Abstract
The millimeter-scale capsules for oil encapsulation have been used successfully in food, cosmetic and pharmaceutical engineering. However, aqueous core capsules still have poor barrier properties and a short storage life. In this work, a rapid and mild coating method was applied on millimeter-scale calcium alginate aqueous core capsules (mm-CaSA-Caps). A layer of poly(ethyl-cyanoacrylate) (PECA) was coated on the surface of the mm-CaSA-Caps using interfacial polymerization. The PECA-coated mm-CaSA-Caps (PECA@CaSA-Caps) prepared in the trichloromethane maintained a spherical shape in wet and dry conditions. Compared with the mm-CaSA-Caps, the weight loss ratio of water in the PECA@CaSA-Caps decreased by 38.59%. The release rates of dye in PECA@CaSA-Caps greatly reduced in neutral (pH 7.0), alkaline (pH 10.0) and acidic (pH 1.0) conditions. This work provides a new way to improve the barrier properties and storage life of hydrogel capsules.
1. Introduction
The fish-egg-like capsules with a liquid core on the millimeter scale (mm-Caps), because of their simple setup, high encapsulation efficiency and loading capacity, have the most successful applications in food, nutrition, cosmetic and agriculture, such as in commercialized vitamin capsules, fish oil capsules, essence capsules and the popular Boba tea [1]. With the demand for health and safety, biomass materials, such as gelatin, xanthan gum, alginate, chitosan, cellulose and its derivatives, which have a high gelling capacity, biocompatibility and low toxicity and are better choices as barrier materials, are used for oil encapsulation [2]. But encapsulation and storage of the water-based materials are still a challenge due to their good water-solubility and water-swelling properties even after crosslinking [3]. The aqueous core mm-caps can only be stored in liquid media like tapioca balls, or they will collapse in the air because of water evaporation from the hydrophilic semipermeable capsule membrane [4].
An aqueous core encapsulated in calcium alginate (CaSA) membrane with the extrusion method, which is characterized by its biomass material and mild preparation conditions, is beneficial to the stability of bioactive products [5,6]. Because CaSA only slightly dissolves in acidic gastric juices and rapidly degrades in alkaline intestinal juices, it can be used as an intestinal delivery agent by taking advantage of its pH-selective controlled release properties [7]. In addition, the degradation rate of CaSA is significantly accelerated at 60 °C. Using its temperature sensitivity, CaSA degrades, resulting in the slow release of the drug, which can be used for photothermal therapy and controlled chemotherapy of tumors [8]. However, due to its pH and temperature sensitivity [9], strong and long-term chemical reactions will lead to the degradation and collapse of CaSA. Therefore, a lot of research studies have reported mild modification methods to improve the barrier properties of CaSA-based mm-capsules, such as physical blending [10,11], crosslinking [12], interfacial precipitation and layer-by-layer assembly technique [13,14,15].
The poly(alkylcyanoacrylates) are a family of biocompatible and biodegradable polymers which have been used to prepare particles and capsules for drug delivery [16,17]. Poly(alkylcyanoacrylates) particles and capsules can be prepared through emulsion polymerization, interfacial polymerization and emulsifier-free emulsion polymerization by the polymerization of alkylcyanoacrylates monomers [18]. Among them, ethyl-cyanoacrylate (ECA) with strong reactivity displays a remarkable tendency to polymerize in the presence of weak bases of water, alcohol, amino acid and anions of I−, CH3COO−, Br− and OH− at ambient temperatures, which has been successfully used in pharmaceutical carriers and tissue adhesive [19,20,21,22]. Tuncel et al. [22] synthesized a monosize, biodegradable poly(ethylcyanoacry1ate) (PECA) microparticle using dispersion polymerization. Fu et al. [21] prepared a phase change microcapsule based on sodium thiosulfate pentahydrate as the core and PECA as the shell using interfacial polymerization in a water-in-oil emulsion system. Due to its fast reactivity and the mild conditions, ECA was also used as a surface modifier to package liquid marble using moisture curing. The PECA-coated liquid marble had good water vapor barrier properties and mechanical properties [23]. However, the moisture-curing method was not suitable for the industrial production of the capsules.
In order to control the releasing properties and increase the stability of aqueous core mm-Caps, here, ECA was initiated as the coating agent with trace water on the surface of millimeter-scale calcium alginate aqueous core capsules (mm-CaSA-Caps), which was in-situ reacted at the interface of the mm-CaSA-Caps and the chloromethane with interfacial polymerization. The effects of the type of chloromethane, the amount of ECA and the reaction time on the morphology, size, sphericity and controlled releasing properties of the PECA-coated mm-CaSA-Caps (PECA@CaSA-Caps) are discussed. The synthesis method is convenient and efficient and provides a general approach to modify the hydrogel capsules for industrial production.
2. Experimental Section
2.1. Materials
Sodium alginate (SA, 10 g·L−1, 20 °C, >0.02 Pa·s) was dissolved in deionized water for a gelation bath. Sodium carboxymethyl cellulose (CMC, 20 g·L−1, 25 °C, 800 mPa·s–1200 mPa·s) was used as a non-gelling thickener agent to increase the viscosity of the aqueous core solution. Calcium chloride (CaCl2), dichloromethane, trichloromethane, tetrachloromethane, sodium hydroxide (NaOH, 0.0001 mol·L−1) and hydrochloric acid (HCl, 0.1 mol·L−1) of analytic grade were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Ethyl-cyanoacrylate (ECA) was purchased from Shenzhen Fu kang glue Co., Ltd., Shenzhen, China. Reactive Red X-3B was purchased from Nanjing Dulai Bio-technology Co., Ltd., Nanjing, China.
2.2. Preparation of mm-CaSA-Caps
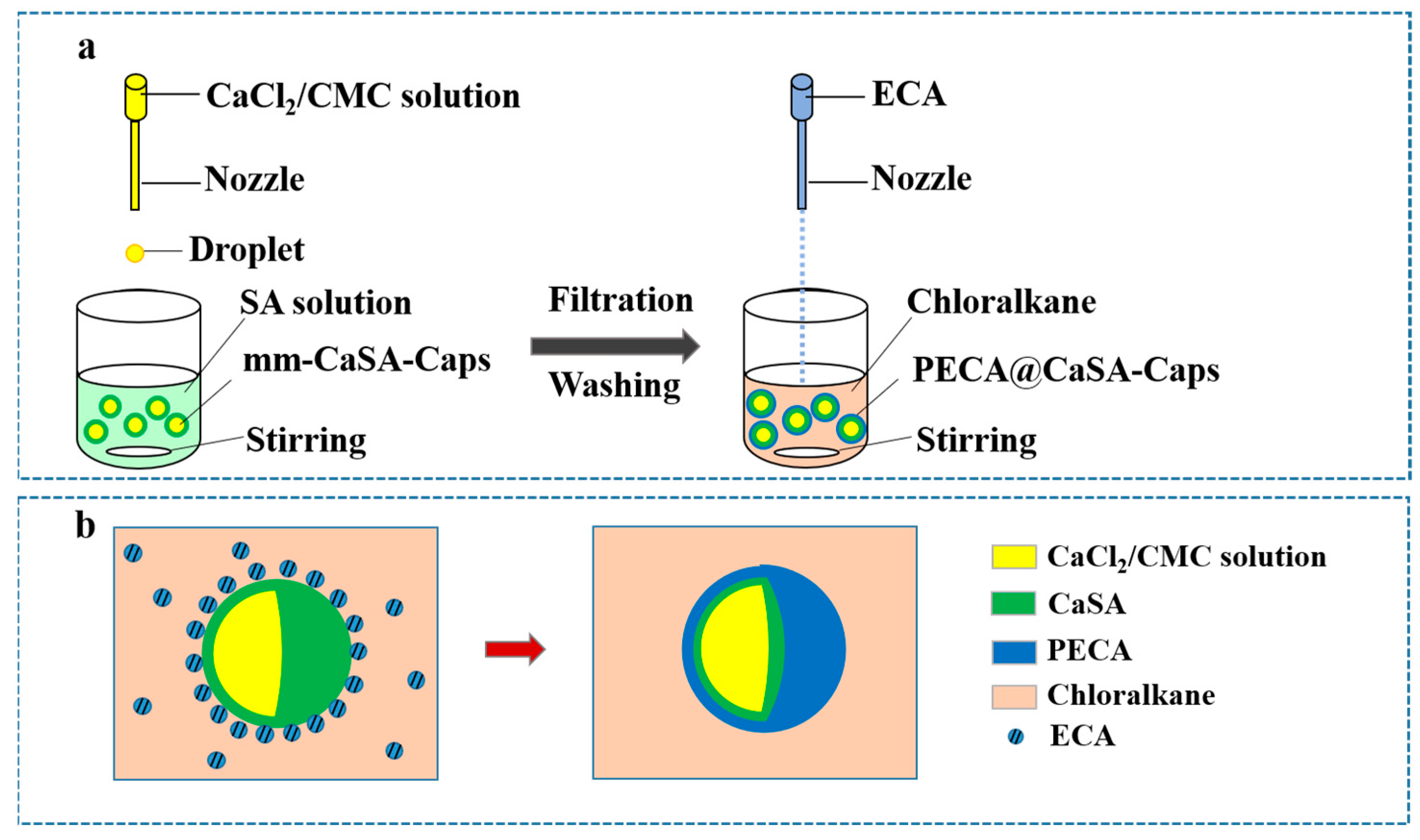
The mm-CaSA-Caps were prepared with an inverse extrusion dripping method according to our previous work [5], as shown in Figure 1a. In detail, 2.01 g SA was added to 400 mL deionized water, swelled at room temperature for 4 h, mechanically stirred at 55 °C for 6 h, then cooled to ambient temperature and stood for 24 h for defoaming; the SA solution (0.5%) was obtained. An amount of 6.091 g CMC was added to 400 mL deionized water, swelled at room temperature for 4 h, mechanically stirred at 90 °C for 6 h, then cooled to ambient temperature and stood for 24 h for defoaming; the CMC solution (1.5%) was obtained. A total of 4.04 g CaCl2 was dissolved in the 1.5% CMC solution. The 1.5% CMC/1.0% CaCl2 mixture was obtained. The mixture was loaded into a plastic syringe (20 mL), pumped dropwise with a flow rate of 0.5 mL·min−1 with a dripping nozzle into the gelation bath containing the SA solution (0.5%, 200 mL). The inner and outer diameters of the nozzle were 0.41 mm and 0.71 mm, respectively. The distance between the dripping nozzle and the surface of the SA solution was fixed at 10 cm. After a 10 min dripping process, the solution was stirred for 20 min, and the obtained capsules were washed with deionized water three times. Then, an additional crosslinking process for the capsules was conducted for 15 min in a CaCl2 solution (2.0%). Finally, the obtained mm-CaSA-Caps were stored in deionized water before modification.
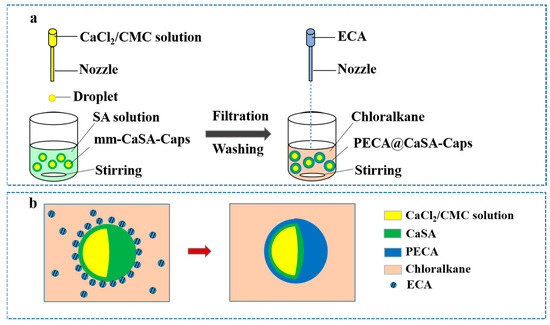
Figure 1.
(a) Preparation of PECA@CaSA-Caps. (b) The schematic of the reaction process.
2.3. Preparation of PECA@CaSA-Caps
The mm-CaSA-Caps were coated with the PECA as shown in Figure 1a. In detail, 100 mm-CaSA-Caps were randomly selected and added to 50 mL chloromethane using magnetic stirring at 650 rpm-750 rpm. A certain amount of ECA was loaded into a plastic syringe (20 mL) and vertically dripped into the abovementioned mixture by an injection pump with a flow rate of 0.5 mL·min−1. The distance between the dripping nozzle and the surface of chloromethane was fixed at 10 cm. The dripping nozzle had an internal and external diameter of 0.41/0.71 mm. During the reaction process, the ECA which was dissolved in chloromethane was initiated with the water on the surface of the mm-CaSA-Caps as shown in Figure 1b. The PECA coating on the mm-CaSA-Caps was formed continuously with in-situ polymerization. After a certain reaction time, the PECA@CaSA-Caps were obtained.
In single-factor experiments, the chloromethanes were dichloromethane, trichloromethane and tetrachloromethane. The amounts of ECA were 0, 1 mL, 2 mL, 3 mL, 4 mL, 5 mL and 6 mL. The reaction times were 20 min, 30 min, 60 min and 120 min.
2.4. Characterizations
The digital images of the capsules in air and water were captured with a digital camera (BL-SM500, Jinhua Oushilang Trade Co., Ltd., Jinhua, China). The diameter of the capsule was measured according to the digital image. The data were the average of 20 measurements. The surface and cross-section morphologies of capsules were observed with a scanning electron microscope (SEM, JSM-IT300, JEOL Ltd., Tokyo, Japan). The capsules were selected randomly and vacuum freeze-dried. The cross-section of the capsule was prepared by breaking the freeze-dried sample and taking the slices.
The sphericity factor (SF) of the capsule was calculated according to Equation (1) [24],
where Dmax is the longest diameter length, and Dmin is the shortest diameter length perpendicular to Dmax. The capsule was considered spherical when SF < 0.07. To determine SF, 15 capsules for each condition were randomly chosen.
The water barrier property of the capsule was measured using analytical balance (ME104E, Mettler Toledo, Greifensee, Switzerland). The weight change of water in the capsules was calculated using Equation (2). Twenty capsules were randomly selected and placed in the oven at 60 °C.
where m0 is the initial weight, m1 is the dry weight, mn is the weight measured at n minute.
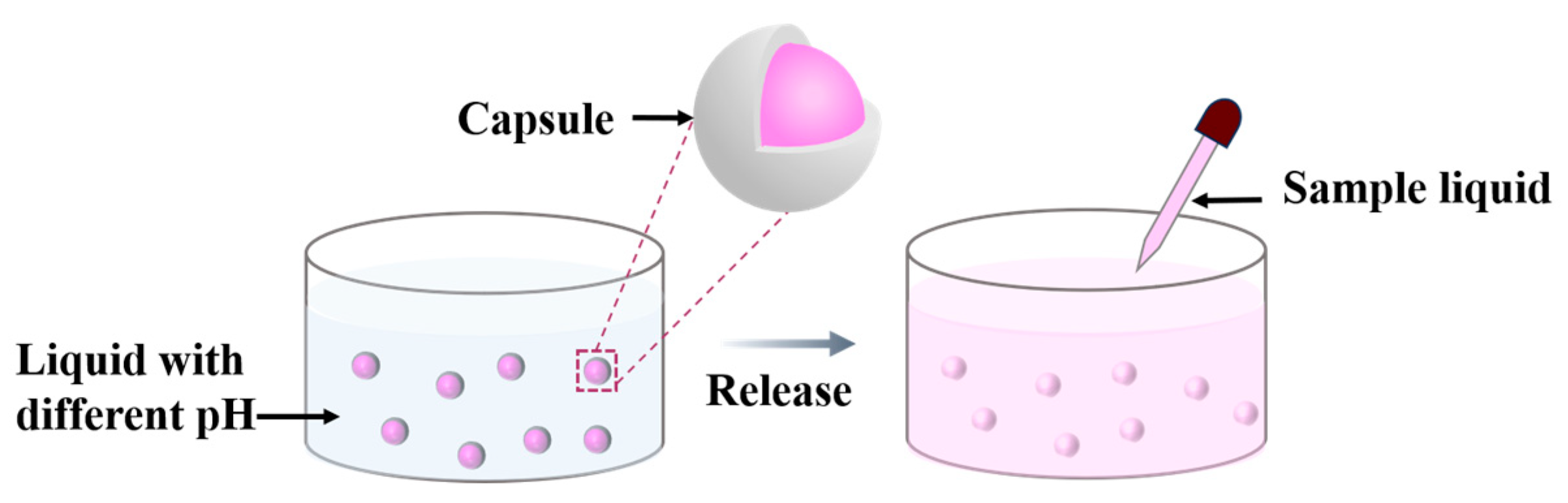
Simulated experiments to test the controlled release characteristics of the capsules at different pH were performed as shown in Figure 2. In detail, the preparation of the mm-CaSA-Caps with an aqueous core containing red dye was performed as written in Section 2.2. An amount of 2.5 g∙L−1 Reactive Red X-3B was added to the SA solution. The PECA@CaSA-Caps were prepared as written in Section 2.3 with 6 mL ECA in trichloromethane and a reaction time of 60 min. Then 20 of the prepared capsules were randomly selected and their surface water was wiped with filter paper. Then the capsules were added to 500 mL of liquid under magnetic stirring. An amount of 5 mL of sample liquid was taken in a cuvette every 5 min, and the absorbance of the sample liquid was measured with a UV-Vis spectrophotometer (756PC, Tianjin Tuopu Instrument Co., Ltd., Tianjin, China) at 540 nm (the maximum wavelength of absorption of the Reactive Red X-3B). Three kinds of liquid, deionized water, 0.1 mol·L−1 HCl and 0.0001 mol·L−1 NaOH, were used, the pH of which were 7.0, 1.0 and 10.0, respectively. The concentration of the liquid containing the Reactive Red X-3B (c) was calculated according to Equation (3).
where A is the absorbance, a is the absorption coefficient and b is the distance of the test light traveled in solution. In the experiment, a and b were identical; thus c is proportional to A.
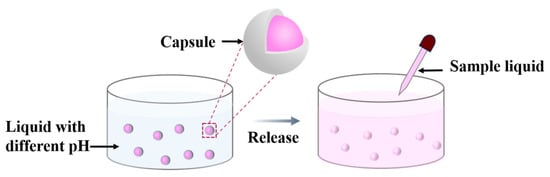
Figure 2.
Tests of the controlled release characteristics of the capsules at different pH.
3. Results and Discussion
In anionic polymerization, ECA is very active for its strong electronic drawing group and can be initiated with weak initiators such as water and alcoholate at room temperature without additives [25]. In this work, the mm-CaSA-Caps were dispersed in the chloromethane which were insoluble or nearly insoluble in water but could dissolve the ECA. The ECA was initiated with the water on the surface of mm-CaSA-Caps and in-situ polymerized at the interface of mm-CaSA-Caps and the chloromethane.
3.1. Effect of the Reaction Time
The effect of the reaction time on the morphology of PECA@CaSA-Caps is shown in Table 1. In Table 1 Group a, with a prolonged reaction time, the capsules change from colorless and transparent to translucent at 60 min and white at 120 min. The anionic polymerization of ECA is initiated by the water on the surface of the mm-CaSA-Caps. And a uniform and white PECA coating is formed on the surface of mm-CaSA-Caps. The changes in capsule morphology are more obvious in water (Table 1 Group b). Compared with the mm-CaSA-Caps, the light transmittance of PECA@CaSA-Caps decreases as the reaction time increases. Finally, the outline of the membrane disappears due to the growth of the PECA coating. As the reaction lasted for 120 min, the capsule remained spherical and intact, indicating that the reaction condition was mild and did not cause the decomposition of the mm-CaSA-Caps. In Table 1 Group c, after dehydration at 60 °C, the PECA@CaSA-Caps prepared with different times all collapse. However, the PECA coating does not separate from the surface of the mm-CaSA-Caps, indicating that the in-situ polymerized PECA coating had a good binding force with the membrane of the capsules. In general, PECA@CaSA-Caps with an obvious PECA coating and good sphericity can be successfully prepared with the interfacial polymerization of ECA.

Table 1.
Appearances of the PECA@CaSA-Caps with different reaction times. Group a, the prepared PECA@CaSA-Caps at ambient temperature; Group b, the mm-CaSA-Caps (left) and the prepared PECA@CaSA-Caps (right) in water; Group c, the PECA@CaSA-Caps after dehydration at 60 °C. (The amount of ECA was 1 mL. The solvent is trichloromethane).
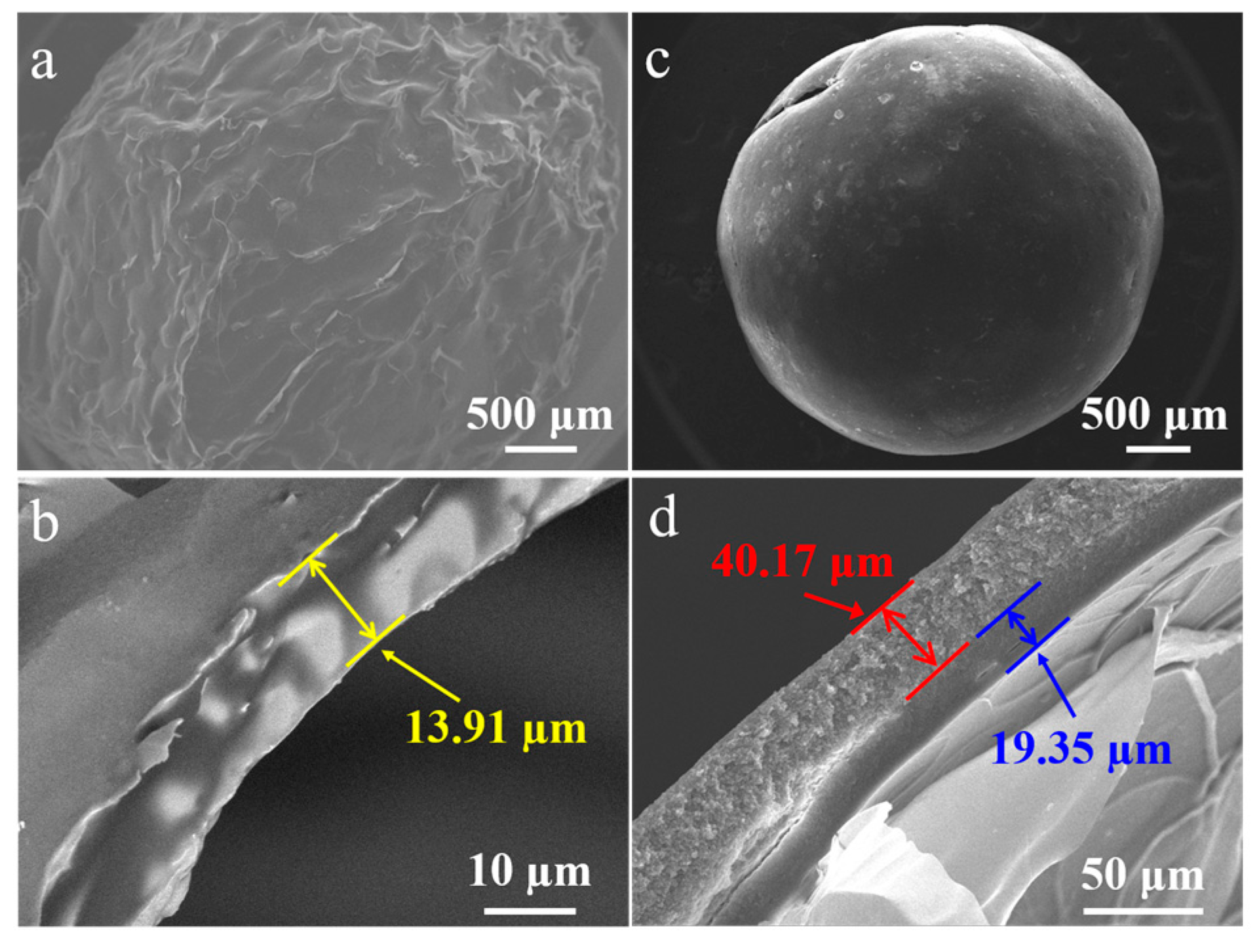
Figure 3 demonstrates the morphology of mm-CaSA-Caps and PECA@CaSA-Caps. After vacuum freeze-drying, the mm-CaSA-Caps present a wrinkled surface ascribed to freezing and expanding in the cold, then thawing and shrinking (Figure 3a). The thickness of the membrane of the mm-CaSA-Caps is 13.91 μm (Figure 3b). After coating with PECA, the structure of the PECA@CaSA-Caps is stable and remains spherical during vacuum freeze-drying (Figure 3c). In Figure 3d, the PECA layer which covers the membrane of the mm-CaSA-Caps tightly is 40.17 μm. The thickness of the membrane of the mm-CaSA-Caps in Figure 3d is 19.35 μm, which is thicker than that in Figure 3b, because the inner CaSA layer protected by the PECA coating is unexpanded.
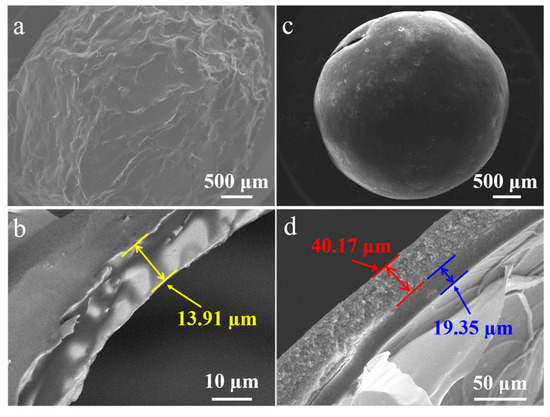
Figure 3.
SEM images of (a) the mm-CaSA-Caps and (b) its cross-section and (c) the PECA@CaSA-Caps and (d) its cross-section prepared in 120 min.
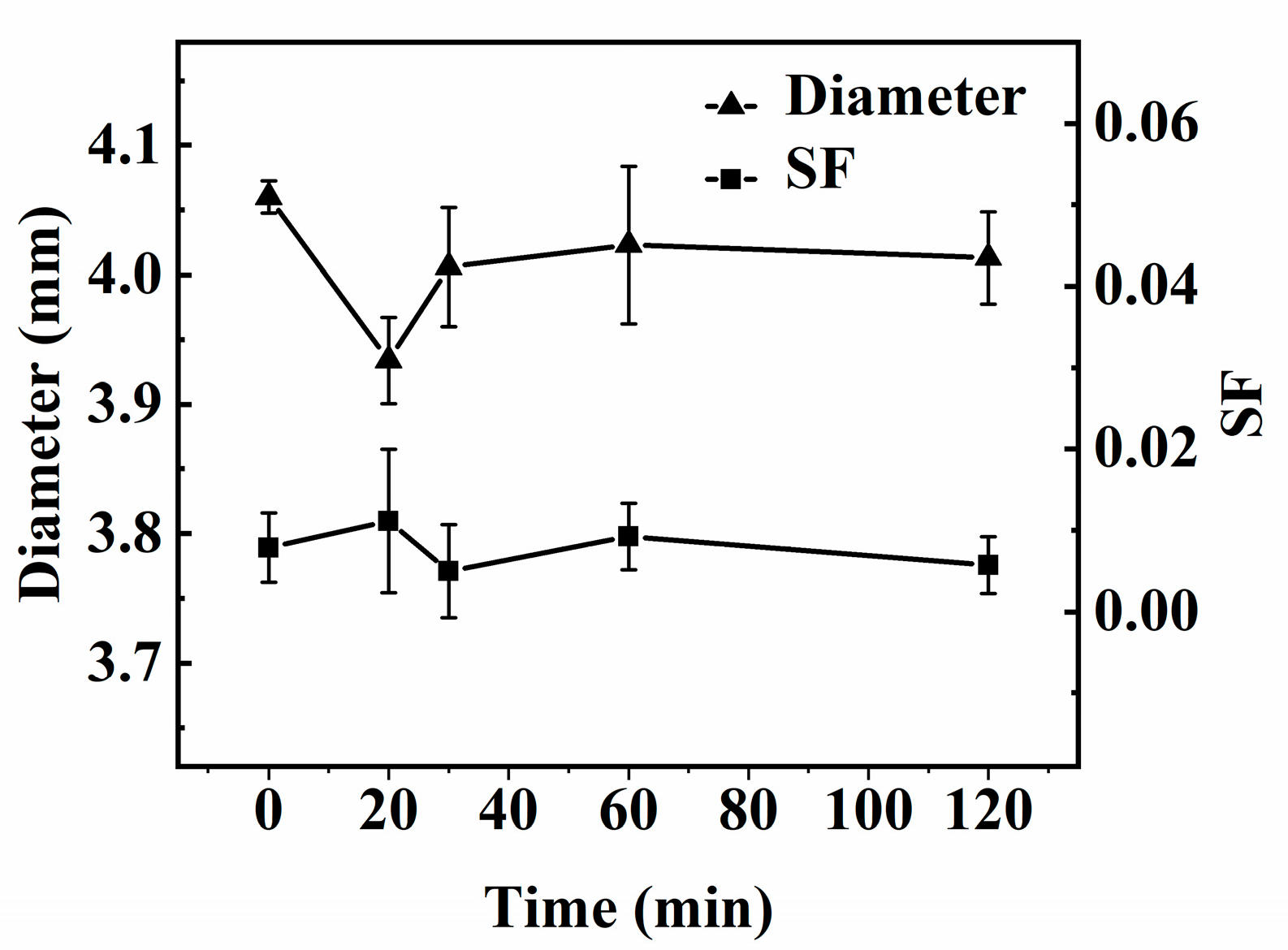
The diameter of the capsule is shown in Figure 4. The diameter of the mm-CaSA-Caps is 4.06 mm. When the interfacial polymerization occurs, the diameter of the PECA@CaSA-Caps decreases to 3.93 mm at 20 min, then increases gradually to 4.02 mm at 60 min. The diameter of the PECA@CaSA-Caps is 4.01 mm at 120 mm, which is still lower than the diameter of the mm-CaSA-Caps. Since the ECA monomer dissolved in trichloromethane undergoing the anionic polymerization reaction was initialized by the water in the surface of mm-CaSA-Caps, part of the bond water diffuses outward from the membrane of mm-CaSA-Caps. Then the mm-CaSA-Caps undergo a certain degree of shrinkage in volume, leading to a reduction in the diameter of the capsule. With the increase in reaction time, the diameter of the capsule increases, because the PECA coating grows constantly on the surface of mm-CaSA-Caps. The sphericity of the capsule is also shown in Figure 4. The SF value of the mm-CaSA-Caps is 0.008. The SF values of PECA@CaSA-Caps that are between 0.005 and 0.011 are also much less than 0.07. That is, under mild reaction conditions, the PECA@CaSA-Caps maintain an intact structure and a good spherical shape. It indicates that the rapid and mild coating process has no obvious effect on the sphericity of the capsule, which is consistent with the digital photos of the capsules in Table 1.
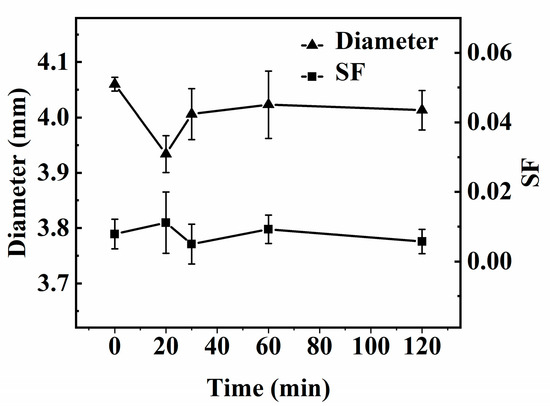
Figure 4.
Effect of the reaction time on the diameter and SF of capsules.
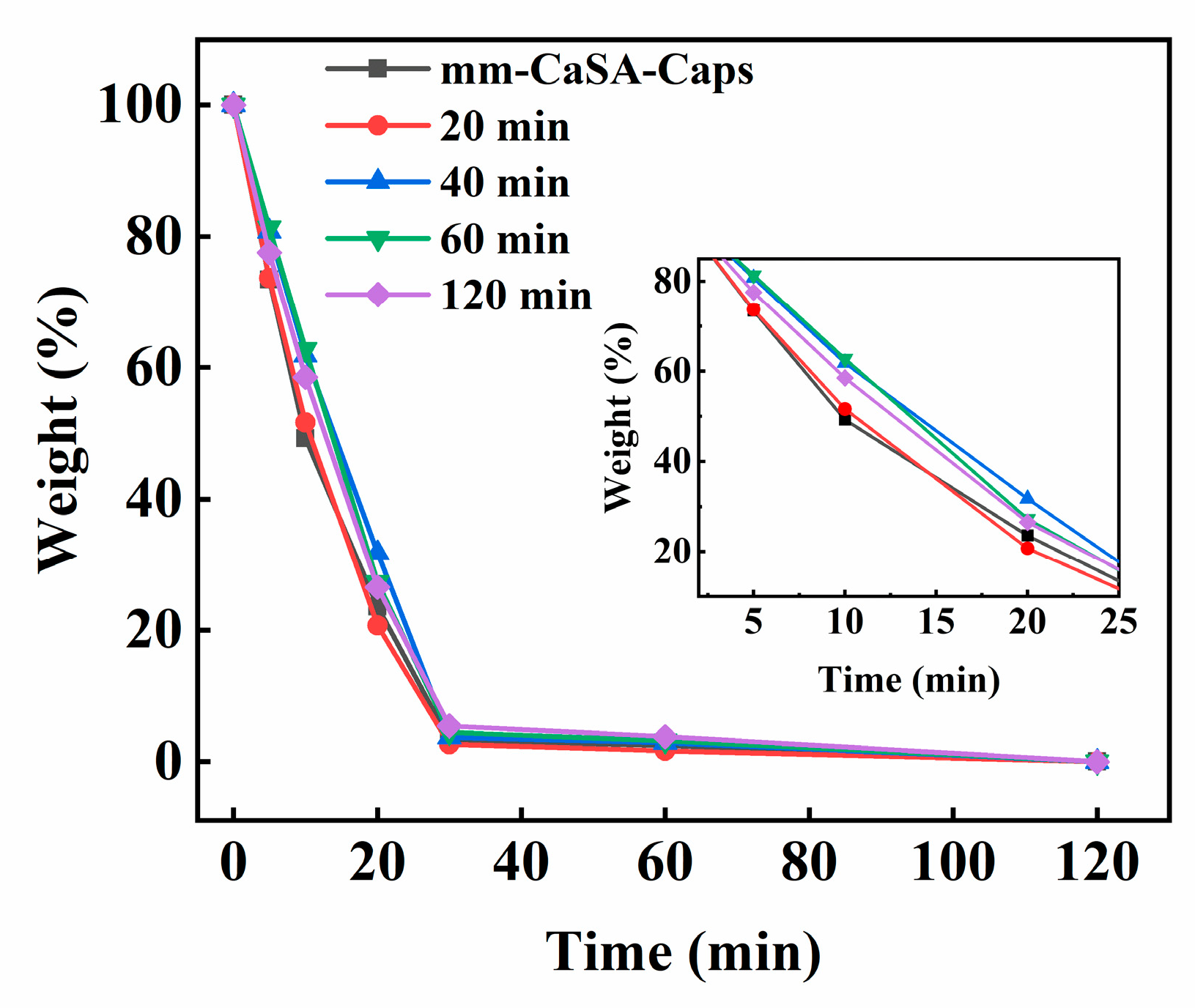
Figure 5 shows the change in water weight in the capsules tested at 60 °C. As the test time increases, the water weight ratio in all capsules decreases until it becomes zero at 120 min. At 10 min, the water weight ratio of mm-CaSA-Caps and PECA@CaSA-Caps prepared in 20 min, 40 min, 60 min and 120 min are 49.3%, 51.6%, 61.8%, 62.8% and 58.5%, respectively. Compared with the mm-CaSA-Caps, the water weight ratio of PECA@CaSA-Caps prepared in 20 min shows little difference. The water weight ratio of PECA@CaSA-Caps prepared in 40 min, 60 min and 120 min decreases by 12.5%, 13.5% and 9.2%, respectively. Since the Ca-SA membrane is a hydrophilic and semipermeable membrane, the mm-CaSA-Caps cannot effectively control the release of water. As the PECA coating forms on the mm-CaSA-Caps, it retards the permeation of water. When the reaction time is 20 min, the coated PECA layer on the surface of mm-CaSA-Caps is thin, which makes it difficult to achieve the ideal barrier effect. As the reaction time increases, anionic polymerization of the ECA constantly occurs on the surface of capsules, and the produced PECA coating becomes thicker; thus the permeation of water from the PECA@CaSA-Caps decreases. The water weight ratio of PECA@CaSA-Caps prepared in 40 min, 60 min and 120 min obviously increases. It indicates that the PECA coating can effectively improve the barrier property of mm-CaSA-Caps.
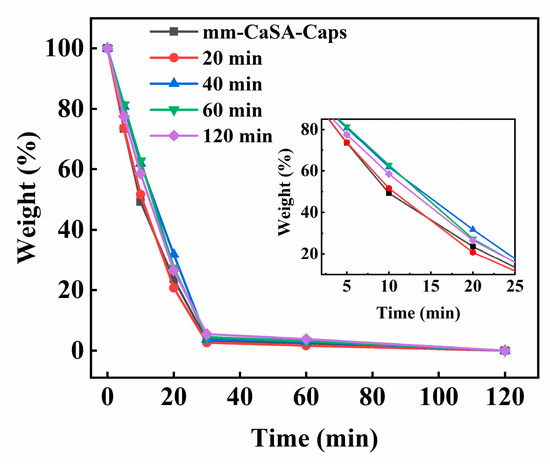
Figure 5.
Effect of reaction time on the weight change of water in the capsules. The inset shows the difference in the weight change of water in the capsules from 2 min to 25 min.
3.2. Effect of the Amount of ECA
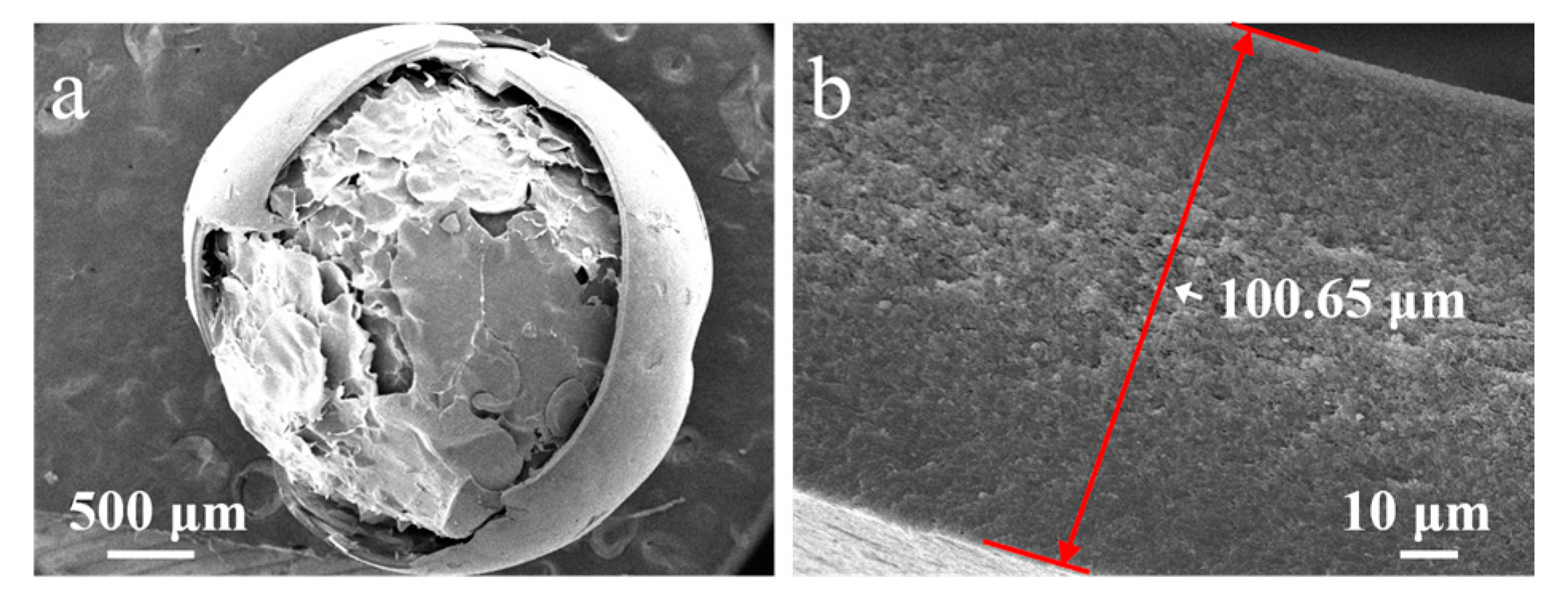
The effect of the ECA amount on the morphology of PECA@CaSA-Caps is discussed. As shown in Table 2 Group a, a uniform and white PECA layer is formed on the surface of mm-CaSA-Caps. As the amount of ECA increased, the PECA@CaSA-Caps gradually changed from translucent white to opaque white. The color of the PECA@CaSA-Caps displayed a deepened white when the ECA was 6 mL. As shown in Table 2 Group b, compared with the mm-CaSA-Caps, the PECA@CaSA-Caps prepared with different amounts of ECA maintain good sphericity. As the amount of ECA is above 2 mL, the PECA@CaSA-Caps do not transfer the backlight, indicating that the thickness of the PECA coating increases with the increase in the amount of ECA. In Table 2 Group c, the PECA@CaSA-Caps collapse after dehydration as the amount of ECA is less than 6 mL. When the ECA is 6 mL, there is a small dent in the shell of the PECA@CaSA-Caps. The structure of PECA@CaSA-Caps does not damage since the mm-CaSA-Caps are coated with a thick layer of PECA coating (Figure 6a). The thickness of the PECA layer increases from 40.17 µm (Figure 3d) to 100.65 µm (Figure 6b) as the amount of ECA increases from 1 mL to 6 mL, allowing the PECA@CaSA-Caps to maintain its spherical appearance after dehydration. This advantage ensures that the appearance of the capsules does not change during use.

Table 2.
Appearance of the PECA@CaSA-Caps with different amounts of ECA. Group a, the prepared PECA@CaSA-Caps at ambient temperature; Group b, the mm-CaSA-Caps (left) and the prepared PECA@CaSA-Caps (right) in water; Group c, the PECA@CaSA-Caps after dehydration at 60 °C. (The reaction time is 60 min. The solvent is trichloromethane).
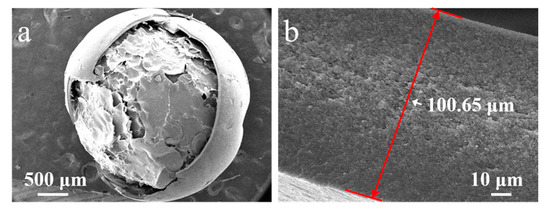
Figure 6.
SEM images of (a) the PECA@CaSA-Caps and (b) its cross-section prepared with 6 mL ECA.
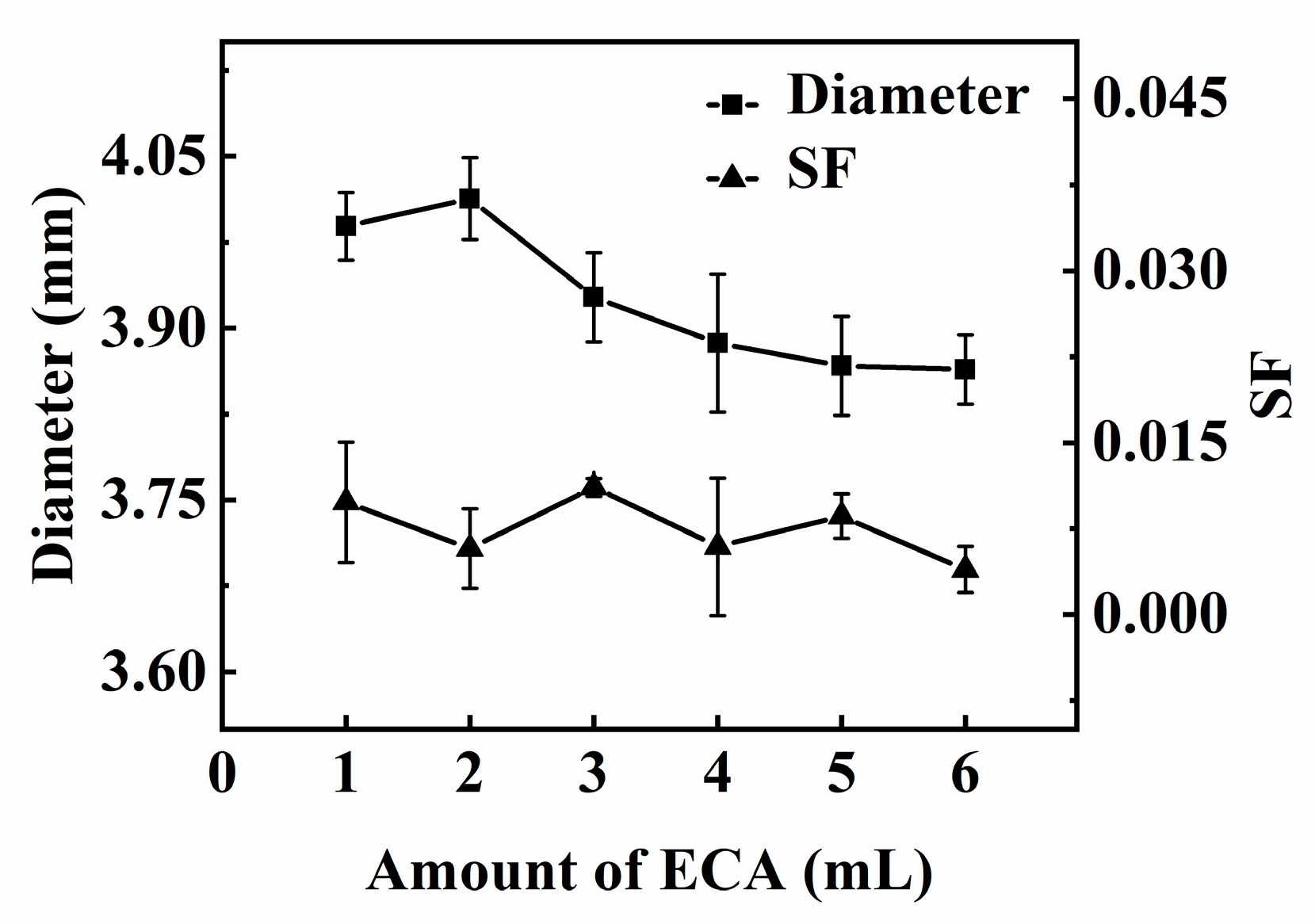
In Figure 7, the effect of the amount of ECA on the diameter and sphericity of PECA@CaSA-Caps is discussed. As the amount of ECA increases from 1 mL to 2 mL, the diameter of the PECA@CaSA-Caps increases slightly from 3.99 mm to 4.01 mm, then decreases gradually to 3.86 mm when the amount of ECA increases to 6 mL. On the one hand, the thickness of the PECA coating increases as the increase in the amount of ECA leads to a rise in the diameter of the PECA@CaSA-Caps. On the other hand, water on the surface and on the inside of the mm-CaSA-Caps diffuses outward and initiates the anionic polymerization of ECA monomers, resulting in the shrinkage of the mm-CaSA-Caps. The result is that the latter generally gets the upper hand. Finally, the diameter of the PECA@CaSA-Caps decreases with the increase in the amount of ECA. The SF of the PECA@CaSA-Caps prepared with different amounts of ECA are also demonstrated in Figure 7. The SF of PECA@CaSA-Caps is between 0.004 and 0.011, which is far below 0.07. That is to say, the PECA@CaSA-Caps keep good sphericity and the coating process has no obvious effect on the sphericity of the capsules, which is consistent with the photos in Table 2.
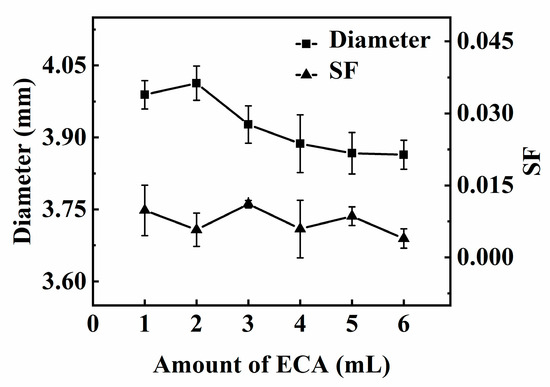
Figure 7.
Effect of the amount of ECA on the diameter and SF of capsules.
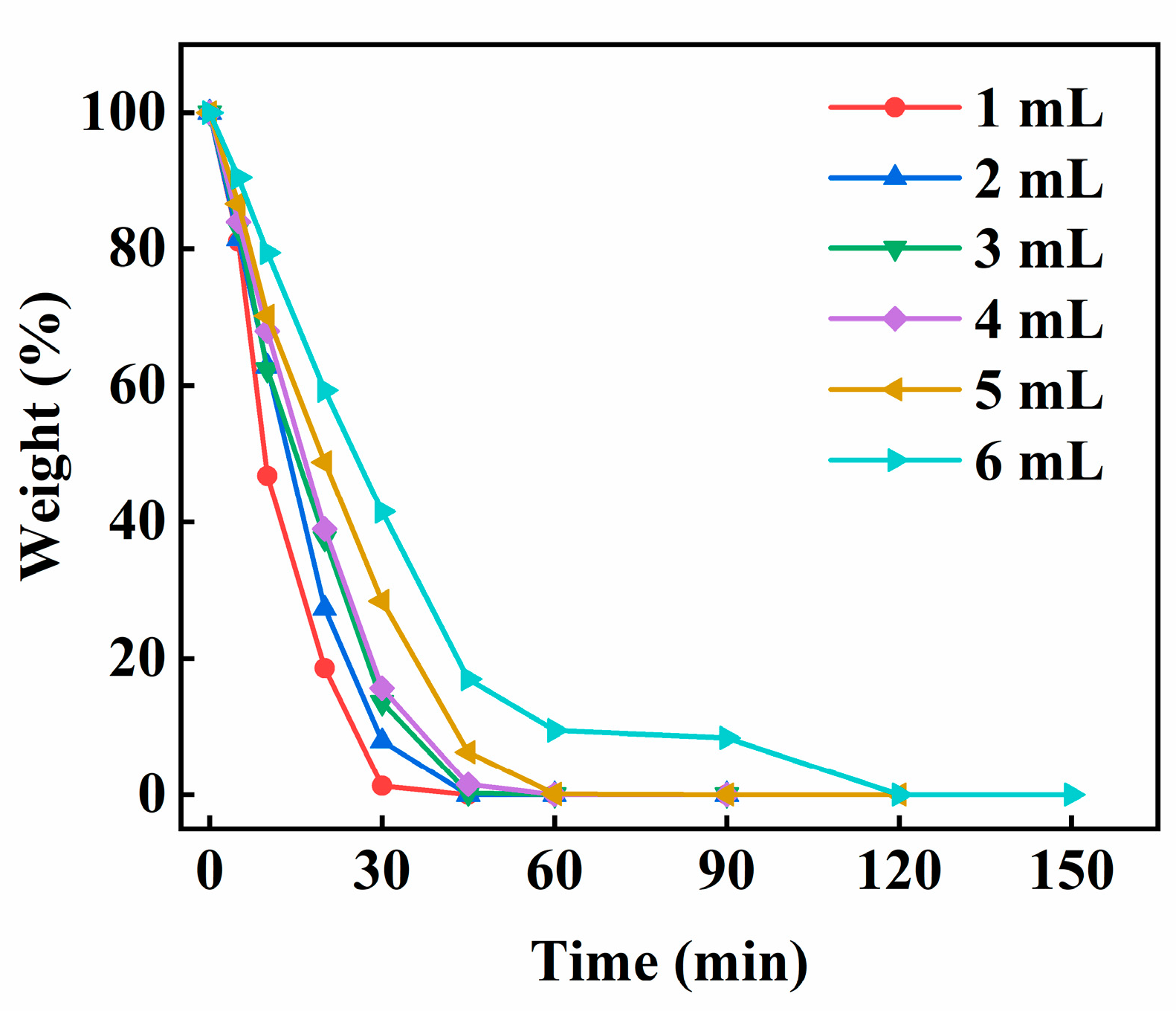
Figure 8 shows the effect of the amount of ECA on the weight change of water in the PECA@CaSA-Caps. As the amount of ECA increases, the weight loss ratio of water in the PECA@CaSA-Caps decreases. At 30 min, the weights of the water in the PECA@CaSA-Caps are 1.28%, 7.93%, 13.57%, 15.65%, 28.4% and 41.5%, respectively, when the amounts of ECA are 1 mL, 2 mL, 3 mL, 4 mL, 5 mL and 6 mL. The reason is that the larger the amount of ECA, the thicker the PECA layer that forms, resulting in its stronger ability to block the permeation of water. When the amount of ECA is 6 mL, the water in PECA@CaSA-Caps releases completely within 120 min, which is nearly three times the releasing time of PECA@CaSA-Caps prepared with 1 mL ECA. In general, the PECA@CaSA-Caps can be successfully prepared with different amounts of ECA. The prepared capsules have good sphericity. When the amount of ECA is 6 mL, the PECA@CaSA-Caps have the best water retention performance.
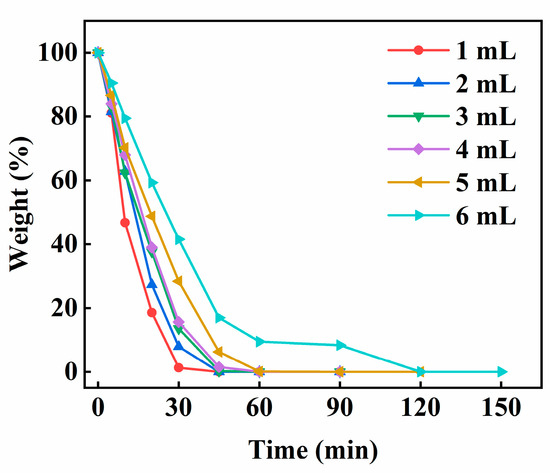
Figure 8.
Effect of the amount of ECA on the weight change of water in the PECA@CaSA-Caps.
3.3. Effect of the Type of Solvent
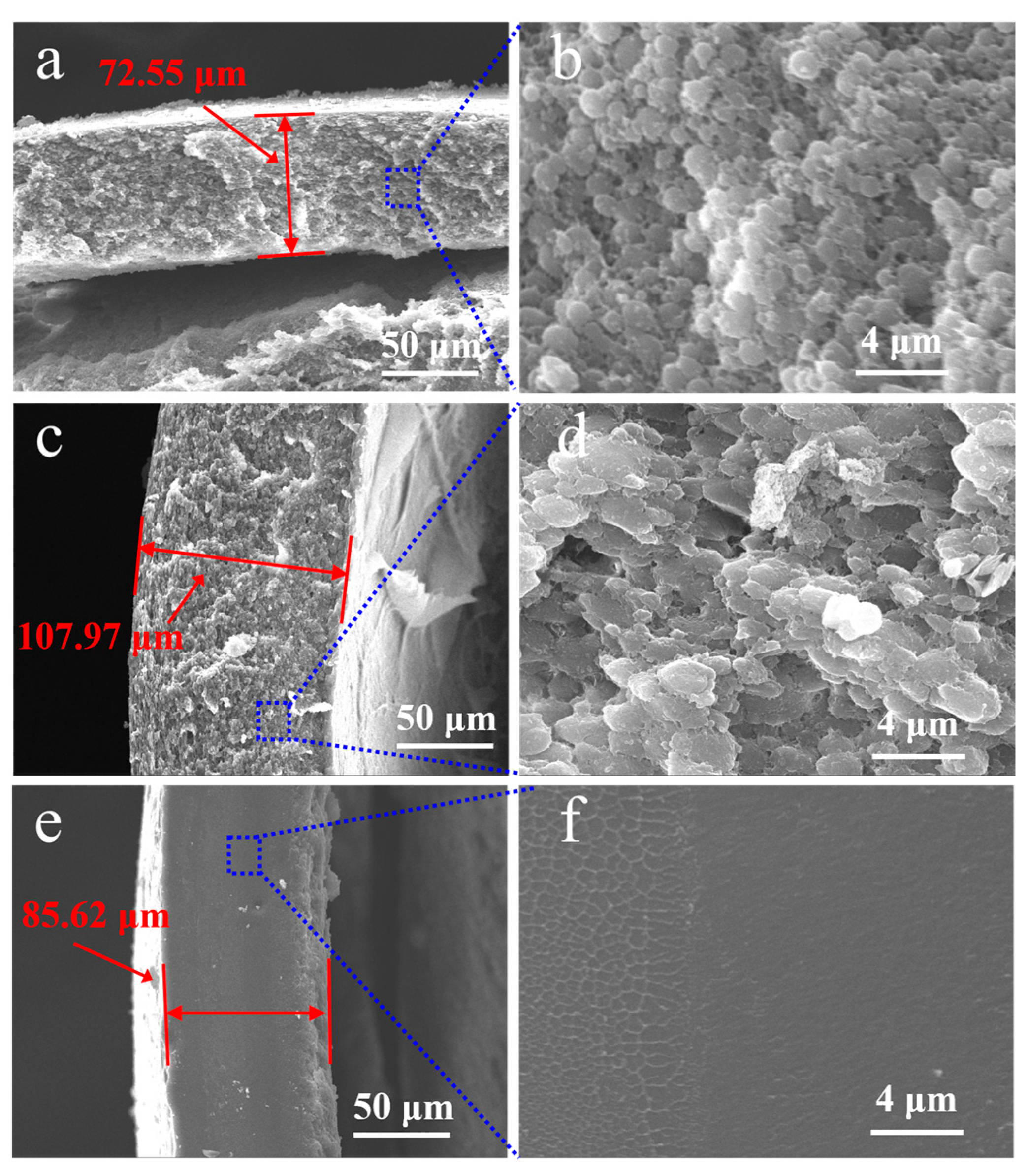
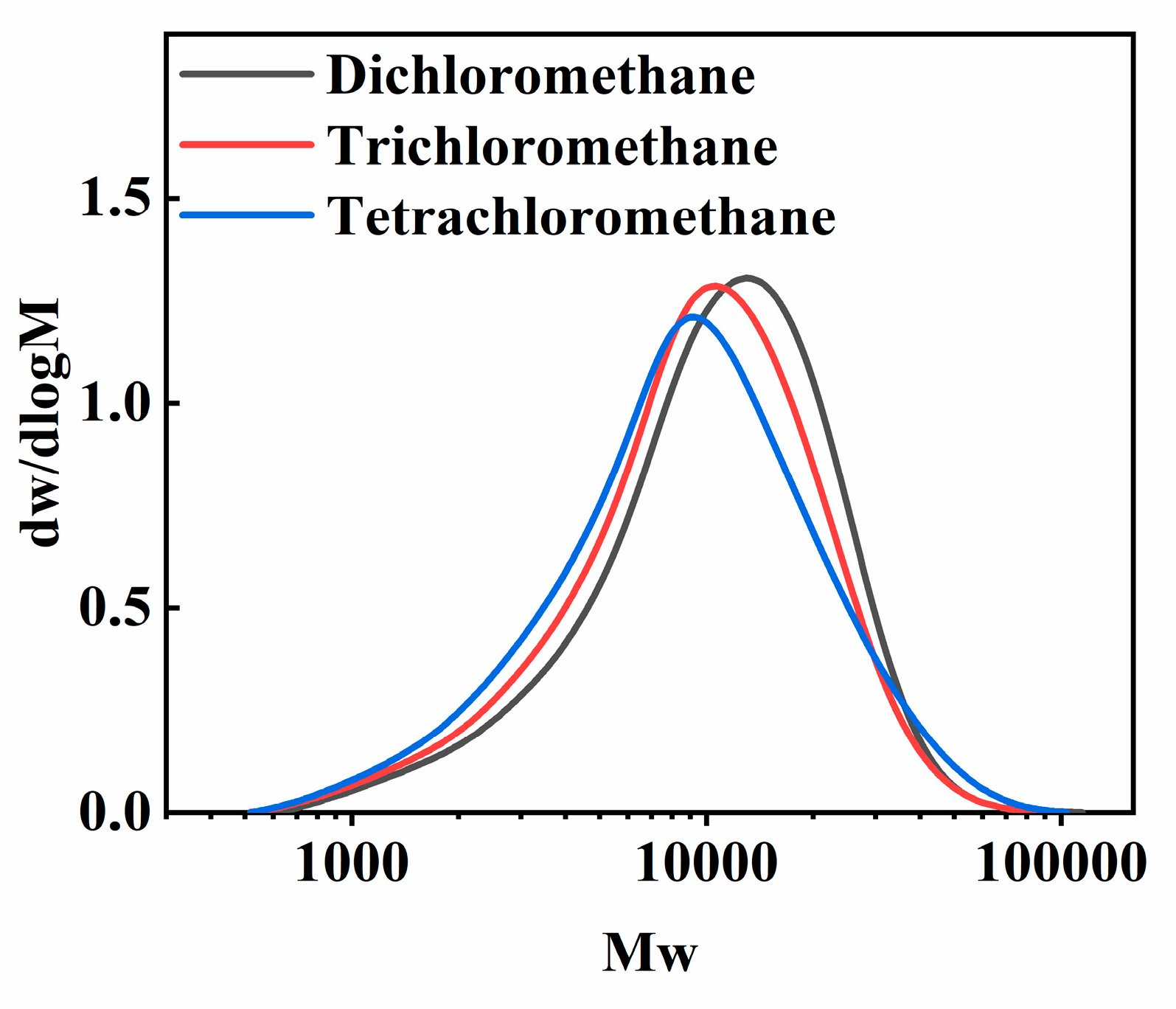
The effect of three types of chloromethane solvents, dichloromethane, trichloromethane and tetrachloromethane, on the morphology of PECA@CaSA-Caps is discussed. As shown in Table 3 Group a, the PECA@CaSA-Caps prepared in the three solvents have similar appearances. A white PECA coating is formed on the surface of prepared capsules, and the PECA@CaSA-Caps maintain good sphericity. As shown in Table 3 Group b, the PECA layers on PECA@CaSA-Caps prepared in the three solvents are all thick enough to shield the backlight. As shown in Table 3 Group c, though most of the PECA@CaSA-Caps prepared in dichloromethane remain intact and spherical after dehydration, a few of them collapse; while in trichloromethane and tetrachloromethane, the PECA@CaSA-Caps have good sphericity and their shells have no obvious changes after dehydration. As shown in Figure 9, from the cross-section of PECA@CaSA-Caps, the thicknesses of the PECA coatings in dichloromethane (Figure 9a), trichloromethane (Figure 9c) and tetrachloromethane (Figure 9e) are 72.55 µm, 107.97 µm and 85.62 µm, respectively. But their shell structures are different. In dichloromethane (Figure 9b) and trichloromethane (Figure 9d), the shells of PECA@CaSA-Caps consist of a number of flaky PECA pieces. And the PECA pieces formed in trichloromethane are bigger than those in dichloromethane. In both of the cross-sections of the PECA@CaSA-Caps, there are abundant pore structures between the PECA lamellae in the capsule membranes. However, in tetrachloromethane (Figure 9f), there is a continuous porous-free PECA membrane. Figure 10 shows the GPC diagram of the PECA shells of the PECA@CaSA-Caps prepared in different chloromethane solvents. The Mw values of the PECA shells of the PECA@CaSA-Caps prepared in dichloromethane, trichloromethane and tetrachloromethane decrease in turn, which are 13,280, 12,037 and 11,954, respectively. According to the reference [26], the chain transfer constants of ECA in dichloromethane, trichloromethane and tetrachloromethane increase in turn, resulting in a decrease in the Mw of PECA in turn. The smaller the Mw of PECA, the easier it crystallizes, leading to the regular structure of the PECA layer in tetrachloromethane.

Table 3.
Appearance of the PECA@CaSA-Caps with different solvents. Group a, the prepared PECA@CaSA-Caps at ambient temperature; Group b, the mm-CaSA-Caps (left) and the prepared PECA@CaSA-Caps (right) in water; Group c, the PECA@CaSA-Caps after dehydration at 60 °C. (The ECA is 6 mL. The reaction time is 60 min.).
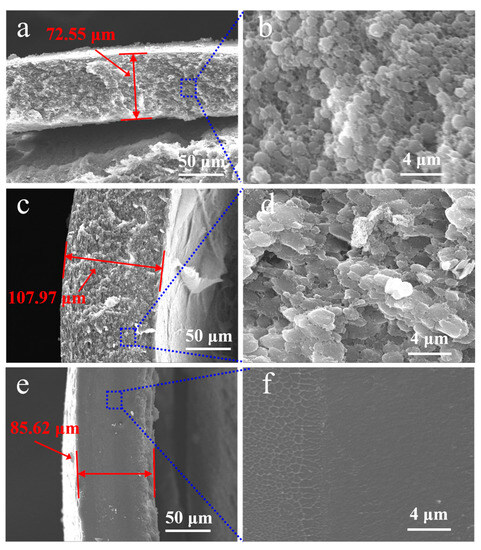
Figure 9.
Cross-section images of the PECA@CaSA-Caps prepared in dichloromethane ((a,b) high magnification image); trichloromethane ((c,d) high magnification image); tetrachloromethane ((e,f) high magnification image).
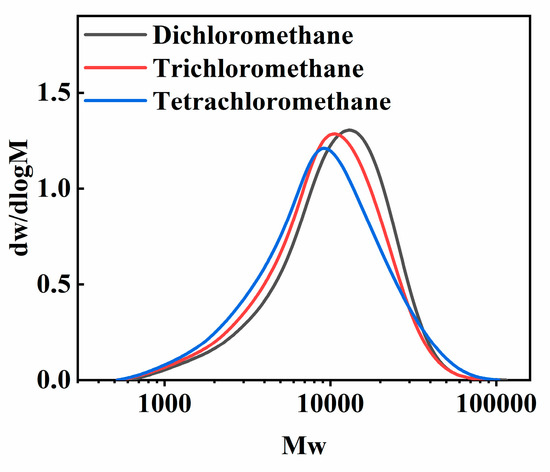
Figure 10.
The GPC diagram of the PECA shells of PECA@CaSA-Caps prepared in different chloromethane solvents.
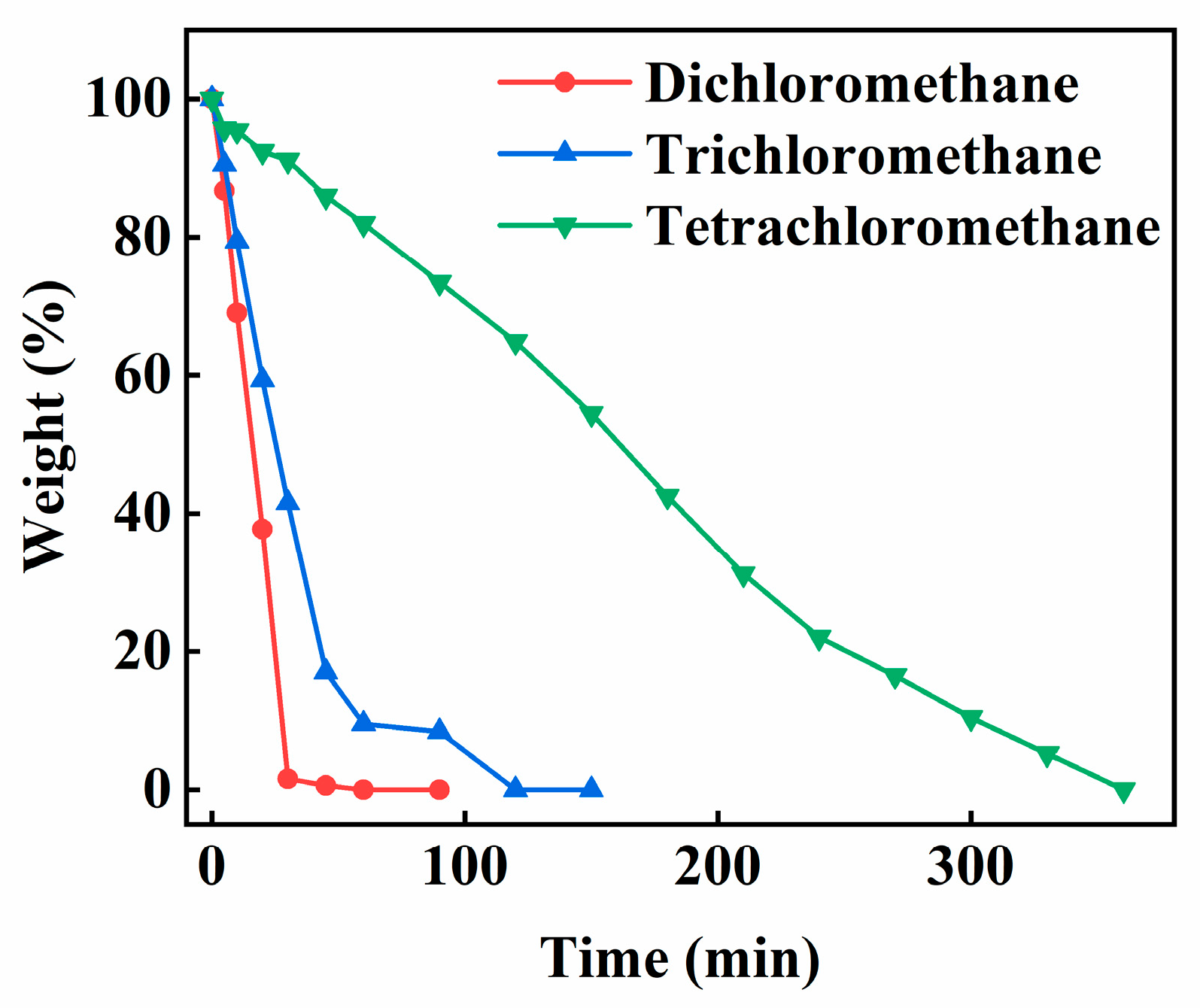
Figure 11 shows the weight change of water in the PECA@CaSA-Caps prepared in different chloromethane solvents. The water loss ratio of the PECA@CaSA-Caps decreases in the order of dichloromethane, trichloromethane and tetrachloromethane. The dehydration times of the PECA@CaSA-Caps prepared in dichloromethane, trichloromethane and tetrachloromethane are 60 min, 120 min and 360 min. Based on the SEM images in Figure 8, the PECA@CaSA-Caps prepared in dichloromethane and trichloromethane have porous structures caused by irregular flaky PECA stacking. And the thickness of the PECA coating formed in trichloromethane is higher than that formed in dichloromethane. Compared with the lamellar porous structure, the continuous non-porous structure of the PECA@CaSA-Caps prepared in tetrachloromethane can effectively reduce the evaporation of water in the core of the capsules [22], leading to the excellent water barrier performance of the PECA@CaSA-Caps. In general, the PECA@CaSA-Caps prepared in the three chloromethane solvents have a uniform diameter and good sphericity. The PECA@CaSA-Caps prepared in tetrachloromethane have the best water barrier performance and storage stability, which extends the application of hydrogel capsules [27].
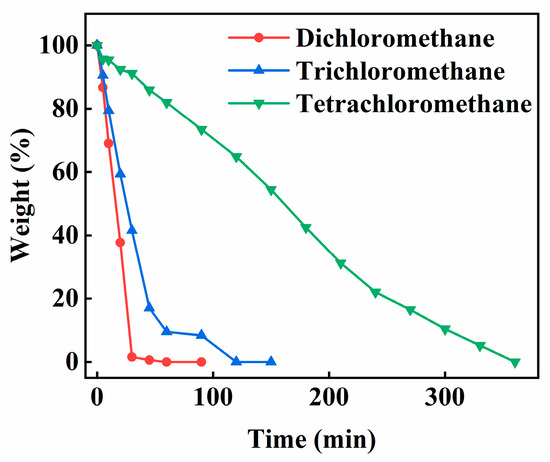
Figure 11.
Effect of solvent on the weight change of water in the PECA@CaSA-Caps.
3.4. Effect of pH on the Controlled Release Characteristics of the Capsules
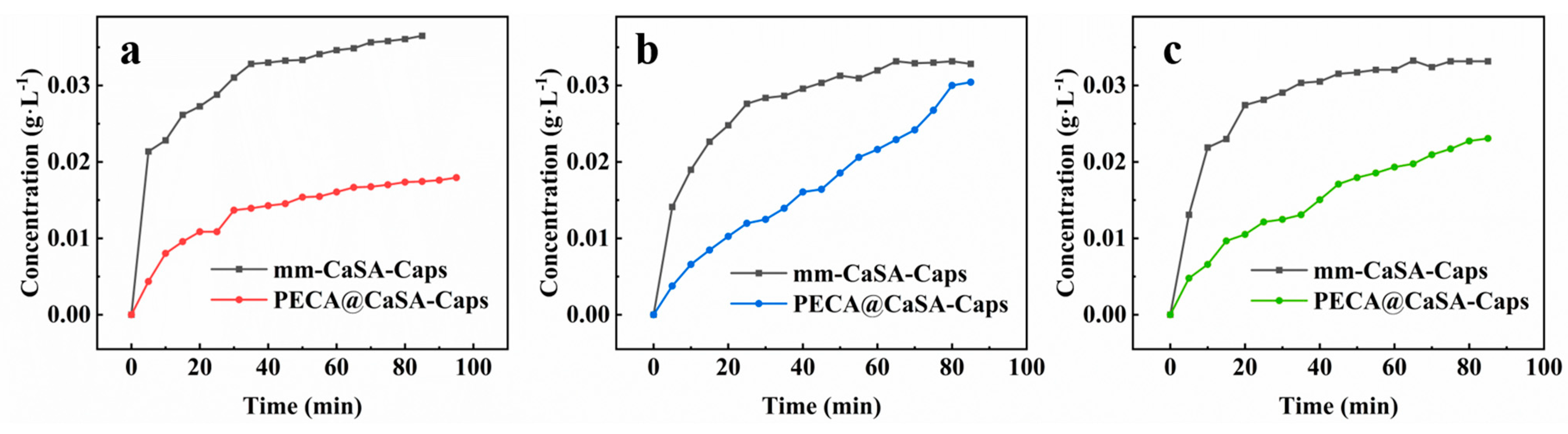
In order to verify the effect of PECA coating on the PECA@CaSA-Caps, the controlled release properties of the prepared capsules at different pH conditions were investigated. As shown in Figure 12, the sudden release of dye released from the mm-CaSA-Caps occurs at the beginning in acidic (pH 1.0) (Figure 12a), neutral (pH 7.0) (Figure 12b) and alkaline (pH 10.0) (Figure 12c) environments. At 30 min, the concentrations of dye released from the mm-CaSA-Caps are 0.031 g·L−1, 0.028 g∙L−1 and 0.029 g∙L−1 in the acidic (pH 1.0), neutral (pH 7.0) and alkaline (pH 10.0) environments, respectively. The release rates of the dye released from the mm-CaSA-Caps increase in order the of neutral (pH 7.0), alkaline (pH 10.0) and acidic (pH 1.0) conditions. Because the mm-CaSA-Caps are pH sensitive, they swell in acidic gastric solution (pH 2.0) but degrade in alkaline intestinal solution (pH 7.5) [1]. However, the release rates of dye released from the PECA@CaSA-Caps decrease dramatically compared with those of the mm-CaSA-Caps under the three pH conditions. There is no sudden release phenomenon of the PECA@CaSA-Caps under the three pH conditions. At 30 min, the concentrations of dye released from the PECA@CaSA-Caps are 0.014 g∙L−1, 0.013 g∙L−1 and 0.012 g∙L−1 in the acidic (pH 1.0) (Figure 12a), neutral (pH 7.0) (Figure 12b) and alkaline (pH 10.0) (Figure 12c) environments, respectively. At 60 min, the concentrations of dye released from the PECA@CaSA-Caps are 0.016 g∙L−1, 0.022 g∙L−1 and 0.019 g∙L−1 in the acidic (pH 1.0), neutral (pH 7.0) and alkaline (pH 10.0) environments, respectively. The release rates of dye released from the PECA@CaSA-Caps are faster later in the neutral (pH 7.0) and alkaline (pH 10.0) environments than in the acidic (pH 1.0) environment. It is indicated that the PECA coating has a good barrier effect which can delay the release rates of the dye in the PECA@CaSA-Caps in neutral (pH 7.0), alkaline (pH 10.0) and acidic (pH 1.0) conditions and achieves the effect of controlled release according to pH.
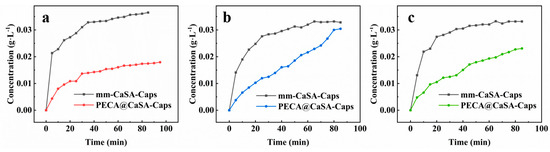
Figure 12.
Controlled release properties of the mm-CaSA-Caps and the PECA@CaSA-Caps in (a) 0.1 mol·L−1 HCl with pH 1.0; (b) deionized water with pH 7.0; (c) 0.0001 mol·L−1 NaOH with pH 10.0.
4. Conclusions
Based on the high reactivity, mild reaction conditions and biocompatibility of ECA, the PECA@CaSA-Caps were successfully prepared with the in-situ polymerization of ECA at the interface of the mm-CaSA-Caps and the chloromethane solvents. A uniform and white PECA coating layer was formed on the surface of the mm-CaSA-Caps. The effects of reaction time, amount of ECA and type of solvent on the morphology and properties of the PECA@CaSA-Caps were investigated. During the coating process, PECA was rapidly forming on the surface of the mm-CaSA-Caps. As the reaction time increased, the thickness of the PECA coating increased. And the morphology of PECA@CaSA-Caps remained spherical. When the amount of ECA was 6 mL, the PECA@CaSA-Caps maintained an intact appearance. The PECA@CaSA-Caps prepared in tetrachloromethane had the best water barrier performance. Meanwhile, the PECA coating dramatically enhanced the stability of the PECA@CaSA-Caps in neutral (pH 7.0), alkaline (pH 10.0) and acidic (pH 1.0) conditions. The PECA@CaSA-Caps have a spherical appearance, good biocompatibility and excellent barrier properties, which have great application prospects in the fields of food, nutrition, cosmetic and agricultural engineering.
Author Contributions
Conceptualization, Y.Z. and Q.D.; Methodology, J.Z. and L.H.; Formal analysis, S.G., W.H. and C.P.; Investigation, W.H. and C.P.; Data curation, S.G., W.H. and C.P.; Writing—review & editing, S.G. and J.Z.; Supervision, J.Z. and L.H.; Project administration, Y.Z. and Q.D.; Funding acquisition, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, P.; Li, M.; Wei, D.; Ding, M.; Tao, L.; Liu, X.; Zhang, F.; Tao, N.; Wang, X.; Gao, M.; et al. Electrosprayed soft capsules of millimeter size for specifically delivering fish oil/nutrients to the stomach and intestines. ACS Appl. Mater. Interfaces 2020, 12, 6536–6545. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhao, G.; Zheng, H.; He, G.; Li, J.; Zhang, J.; Chen, Y.; Wang, Y. Chitosan capsules with hydrogel core for encapsulation and controlled-release of small molecule materials. Mater. Lett. 2020, 278, 128348. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lomora, M.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Q.; Wei, H.; Zhang, T.; Wang, J.; Zhang, Y.; Huang, L.; Tang, Y. Shape tuning and size prediction of millimeter-scale calcium-alginate capsules with aqueous core. Polymers 2020, 12, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Mu, X.; Huang, W.; Guo, Q.; Zhao, J. Versatile surface modification of millimeter-scale “aqueous pearls” with nanoparticles via self-polymerization of dopamine. Polym. Adv. Technol. 2021, 32, 3059–3069. [Google Scholar] [CrossRef]
- Han, M.R.; Kwon, M.C.; Lee, H.Y.; Kim, J.C.; Kim, J.D.; Yoo, S.K.; Sin, I.S.; Kim, S.M. pH-dependent release property of alginate beads containing calcium carbonate particles. J. Microencapsul. 2007, 24, 787–796. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Dong, K.; Luo, J.; Zhang, Q.; Cheng, Y. Injectable and responsively degradable hydrogel for personalized photothermal therapy. Biomaterials 2016, 104, 129–137. [Google Scholar] [CrossRef]
- Zou, Q.; Hou, F.; Wang, H.; Liao, Y.; Wang, Q.; Yang, Y. Microfluidic one-step preparation of alginate microspheres encapsulated with in situ-formed bismuth sulfide nanoparticles and their photothermal effect. Eur. Polym. J. 2019, 115, 282–289. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jin, Y.; Xie, R.; Liu, J.Y.; Ju, X.J.; Meng, T.; Chu, L.Y. Novel calcium-alginate capsules with aqueous core and thermo-responsive membrane. J. Colloid. Interface Sci. 2011, 353, 61–68. [Google Scholar] [CrossRef]
- Dembczynski, R.; Jankowski, T. Growth characteristics and acidifying activity of Lactobacillus rhamnosus in alginate/starch liquid-core capsules. Enzym. Microb. Technol. 2002, 31, 111–115. [Google Scholar] [CrossRef]
- Taqieddin, E.; Amiji, M. Enzyme immobilization in novel alginate–chitosan core-shell microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Borges, J.; Costa, A.M.S.; Gaspar, V.M.; Bermudez, V.Z.; Mano, J.F. Preparation of well-dispersed chitosan/alginate hollow multilayered microcapsules for enhanced cellular internalization. Molecules 2018, 23, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.-L.; Wang, W.; Li, Z.-L.; Ju, X.-J.; Xie, R.; Deng, N.-N.; Wei, J.; Liu, Z.; Chu, L.-Y. Trojan-horse-like stimuli-responsive microcapsules. Adv. Sci. 2018, 5, 1700960. [Google Scholar] [CrossRef]
- Johnston, A.P.R.; Cortez, C.; Angelatos, A.S.; Caruso, F. Layer-by-layer engineered capsules and their applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 203–209. [Google Scholar] [CrossRef]
- Andrieux, K.; Couvreur, P. Polyalkylcyanoacrylate nanoparticles for delivery of drugs across the blood-brain barrier. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 463–474. [Google Scholar] [CrossRef]
- Yao, J.J.; Zeng, D.C.; Zhang, Y.X.; Sun, D.D.; Yang, E.D.; Liu, H.P.; Guo, F.; Wang, W.Y. Effect of solvents on forming poly(butyl-2-cyanoacrylate) encapsulated paeonol nanocapsules. J. Biomater. Sci. Polym. Ed. 2017, 28, 240–256. [Google Scholar] [CrossRef]
- Xu, Q.L.; Li, H.X.; Wang, G.C. Preparation of polyalkylcyanoacrylate nanoparticles with various morphologies. Chin. J. Polym. Sci. 2011, 29, 336–341. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, C.; Mayer, C. Permeability profile of poly(alkyl cyanoacrylate) nanocapsules. J. Colloid. Interface Sci. 2016, 478, 394–401. [Google Scholar] [CrossRef]
- Fu, W.; Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Characterization and thermal performance of microencapsulated sodium thiosulfate pentahydrate as phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 193, 149–156. [Google Scholar] [CrossRef]
- Tuncel, A.; Çiçek, H.; Hayran, M.; Pişkin, E. Monosize poly(ethylcyanoacrylate) microspheres: Preparation and degradation properties. J. Biomed. Mater. Res. 1995, 29, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wei, W.; Liu, H.; Ye, Z.; Jing, L.; Xiao-Ya, L. Water-bearing capsule in situ prepared from liquid marble and lts performance. J. Funct. Polym. 2018, 31, 121–127. [Google Scholar] [CrossRef]
- Lee, B.-B.; Chan, E.-S.; Ravindra, P. Calcium pectinate beads formation: Shape and size analysis. J. Eng. Technol. Sci. 2014, 46, 78–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Bei, F.; Ji, H.; Yang, X.; Lu, L.; Wang, X. Property and quantum chemical investigation of poly(ethyl α-cyanoacrylate). J. Mol. Struct. 2005, 737, 117–123. [Google Scholar] [CrossRef]
- Moghadam, N.; Srinivasan, S.; Grady, M.C.; Rappe, A.M.; Soroush, M. Theoretical study of chain transfer to solvent reactions of alkyl acrylates. J. Phys. Chem. A 2014, 118, 5474–5487. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Linares-Molinero, F.; Gallardo, V.; Delgado, Á.V. Study of carbonyl iron/poly(butylcyanoacrylate) (core/shell) particles as anticancer drug delivery systems: Loading and release properties. Eur. J. Pharm. Sci. 2008, 33, 252–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).