Evolution of Microstructure and Mechanical Properties of Novel Al-Mg-Mn-Ag-Cr-Zr Alloy
Abstract
1. Introduction
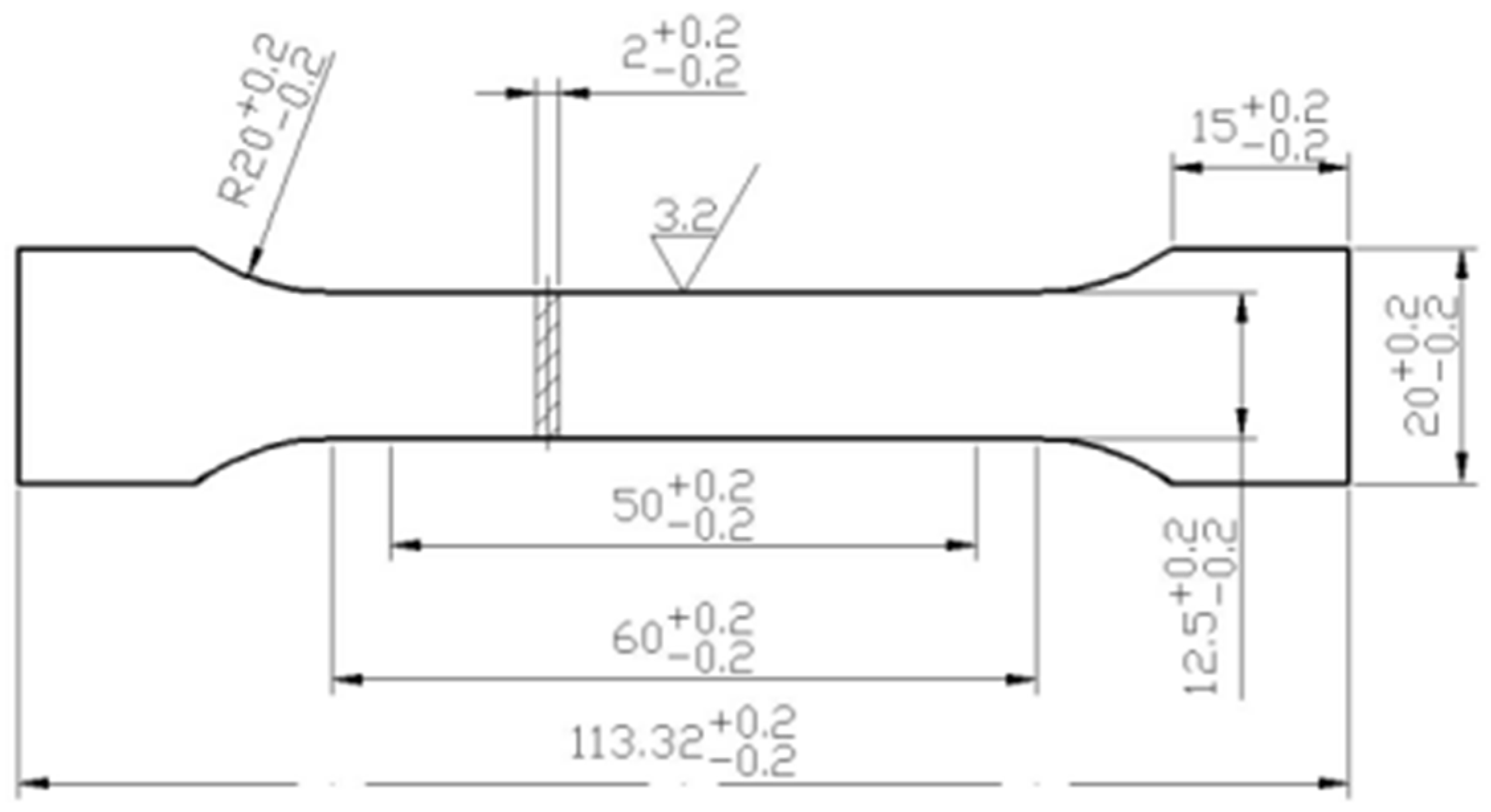
2. Experimental
3. Results
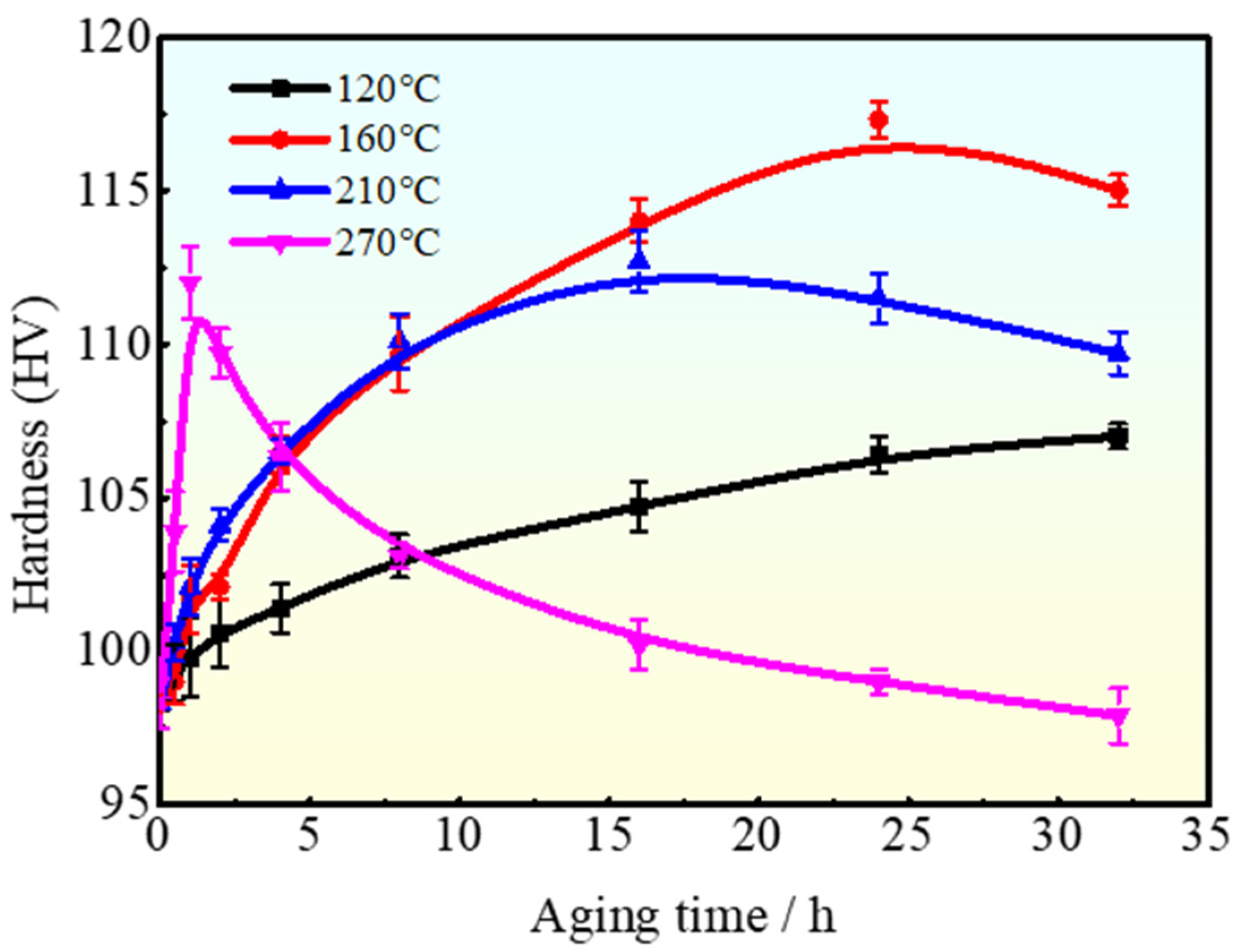
3.1. Microstructure
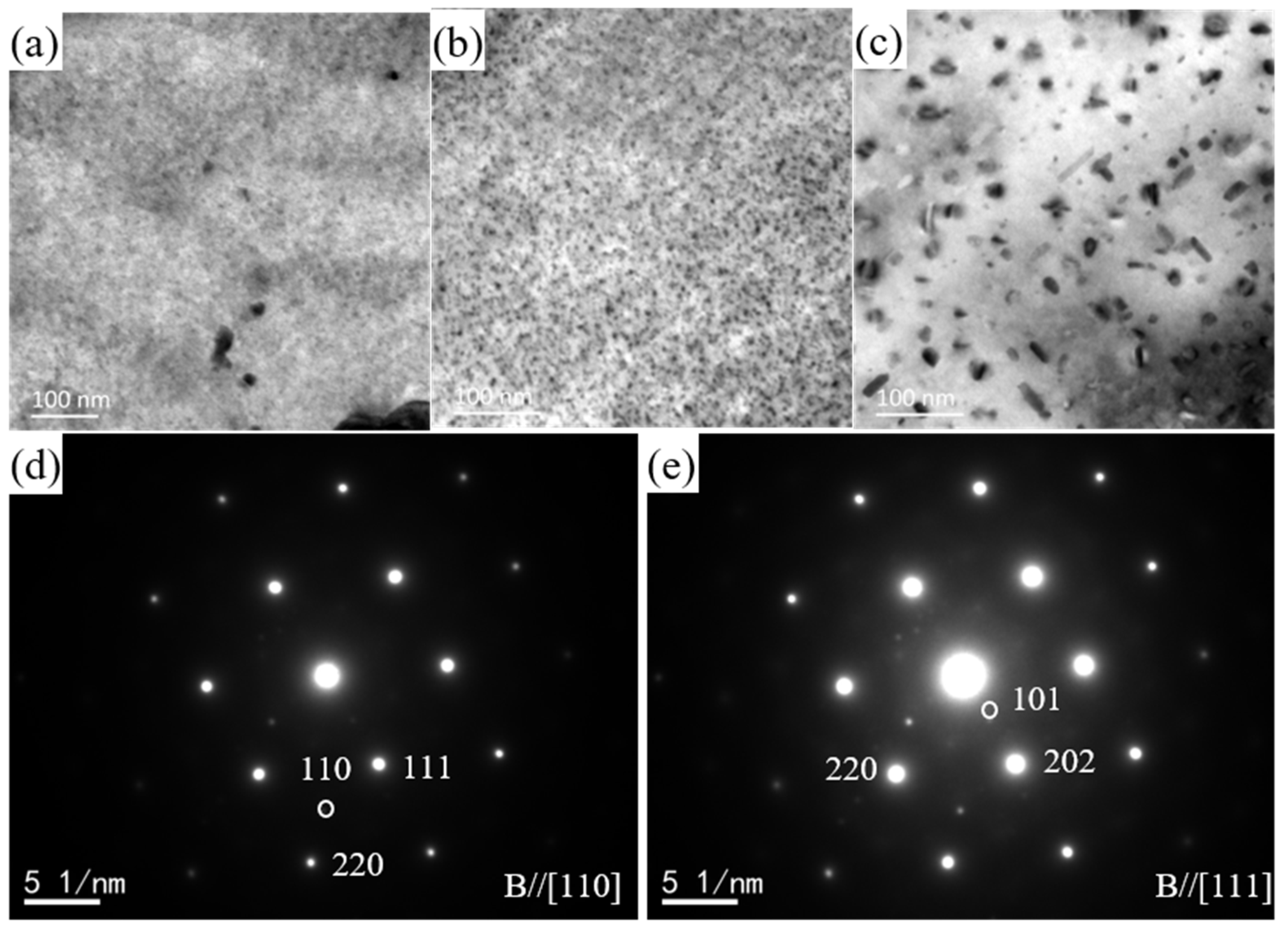
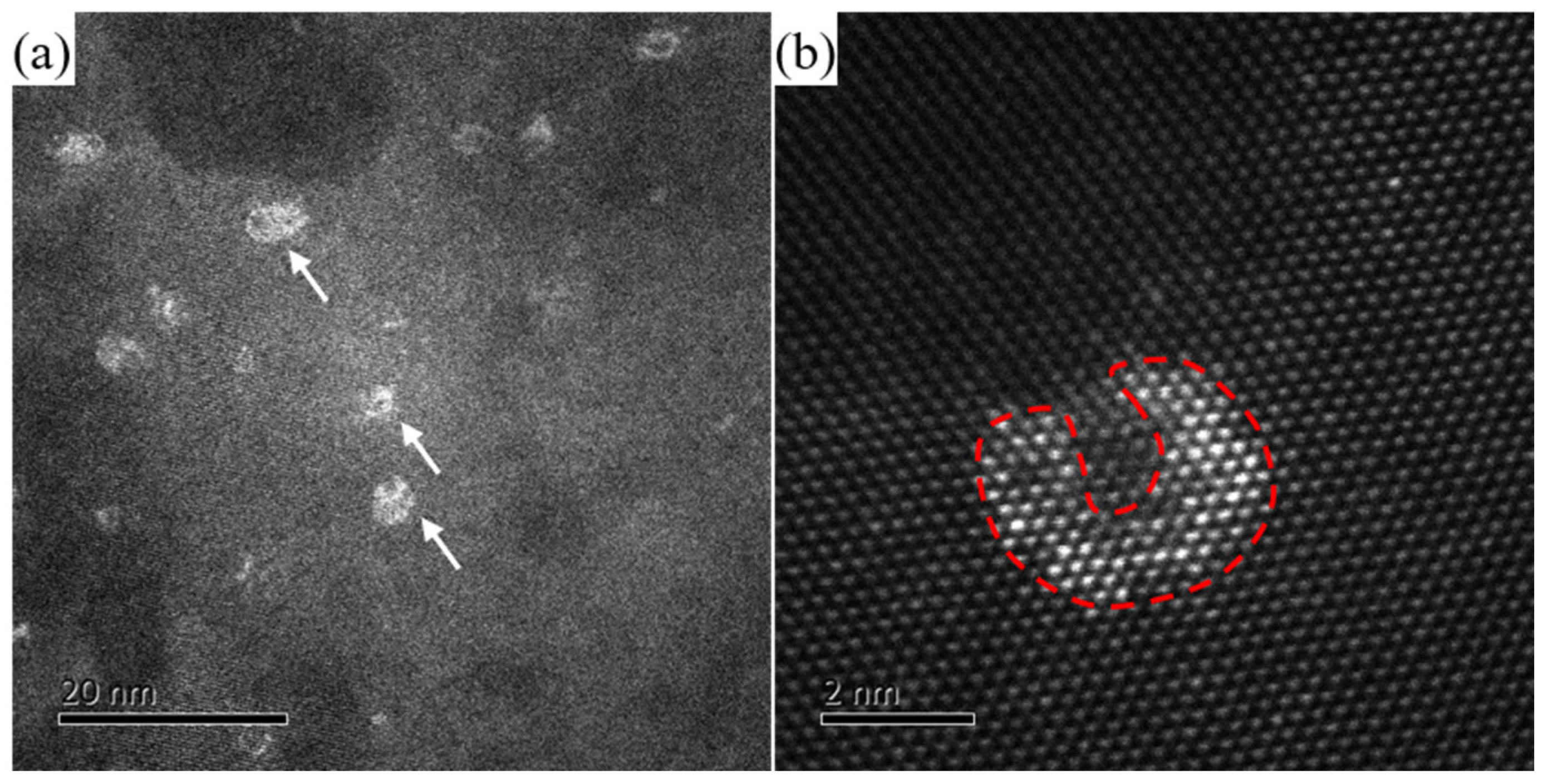
3.2. Precipitation Behavior of the Investigated Al-Mg-Mn-Ag-Cr-Zr Alloy
4. Discussion
5. Conclusions
- (1)
- In regard to the as-cast alloy, the microstructure contained a large number of dendrites with significant diameter segregation. The second phase consisted of the T-Mg32(Al, Ag)49 phase and the Al6(Mn, Fe) phase.
- (2)
- After homogenization, the dendritic segregation was eliminated and numerous nanoscale phases formed in the Al-matrix. The microstructure contained typical fiber structures and deformation textures after cold rolling. The fiber structures transformed into fine equiaxed grains after solute treatment.
- (3)
- During the aging procedure, the addition of Ag assisted the precipitation of the β″ phase, which nucleated on the Mg-Ag clusters. The β″ phase had a L12-type lattice structure and presented a completely coherent relevance with the Al-matrix. The lattice parameter, a, of the β″ phase was 0.408 nm. The β″ phase presented a “core-shell” structure as Ag atoms diffused towards the edges of the precipitates, while Mg atoms were distributed in the center of the precipitates.
- (4)
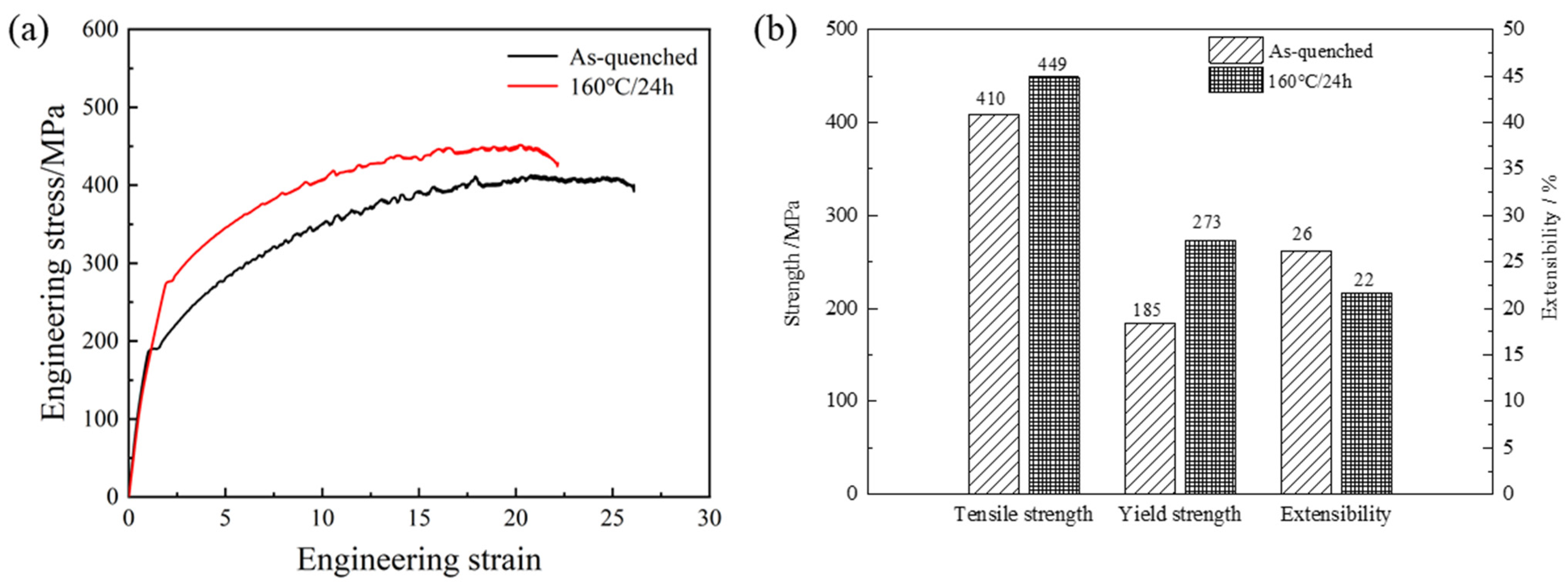
- The mechanical properties of the alloy in the peak-aged period improved greatly as compared to that of the as-quenched alloy. The tensile strength grew from 410 MPa to 449 MPa while the yield strength increased from 185 MPa to 273 MPa, an increase of 9.5% and 47.5%, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, L.Z.; Li, X.H.; Liu, X.T.; Wang, X.J.; Zhang, H.T.; Cui, J.Z. Effects of homogenization on microstructures and properties of a new type Al-Mg-Mn-Zr-Ti-Er alloy. Mater. Sci. Eng. A 2010, 527, 7510–7518. [Google Scholar] [CrossRef]
- Preston, G.D. The Diffraction of X-rays by age-hardening aluminum copper alloys. Proc. R. Soc. A 1938, 167, 526–538. [Google Scholar]
- Sato, T.; Kojima, Y.; Takahashi, T. Modulated structures and G.P. zones in Al-Mg alloys. Metall. Trans. A 1982, 13, 1373–1378. [Google Scholar] [CrossRef]
- Bouchear, M.; Hamana, D.; Laoui, T. GP zones and precipitate morphology in aged Al-Mg alloys. Philos. Mag. A 1996, 73, 1733–1740. [Google Scholar] [CrossRef]
- Yang, W.C.; Wang, M.P.; Jia, Y.L.; Zhang, R.R. Studies of orientations of β′′ precipitates in Al-Mg-Si-(Cu) alloys by electron diffraction and transition matrix analysis. Metall. Mater. Trans. A 2011, 42, 2917–2929. [Google Scholar] [CrossRef]
- Jeong, H.T.; Kim, W.J. Strain hardening behavior and strengthening mechanism in Mg-rich Al-Mg binary alloys subjected to aging treatment. Mater. Sci. Eng. A 2020, 794, 139862. [Google Scholar] [CrossRef]
- Fukui, K.; Watanabe, R.; Takeda, M. Morphology and thermal stability of metastable precipitates formed in an Al-Mg alloy aged at 373K and 473K. Appl. Mech. Mater. 2015, 799, 212–216. [Google Scholar] [CrossRef]
- Yang, M.J.; Chen, H.N.; Orekhov, A.; Lu, Q.; Lan, X.Y.; Li, K.; Zhang, S.Y.; Song, M.; Kong, Y.; Schryvers, D.; et al. Quantified contribution of β′′ and β′ precipitates to the strengthening of an aged Al-Mg-Si alloy. Mater. Sci. Eng. A 2020, 774, 138776. [Google Scholar] [CrossRef]
- Ninive, P.H.; Strandlie, A.; Gulbrandsen-Dahl, S.; Lefebvre, W.; Marioara, C.D.; Andersen, S.J.; Friis, J.; Holmestad, R.; Løvvik, O.M. Detailed atomistic insight into the β′′ phase in Al-Mg-Si alloys. Acta Mater. 2014, 69, 126–134. [Google Scholar] [CrossRef]
- Wei, Z.J.; Jiang, W.; Zou, C.M.; Wang, H.W.; Zhao, W.Q. Microstructural evolution and mechanical strengthening mechanism of the high pressure heat treatment (HPHT) on Al-Mg alloy. J. Alloys Compd. 2017, 692, 629–633. [Google Scholar] [CrossRef]
- Ozturk, F.; Sisman, A.; Toros, S.; Kilic, S.; Picu, R.C. Influence of aging treatment on mechanical properties of 6061 aluminum alloy. Mater. Des. 2010, 31, 972–975. [Google Scholar] [CrossRef]
- Chen, M.C.; Chung, T.F.; Tai, C.L.; Chen, Y.H.; Yang, J.R.; Lee, S.L.; Hsiao, C.N.; Tsao, C.S.; Chou, C.M. Quantitative evaluation of the effect of Ag-addition on the concurrently-existing precipitation kinetics in the aged Al-Cu-Mg (-Ag) alloys. Mater. Des. 2023, 227, 111766. [Google Scholar] [CrossRef]
- Mihara, M.; Marioara, C.D.; Andersen, S.J.; Holmestad, R.; Kobayashi, E.; Sato, T. Precipitation in an Al-Mg-Cu alloy and the effect of a low amount of Ag. Mater. Sci. Eng. A 2016, 658, 91–98. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.T.; Wu, Z.B.; Wang, D.T.; Li, B.M.; Cui, J.D. Effects of Ag on the age hardening response and intergranular corrosion resistance of Al-Mg alloys. Mater. Charact. 2019, 147, 84–92. [Google Scholar] [CrossRef]
- Kubota, M.; Muddle, B.C. Effect of trace additions of Ag on precipitation in Al-Mg alloys. Mater. Trans. 2005, 46, 2968–2974. [Google Scholar] [CrossRef]
- Kubota, M.; Feng, N.J.; Chales, M.B. Characterisation of precipitation hardening response and as-quenched microstructure in Al-Mg(-Ag) Alloys. Mater. Trans. 2004, 45, 3256–3263. [Google Scholar] [CrossRef][Green Version]
- Yi, G.; Littrell, K.C.; Poplawsky, J.D.; Cullen, D.A.; Sundberg, E.; Freea, M.L. Characterization of the effects of different tempers and aging temperatures on the precipitation behavior of Al-Mg (5.25 at.%)-Mn alloys. Mater. Des. 2017, 118, 22–35. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.T.; Wu, Z.B.; Shen, X.D.; Wang, P.; Li, B.M.; Cui, J.Z.; Nagaumi, H. An atomic-resolution investigation of precipitation evolution in Al-Mg-Ag alloys. Mater. Lett. 2019, 248, 231–235. [Google Scholar] [CrossRef]
- Ercetin, A. Application of the hot press method to produce new Mg alloys: Characterization, mechanical properties, and effect of Al addition. J. Mater. Eng. Perform. 2021, 30, 4254–4262. [Google Scholar] [CrossRef]
- Wen, H.; Topping, T.; Isheim, D.; Seidman, D.; Lavernia, E. Strengthening mechanisms in a high-strength bulk nanostructured Cu-Zn-Al alloy processed via cryomilling and spark plasma sintering. Acta Mater. 2013, 61, 2769–2782. [Google Scholar] [CrossRef]
- Sahoo, B.; Das, D.; Chaubey, A. Strengthening mechanisms and modelling of mechanical properties of submicron-TiB2 particulate reinforced Al 7075 metal matrix composites. Mater. Sci. Eng. A 2021, 825, 141873. [Google Scholar] [CrossRef]
- Sato, T. Nanocluster Control for Achieving High Strength Aluminum Alloys. Mater. Trans. 2018, 59, 861–869. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wu, X.D.; Cao, L.F.; Tong, X.; Zou, Y.; Zhu, Q.Q.; Tang, S.B.; Song, H.; Guo, M.X. Effect of Ag on aging precipitation behavior and mechanical properties of aluminum alloy 7075. Mater. Sci. Eng. A 2021, 804, 140515. [Google Scholar] [CrossRef]
- Maeng, D.Y.; Lee, J.H.; Hong, S.I. The effect of transition elements on the superplastic behavior of Al-Mg alloys. Mater. Sci. Eng. A 2003, 357, 188–195. [Google Scholar] [CrossRef]
- Wang, J.; Shin, S.; Nobakht, A.; Shyam, A. Structural deformation and transformation of θ′-Al2Cu precipitate in Al matrix via interfacial diffusion. Comput. Mater. Sci. 2019, 156, 111–120. [Google Scholar] [CrossRef]
- Senapati, S. Evolution of Lamellar Structures in Al-Ag Alloys. Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2005. [Google Scholar]
- Kubota, M.; Nie, J.F.; Muddle, B.C. Identification of metastable rod-like particles in an isothermally aged Al-10Mg-0.5Ag (mass%) alloy. Mater. Trans. 2005, 46, 1288–1294. [Google Scholar] [CrossRef][Green Version]
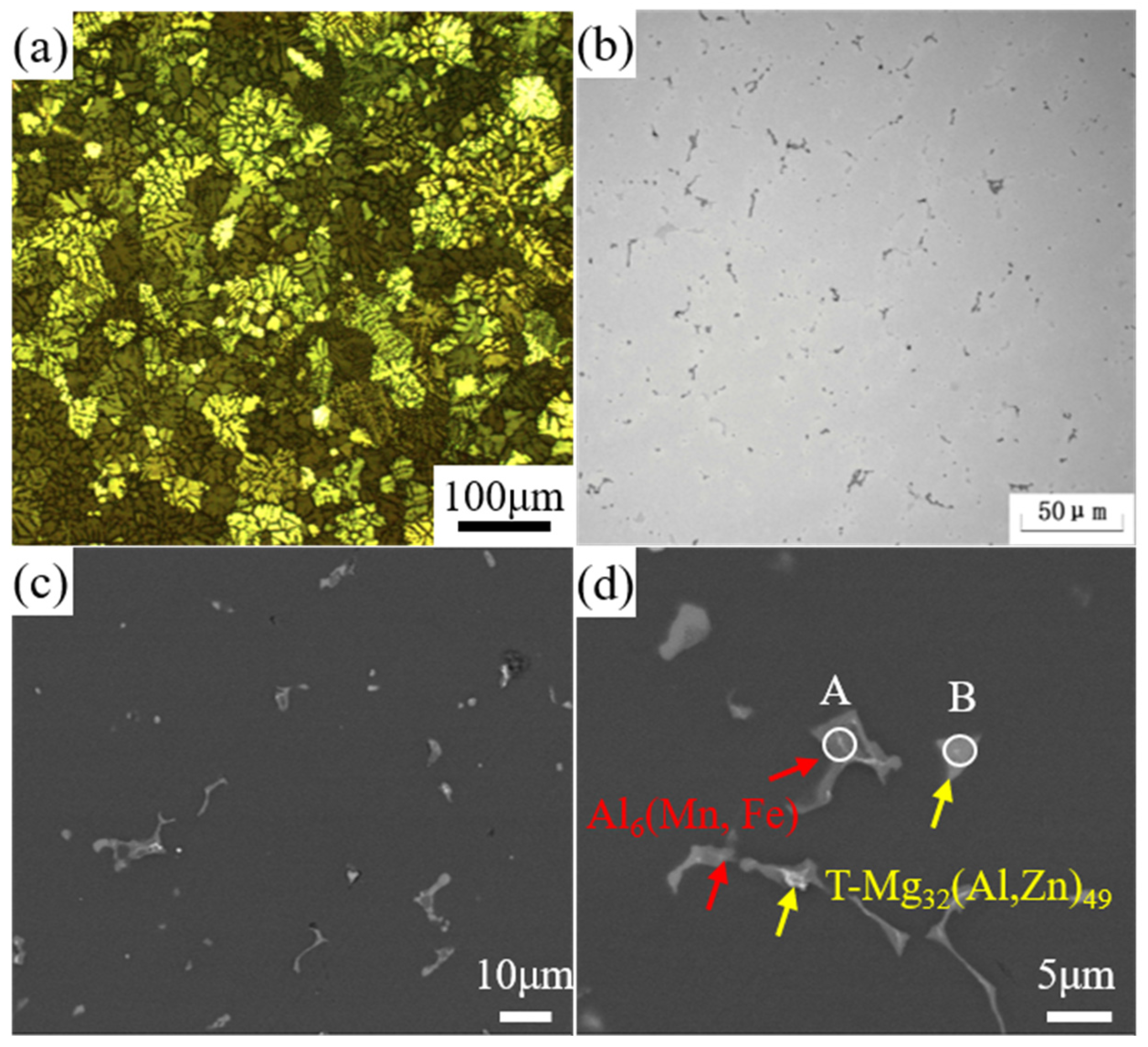
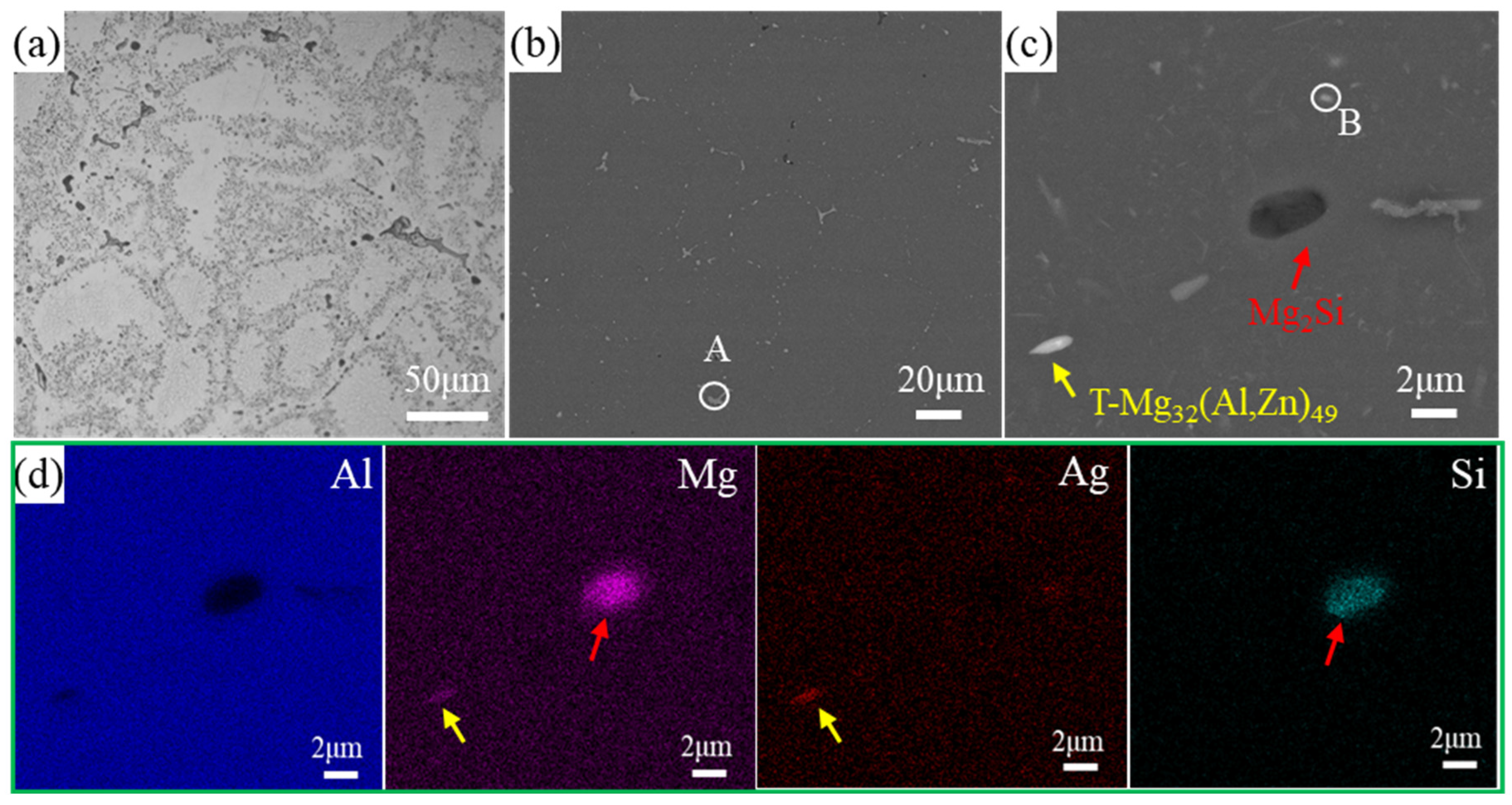
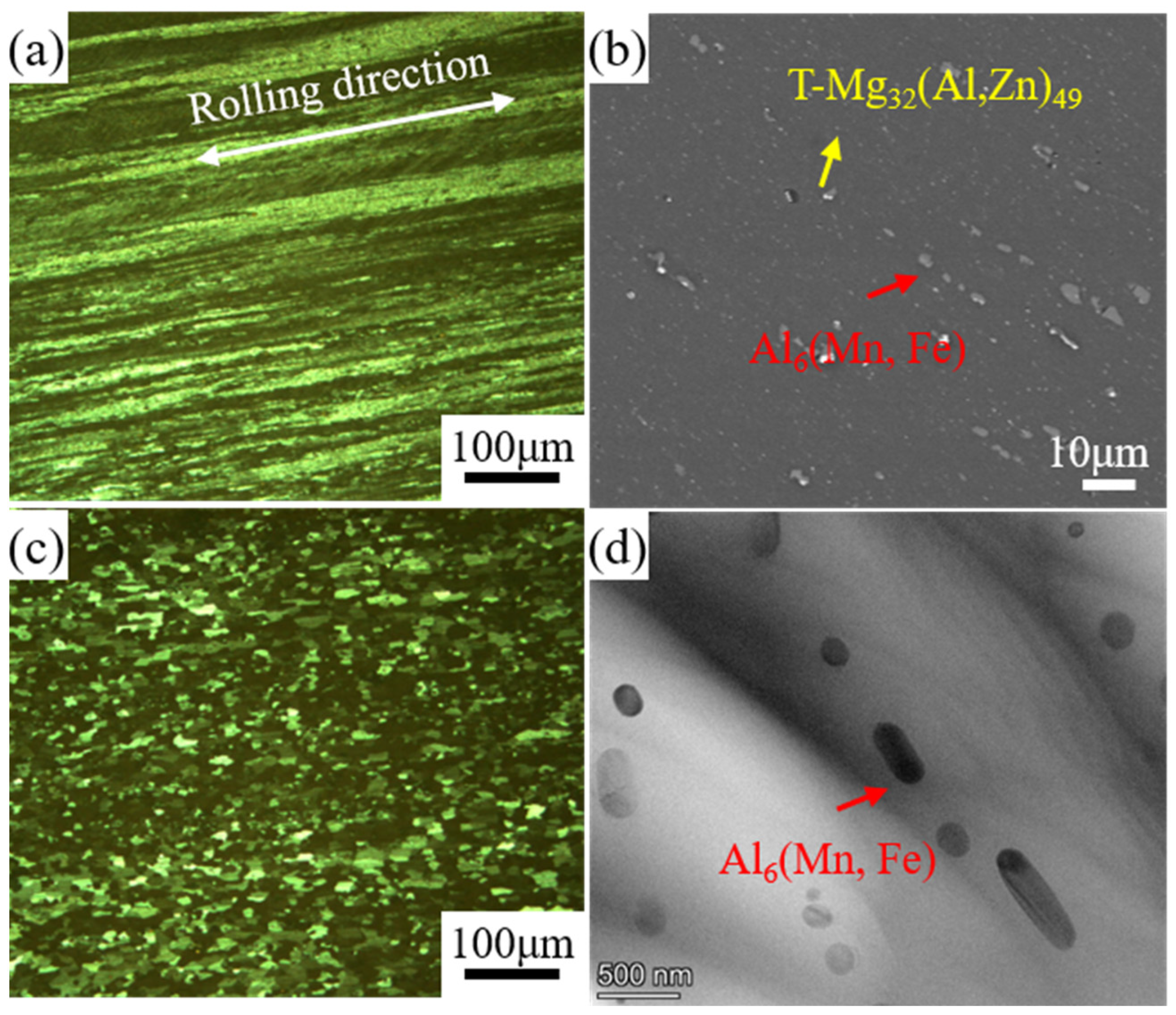
| Phase | Si | Mg | Mn | Cr | Fe | Ag | Al |
|---|---|---|---|---|---|---|---|
| A | 0.9 | 2.3 | 15.4 | 0.9 | 10.6 | 1.2 | Balance |
| B | 2.1 | 24.3 | - | - | - | 21.2 | Balance |
| Phase | Mg | Mn | Fe | Ag | Al |
|---|---|---|---|---|---|
| A | 0.6 | 9.9 | 13.3 | 0.6 | Balance |
| B | 8.1 | - | - | 12.9 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, T.; Wu, Y.; Guo, C. Evolution of Microstructure and Mechanical Properties of Novel Al-Mg-Mn-Ag-Cr-Zr Alloy. Coatings 2024, 14, 134. https://doi.org/10.3390/coatings14010134
Wang H, Liu T, Wu Y, Guo C. Evolution of Microstructure and Mechanical Properties of Novel Al-Mg-Mn-Ag-Cr-Zr Alloy. Coatings. 2024; 14(1):134. https://doi.org/10.3390/coatings14010134
Chicago/Turabian StyleWang, Huan, Tao Liu, Yanli Wu, and Cheng Guo. 2024. "Evolution of Microstructure and Mechanical Properties of Novel Al-Mg-Mn-Ag-Cr-Zr Alloy" Coatings 14, no. 1: 134. https://doi.org/10.3390/coatings14010134
APA StyleWang, H., Liu, T., Wu, Y., & Guo, C. (2024). Evolution of Microstructure and Mechanical Properties of Novel Al-Mg-Mn-Ag-Cr-Zr Alloy. Coatings, 14(1), 134. https://doi.org/10.3390/coatings14010134




