Electrochemical Properties of LaMO3(M=Cr, Mn, and Co) Perovskite Materials
Abstract
1. Introduction
2. Experimental Section
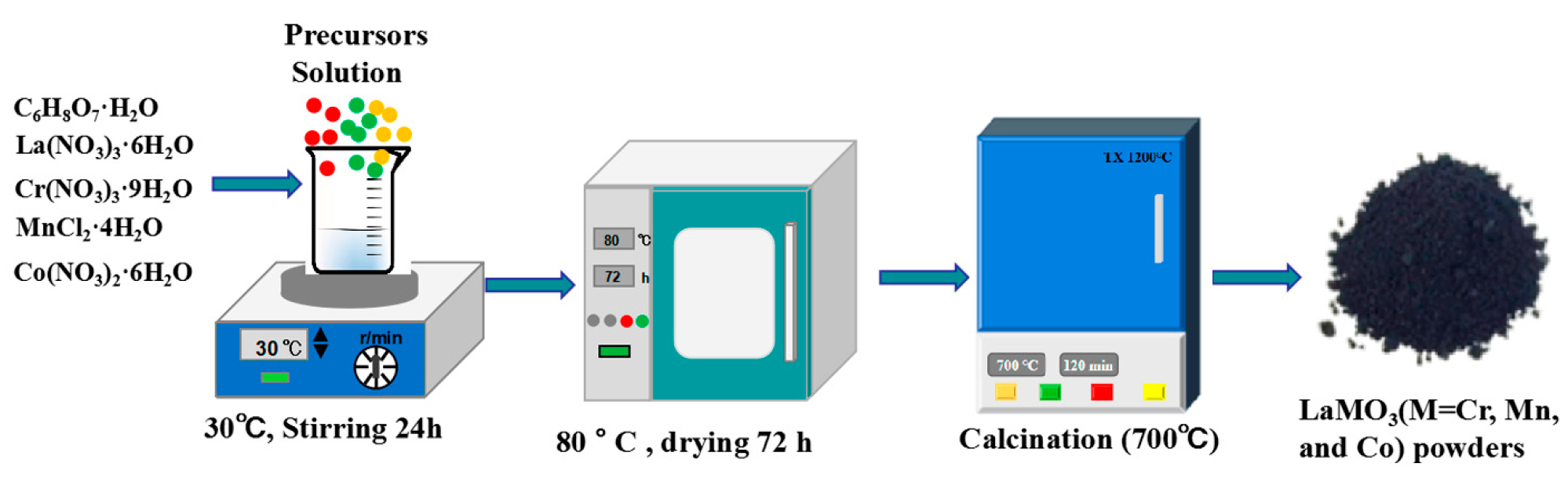
2.1. Synthesis of LaMO3(M=Cr, Mn, and Co) Powders
2.2. Characterization of LaMO3(M=Cr, Mn and Co) Powders
2.3. Electrochemical Test
3. Results and Discussion
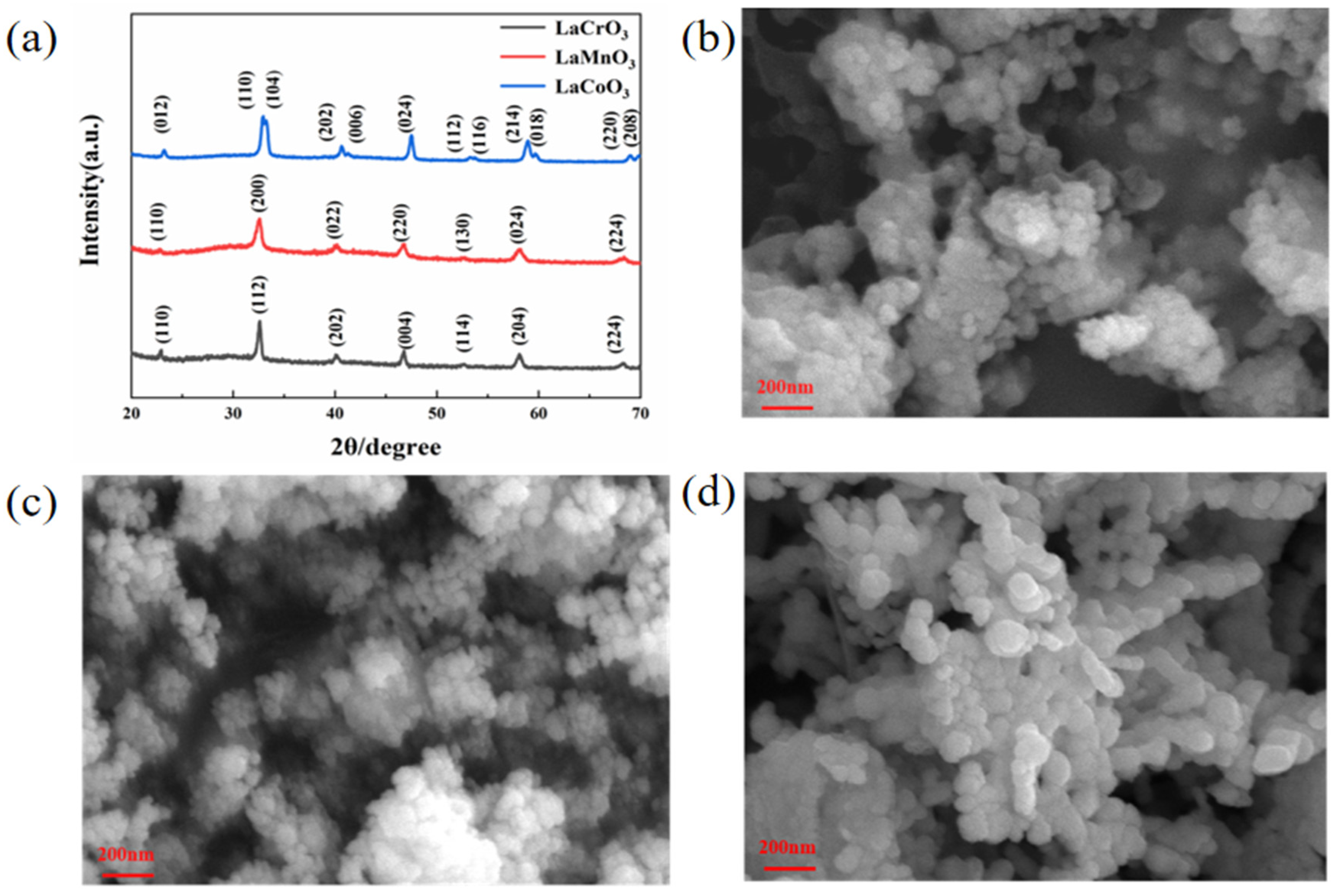
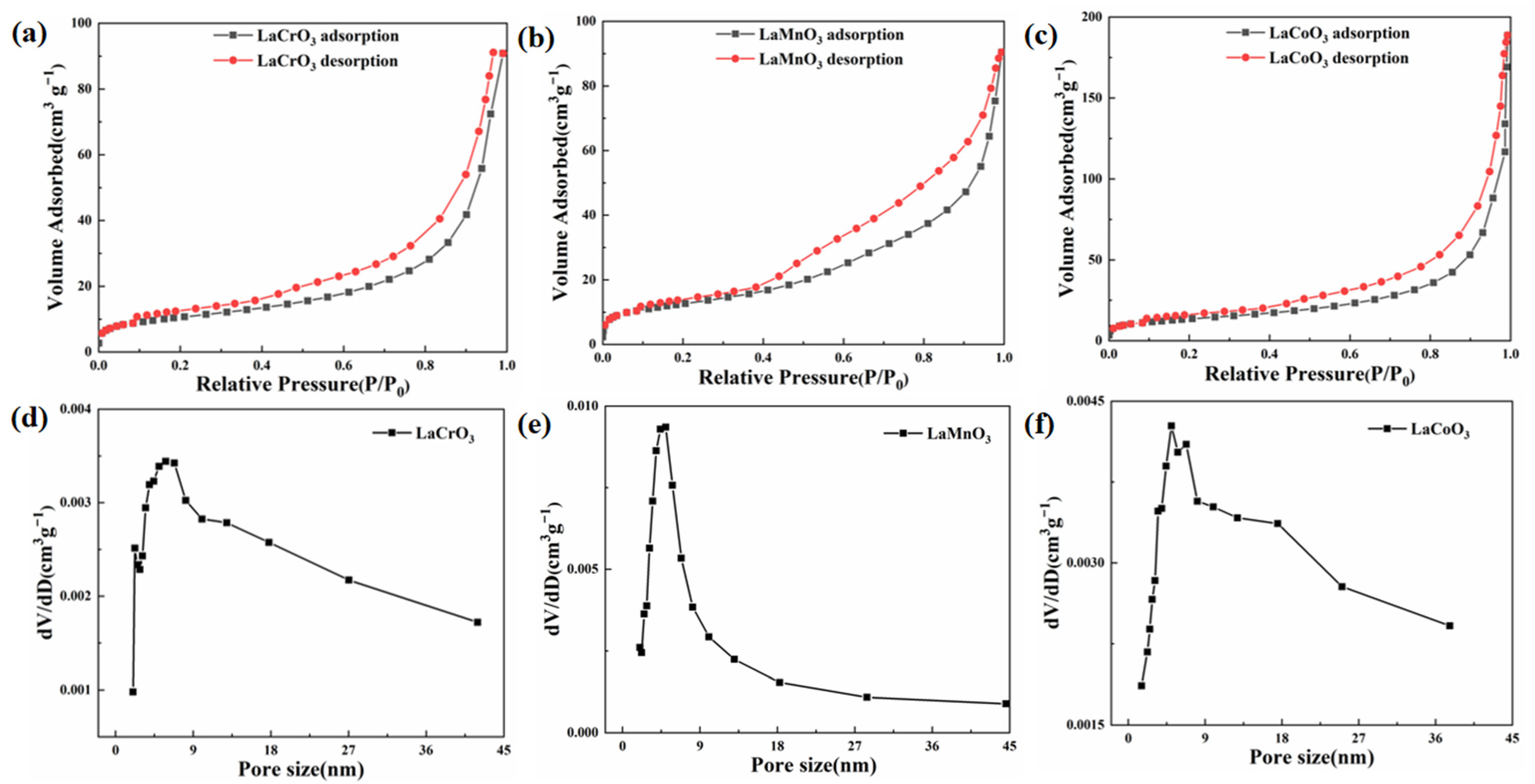
3.1. Microstructure and Morphology
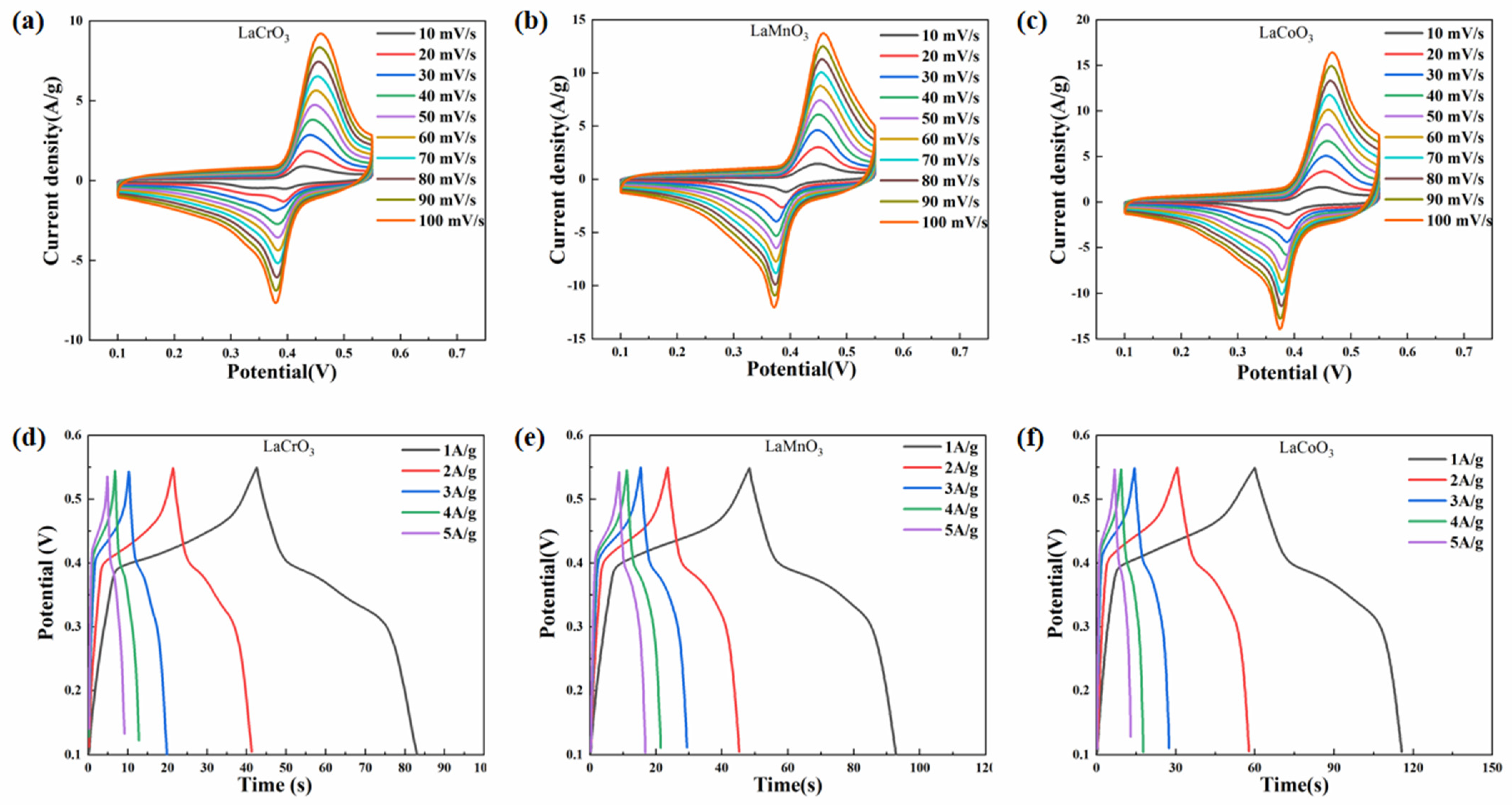
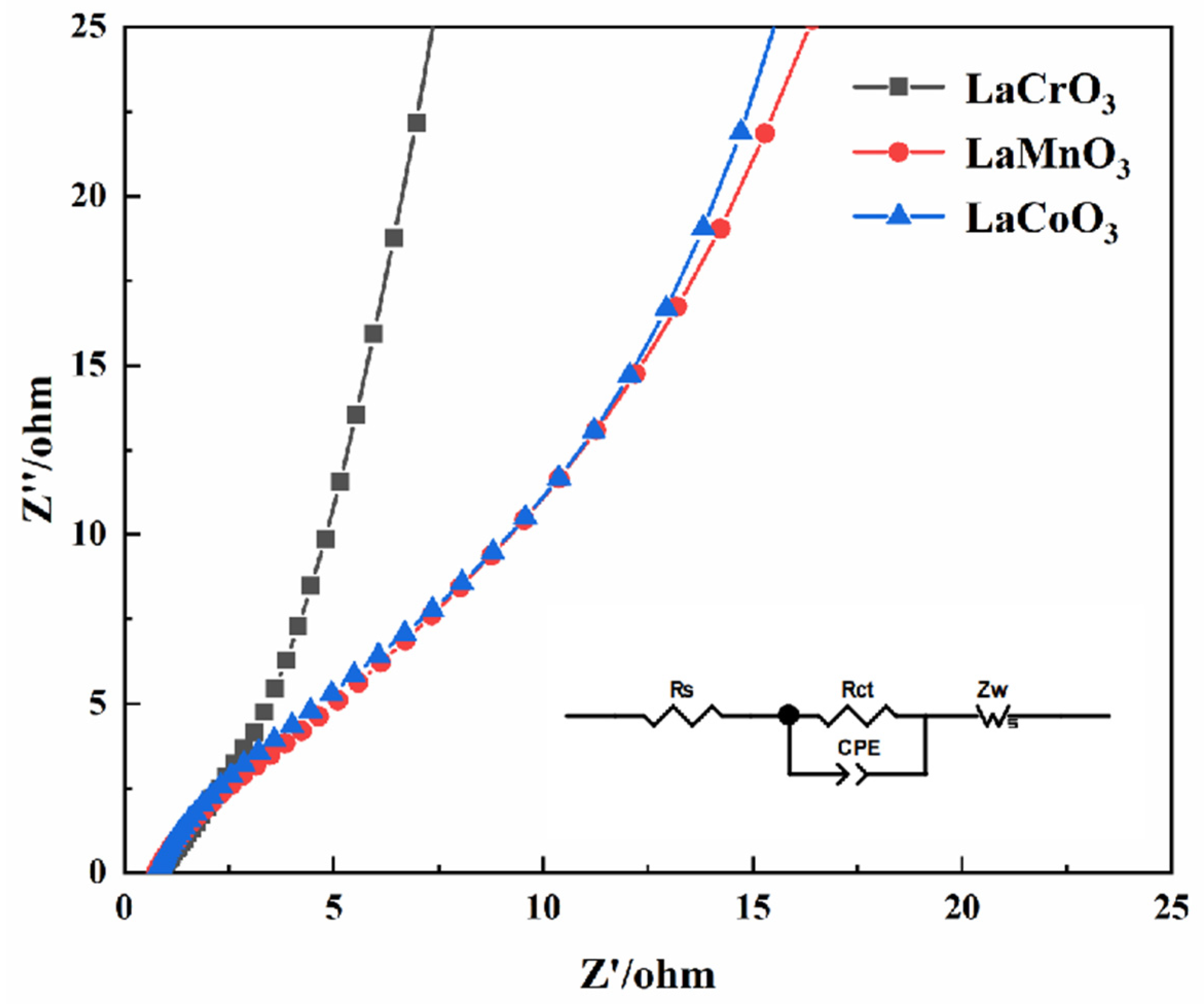
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaheen, I.; Hussain, I.; Zahra, T.; Javed, M.S.; Shah, S.S.A.; Khan, K.; Hanif, M.B.; Assiri, M.A.; Said, Z.; Arifeen, W.U.; et al. Recent advancements in metal oxides for energy storage materials: Design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 2023, 72, 108719. [Google Scholar] [CrossRef]
- Ahangari, M.; Mostafaei, J.; Sayyah, A.; Mahmoudi, E.; Asghari, E.; Coruh, A.; Delibas, N.; Niaei, A. Investigation of structural and electrochemical properties of SrFexCo1-xO3-δ perovskite oxides as a supercapacitor electrode material. J. Energy Storage 2023, 63, 107034. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, X.; Sun, Y.; Bai, Z.; Zhang, G.; Shen, Y.; Liang, Q.; Zhang, Y. Au-Embedded ZnO/NiO Hybrid with Excellent Electrochemical Performance as Advanced Electrode Materials for Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, W.-K.; Rong, Q.; Jiang, J.; Zhang, Y.-J.; HanQing, Y. Porous ZnO-coated Co3O4 nanorod as a high-energy-density supercapacitor material. ACS Appl. Mater. Interfaces 2018, 10, 2316–23173. [Google Scholar] [CrossRef] [PubMed]
- Tabari, T.; Singh, D.; Calisan, A.; Ebadi, M.; Tavakkoli, H.; Caglar, B. Microwave assisted synthesis of La1− xCaxMnO3 (x = 0, 0.2 and 0.4): Structural and capacitance properties. Ceram. Int. 2017, 43, 15970–15977. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J.F.; Huang, Y.; Zhu, M.S.; Pei, Z.X.; Li, H.F.; Wang, Y.K.; Li, N.; Zhang, H.Y.; Zhi, C.Y. Recent progress on flexible and wearable supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef]
- Jayakumar, S.; Santhosh, P.C.; Mohideen, M.M.; Radhamani, A. A comprehensive review of metal oxides (RuO2, Co3O4, MnO2 and NiO) for supercapacitor applications and global market trends. J. Alloys Compd. 2023, 976, 173170. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Wei, D.; Cai, Z.; Song, Y.; Wang, X. Design and synthesis of K-doped tremella-like δ-MnO2 for high-performance supercapacitor. J. Energy Storage 2023, 72, 108468. [Google Scholar] [CrossRef]
- Zhong, J.H.; Wang, A.L.; Li, G.R.; Wang, J.W.; Ou, Y.N.; Tong, Y.X. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J. Mater. Chem. 2012, 22, 5656–5665. [Google Scholar] [CrossRef]
- Patel, M.N.; Wang, X.; Slanac, D.A.; Ferrer, D.A.; Dai, S.; Johnston, K.P.; Stevenson, K.J. High pseudocapacitance of MnO2 nanoparticles in graphitic disordered mesoporous carbon at high scan rates. J. Mater. Chem. 2012, 22, 3160–3169. [Google Scholar] [CrossRef]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.P.; Wang, Z.L. Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef]
- Yesuraj, J.; Ramesh, M.; Kim, K.; Biswas, K. Fundamental aspects of perovskite oxides as an emerging material for supercapacitor applications and its anion intercalation mechanism—Review. J. Energy Storage 2024, 78, 109968. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, J.; Li, X.; Yue, L.; Liu, Q.; Lu, S.; Asiri, A.M.; Hu, J.; Luo, Y.; Sun, X. Recent advances in perovskite oxides as electrode materials for supercapacitors. Chem. Commun. 2021, 57, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Sundriyal, P.G.S.; Vishal, S.; Unita, M.; Deepak, P.D.; Ki-Hyun, K.; Akash, D. Perovskite materials as superior and powerful platforms for energy conversion and storage applications. Nano Energy 2021, 80, 105552. [Google Scholar]
- Kumar, T.A.; Akanksha, J.; Gurmeet, S.; Kishore, S.R. Perovskite oxides as supercapacitive electrode: Properties, design and recent advances. Coord. Chem. Rev. 2021, 431, 213680. [Google Scholar]
- Nan, H.-S.; Hu, X.-Y.; Tian, H.-W. Recent advances in perovskite oxides for anion-intercalation supercapacitor: A review. Mater. Sci. Semicond. Process. 2019, 94, 35–50. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.K.; Singh, G.; Sharma, R.K. Charge storage characteristics of mesoporous strontium titanate perovskite aqueous as well as flexible solid-state supercapacitor cell. J. Power Sources 2019, 426, 223–232. [Google Scholar] [CrossRef]
- Tomar, A.K.; Singh, G.; Sharma, R.K. Fabrication of a Mo-Doped Strontium Cobaltite Perovskite Hybrid Supercapacitor Cell with High Energy Density and Excellent Cycling Life. ChemSusChem 2018, 11, 4123–4130. [Google Scholar] [CrossRef]
- George, G.; Jackson, S.L.; Luo, C.Q.; Fang, D.; Luo, D.; Hu, D.; Wen, J.; Luo, Z. Effect of doping on the performance of high-crystalline SrMnO3 perovskite nanofibers as a supercapacitor electrode. Ceram. Int. 2018, 44, 21982–21992. [Google Scholar] [CrossRef]
- Oloore, L.E.; Gondal, M.A.; Popoola, A.; Popoola, I. Pseudocapacitive contributions to enhanced electrochemical energy storage in hybrid perovskite-nickel oxide nanoparticles composites electrodes. Electrochim. Acta 2020, 361, 137082. [Google Scholar] [CrossRef]
- Verma, S.; Arya, S.; Gupta, V.; Mahajan, S.; Furukawa, H.; Khosla, A. Performance analysis, challenges and future perspectives of nickel based nanostructured electrodes for electrochemical supercapacitors. J. Mater. Res. Technol. 2021, 11, 564–599. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Yang, F.; Shao, H.; Wang, J.; Ji, L.; Jia, Y.; Wang, Y.; Liu, J. Facile synthesis and electrochemical properties of perovskite-type CeMnO3 nanofibers. ChemistrySelect 2019, 4, 11903–11912. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Mary, A.J.C.; Bose, A.C. Electrochemical performance of ANiO3 (A = La, Ce) perovskite oxide material and its device performance for super-capattery application. Electrochim. Acta 2020, 362, 137095. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Shao, H.; Yang, F.; Wang, J.; Wang, Y.; Liu, J. Facile syntheses of cerium-based CeMO3 (M = Co, Ni, Cu) perovskite nanomaterials for high-performance supercapacitor electrodes. J. Mater. Sci. 2020, 55, 8421–8434. [Google Scholar] [CrossRef]
- Ye, X.; Dong, S.; Jin, X.; Wei, J.; Wang, L.; Zhang, Y. Enhancement in the Electrochemical Performance of Strontium (Sr)-Doped LaMnO3 as Supercapacitor Materials. Coatings 2022, 12, 1739. [Google Scholar] [CrossRef]
- Dong, S.; Jin, X.; Ye, X.; Wei, J.; Wang, L.; Zhang, Y. Effects of Calcium Substitution for La on the Electrochemical Performance of LaMnO3 Nanoparticles. ChemistrySelect 2023, 8, 202203977. [Google Scholar] [CrossRef]
- Dong, S.-T.; Ye, X.; Fu, Z.; Jin, X.; Wei, J.; Wang, L.; Zhang, Y.-M. Effects of strontium substitution for La on the electrochemical performance of LaAlO3 perovskite nanotubes. J. Mater. Res. Technol. 2022, 19, 91–100. [Google Scholar] [CrossRef]
- Shao, T.; You, H.; Zhai, Z.; Liu, T.; Li, M.; Zhang, L. Hollow spherical LaNiO3 supercapacitor electrode synthesized by a facile template-free method. Mater. Lett. 2017, 201, 122–124. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Yuan, C.; Su, Y. Controlled synthesis of perovskite lanthanum ferrite nanotubes with excellent electrochemical properties. RSC Adv. 2017, 7, 12931–12937. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.S.; Ullah, N.; Shaheen, A.; Aslam, N.; Ashraf, I.; Abbas, Y.; Wang, M.; Liu, G.; Qiao, G. Unique hierarchical mesoporous LaCrO3 perovskite oxides for highly efficient electrochemical energy storage applications. Ceram. Int. 2019, 45, 15164–15170. [Google Scholar] [CrossRef]
- Arjun, N.; Pan, G.-T.; Yang, T.C. The exploration of Lanthanum based perovskites and their complementary electrolytes for the supercapacitor applications. Results Phys. 2017, 7, 920–926. [Google Scholar] [CrossRef]
- Deshmukh, V.; Harini, H.; Nagaswarupa, H.; Naik, R.; Ravikumar, C. Development of novel Co3+ doped LaMnO3 perovskite electrodes for supercapacitors and sensors: Mechanism of electrochemical energy storage and oxygen intercalation. J. Energy Storage 2023, 68, 107805. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Shao, H.; Yang, F.; Wang, J.; Wang, Y.; Liu, J. Facile syntheses of perovskite type LaMO3 (M = Fe, Co, Ni) nanofibers for high performance supercapacitor electrodes and lithium-ion battery anodes. J. Alloys Compd. 2021, 852, 157002. [Google Scholar] [CrossRef]
- Hadji, F.; Omari, M.; Mebarki, M.; Gabouze, N.; Layadi, A. Zinc doping effect on the structural and electrochemical properties of LaCoO3 perovskite as a material for hybrid supercapacitor electrodes. J. Alloys Compd. 2023, 942, 169047. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor Performance of Perovskite La1-xSrxMnO3. Dalton Trans. 2017, 46, 13720–13730. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Sr-doped Lanthanum Nickelate Nanofibers for High Energy Density Supercapacitors. Electrochim. Acta 2015, 174, 41–50. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Vijayakumar, S.; Ryu, K.-S. Cerium oxide mixed LaMnO3 nanoparticles as the negative electrode for aqueous asymmetric supercapacitor devices. Mater. Chem. Phys. 2017, 199, 543–551. [Google Scholar] [CrossRef]
- Yan, D.; Wang, W.; Luo, X.; Chen, C.; Zeng, Y.; Zhu, Z. NiCo2O4 with oxygen vacancies as better performance electrode material for supercapacitor. Chem. Eng. J. 2018, 334, 864–872. [Google Scholar] [CrossRef]
- Tian, H.; Lang, X.; Nan, H.; An, P.; Zhang, W.; Hu, X.; Zhang, J. Nanosheet-assembled LaMnO3@NiCo2O4 nanoarchitecture growth on Ni foam for high power density supercapacitors. Electrochim. Acta 2019, 318, 651–659. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Srinivasan, V.; Weidner, J.W. Studies on the Capacitance of Nickel Oxide Films: Effect of Heating Temperature and Electrolyte Concentration. J. Electrochem. Soc. 2000, 147, 880–885. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Mahanty, S. Extraordinarily high pseudocapacitance of metal organic framework derived nanostructured cerium oxide. Chem. Commun. 2014, 50, 11717–11720. [Google Scholar] [CrossRef] [PubMed]

| LaCrO3 | LaMnO3 | LaCoO3 | |
|---|---|---|---|
| D (nm) | 19.76 | 11.82 | 10.70 |
| δ × 10−3 (nm−2) | 2.56 | 7.15 | 8.73 |
| ε × 10−3 | 6.19 | 10.36 | 11.26 |
| LaCrO3 | LaMnO3 | LaCoO3 | |
|---|---|---|---|
| O1 | 49.3 | 47.7 | 45.3 |
| O2 | 38.6 | 47.5 | 47.5 |
| O3 | 12.1 | 6.8 | 7.2 |
| O2/O1 | 0.78 | 0.99 | 1.05 |
| Columbic Efficiency | Available Redox Reaction Active Sites | |
|---|---|---|
| LaCrO3 | 92.7% | 0.0960 |
| LaMnO3 | 90.1% | 0.1104 |
| LaCoO3 | 94.6% | 0.1360 |
| Rs (Ω) | Rct (Ω) | CPE (Ω) | ZW (Ω) | Chi2 | |
|---|---|---|---|---|---|
| LaCrO3 | 0.83 | 5.21 | 1.68 | 0.68 | 0.0030386 |
| LaMnO3 | 0.76 | 4.02 | 0.78 | 0.69 | 0.0012108 |
| LaCoO3 | 0.79 | 2.56 | 0.94 | 0.66 | 0.0009933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhu, Q.; Ye, X.; Wang, L.; Dong, S. Electrochemical Properties of LaMO3(M=Cr, Mn, and Co) Perovskite Materials. Coatings 2024, 14, 147. https://doi.org/10.3390/coatings14010147
Zhao H, Zhu Q, Ye X, Wang L, Dong S. Electrochemical Properties of LaMO3(M=Cr, Mn, and Co) Perovskite Materials. Coatings. 2024; 14(1):147. https://doi.org/10.3390/coatings14010147
Chicago/Turabian StyleZhao, Hongquan, Qiudong Zhu, Xin Ye, Lei Wang, and Songtao Dong. 2024. "Electrochemical Properties of LaMO3(M=Cr, Mn, and Co) Perovskite Materials" Coatings 14, no. 1: 147. https://doi.org/10.3390/coatings14010147
APA StyleZhao, H., Zhu, Q., Ye, X., Wang, L., & Dong, S. (2024). Electrochemical Properties of LaMO3(M=Cr, Mn, and Co) Perovskite Materials. Coatings, 14(1), 147. https://doi.org/10.3390/coatings14010147








