Effect of Elastomeric Coating on the Properties and Performance of Myristic Acid (MA) Phase Change Material (PCM) Used for Photovoltaic Cooling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding of NBR Coating Emulsion
2.3. Preparation of Coated MA PCMs
2.4. Characterization of PCMs
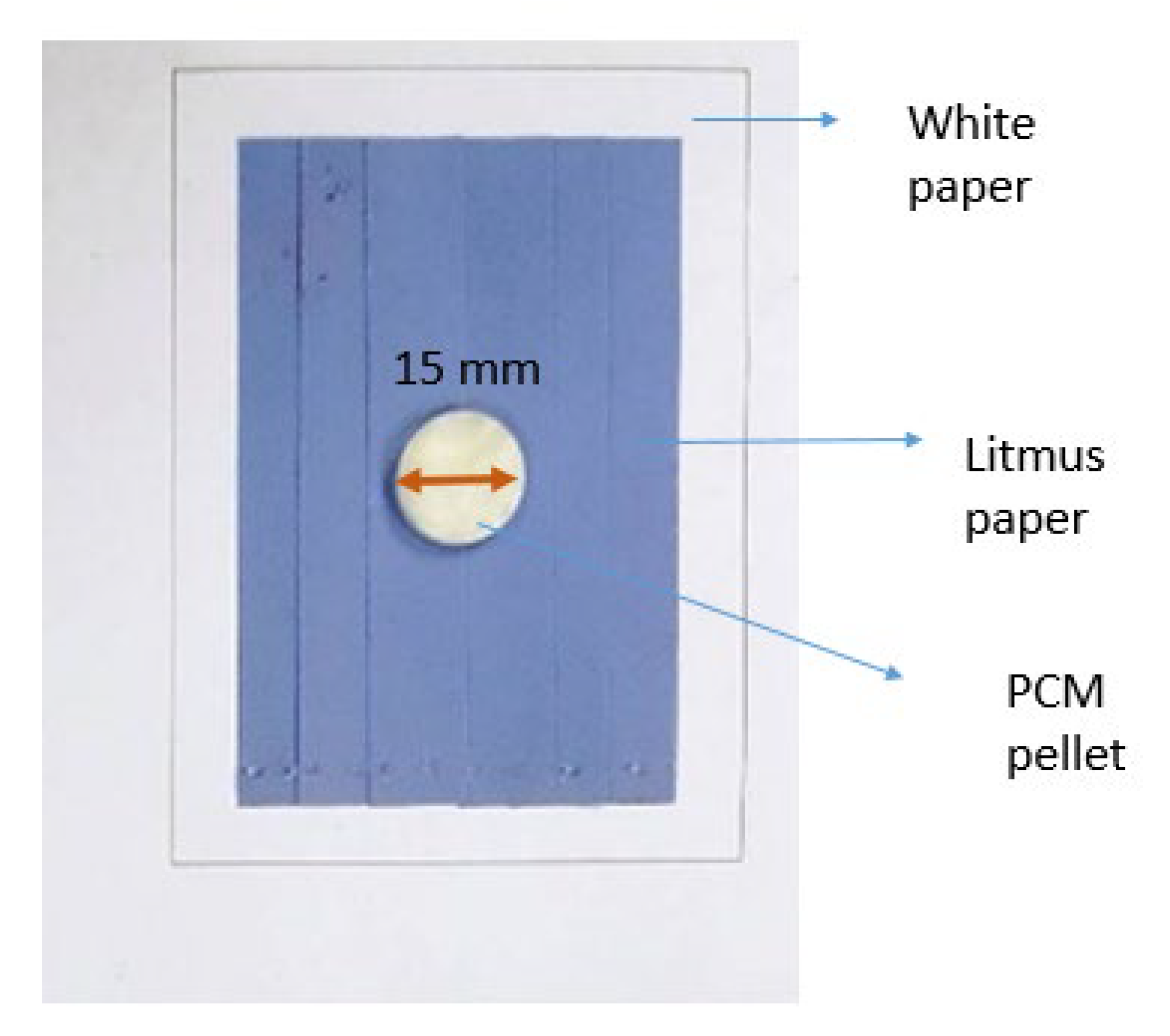
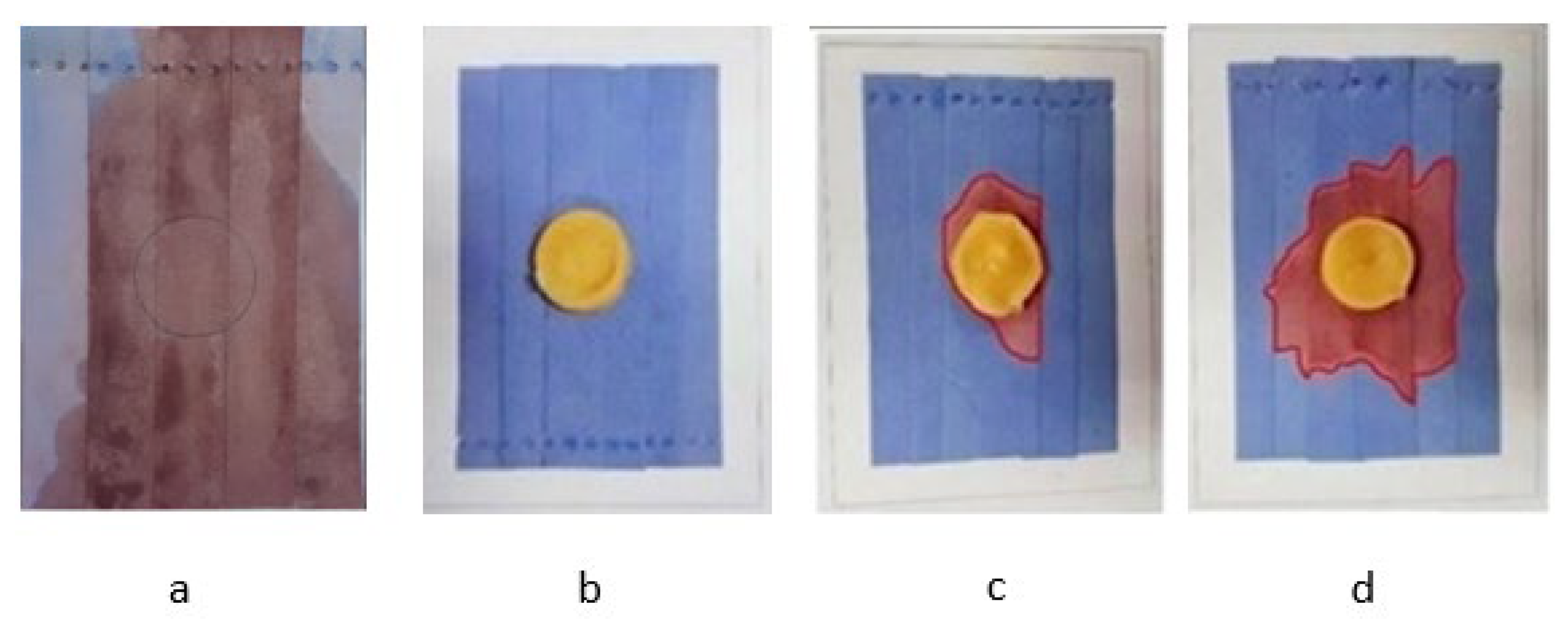
2.4.1. Leakage Test
2.4.2. Crosslink Density Calculation
2.4.3. Thermal Properties Analysis
2.4.4. Tensile Test
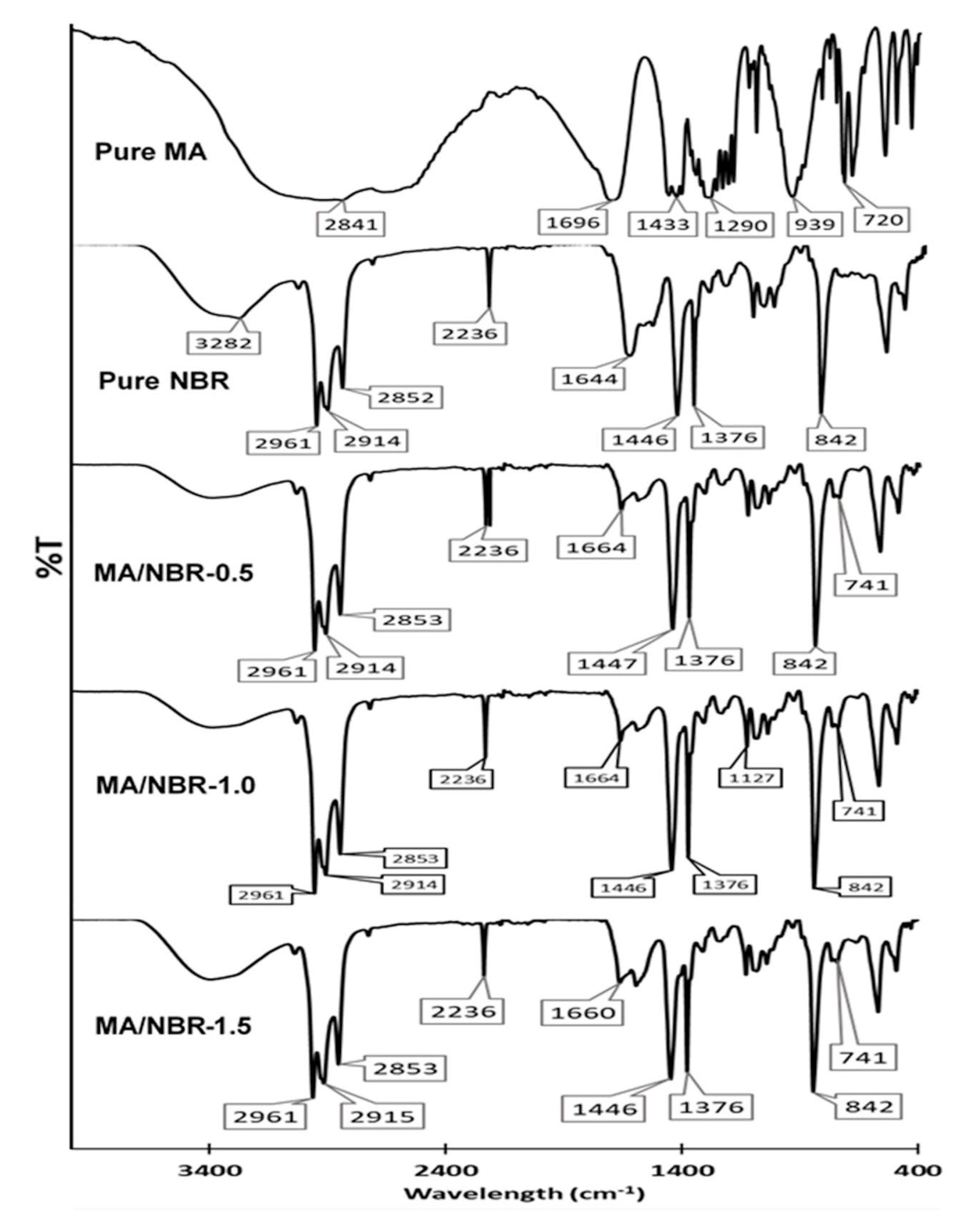
2.4.5. Chemical Functionality of PCM Pellets
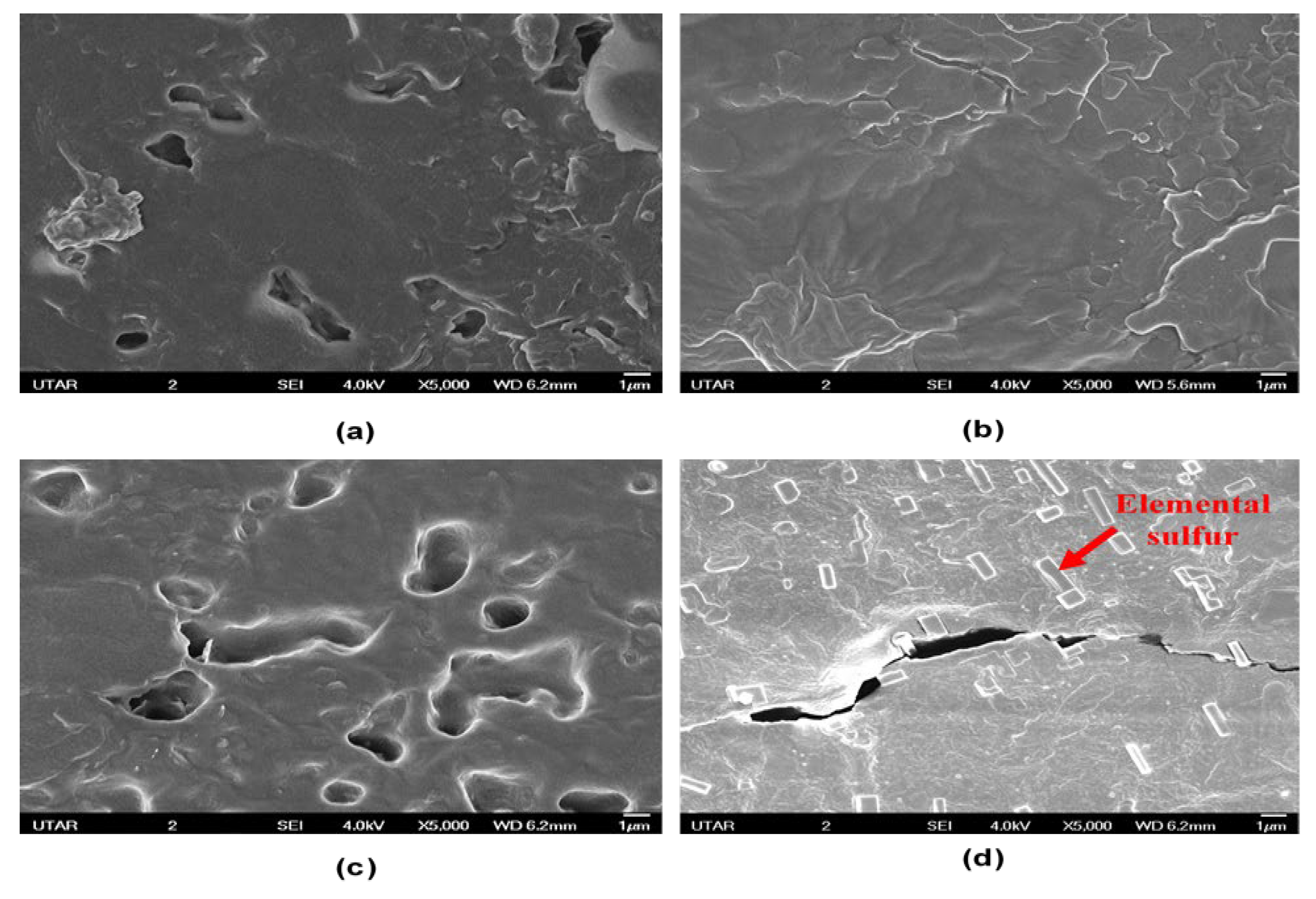
2.4.6. Morphology of Coatings
2.4.7. Photovoltaic Temperature Reduction and Power Generation
3. Results
3.1. Leakage Analysis
3.2. Crosslink Density Calculation
3.3. Tensile Properties of the Coating
3.4. Chemical Functionality of Coatings
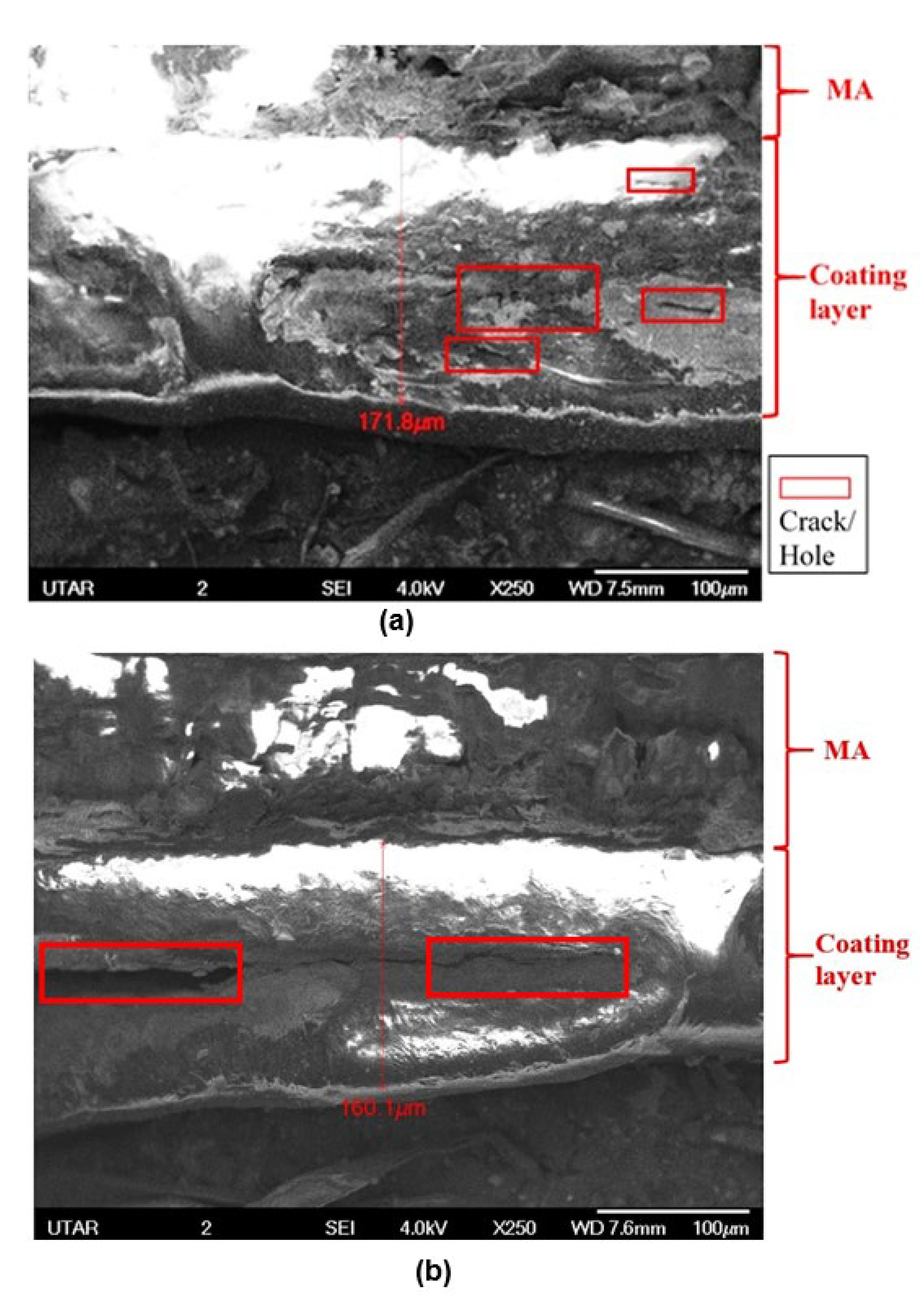
3.5. Surface and Cross-Section Morphology Analysis
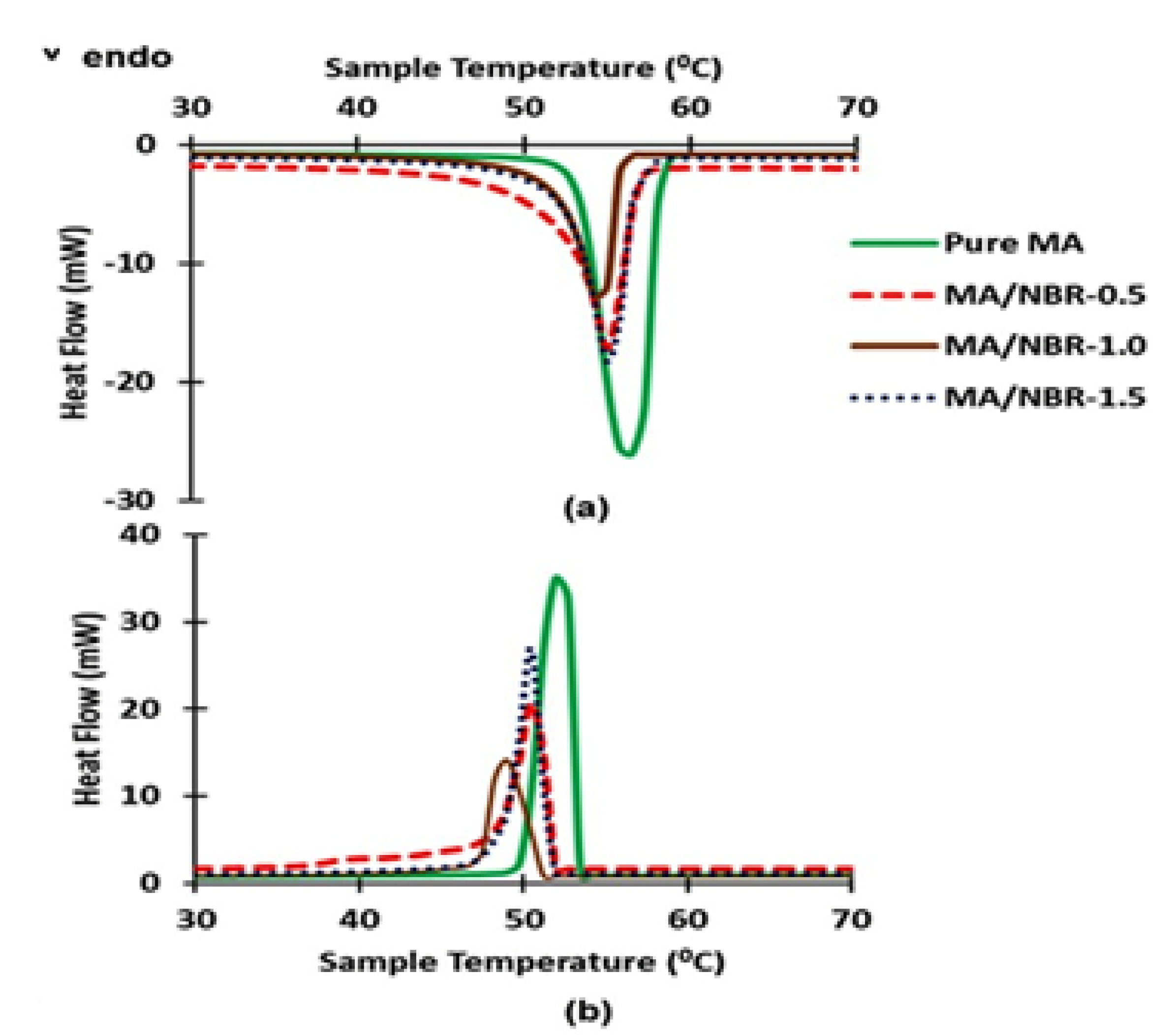
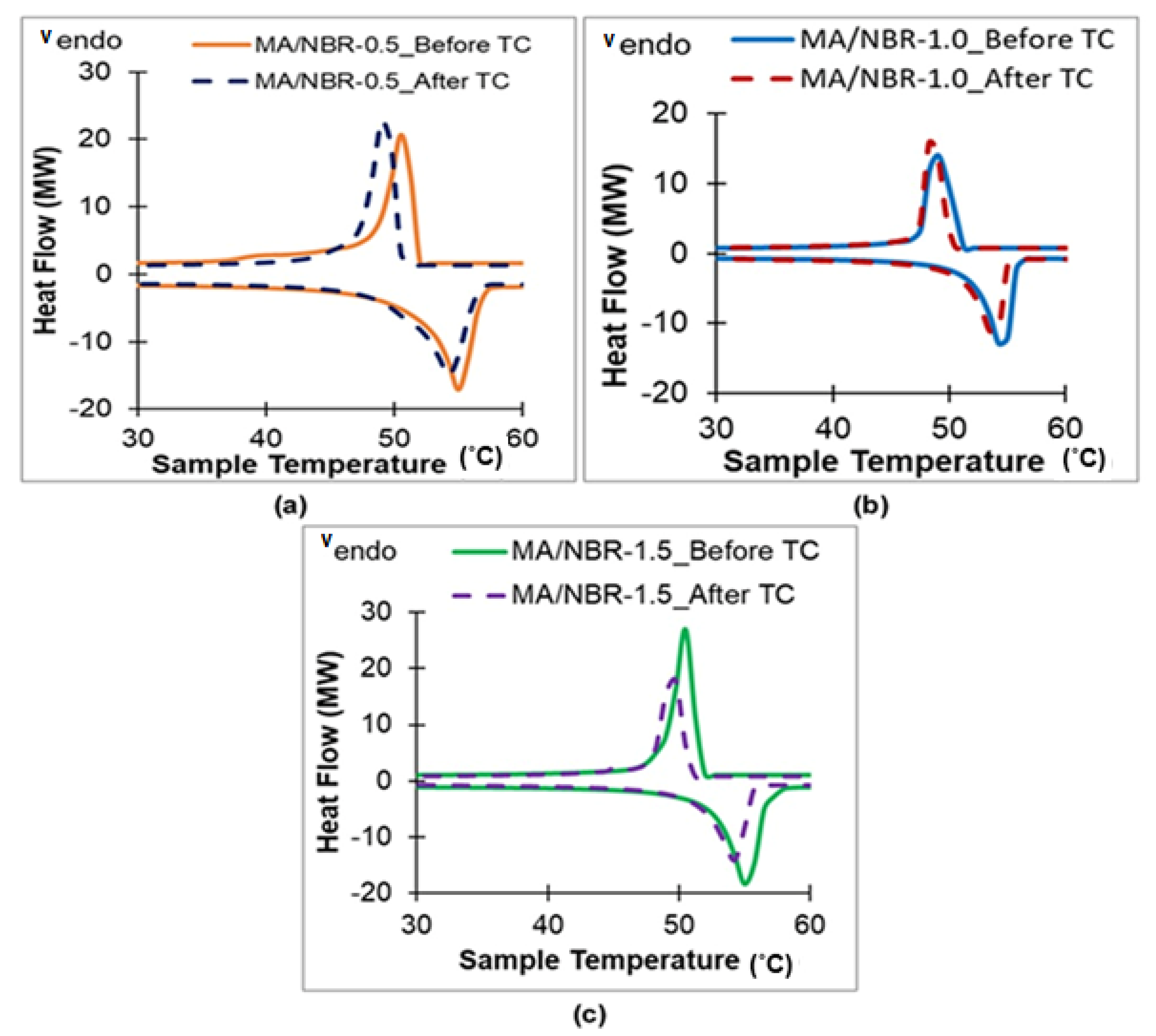
3.6. Thermal Properties of Dip-Coated MA Pellet
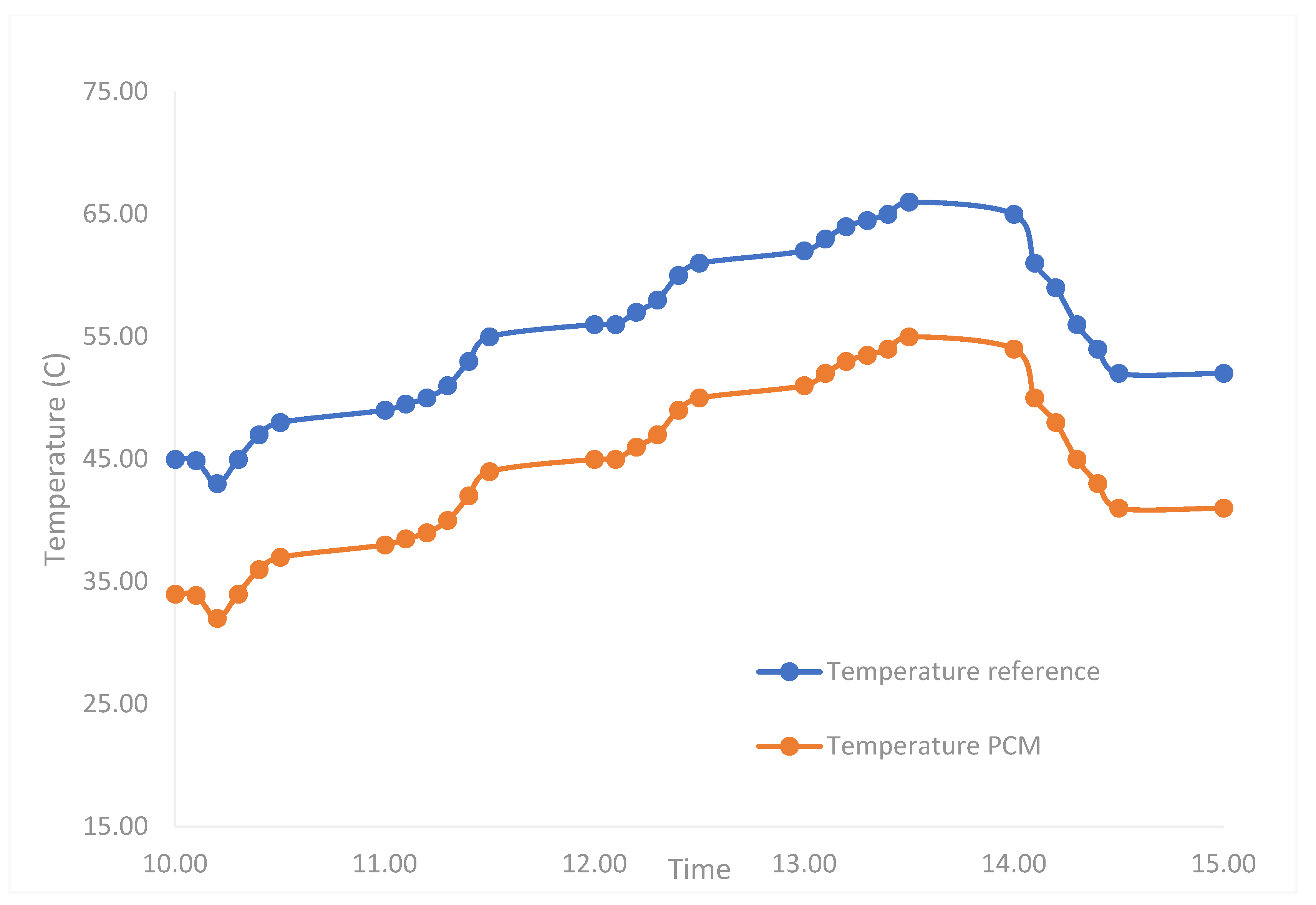
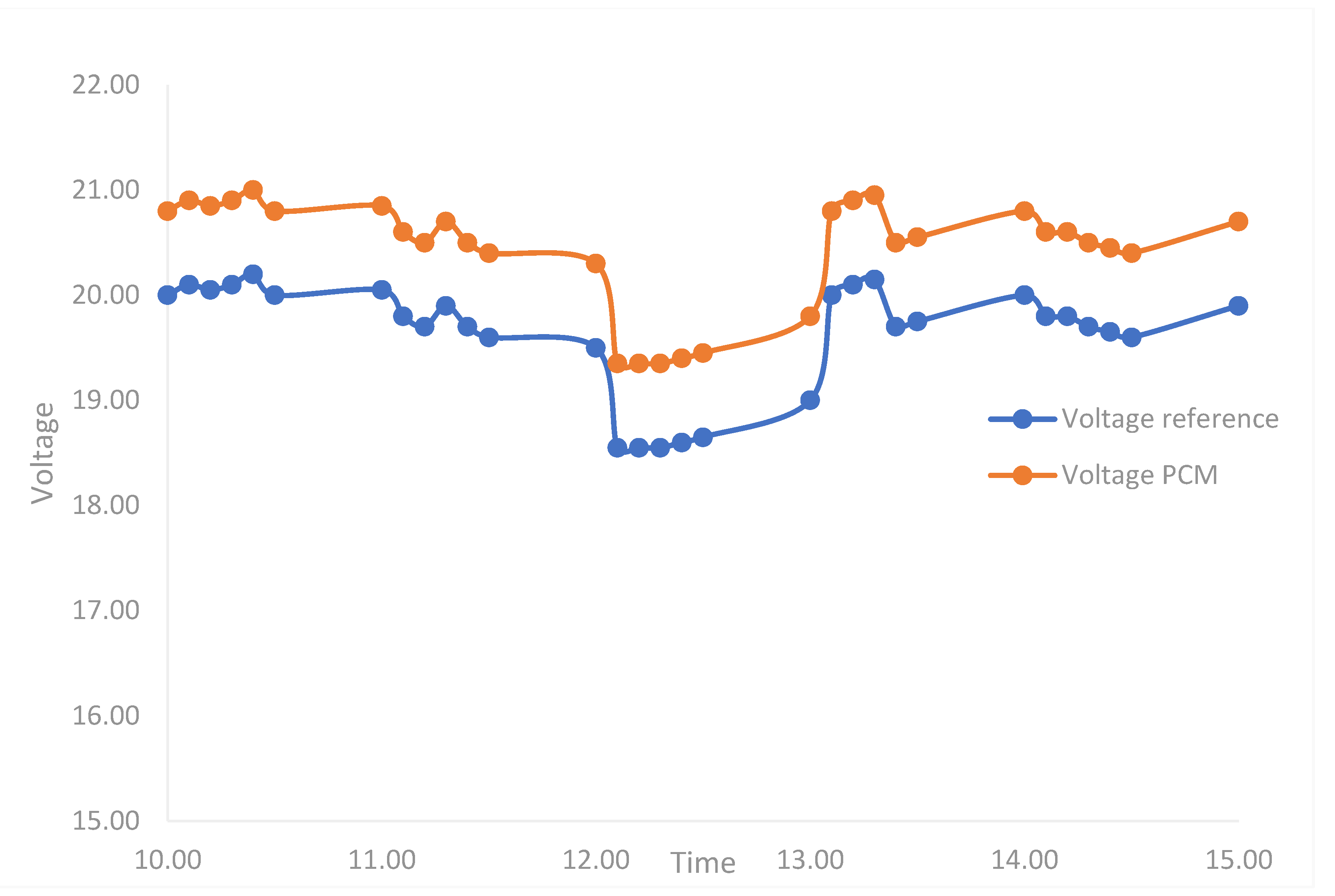
3.7. Effect of Coating Solution on the Performance of the Solar PV Module
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, P.; Sudhakar, K.; Soni, A.; Solomin, E.; Kirpichnikova, I. Advanced cooling techniques of P.V. modules: A state of art. Case Stud. Therm. Eng. 2020, 21, 100674. [Google Scholar] [CrossRef]
- Wang, S.; Shi, J.; Chen, H.-H.; Schafer, S.R.; Munir, M.; Stecker, G.; Pan, W.; Lee, J.-J.; Chen, C.-L. Cooling design and evaluation for photovoltaic cells within constrained space in a CPV/CSP hybrid solar system. Appl. Therm. Eng. 2017, 110, 369–381. [Google Scholar] [CrossRef]
- Ghadikolaei, S.S.C. Solar photovoltaic cells performance improvement by cooling technology: An overall review. Int. J. Hydrogen Energy 2021, 46, 10939–10972. [Google Scholar] [CrossRef]
- Nižetić, S.; Jurčević, M.; Čoko, D.; Arıcı, M. A novel and effective passive cooling strategy for photovoltaic panel. Renew. Sustain. Energy Rev. 2021, 145, 111164. [Google Scholar] [CrossRef]
- Pathak, S.K.; Sharma, P.O.; Goel, V.; Bhattacharyya, S.; Aybar, H.Ş.; Meyer, J.P. A detailed review on the performance of photovoltaic/thermal system using various cooling methods. Sustain. Energy Technol. Assess. 2022, 51, 101844. [Google Scholar] [CrossRef]
- Kaaya, I.; Ascencio-Vásquez, J.; Weiss, K.-A.; Topič, M. Assessment of uncertainties and variations in PV modules degradation rates and lifetime predictions using physical models. Sol. Energy 2021, 218, 354–367. [Google Scholar] [CrossRef]
- Al-Lami, H.; Al-Mayyahi, N.N.; Al-Yasiri, Q.; Ali, R.; Alshara, A. Performance enhancement of photovoltaic module using finned phase change material panel: An experimental study under Iraq hot climate conditions. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 6886–6897. [Google Scholar] [CrossRef]
- Jakhar, S.; Soni, M.S.; Gakkhar, N. Historical and recent development of concentrating photovoltaic cooling technologies. Renew. Sustain. Energy Rev. 2016, 60, 41–59. [Google Scholar] [CrossRef]
- Ahmad, F.F.; Said, Z.; Hachicha, A.A. Experimental performance evaluation of closed loop mist/fog cooling system for photovoltaic module application. Energy Convers. Manag. X 2022, 14, 100226. [Google Scholar]
- PraveenKumar, S.; Agyekum, E.B.; Qasim, M.A.; Alwan, N.T.; Velkin, V.I.; Shcheklein, S.E. Experimental assessment of thermoelectric cooling on the efficiency of PV module. Int. J. Renew. Energy Res. 2022, 12, 1670–1681. [Google Scholar]
- Praveenkumar, S.; Gulakhmadov, A.; Agyekum, E.B.; TAlwan, N.; Velkin, V.I.; Sharipov, P.; Safaraliev, M.; Chen, X. Experimental study on performance enhancement of a photovoltaic module incorporated with CPU heat pipe—A 5E analysis. Sensors 2022, 22, 6367. [Google Scholar] [CrossRef] [PubMed]
- Kazem, H.A.; Al-Waeli, A.H.; Chaichan, M.T.; Al-Waeli, K.H.; Al-Aasam, A.B.; Sopian, K. Evaluation and comparison of different flow configurations PVT systems in Oman: A numerical and experimental investigation. Sol. Energy 2020, 208, 58–88. [Google Scholar] [CrossRef]
- Dorobanţu, L.; Popescu, M.O. Increasing the efficiency of photovoltaic panels through cooling water film. UPB Sci. Bull. Ser. C 2013, 75, 223–232. [Google Scholar]
- Moharram, K.A.; Abd-Elhady, M.S.; Kandil, H.A.; El-Sherif, H. Enhancing the performance of photovoltaic panels by water cooling. Ain Shams Eng. J. 2013, 4, 869–877. [Google Scholar] [CrossRef]
- Mah, C.-Y.; Lim, B.-H.; Wong, C.-W.; Tan, M.-H.; Chong, K.-K.; Lai, A.-C. Investigating the performance improvement of a photovoltaic system in a tropical climate using water cooling method. Energy Procedia 2019, 159, 78–83. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Malek, A.B.M.A.; Islam, M.M.; Pandey, A.K.; Rahim, N.A. Global advancement of cooling technologies for PV systems: A review. Sol. Energy 2016, 137, 25–45. [Google Scholar] [CrossRef]
- Hasan, A.; McCormack, S.; Huang, M.; Sarwar, J.; Norton, B. Increased photovoltaic performance through temperature regulation by phase change materials: Materials comparison in different climates. Sol. Energy 2015, 115, 264–276. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Sopian, K.; Kazem, H.A.; Chaichan, M.T. Evaluation of the electrical performance of a photovoltaic thermal system using nano-enhanced paraffin and nanofluids. Case Stud. Therm. Eng. 2020, 21, 100678. [Google Scholar] [CrossRef]
- Abdelrazik, A.; Al-Sulaiman, F.; Saidur, R. Numerical investigation of the effects of the nano-enhanced phase change materials on the thermal and electrical performance of hybrid PV/thermal systems. Energy Convers. Manag. 2020, 205, 112449. [Google Scholar] [CrossRef]
- Rostami, Z.; Heidari, N.; Rahimi, M.; Azimi, N. Enhancing the thermal performance of a photovoltaic panel using nano-graphite/paraffin composite as phase change material. J. Therm. Anal. Calorim. 2022, 147, 3947–3964. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Saidur, R.; Al-Sulaiman, F.A. A review for phase change materials (PCMs) in solar absorption refrigeration systems. Renew. Sustain. Energy Rev. 2017, 76, 105–137. [Google Scholar] [CrossRef]
- Kallingal, N.; Sobolčiak, P.; Akbar, H.M.; Krupa, I.; Novak, I.; Popelka, A. An Enhancement of Compositional Stability of Phase Change Materials by Lamination with Aluminum Sheet. Coatings 2023, 13, 444. [Google Scholar] [CrossRef]
- Velmurugan, K.; Kumarasamy, S.; Wongwuttanasatian, T.; Seithtanabutara, V. Review of PCM types and suggestions for an applicable cascaded PCM for passive PV module cooling under tropical climate conditions. J. Clean. Prod. 2021, 293, 126065. [Google Scholar] [CrossRef]
- Tang, Y.; Jia, Y.; Alva, G.; Huang, X.; Fang, G. Synthesis, characterization and properties of palmitic acid/high density polyethylene/graphene nanoplatelets composites as form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2016, 155, 421–429. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Tian, B.; Yang, W.; Luo, L.; Wang, J.; Zhang, K.; Fan, J.; Wu, J.; Xing, T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol. Energy 2016, 127, 48–55. [Google Scholar] [CrossRef]
- Saraç, E.G.; Öner, E.; Kahraman, M.V. Microencapsulated organic coconut oil as a natural phase change material for thermo-regulating cellulosic fabrics. Cellulose 2019, 26, 8939–8950. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y. Synthesis and thermal properties of nanoencapsulation of paraffin as phase change material for latent heat thermal energy storage. Energy Built Environ. 2020, 1, 410–416. [Google Scholar] [CrossRef]
- Weinstock, L.; Sanguramath, R.A.; Silverstein, M.S. Encapsulating an organic phase change material within emulsion-templated poly(urethane urea)s. Polym. Chem. 2019, 10, 1498–1507. [Google Scholar] [CrossRef]
- Machotová, J.; Kalendová, A.; Steinerová, D.; Mácová, P.; Šlang, S.; Šňupárek, J.; Vajdák, J. Water-resistant latex coatings: Tuning of properties by polymerizable surfactant, covalent crosslinking and nanostructured ZnO additive. Coatings 2021, 11, 347. [Google Scholar] [CrossRef]
- Khanna, S.; Paneliya, S.; Marathey, P.; Shah, K.; Prajapati, P.; Chaudhari, R.; Vora, J. Investigation of Thermophysical Properties of Synthesized N-Hexacosane-Encapsulated Titania Phase Change Material for Enhanced Thermal Storage Application. In Recent Advances in Mechanical Infrastructure, Proceedings of the ICRAM 2021, Ahmedabad, India, 18–19 January 2021; Springer: Singapore, 2022; pp. 107–118. [Google Scholar]
- Sundaram, P.; Kalaisselvane, A. Cold thermal energy storage performance of graphene nanoplatelets–DI water nanofluid PCM using gum acacia in a spherical encapsulation. J. Therm. Anal. Calorim. 2022, 147, 14973–14985. [Google Scholar] [CrossRef]
- Jacob, R.; Bruno, F. Review on shell materials used in the encapsulation of phase change materials for high temperature thermal energy storage. Renew. Sustain. Energy Rev. 2015, 48, 79–87. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K.-H.; Kim, S.; Tabatabaei, M. Recent advances in polyurethanes as efficient media for thermal energy storage. Energy Storage Mater. 2020, 30, 74–86. [Google Scholar] [CrossRef]
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Kazemian, A.; Ali, H.M. Development and thermal performance of nanoencapsulated PCM/plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727. [Google Scholar] [CrossRef]
- Kizildag, N. Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality. Coatings 2023, 13, 414. [Google Scholar] [CrossRef]
- Tan, S.; Yu, S.; Xu, G.; Zhang, Y. Preparation and properties studies of paraffin/high density polyethylene composites and phase-change coatings. Prog. Org. Coat. 2013, 76, 1761–1764. [Google Scholar] [CrossRef]
- Aung, Q.L.; Chow, W.S.; Yong, Y.P.; Lam, C.N. Nanokaolin reinforced carboxylated nitrile butadiene rubber/polyurethane blend-based latex with enhanced tensile properties and chemical resistance. Prog. Rubber Plast. Recycl. Technol. 2023, 39, 14777606231161368. [Google Scholar] [CrossRef]
- Nilmini, A.; Priyanka, S.; Nilmini, R.; Siriwardene, S.; Priyadarshana, G. The Effect of Hydrogen Peroxide based Hand Sanitizing Chemicals on the Physicomechanical Properties of the NBR Gloves. Adv. Technol. 2021, 1, 546–552. [Google Scholar]
- Kee, S.Y.; Munusamy, Y.; Ong, K.S.; Metselaar, H.S.C.; Chee, S.Y.; Lai, K.C. Thermal performance study of composite phase change material with polyacrylicand conformal coating. Materials 2017, 10, 873. [Google Scholar] [CrossRef]
- Naebpetch, W.; Junhasavasdikul, B.; Saetung, A.; Tulyapitak, T.; Nithi-Uthai, N. Influence of filler type and loading on cure characteristics and vulcanisate properties of SBR compounds with a novel mixed vulcanisation system. Plast. Rubber Compos. 2017, 46, 137–145. [Google Scholar] [CrossRef]
- Paul, D.I. Experimental characterisation of photovoltaic modules with cells connected in different configurations to address nonuniform illumination effect. J. Renew. Energy 2019, 2019, 5168259. [Google Scholar] [CrossRef]
- Kee, S.Y.; Wong, J.L.O.; Munusamy, Y.; Ong, K.S.; Choong, Y.C. Light absorptive polymeric form-stable composite phase change material for thermal storage. Appl. Therm. Eng. 2020, 172, 114673. [Google Scholar] [CrossRef]
- Munusamy, Y.; Lin Onn, J.W.; Alquraish, M.; Kchaou, M.; Sethupathi, S. Thermal Performance of Finned Heat Sinks Embedded with Form-Stable Myristic Acid Phase Change Material in Photovoltaic Cooling for Green Energy Storage. Energies 2021, 14, 6860. [Google Scholar] [CrossRef]
- Lawandy, S.N.; Halim, S.F. Effect of vulcanizing system on the crosslink density of nitrile rubber compounds. J. Appl. Polym. Sci. 2005, 96, 2440–2445. [Google Scholar] [CrossRef]
- Alvarez-Gayosso, C.; Canseco, M.A.; Estrada, R.; Palacios-Alquisira, J.; Hinojosa, J.; Castano, V. Preparation and microstructure of cobalt(III) poly (acrylate) hybrid materials. Int. J. Basic Appl. Sci. 2015, 4, 255–263. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Hezma, A.M.; El-Khodary, A.; Elzayat, A.M. Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Alhareb, A.; Akil, H.; Ahmad, Z. Poly (methyl methacrylate) denture base composites enhancement by various combinations of nitrile butadiene rubber/treated ceramic fillers. J. Thermoplast. Compos. Mater. 2017, 30, 1069–1090. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Xu, L.; Zhang, P. Investigation of aging behavior and mechanism of nitrile-butadiene rubber (NBR) in the accelerated thermal aging environment. Polym. Test. 2016, 54, 59–66. [Google Scholar] [CrossRef]
- Aly, R.O. Influence of gamma irradiation on mechanical and thermal properties of waste polyethylene/nitrile butadiene rubber blend. Arab. J. Chem. 2016, 9, S1547–S1554. [Google Scholar] [CrossRef]
- Mohamed, A.M.O.; Gamal, M.E. A sustainable process for the preparation of sulfur cement for use in public works. In Advances in Sustainable Manufacturing, Proceedings of the 8th Global Conference on Sustainable Manufacturing, Abu Dhabi, United Arab Emirates, 22–24 November 2010; Springer: Berlin/Heidelberg, Germany, 2011; pp. 127–132. [Google Scholar]
- Bakhshandeh, G.R.; Farahani, T.D.; Emamikia, M. Effect of curing system on mechanical properties of NBR/nylon-PET cord composite. e-Polymers 2008, 8, 273–284. [Google Scholar] [CrossRef]
- Howse, S.; Porter, C.; Mengistu, T.; Pazur, R.J. Experimental determination of the quantity and distribution of chemical crosslinks in unaged and aged natural rubber, part 1: Peroxide vulcanization. Polym. Test. 2018, 70, 263–274. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Ternary mixture of fatty acids as phase change materials for thermal energy storage applications. Energy Rep. 2016, 2, 274–279. [Google Scholar] [CrossRef]
- Huang, X.; Guo, J.; He, J.; Gong, Y.; Wang, D.; Song, Z. Novel phase change materials based on fatty acid eutectics and triallyl isocyanurate composites for thermal energy storage. J. Appl. Polym. Sci. 2017, 134, 44866. [Google Scholar] [CrossRef]
- Megariotis, G.; Vogiatzis, G.G.; Sgouros, A.P.; Theodorou, D.N. Slip spring-based mesoscopic simulations of polymer networks: Methodology and the corresponding computational code. Polymers 2018, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.O.; Munusamy, Y.; Yu, G.Q.; Kee, S.Y.; Seng, O.K. Performance of form-stable myristic acid/polymethyl methacrylate composite phase-change material coated with nitrile butadiene rubber/polyacrylic acid layered coatings. J. Appl. Polym. Sci. 2020, 137, 48642. [Google Scholar] [CrossRef]
- Jensen, K.A. Infrared spectra of complex organic selenium compounds. Ann. N. Y. Acad. Sci. 1972, 192, 115–123. [Google Scholar] [CrossRef]
- Ding, J.; Wu, X.; Shen, X.; Cui, S.; Chen, X. A promising form-stable phase change material composed of C/SiO2 aerogel and palmitic acid with large latent heat as short-term thermal insulation. Energy 2020, 210, 118478. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, G.; Sun, H.; Chen, P.; Zhu, Z.; Li, A. Graphene–nickel/n-carboxylic acids composites as form-stable phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2015, 132, 425–430. [Google Scholar] [CrossRef]
- Sahan, N.; Nigon, D.; Mantell, S.C.; Davidson, J.H.; Paksoy, H. Encapsulation of stearic acid with different PMMA-hybrid shell materials for thermotropic materials. Sol. Energy 2019, 184, 466–476. [Google Scholar] [CrossRef]
- Fashandi, M.; Leung, S.N. Preparation and characterization of 100% bio-based polylactic acid/palmitic acid microcapsules for thermal energy storage. Mater. Renew. Sustain. Energy 2017, 6, 14. [Google Scholar] [CrossRef]
- Verma, S.; Mohapatra, S.; Chowdhury, S.; Dwivedi, G. Cooling techniques of the PV module: A review. Mater. Today Proc. 2021, 38, 253–258. [Google Scholar] [CrossRef]
- Li, Z.; Ma, T.; Zhao, J.; Song, A.; Cheng, Y. Experimental study and performance analysis on solar photovoltaic panel integrated with phase change material. Energy 2019, 178, 471–486. [Google Scholar] [CrossRef]
- Said, Z.; Ahmad, F.F.; Radwan, A.M.; Hachicha, A.A. New thermal management technique for PV module using Mist/PCM/Husk: An experimental study. J. Clean. Prod. 2023, 401, 136798. [Google Scholar] [CrossRef]
- Jamil, F.; Khiadani, M.; Ali, H.M.; Nasir, M.A.; Shoeibi, S. Thermal regulation of photovoltaics using various nano-enhanced phase change materials: An experimental study. J. Clean. Prod. 2023, 414, 137663. [Google Scholar] [CrossRef]
- Siahkamari, L.; Rahimi, M.; Azimi, N.; Banibayat, M. Experimental investigation on using a novel phase change material (PCM) in micro structure photovoltaic cooling system. Int. Commun. Heat Mass Transf. 2019, 100, 60–66. [Google Scholar] [CrossRef]



| Materials | Real Compound Weight (g) for Sulfur Formulation of | |||
|---|---|---|---|---|
| 0 phr | 0.5 phr | 1.0 phr | 1.5 phr | |
| NBR | 50.00 | 50.0000 | 50.0000 | 50.0000 |
| ZMBT | - | 0.3051 | 0.3051 | 0.3051 |
| ZDEC | - | 0.5624 | 0.5624 | 0.5624 |
| ZnO | - | 0.6592 | 0.6592 | 0.6592 |
| Sulfur | - | 0.3047 | 0.6094 | 0.9141 |
| KOH | - | 3.0856 | 3.0856 | 3.0856 |
| Sample | Sulfur Formulation (phr) | Leakage Area (%) |
|---|---|---|
| MA | NA | Total leakage |
| MA/NBR-0.5 | 0.5 | 0.00 |
| MA/NBR-1.0 | 1.0 | 13.55 |
| MA/NBR-1.5 | 1.5 | 31.40 |
| Sulfur Formulation (phr) | Crosslink Density (×10−4 mol/cm3) |
|---|---|
| 0.5 | 6.3106 ± 0.0025 |
| 1.0 | 6.4714 ± 0.0032 |
| 1.5 | 6.6005 ± 0.0038 |
| Sample | Latent Heat of Melting (J/g) | Melting Point (°C) | Latent Heat of Freezing (J/g) | Freezing Point (°C) | Latent Heat Reduction (%) |
|---|---|---|---|---|---|
| Pure MA | 210.02 ± 2.46 | 56.16 ± 0.08 | 209.95 ± 2.38 | 52.27 ± 0.07 | NA |
| MA/NBR-0.5 | 142.30 ± 1.38 | 54.60 ± 0.08 | 139.47 ± 1.23 | 50.51 ± 0.08 | 32.24 |
| MA/NBR-1.0 | 127.51 ± 1.37 | 54.14 ± 0.11 | 124.75 ± 1.29 | 49.25 ± 0.10 | 39.27 |
| MA/NBR-1.5 | 124.64 ± 1.42 | 54.56 ± 0.09 | 123.28 ± 1.38 | 50.70 ± 0.09 | 40.65 |
| Sample | Melting Point (°C) | Freezing Point (°C) | ||||
|---|---|---|---|---|---|---|
| Before | After | Change Percentage (%) | Before | After | Change Percentage (%) | |
| MA/NBR-0.5 | 54.60 ± 0.08 | 53.85 ± 0.09 | 1.37 | 50.51 ± 0.08 | 49.05 ± 0.07 | 2.89 |
| MA/NBR-1.0 | 54.14 ± 0.11 | 53.52 ± 0.11 | 1.15 | 49.25 ± 0.10 | 48.27 ± 0.10 | 1.99 |
| MA/NBR-1.5 | 54.56 ± 0.09 | 53.88 ± 0.14 | 1.25 | 50.70 ± 0.09 | 49.91 ± 0.13 | 1.56 |
| Sample | Latent Heat of Melting (J/g) | Latent Heat of Freezing (J/g) | ||||
|---|---|---|---|---|---|---|
| Before | After | Change Percentage (%) | Before | After | Change Percentage (%) | |
| MA/NBR-0.5 | 142.30 ± 1.38 | 139.96 ± 3.16 | 1.64 | 139.47 ± 1.23 | 137.01 ± 2.84 | 1.76 |
| MA/NBR-1.0 | 127.51 ± 1.37 | 116.38 ± 3.84 | 8.73 | 124.75 ± 1.29 | 111.00 ± 3.37 | 11.02 |
| MA/NBR-1.5 | 124.64 ± 1.42 | 98.01 ± 4.59 | 21.37 | 123.28 ± 1.38 | 93.22 ± 4.04 | 24.38 |
| No. | Material and Method | Pure PCM | Form-stable PCM | Reference | |||
|---|---|---|---|---|---|---|---|
| Melting Point (°C) | Latent Heat of Melting (J/g) | Melting Point (°C) | Latent Heat of Melting (J/g) | Latent Heat Reduction (%) | |||
| 1. | PCM: Myristic acid Supporting material: Nitrile rubber latex Method: Dip coating. | 56.16 ± 0.08 | 210.02 ± 2.46 | 54.60 ± 0.08 | 142.30 ± 1.38 J/g | 32.24 ± 0.11 | Current study |
| 2. | PCM: Palmitic acid Supporting material: Pinecone biochar Method: Vacuum impregnation. | 62.07 | 219.63 | 59.25 | 84.74 | 61.42 | [59] |
| 3. | PCM: Myristic acid Supporting material: Superoleophilic graphene-nickel foam Method: Direct impregnation. | 55.8 | 207.69 | 59.8 | 102.54 | 50.63 | [60] |
| 4. | PCM: Stearic acid Supporting material: Poly(methyl methacrylate) and methacrylic acid Method: Emulsion polymerization. | 68–70 | 189 | 66–86 | 73.5 | 61.11 | [61] |
| 5. | PCM: Palmitic acid Supporting material: Bio-based polylactic acid Method: Solvent evaporation. | 62.8 | 167.3 | 62.1 | 70.1 | 58.10 | [62] |
| No | PCM Type and System | Electrical Performances (Power Generation Efficiency) | Thermal Performance (Temperature Reduction) | Reference |
|---|---|---|---|---|
| 1 | MA coated with NBR (current study) molded into tablets and fixed at the rear part of PV module. | Increase by 5.54% | 17 °C. | NA |
| 2 | OM-29 | Increase by 2% | 10.35 °C | [63] |
| 3 | Paraffin wax in container with coil heat exchanger. | Increase by 5.18% | 23 °C | [64] |
| 4 | Paraffin wax poured between stainless steel insert and PV panel rear part. The system also cooled using additional mist spray on the front surface of PV. | Increase by 6.61% | NA | [65] |
| 5 | Nano-enhanced PCM with at 0.5 wt% nanofillers concentration. Assembled together with heat pipes. The PCM used is organic-type paraffin wax (PT58). | Increase by 7.6% | 9.74 °C | [66] |
| 6 | Sheep fat novel PCM with CuO nanoparticles. Copper microchannel tubes are also inserted at the rear part of PV module. Water flows through this channel to extract heat from PCM. Active system. | Increase by 26.2% | 13.3 °C | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldawood, F.K.; Munusamy, Y.; Kchaou, M.; Alquraish, M. Effect of Elastomeric Coating on the Properties and Performance of Myristic Acid (MA) Phase Change Material (PCM) Used for Photovoltaic Cooling. Coatings 2023, 13, 1606. https://doi.org/10.3390/coatings13091606
Aldawood FK, Munusamy Y, Kchaou M, Alquraish M. Effect of Elastomeric Coating on the Properties and Performance of Myristic Acid (MA) Phase Change Material (PCM) Used for Photovoltaic Cooling. Coatings. 2023; 13(9):1606. https://doi.org/10.3390/coatings13091606
Chicago/Turabian StyleAldawood, Faisal Khaled, Yamuna Munusamy, Mohamed Kchaou, and Mohammad Alquraish. 2023. "Effect of Elastomeric Coating on the Properties and Performance of Myristic Acid (MA) Phase Change Material (PCM) Used for Photovoltaic Cooling" Coatings 13, no. 9: 1606. https://doi.org/10.3390/coatings13091606
APA StyleAldawood, F. K., Munusamy, Y., Kchaou, M., & Alquraish, M. (2023). Effect of Elastomeric Coating on the Properties and Performance of Myristic Acid (MA) Phase Change Material (PCM) Used for Photovoltaic Cooling. Coatings, 13(9), 1606. https://doi.org/10.3390/coatings13091606







