ti) by surface mechanical attrition treatment (SMAT). Tribol. Int. 2018, 119, 744–752. [Google Scholar] [CrossRef]
- Li, C.; Cui, W.F.; Zhang, Y.S. Surface self-nanocrystallization of α+β titanium alloy by surface mechanical grinding treatment. Met. Mater. Int. 2017, 23, 512–518. [Google Scholar] [CrossRef]
- Qin, Z.B.; Zhang, Q.; Luo, Q.; Zhong, W.; Shen, B.; Liu, L.; Hu, W.B. Microstructure design to improve the corrosion and cavitation corrosion resistance of a nickel-aluminum bronze. Corros. Sci. 2018, 139, 255–266. [Google Scholar] [CrossRef]
- Dang, K.X.; Wang, K.H.; Chen, W.T.; Liu, G. Study on fast gas forming with in-die quenching for titanium alloys and the strengthening mechanisms of the components. J. Mater. Res. Technol. 2022, 18, 3916–3932. [Google Scholar] [CrossRef]
- Qin, Z.B.; Xia, D.H.; Zhang, Y.W.; Wu, Z.; Liu, L.; Lv, Y.T.; Liu, Y.C.; Hu, W.B. Microstructure modification and improving corrosion resistance of laser surface quenched nickel–aluminum bronze alloy. Corros. Sci. 2020, 174, 108744. [Google Scholar] [CrossRef]
- Jian, S.C.; Wang, J.X.; Xu, D.; Ma, R.; Huang, C.W.; Lei, M.; Liu, D.; Wan, M.P. Gradient microstructure and mechanical properties of Ti-6Al-4V titanium alloy fabricated by high-frequency induction quenching treatment. Mater. Des. 2022, 222, 111031. [Google Scholar] [CrossRef]
- Teixeira, J.; Denand, B.; Aeby-Gautier, E.; Denis, S. Simulation of coupled temperature, microstructure and internal stresses evolutions during quenching of a β-metastable titanium alloy. Mater. Sci. Eng. A 2016, 651, 615–625. [Google Scholar] [CrossRef]
- Lytvynenko, I.V.; Maruschak, P.O. Analysis of the state of the modified nanotitanium surface with the use of the mathematical model of a cyclic random process. Optoelectron. Instrum. 2015, 51, 254–263. [Google Scholar] [CrossRef]
- He, Q.W.; Du, Z.X.; Wang, X.P.; Cui, X.M.; Liu, F.; Chen, Y.F.; Cheng, J.; Zhao, X.P.; Chen, Y.Y. Effect of cold rolling reduction on grain growth kinetics of TB8 titanium alloy during annealing heat treatment. Mater. Res. Express 2019, 6, 116586. [Google Scholar] [CrossRef]
- Wang, K.; Wu, M.Y.; Ren, Z.; Zhang, Y.; Xin, R.L.; Liu, Q. Static globularization and grain morphology evolution of α and β phases during annealing of hot-rolled TC21 titanium alloy. Trans. Nonferr. Met. Soc. 2021, 31, 2664–2676. [Google Scholar] [CrossRef]
- Huang, Z.R.; Xiao, H.; Yu, J.X.; Zhang, H.Y.; Huang, H.G.; Yu, K.; Zhou, R.F. Effects of different annealing cooling methods on the microstructure and properties of TA10 titanium alloys. J. Mater. Res. Technol. 2022, 18, 4859–4870. [Google Scholar] [CrossRef]
- Kumar, U.; Chattopadhyaya, S.; Das, K.A.; Seikh, A.; Sharma, S.; Dwivedi, P.S.; Nagai, K.; Kumar, A.; Agrawal, A.; Singh, S. Effect of Pulsation in Microstructure and Mechanical Properties of Titanium Alloy-Annealed Welded Joints at Different Temperatures. Photonics 2023, 10, 372. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Yun, X.B.; Fu, H.W. Effect of annealing process on the organization and impact properties of TC10 titanium alloy. Rare Met. Mat. Eng. 2023, 52, 3106–3115. [Google Scholar]
- Duan, H.Q.; Han, Y.F.; Lu, W.J.; Mao, J.W.; Wang, L.Q.; Zhang, D. Effect of solid carburization on surface microstructure and hardness of Ti-6Al-4V alloy and (TiB+La2O3)/Ti-6Al-4V composite. Trans. Nonferr. Met. Soc. 2016, 26, 1871–1877. [Google Scholar] [CrossRef]
- Jacek, G.; Damian, B.; Witold, K.; Bartosz, P.; Bartłomiej, J.; Barbara, B.; Małgorzata, C.; Marcin, M.; Piotr, N. Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties. Materials 2020, 13, 5192–5207. [Google Scholar]
- Zhao, Z.Y.; Hui, P.F.; Wang, T.; Wang, X.; Xu, Y.H.; Zhong, L.S.; Zhao, M.X. New strategy to grow TiC coatings on titanium alloy: Contact solid carburization by cast iron. J. Alloys Compd. 2018, 745, 637–643. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.T.; Wang, F.; Du, K.F.; Zhang, Z.F.; Guo, Y.Z.; Yin, H.Y.; Wang, D.H. A durable and pH-universal self-standing MoC–Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12, 6776–6785. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Deng, B.W.; Du, K.F.; Chen, D.; Gao, M.X.; Wang, D.H. Modulating carbon growth kinetics enables electrosynthesis of graphite derived from CO2 via a liquid–solid–solid process. Carbon 2021, 184, 426–436. [Google Scholar] [CrossRef]
- Song, Q.S.; Xu, Q.; Xu, L.; Ning, Z.Q.; Lou, T.P.; Xie, H.W.; Qi, Y.; Yu, K. Synthesis of Ni-TiC composite powder electrochemically in molten chlorides. J. Alloys Compd. 2017, 690, 116–122. [Google Scholar] [CrossRef]
- Mao, Y.; Xie, H.W.; Chen, X.; Zhao, Y.; Qu, J.K.; Song, Q.S.; Ning, Z.Q.; Xing, P.F.; Yin, H.Y. A combined leaching and electrochemical activation approach to converting coal to capacitive carbon in molten carbonates. J. Clean. Prod. 2020, 248, 119218. [Google Scholar] [CrossRef]
- Jiang, R.; Gao, M.X.; Mao, X.H.; Wang, D.H. Advancements and potentials of molten salt CO2 capture and electrochemical transformation (MSCC-ET) process. Curr. Opin. Electrochem. 2019, 17, 38–46. [Google Scholar] [CrossRef]
- Liu, J.C.; Dolan, P.K.; Liu, J.X.; Wang, J.Q.; Long, D.W. Dense carbon film coated 316L via In-Situ synthesized CaC2 in FLiNaK molten salts and its high performance of anti-corrosion property. Electrochim. Acta 2019, 317, 232–239. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Du, P.; Liu, W.; Du, K.F.; Ma, Y.S.; Yin, H.Y.; Wang, D.H. Anodic carbidation of tantalum in molten CaCl2-CaC2. J. Solid State Electrochem. 2022, 26, 791–798. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Ma, Y.S.; Zhang, Y.; Liu, X.L.; Sun, H.O.; Liang, R.H.; Yin, H.Y.; Wang, D.H. An efficient salt-thermo-carburizing method to prepare titanium carbide coating. Surf. Coat. Technol. 2023, 465, 129546. [Google Scholar] [CrossRef]
- Sveidy, V.; Oscar, P.; Rodríguez, A.G.; Laura, N.S.; Naser, Q.; Laura, O. Photolithographically-patterned C-MEMS graphene by carbon diffusion through nickel. Nanotechnology 2021, 32, 265302. [Google Scholar]
- Dariel, M.; Klein, O.; Frage, N. Enhanced Mass Transport In Titanium Carbide At Large Departures From Stoichiometry. Powder Metall. Met. C. 2003, 42, 460–467. [Google Scholar] [CrossRef]
- Wu, X.; Guan, J.; Liu, J.; Li, K.M.; Yang, F.; Dai, Y. Corrosion behavior of TA2 titanium alloy vacuum induction carburized layer in fluorinated mixed acid. Surf. Technol. 2019, 48, 304–311. (In Chinese) [Google Scholar]
- Jiang, Y.; Sun, N.; Peng, Y.W.; Gong, J.M. Stability of low-temperature-gaseous-carburization layer in AISI316L stainless steel at high temperature. Surf. Interfaces 2021, 23, 100898. [Google Scholar] [CrossRef]
- Amar, T.; Zine, M.T.; Kamel, F. Effect of gaseous carburizing thermochemical treatment on tribological behavior of Ti-6Al-4V alloy. Frat. Integrita. Strut. 2021, 58, 179–190. [Google Scholar]
- Xue, Y.J.; Yan, Y.M.; Yu, W.C.; Liu, K.; Shi, J.; Wang, M.Q. Variation of hydrogen content in gear steel after carburizing and heat treatment and its effect on fatigue properties. Int. J. Fatigue 2023, 177, 107967. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Q.L.; Xing, Y.Z.; Jiang, C.P.; Li, X.H.; Zhao, Z.Y. Evaluation of microstructure and wear properties of Ti-6Al-4V alloy plasma carbonized at different temperatures. J. Wuhan Univ. Technol. 2015, 30, 631–638. [Google Scholar] [CrossRef]
- Yang, W.L.; He, X.J.; Li, H.P.; Dong, J.; Chen, W.; Xin, H.; Jin, Z.M. A tribological investigation of SLM fabricated TC4 titanium alloy with carburization pre-treatment. Ceram. Int. 2020, 46, 3043–3050. [Google Scholar] [CrossRef]
- Dong, B.Z.; Guo, X.H.; Zhang, K.D.; Zhang, Y.P.; Li, Z.H.; Wang, W.S.; Cai, C. Combined effect of laser texturing and carburizing on the bonding strength of DLC coatings deposited on medical titanium alloy. Surf. Coat. Technol. 2022, 429, 127951. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhong, J.; Lv, X.P.; Zhao, Y.J.; Zhou, W.; Zhang, Y.X. Microstructure and performance of functionally graded Ti (C, N)-based cermets prepared by double-glow plasma carburization. Int. J. Refract. Met. Hard 2014, 44, 109–112. [Google Scholar] [CrossRef]
- Semboshi, S.; Iwase, A.; Takasugi, T. Surface hardening of age-hardenable Cu–Ti alloy by plasma carburization. Surf. Coat. Technol. 2015, 283, 262–267. [Google Scholar] [CrossRef]
- Oliveira, D.A.C.M.V.; Silva, D.L.C.M.; Pinto, G.C.; Suzuki, A.P.; Machado, B.P.J.; Chad, M.V.; Barboza, R.J.M. Short-term creep properties of Ti-6Al-4V alloy subjected to surface plasma carburizing process. J. Mater. Res. Technol. 2015, 4, 359–366. [Google Scholar] [CrossRef]
- Zhou, Y.; Jing, Z.; Jun, H. Study on surface properties of nanosecond laser textured plasma nitrided titanium alloy. Mater. Today Commun. 2022, 31, 103746. [Google Scholar]
- Peng, T.T.; Dai, M.Y.; Cai, W.; Wei, W.; Wei, K.X.; Hu, J. The enhancement effect of salt bath preoxidation on salt bath nitriding for AISI 1045 steel. Appl. Surf. Sci. 2019, 484, 610–615. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, W.; Zuo, D.; Cao, D. A novel rare earth-salt bath nitriding of TC21-DT titanium alloy. Surf. Eng. 2018, 34, 128–131. [Google Scholar] [CrossRef]
- Deepak, J.; Raja, B.V.; Kumar, A.K.; Radhakrishnan, V.; Thomas, S.S.H. Salt Bath Nitriding of CP Titanium Grade-2 and TI-6AL-4V Grade-5. IOP Conf. Ser. Mater. Sci. Eng. 2017, 197, 012066. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Yang, L.; Duan, Z.G.; Yang, Y.X. Study on corrosion performance of TC4 titanium alloy treated by low pressure vacuum nitriding. Foundry Technol. 2014, 35, 503–505. (In Chinese) [Google Scholar]
- Ye, M.; Song, X.L.; Liu, Q.; Liu, X.F. Research on surface heat treatment technology of titanium alloy. Met. Process. 2010, 19, 31–32+34. (In Chinese) [Google Scholar]
- Chen, Z.L.; Wang, Z.G.; Liu, J.Q.; Ye, Z.P.; Hu, Y.; Wu, J.H.; Liu, K.Z.; Cai, Z.B. Enhancing the tribological properties of TA1 pure titanium by modulating the energy of pulsed laser nitriding. Opt. Laser Technol. 2024, 169, 110118. [Google Scholar] [CrossRef]
- Xin, Z.D.; Ren, N.F.; Ren, Y.P.; Yue, X.L.; Han, Q.; Zhou, W.F.; Tao, Y.F.; Ye, Y.X. In-Situ nitriding on the textured titanium alloy using femtosecond laser. J. Mater. Res. Technol. 2022, 19, 466–471. [Google Scholar] [CrossRef]
- Kang, J.J.; Wang, M.Z.; Yue, W.; Fu, Z.Q.; Zhu, L.N.; She, D.S.; Wang, C.B. Tribological Behavior of Titanium Alloy Treated by Nitriding and Surface Texturing Composite Technology. Materials 2019, 12, 301. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, S.Z.; Feng, Z.H.; Qi, H.B.; Fu, H.; Liu, R.P. Improving vacuum gas nitriding of a Ti-based alloy via surface solid phase transformation. Vacuum 2021, 197, 110860. [Google Scholar] [CrossRef]
- Liu, J.; Suslov, S.; Vellore, A.; Ren, Z.C.; Amanov, A.; Pyun, Y.; Martini, A.; Dong, Y.L.; Ye, C. Surface Nanocrystallization by Ultrasonic Nanocrystal Surface Modification and its Effect on Gas Nitriding of Ti6Al4V Alloy. Mater. Sci. Eng. A 2018, 736, 335–343. [Google Scholar] [CrossRef]
- Li, Q.X.; Zhang, X.F.; Chen, M.; Li, W.; Luo, X.F. Development of gaseous element diffusion wear-resistant treatment technology for titanium surfaces. Iron Steel Vanadium Titan. 2021, 42, 28–35. (In Chinese) [Google Scholar]
- Wang, M.Z. Effects of Ion Nitriding/Laser Surface Weaving Composite Treatment on the Organizational Structure of Titanium Alloys and Their Vacuum Tribological Properties. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2018. [Google Scholar]
- Harse, S.; Guan, F.Y.; Syedul, B.H.; Muhammad, I.; Muzamil, I.; Muhammad, B.A.; Guo, L.B.; Luo, W. Investigating the impact of plasma nitriding on Ti6Al4V surface, structural, and mechanical properties and their simultaneous evaluation via laser opto-ultrasonic dual detection (LOUD) approach. Appl. Surf. Sci. 2024, 642, 158539. [Google Scholar]
- Morgiel, J.; Szymkiewicz, K.; Maj, Ł.; Tarnowski, M.; Wierzchoń, T. TEM studies of low temperature cathode-plasma nitrided Ti6Al7Nb alloy. Surf. Coat. Technol. 2019, 359, 183–189. [Google Scholar] [CrossRef]
- Szymkiewicz, K.; Morgiel, J.; Maj, Ł.; Pomorska, M.; Tarnowski, M.; Tkachuk, O.; Pohrelyuk, I.; Wierzchoń, T. Effect of nitriding conditions of Ti6Al7Nb on microstructure of TiN surface layer. J. Alloys Compd. 2020, 845, 156320. [Google Scholar] [CrossRef]
- Takesue, S.; Kikuchi, S.; Misaka, Y.; Morita, T.; Komotori, J. Rapid nitriding mechanism of titanium alloy by gas blow induction heating. Surf. Coat. Technol. 2020, 399, 126160. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.W.; Shao, M.H.; Zhang, Z.H.; Wang, C.X.; Yan, J.W.; Lu, J.P.; Zhang, L.; Xie, B.; He, Y.Y.; et al. Characterization and electrochemical behavior of a multilayer-structured Ti–N layer produced by plasma nitriding of electron beam melting TC4 alloy in Hank’s solution. Vacuum 2023, 208, 111737. [Google Scholar] [CrossRef]
- Farokhzadeh, K.; Edrisy, A. Plasma Nitriding of Titanium Alloys. In Plasma Science and Technology; Institute of Plasma Physis: Hefei, China, 2016; pp. 67–105. [Google Scholar]
- Zhang, H.; Qin, H.F.; Ren, Z.C.; Zhao, J.Y.; Hou, X.N.; Doll, G.; Dong, Y.L.; Ye, C. Low-temperature nitriding of nanocrystalline Inconel 718 alloy. Surf. Coat. Technol. 2017, 330, 10–16. [Google Scholar] [CrossRef]
- She, D.S.; Yue, W.; Fu, Z.Q.; Wang, C.B.; Yang, X.K.; Liu, J.J. Effects of nitriding temperature on microstructures and vacuum tribological properties of plasma-nitrided titanium. Surf. Coat. Technol. 2015, 264, 32–40. [Google Scholar] [CrossRef]
- Zhang, C.W.; Wen, K.; Gao, Y. Columnar and nanocrystalline combined microstructure of the nitrided layer by active screen plasma nitriding on surface-nanocrystalline titanium alloy. Appl. Surf. Sci. 2023, 617, 156614. [Google Scholar] [CrossRef]
- Saeed, A.; Khan, A.; Jan, F.; Abrar, M.; Khalid, M.; Zakaullah, M. Validity of “sputtering and re-condensation” model in active screen cage plasma nitriding process. Appl. Surf. Sci. 2013, 273, 173–178. [Google Scholar] [CrossRef]
- Yao, Q.T.; Sun, J.; Shen, D.P.; Tong, W.P.; Zuo, L. Large-Scale Synthesis of Nanostructured Nitride Layer on Ti Plate Using Mechanical Shot Peening and Low-Temperature Nitriding. Adv. Eng. Mater. 2017, 19, 1700157. [Google Scholar] [CrossRef]
- Wen, K.; Zhang, C.W.; Gao, Y. Influence of gas pressure on the low-temperature plasma nitriding of surface-nanocrystallined TC4 titanium alloy. Surf. Coat. Technol. 2022, 436, 128327. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Qiu, Y.; Hu, T.T.; Clare, A.T.; Li, Y.; Zhang, L.C. Microstructures and mechanical behavior of beta-type Ti-25V-15Cr-0.2Si titanium alloy coating by laser cladding. Mater. Sci. Eng. A 2020, 796, 140063. [Google Scholar] [CrossRef]
- Arash, F.A.; Maryam, M. A review of functionalizing plasma electrolytic oxidation (PEO) coatings on titanium substrates with laser surface treatments. Appl. Surf. Sci. Adv. 2023, 18, 100506. [Google Scholar]
- Wu, G.L.; Wang, Y.; Sun, M.; Zhang, Q.L.; Yao, J.H. Influence of microstructure of TC4 substrate on the MAO coating. Surf. Eng. 2019, 36, 827–836. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhai, D.J.; Feng, K.Q. Effect of the Microstructure of a Titanium Alloy Fabricated Using Selective Laser Melting on Microarc Oxidation Film. Metall. Mater. Trans. A 2021, 52, 4691–4702. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.H.; Wang, W.L.; Liu, S.F.; Chen, Y.Q.; Tang, H.P. Comparison of two-step surface treatment on surface roughness and corrosion resistance of TC4 alloy parts prepared by SLM and SEBM. J. Alloys Compd. 2022, 921, 165929. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Laçin, M.; Minneboo, M.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. The effects of plasma electrolytically oxidized layers containing Sr and Ca on the osteogenic behavior of selective laser melted Ti6Al4V porous implants. Mater. Sci. Eng. C 2021, 124, 112074. [Google Scholar] [CrossRef]
- Vargas, C.A.; Zuleta, A.A.; Botero, C.A.; Baena, L.M.; Castaño, J.G.; Gómez, M.A.; Tamayo, J.A. Morphological analysis of plasma electrolytic oxidation coatings formed on Ti6Al4V alloys manufactured by electron beam powder bed fusion. Heliyon 2023, 9, e19289. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.J.; Wu, X.Q.; Xie, F.Q.; He, P.; Li, Z.; He, J.Y. Fretting wear properties and microstructure evolution in micro-arc oxidation bioceramic coating pretreated using laser remelting. Ceram. Int. 2023, 49, 4979–4986. [Google Scholar] [CrossRef]
- Yu, F.L.; Zhang, Y.; Kong, C.L.; Yu, H.L. Microstructure and mechanical properties of Ti-6Al-4V alloy sheets via room-temperature rolling and cryorolling. Mater. Sci. Eng. A 2022, 834, 142600. [Google Scholar] [CrossRef]
- Yu, F.L.; Zhang, Y.; Kong, C.L.; Yu, H.L. High strength and toughness of Ti-6Al-4V sheets via cryorolling and short-period annealing. Mater. Sci. Eng. A 2022, 854, 143766. [Google Scholar] [CrossRef]
- Chen, P.H.; Li, B.C.; Liu, Z.; Zhou, Y.H.; Li, R.Q.; Zhang, Y. Understanding high-temperature oxidation behaviors and mechanical properties of TiAlCrMn HEAs during heat treatment. Trans. Nonferr. Metals Soc. 2023; in press. [Google Scholar]
- Zhang, Y.; Lei, G.; Luo, K.G.; Chen, P.H.; Kong, C.L.; Yu, H.L. Tribological behavior of high-entropy alloy particle reinforced aluminum matrix composites and their key impacting factors. Tribol. Int. 2022, 175, 107868. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, K.G.; Lei, G.; Yu, H.L. Interfacial characteristics and enhanced mechanical properties of Al0.5CoCrFeNi high-entropy alloy particles reinforced Al matrix composites. Metall. Mater. Trans. A 2022, 53, 4161–4167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).