In-Depth Understanding of the Chemical Stripping Mechanism of AlSiY Coatings on Nickel Superalloys by First-Principles Calculation
Abstract
1. Introduction
2. Experimental and Calculation Details
2.1. Preparation of Aluminide Coatings
2.2. Chemical Stripping Procedures
2.3. Characterization
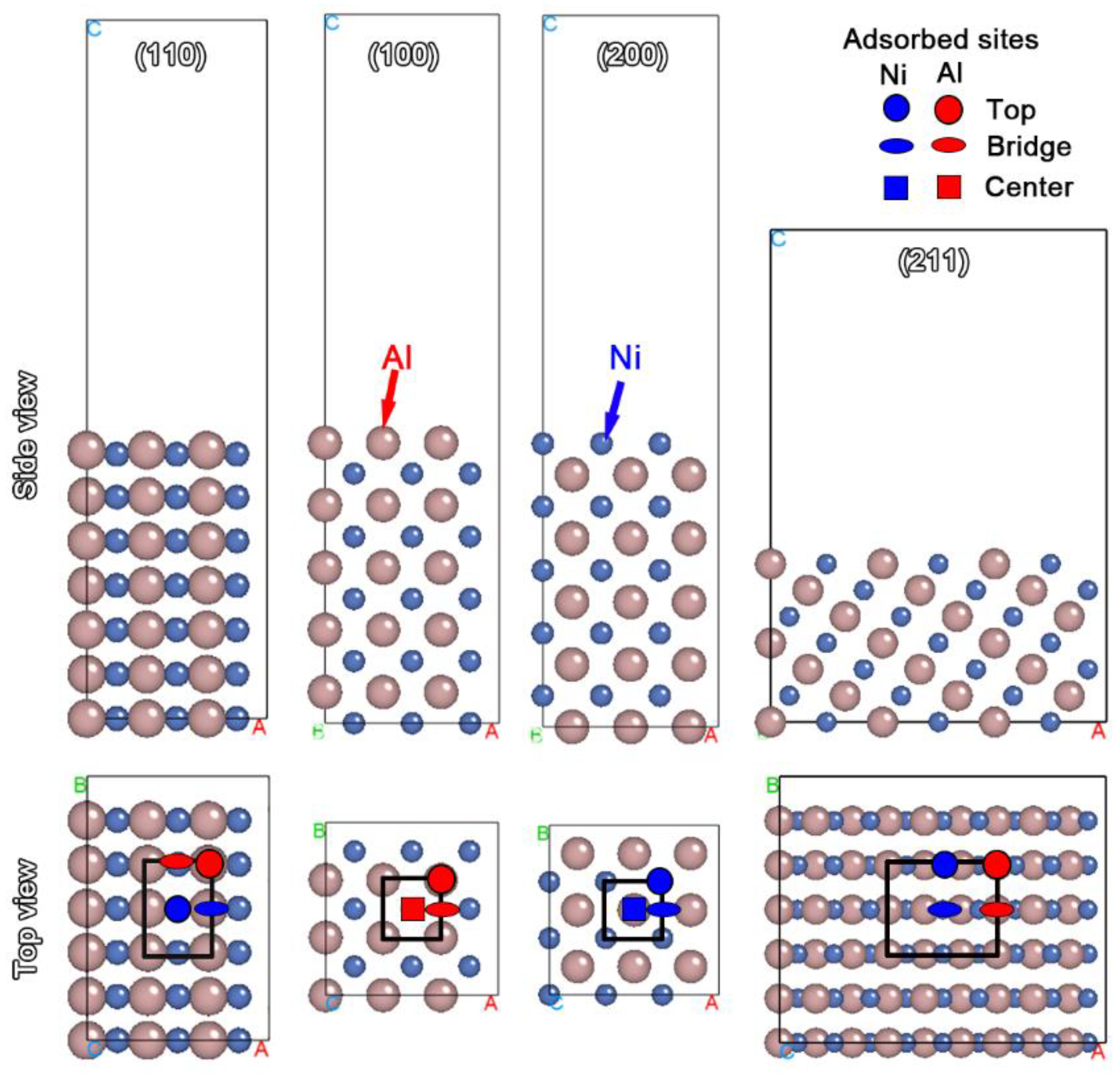
2.4. First-Principles Calculation
3. Results and Discussion
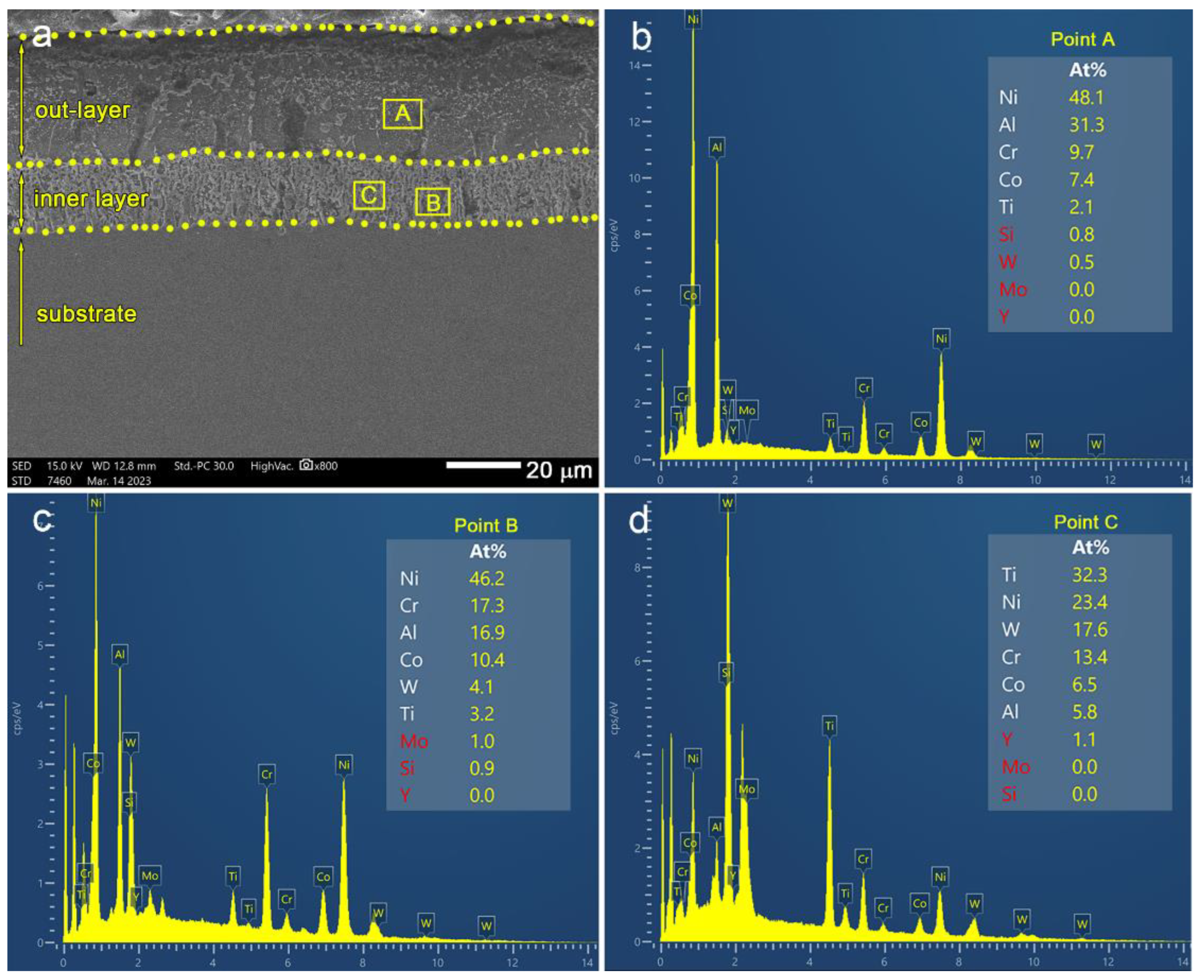
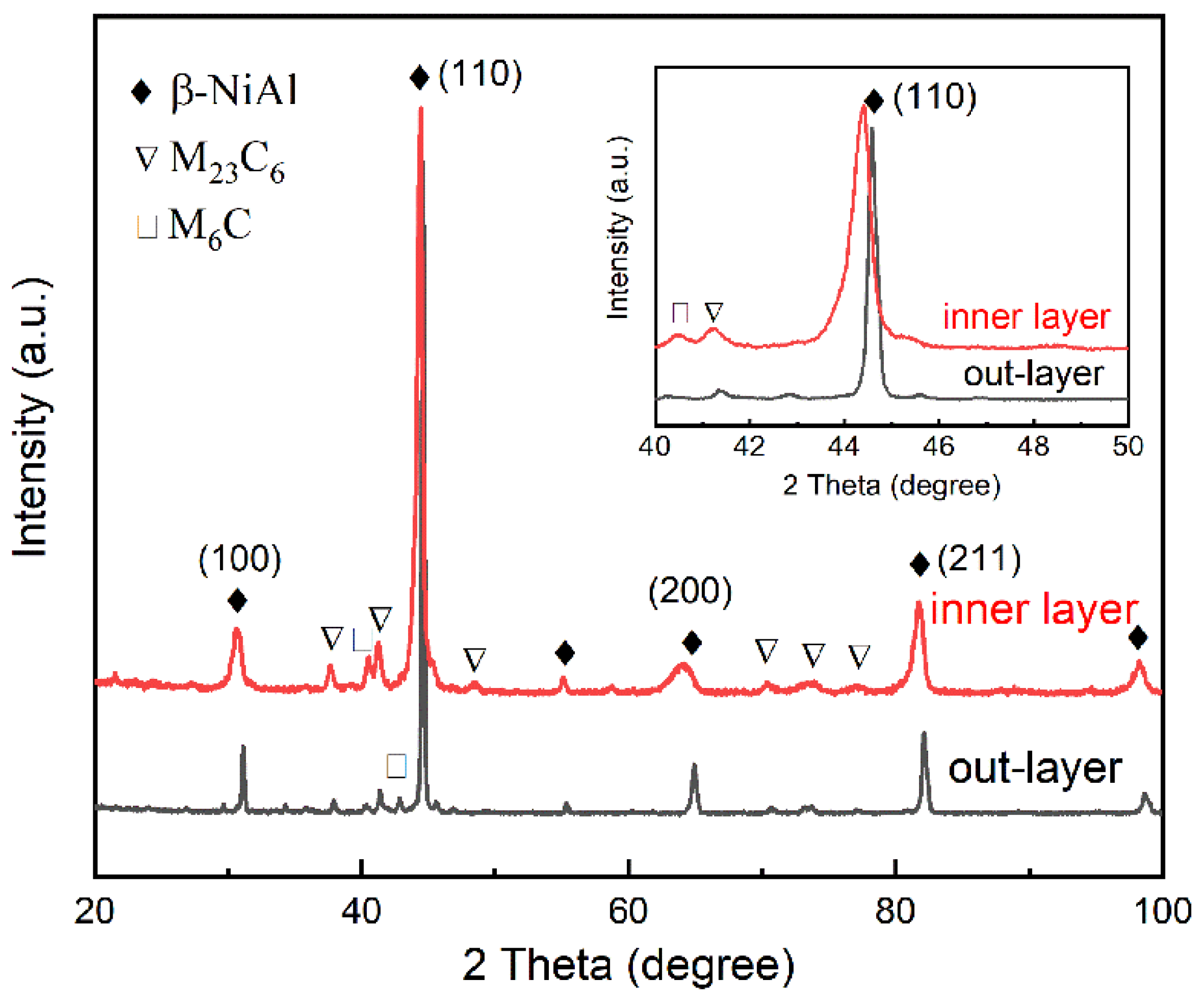
3.1. Microstructure of the AlSiY Coating
3.2. Microstructure of the AlSiY Coating after Chemical Stripping
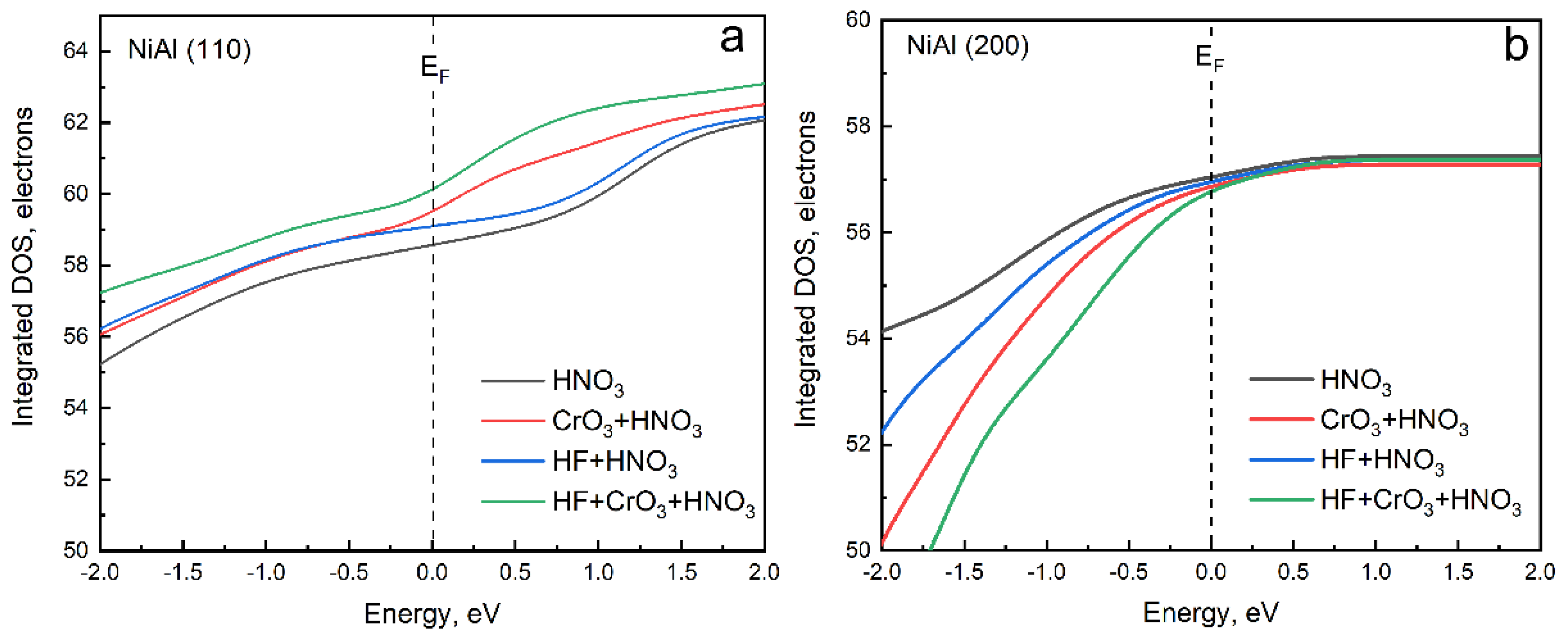
3.3. Explanation of Uniform Stripping by First-Principles Calculation
3.4. Stripping Mechanism of Aluminide Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Fan, Q.; Li, J.; Ma, D.; Gong, J.; Sun, C. Effect of Si addition to improve the performance of type II and type I hot corrosion resistance of aluminide coating. Corros. Sci. 2023, 212, 110937. [Google Scholar] [CrossRef]
- Bobzin, K.; Brögelmann, T.; Kalscheuer, C.; Liang, T. Al-Si and Al-Si-Y coatings deposited by HS-PVD for the oxidation protection of γ-TiAl. Surf. Coat. Technol. 2018, 350, 587–595. [Google Scholar] [CrossRef]
- Abraimov, N.V.; Lukina, V.V.; Zarypov, M.S. Effect of the Composition and structure of Al–Si–Y and Ni–Cr–Al–Y coatings on gas-turbine blades on the service life. Russ. Metall. 2020, 2020, 1404–1410. [Google Scholar] [CrossRef]
- Jiang, S.M.; Xu, C.Z.; Li, H.Q.; Liu, S.C.; Gong, j.; Sun, C. Preparation and oxidation behavior of an Al-Si-Y diffusion coating on a Ni-based single crystal superalloys. Corros. Sci. 2010, 52, 435–440. [Google Scholar] [CrossRef]
- Alam, M.Z.; Sarkar, S.B.; Das, D.K. Refurbishment of thermally degraded diffusion Pt-aluminide (PtAl) bond coat on a Ni-base superalloy. Surf. Coat. Technol. 2018, 354, 101–111. [Google Scholar] [CrossRef]
- Fan, Q.; Yu, H.; Wang, T.; Liu, Y. Microstructure and oxidation resistance of a Si doped platinum modified aluminide coating deposited on a single crystal superalloy. Coatings 2018, 8, 264. [Google Scholar] [CrossRef]
- Littner, A.; Pedraza, F.; Kennedy, A.D.; Moretto, P.; Peich, L.; Webera, T.; Schuetze, M. Performance and thermal stability of Pt-modified Al-diffusion coatings for superalloys under cyclic and isothermal conditions. Mater. High Temp. 2005, 22, 411–420. [Google Scholar] [CrossRef][Green Version]
- Lü, K.; Yuan, X.; Li, D.; Lü, J.; Song, P.; Li, Q.; Li, C.; Huang, T.; Chen, R.; Zheng, B.; et al. Cyclic oxidation behaviour of Pt-doped aluminide coating on DZ125 containing Hf. Mater. Res. Express 2019, 6, 126536. [Google Scholar] [CrossRef]
- Bozorg Nezhad-Nobijari, M.; Isakhani-Zakaria, M.; Bakhshi, A. Evaluation of the aluminide coating on cleaned internal passages of used gas turbine blades. Surf. Coat. Technol. 2016, 289, 206–212. [Google Scholar] [CrossRef]
- Shipway, P.H.; Bromley, J.P.D.; Weston, D.P. Removal of coatings from polymer substrates by solid particle blasting to enhance reuse or recycling. Wear 2007, 263, 309–317. [Google Scholar] [CrossRef]
- Boulesteix, C.; Gregoire, B.; Pedraza, F. Oxidation performance of repaired aluminide coatings on austenitic steel substrates. Surf. Coat. Technol. 2017, 326, 224–237. [Google Scholar] [CrossRef]
- Amirkhanova, N.A.; Nevyantseva, R.R.; Bybin, A.A.; Semenova, I.P.; Smolnikova, O.G. On the removal of aluminide coating from blades of gas turbine engine. Zashchita Met. 2003, 39, 538–541. [Google Scholar] [CrossRef]
- Le Guevel, Y.; Grégoire, B.; Bouchaud, B.; Bilhé, P.; Pasquet, A.; Thiercelin, M.; Pedraza, F. Influence of the oxide scale features on the electrochemical descaling and stripping of aluminide coatings. Surf. Coat. Technol. 2016, 292, 1–10. [Google Scholar] [CrossRef]
- Bouchaud, B.; Creus, J.; Rébéré, C.; Balmain, J.; Pedraza, F. Controlled stripping of aluminide coatings on nickel superalloys through electrolytic techniques. J. Appl. Electrochem. 2008, 38, 817–825. [Google Scholar] [CrossRef]
- Poupard, S.; Martinez, J.F.; Pedraza, F. Soft chemical stripping of aluminide coatings and oxide products on Ni superalloys. Surf. Coat. Technol. 2008, 202, 3100–3108. [Google Scholar] [CrossRef]
- Segall, M.; Lindan, P.J.; Probert, M.; Pickard, C.; Hasnip, P.; Clark, S.; Payne, M. First-Principles Simulation: Ideas, Illustrations and the CASTEP Code. J. Phys. Condens. Matter. 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Laasonen, K.; Pasquarello, A.; Car, R.; Lee, C.; Vanderbilt, D. Car-Parrinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials. Phys. Rev. B 1993, 47, 10142. [Google Scholar] [CrossRef]
- Wen, Z.; Zhao, Y.; Hou, H.; Tian, J.; Han, P. First-principles study of Ni-Al intermetallic compounds under various temperature and pressure. Superlatt. Microstruct. 2017, 103, 9–18. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, C.; Zhan, H.; Huang, W.; Ding, Z.; Chen, D.; Cui, G. High-Efficient Gas Nitridation of AISI 316L Austenitic Stainless Steel by a Novel Critical Temperature Nitriding Process. Coatings 2023, 13, 1708. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Yan, M.F. Electronic structure and properties of (Fe1−xNix)4N (0 < x < 1.0). Phys. B 2010, 405, 2700–2705. [Google Scholar] [CrossRef]
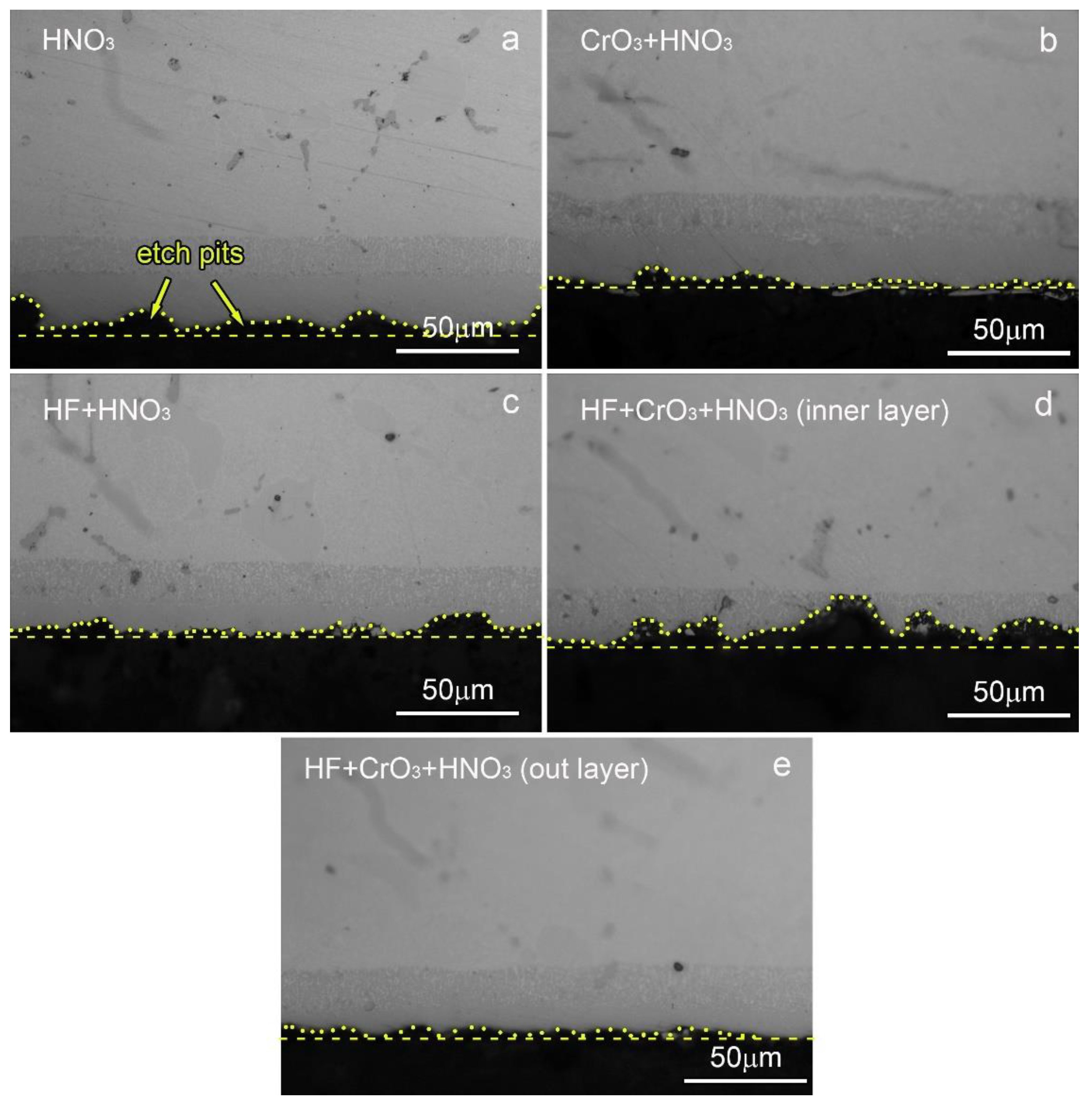
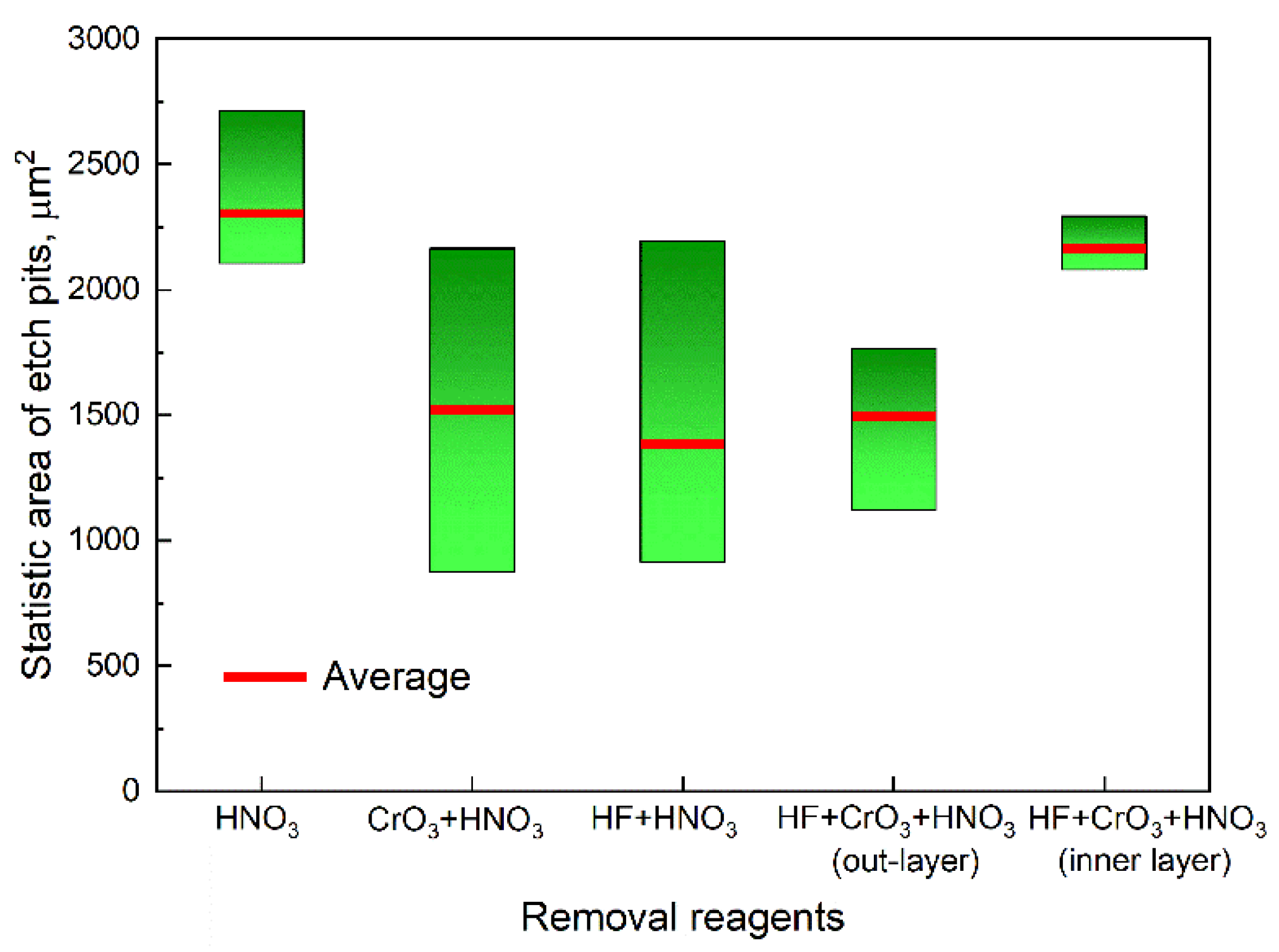
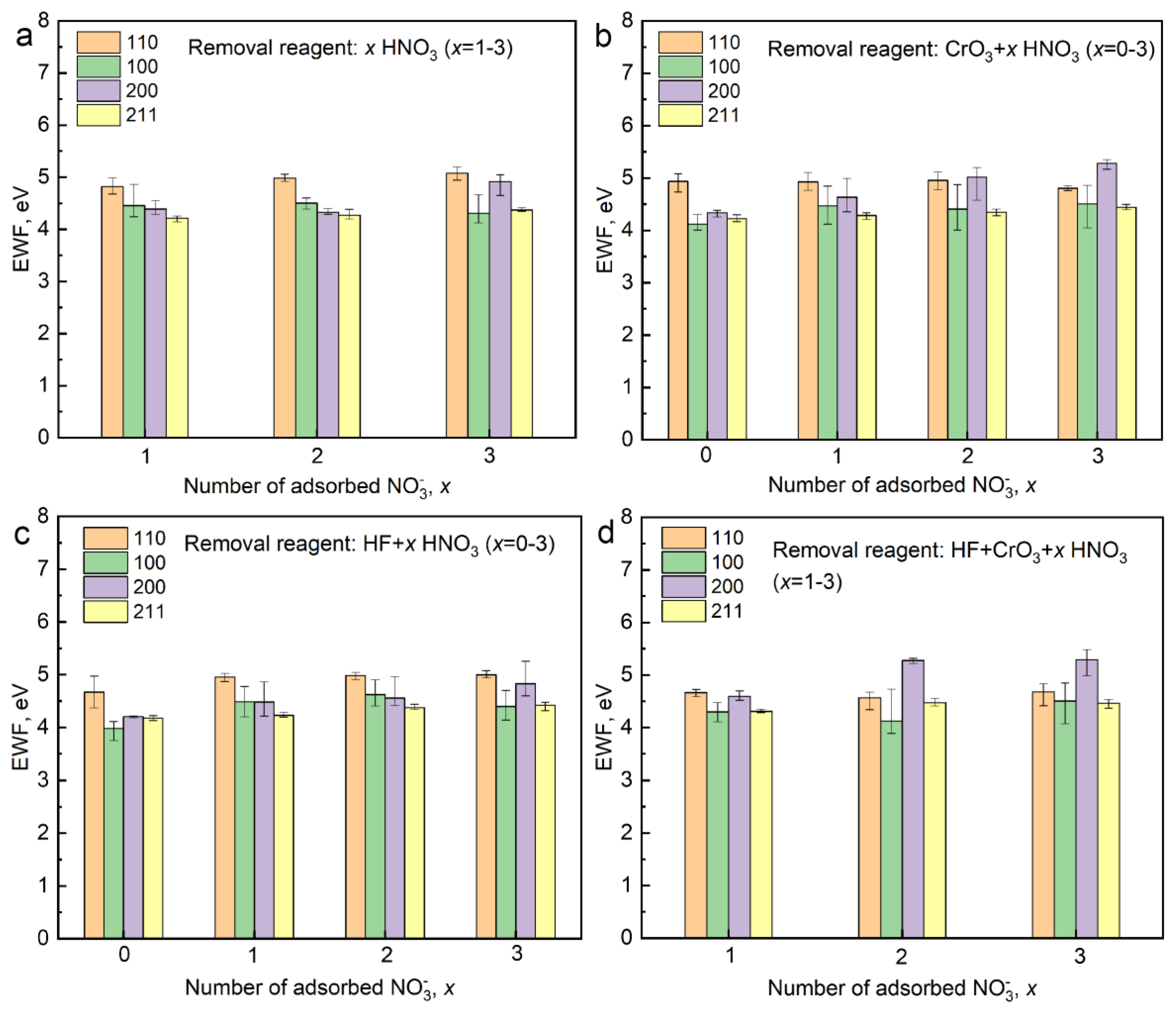
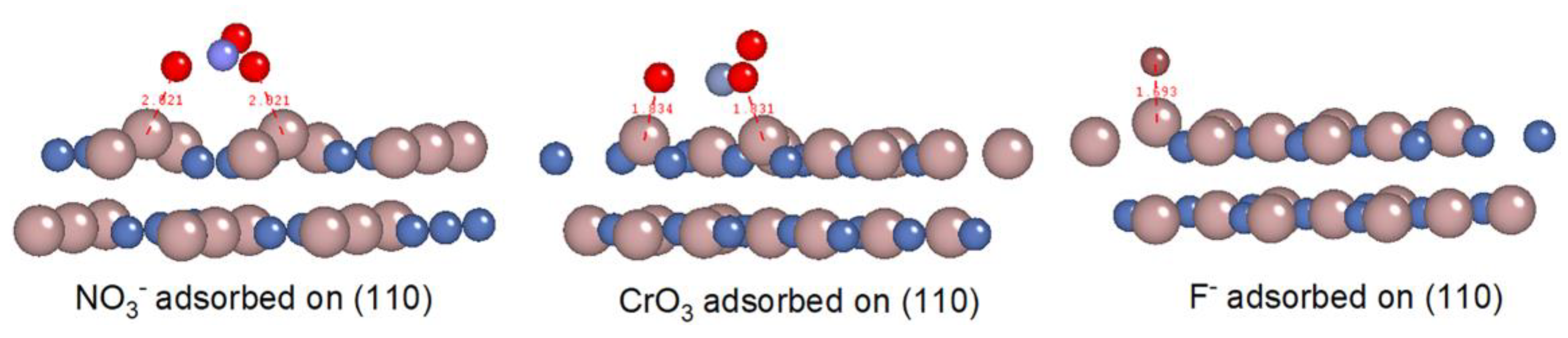
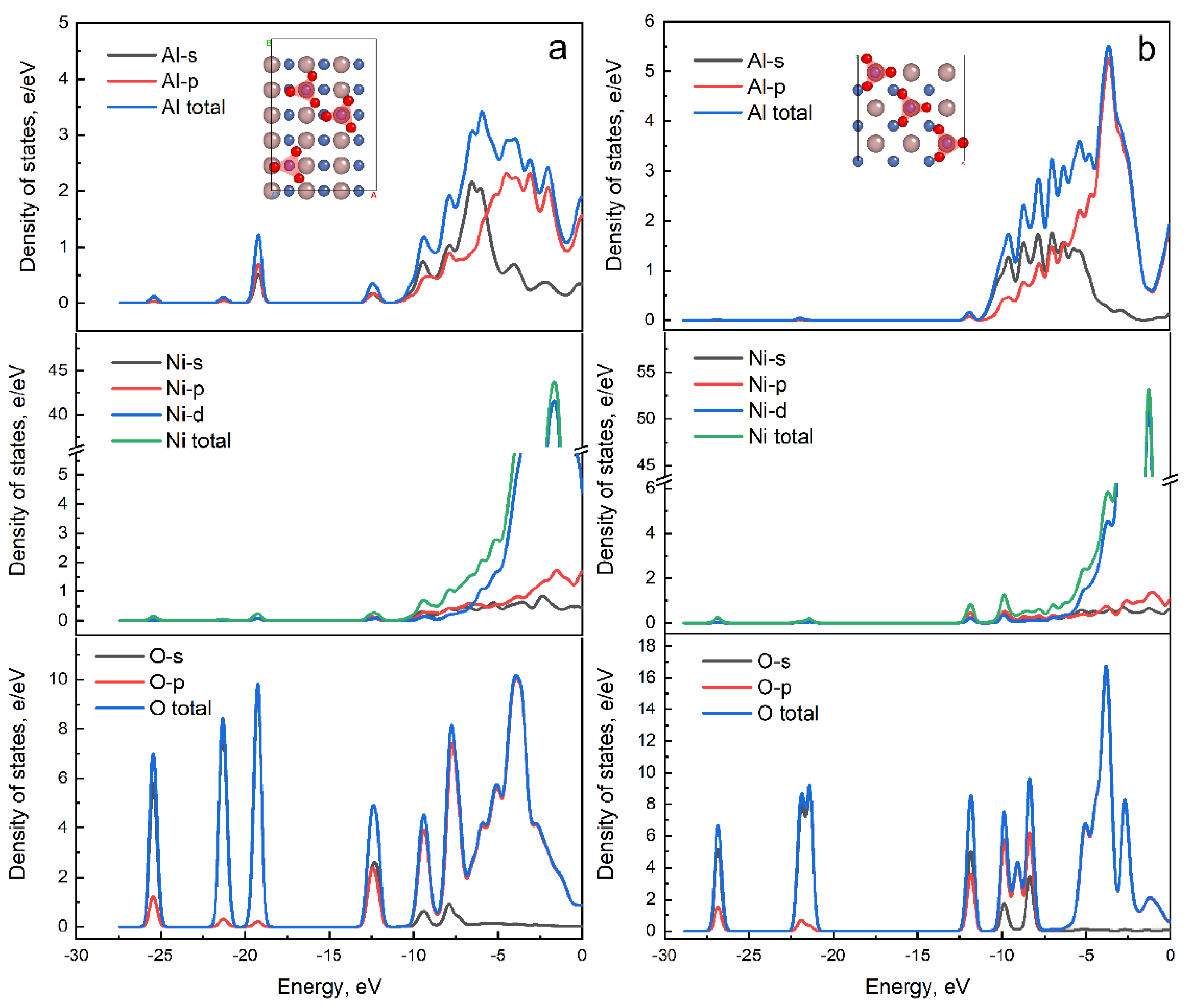
| Combinations | HNO3 | CrO3 | HF |
|---|---|---|---|
| HNO3 | 120 | - | - |
| CrO3+HNO3 | 120 | 8 | - |
| HF+HNO3 | 120 | - | 11 |
| HF+CrO3+HNO3 | 120 | 8 | 11 |
| Surface | Adsorbed Site | Ion Species | |||||
|---|---|---|---|---|---|---|---|
| NO3− | F− | CrO3 | |||||
| Bonding Type | |||||||
| Al-O | Ni-O | Al-F | Ni-F | Al-O | Ni-O | ||
| (110) | Ni-top | 0.3 | - | - | 0.47 | 0.47 | - |
| Al-top | 0.46 | - | 0.49 | - | 0.43 | 0.22 | |
| Ni-bridge | 0.46 | 0.34 | 0.04 | 0.16 | 0.45 | 0.34 | |
| Al-bridge | 0.39 | 0.39 | 0.22 | - | 0.48 | - | |
| (100) | Al-top | 0.59 | - | 0.53 | - | 0.54 | - |
| Al-bridge | 0.49 | - | 0.27 | - | 0.46 | - | |
| Al-center | 0.56 | - | 0.07 | - | 0.47 | - | |
| (200) | Ni-top | - | 0.35 | - | 0.52 | - | 0.41 |
| Ni-bridge | - | 0.39 | - | 0.31 | - | 0.45 | |
| Ni-center | - | 0.39 | - | 0.14 | - | 0.42 | |
| (211) | Ni-top | 0.42 | - | - | 0.51 | 0.59 | - |
| Al-top | 0.35 | 0.26 | 0.49 | - | 0.59 | - | |
| Ni-bridge | - | 0.29 | 0.30 | - | 0.48 | 0.27 | |
| Al-bridge | 0.43 | 0.38 | 0.24 | - | 0.45 | 0.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Luo, C.; Zhang, C.; Wu, L.; Zhou, X.; Zhang, C.; Wang, Y.; Wang, Z. In-Depth Understanding of the Chemical Stripping Mechanism of AlSiY Coatings on Nickel Superalloys by First-Principles Calculation. Coatings 2024, 14, 135. https://doi.org/10.3390/coatings14010135
Li H, Luo C, Zhang C, Wu L, Zhou X, Zhang C, Wang Y, Wang Z. In-Depth Understanding of the Chemical Stripping Mechanism of AlSiY Coatings on Nickel Superalloys by First-Principles Calculation. Coatings. 2024; 14(1):135. https://doi.org/10.3390/coatings14010135
Chicago/Turabian StyleLi, Hongying, Chaoyong Luo, Ce Zhang, Lei Wu, Xiaolong Zhou, Chengsong Zhang, Yang Wang, and Zhiwu Wang. 2024. "In-Depth Understanding of the Chemical Stripping Mechanism of AlSiY Coatings on Nickel Superalloys by First-Principles Calculation" Coatings 14, no. 1: 135. https://doi.org/10.3390/coatings14010135
APA StyleLi, H., Luo, C., Zhang, C., Wu, L., Zhou, X., Zhang, C., Wang, Y., & Wang, Z. (2024). In-Depth Understanding of the Chemical Stripping Mechanism of AlSiY Coatings on Nickel Superalloys by First-Principles Calculation. Coatings, 14(1), 135. https://doi.org/10.3390/coatings14010135





