Characterization and Electrochemical Investigation of Heterogeneous Sb-Cu Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Electrodeposition of Sb-Cu Layers
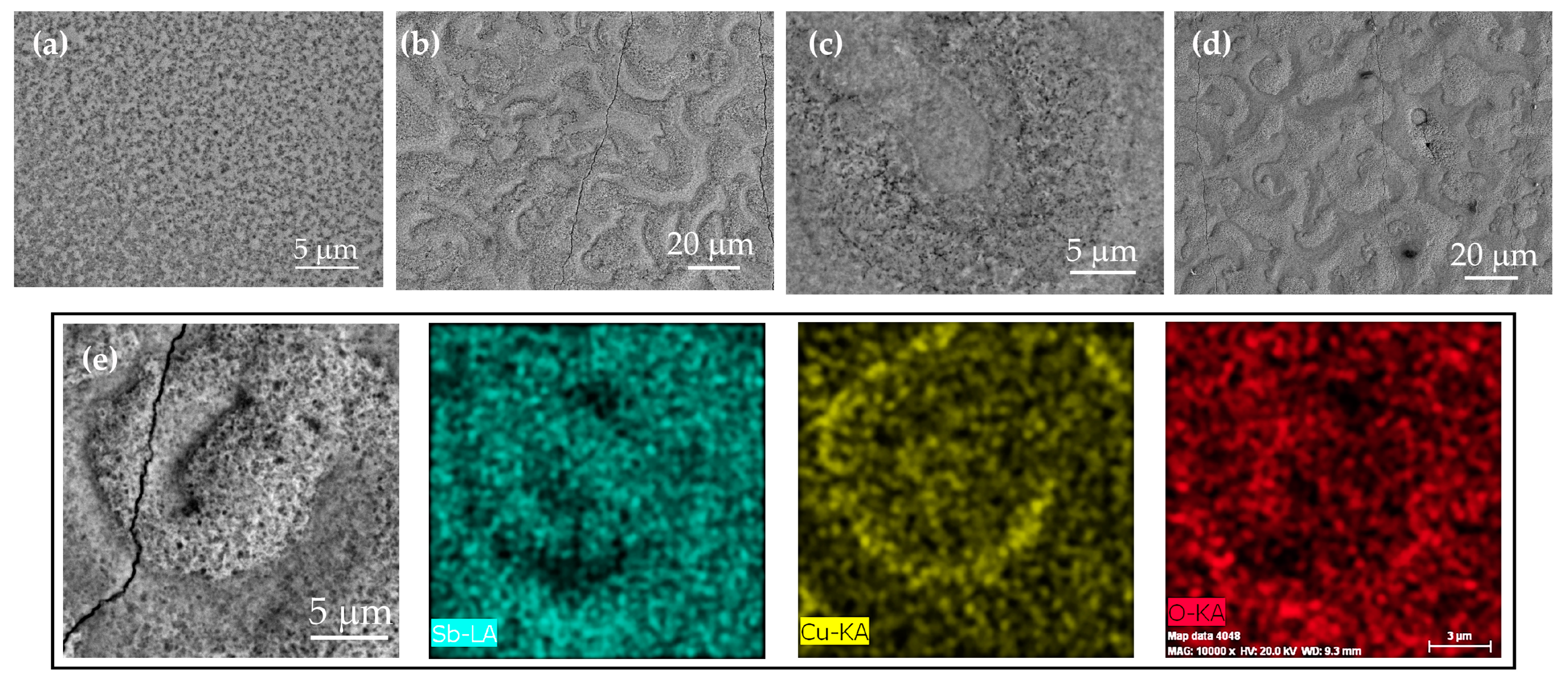
3.2. Characterization of Sb-Cu Layers
3.3. Electrochemical Investigation of Sb-Cu Layers in Sulfuric Acid Solution
3.4. Sb-Cu Surface Observation after Polarization Test in Sulfuric Acid Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Chu, S.; Shen, H.; Xia, Q.; Robertson, A.W.; Masa, J.; Siddiqui, U.; Sun, Z. Achieving Highly Selective Electrocatalytic CO2 Reduction by Tuning CuO-Sb2O3 Nanocomposites. ACS Sustain. Chem. Eng. 2020, 8, 4948–4954. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Li, W.; Zhang, W.; Liu, M.; Weng, Z.; Huo, S.; Zhang, J. Boosting carbon monoxide production during CO2 reduction reaction via Cu-Sb2O3 interface cooperation. J. Colloid Interface Sci. 2021, 601, 661–668. [Google Scholar] [CrossRef]
- Mou, S.; Li, Y.; Yue, L.; Liang, J.; Luo, Y.; Liu, Q.; Li, T.; Lu, S.; Asiri, A.M.; Xiong, X.; et al. Cu2Sb decorated Cu nanowire arrays for selective electrocatalytic CO2 to CO conversion. Nano Res. 2021, 14, 2831–2836. [Google Scholar] [CrossRef]
- Zeng, J.; Fiorentin, M.R.; Fontana, M.; Castellino, M.; Risplendi, F.; Sacco, A.; Cicero, G.; Farkhondehfal, M.A.; Drago, F.; Pirri, C.F. Novel Insights into Sb-Cu Catalysts for Electrochemical Reduction of CO2. Appl. Catal. B Environ. 2022, 306, 121089. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, C.-J.; Wang, Y.; Zhu, J.-W.; Lu, J.-X.; Wang, H. Nitrogen-doped mesoporous carbon supported CuSb for electroreduction of CO2. RSC Adv. 2022, 12, 12997–13002. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Dong, X.; Ding, Y.; Hu, S.; Zhang, R.; Dai, Y.; Cui, P.; Xiao, Z.; Zhao, D.; et al. Selective CO2 electrolysis to CO using isolated antimony alloyed copper. Nat. Commun. 2023, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, D.; Fu, Q.; Zhan, D.; Zhou, H.; Tong, Z.; Zhao, Y.; Han, C. Bimetal (Cu, Sb) nanoparticles-decorated BiVO4 enhances photoelectrochemical properties. Mater. Sci. Semicond. Process. 2023, 165, 107668. [Google Scholar] [CrossRef]
- Vivekanandan, A.K.; Muthukutty, B.; Chen, S.-M.; Sivakumar, M.; Chen, S.-H. Intermetallic Compound Cu2Sb Nanoparticles for Effective Electrocatalytic Oxidation of an Antibiotic Drug: Sulphadiazine. ACS Sustain. Chem. Eng. 2020, 8, 17718–17726. [Google Scholar] [CrossRef]
- Chalapathi, U.; Bhaskar, P.U.; Cheruku, R.; Sambasivam, S.; Park, S.-H. Evolution of large-grained CuSbS2 thin films by rapid sulfurization of evaporated Cu–Sb precursor stacks for photovoltaics application. Ceram. Int. 2023, 49, 4758–4763. [Google Scholar] [CrossRef]
- Aimei, Z.; Yanping, W.; Bing, L.; Dongmei, X.P.; Yujie, Z.Y.; Yupeng, X.; Liyong, Y.; Jinlian, B.; Wei, L. Sulfurization of Electrodeposited Sb/Cu Precursors for CuSbS2: Potential Absorber Materials for Thin-Film Solar Cells. Front. Mater. 2022, 8, 818596. [Google Scholar] [CrossRef]
- Abouabassi, K.; Atourki, L.; Sala, A.; Ouafi, M.; Boulkaddat, L.; Ait Hssi, A.; Labchir, N.; Bouabid, K.; Almaggoussi, A.; Gilioli, E.; et al. Annealing Effect on One Step Electrodeposited CuSbSe2 Thin Films. Coatings 2022, 12, 75. [Google Scholar] [CrossRef]
- Wang, X. Wetting characteristics of Sn-5Sb-CuNiAg lead-free solders on the copper substrate. Solder. Surf. Mt. Technol. 2022, 34, 96–102. [Google Scholar] [CrossRef]
- Bryngelsson, H.; Eskhult, J.; Nyholm, L.; Edström, K. Thin films of Cu2Sb and Cu9Sb2 as anode materials in Li-ion batteries. Electrochim. Acta 2008, 53, 7226–7234. [Google Scholar] [CrossRef]
- Jackson, E.D.; Prieto, A.L. Copper Antimonide Nanowire Array Lithium Ion Anodes Stabilized by Electrolyte Additives. ACS Appl. Mater. Interfaces 2016, 8, 30379–30386. [Google Scholar] [CrossRef]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-Dimensional Printing of a Complete Lithium Ion Battery with Fused Filament Fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Luo, W.; Ren, J.; Feng, W.; Chen, X.; Yan, Y.; Zahir, N. Engineering Nanostructured Antimony-Based Anode Materials for Sodium Ion Batteries. Coatings 2021, 11, 1233. [Google Scholar] [CrossRef]
- Hu, P.; Dong, Y.; Yang, G.; Chao, X.; He, S.; Zhao, H.; Fu, Q.; Lei, Y. Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage. Batteries 2023, 9, 238. [Google Scholar] [CrossRef]
- Chu, P.; Wang, J.; Xie, H.; Zhang, Q.; Feng, J.; Li, Z.; Yang, Z.; Zhao, H. Sb-Cu alloy cathode with a novel lithiation mechanism of ternary intermetallic formation: Enabling high energy density and superior rate capability of liquid metal battery. J. Energy Chem. 2023, 78, 393–400. [Google Scholar] [CrossRef]
- Fürtauer, S.; Flandorfer, H. A new experimental phase diagram investigation of Cu–Sb. Monatsh. Chem. 2012, 143, 1275–1287. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Sasaki, H.; Kamiko, M.; Yoshikawa, T.; Maeda, M. Passivation of Cu–Sb anodes in H2SO4−CuSO4 aqueous solution observed by the channel flow double electrode method and optical microscopy. Electrochim. Acta 2019, 309, 300–310. [Google Scholar] [CrossRef]
- Grasser, M.A.; Müller, U.; Ruck, M. Low-Temperature Synthesis of NiSb2, Cu2Sb, InSb and Sb2Te3 Starting from the Elements. Z. Anorg. Allg. Chem. 2022, 648, e202200195. [Google Scholar] [CrossRef]
- Rodríguez-Lazcano, Y.; Barrios-Salgado, E.; Flores-Castañeda, M.; Camacho-López, S.; Pérez-Alvarez, J.; Chávez-Chávez, A.; Quiñones-Galván, J.G. Physical properties of Sb2S3–Cu nanocomposite thin films synthesized by chemical bath deposition and laser ablation of solids in liquids. J. Mater. Res. Technol. 2023, 24, 6604–6613. [Google Scholar] [CrossRef]
- Schulze, M.C.; Schulze, R.K.; Prieto, A.L. Electrodeposited thin-film CuxSb anodes for Li-ion batteries: Enhancement of cycle life via tuning of film composition and engineering of the film-substrate interface. J. Mater. Chem. A 2018, 6, 12708–12717. [Google Scholar] [CrossRef]
- Oubakalla, M.; Beraich, M.; Taibi, M.; Majdoubi, H.; Aichi, Y.; Guenbour, A.; Bellaouchou, A.; Bentiss, F.; Zarrouk, A.; Fahoume, M. The choice of the copper concentration favoring the production of stoichiometric CuSbS2 and Cu12Sb4S13 thin films co-electrodeposited on FTO. J. Alloys Compd. 2022, 908, 164618. [Google Scholar] [CrossRef]
- Arpacık, M.; Biçer, M.; Şişman, İ. Influence of Microstructure on the Electrochemical Performance of Sn-Sb-Cu Nanostructures as Anode Materials for Lithium-Ion Battery. J. Electrochem. Soc. 2013, 160, A2251. [Google Scholar] [CrossRef]
- Mosby, J.M.; Prieto, A.L. Direct electrodeposition of Cu2Sb for lithium-ion battery anodes. J. Am. Chem. Soc. 2008, 130, 10656–10661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, K.-Q.; Zhou, W.; Lu, X.-F.; Li, R.; Li, G. Copper–Antimony Alloy–Nanoparticle clusters supported on porous cu networks for electrochemical energy storage. Part. Part. Syst. Charact. 2016, 33, 553–559. [Google Scholar] [CrossRef]
- Sadan, Y.N.; Singh, J.P.; Kumar, R. Electrodeposition of antimony and antimony alloys—A review. Surf. Technol. 1985, 24, 319–353. [Google Scholar] [CrossRef]
- Verbruggen, F.; Prévoteau, A.; Bonin, L.; Marcoen, K.; Hauffman, T.; Hennebel, T.; Rabaey, K.; Moats, M.S. Electrochemical codeposition of copper-antimony and interactions with electrolyte additives: Towards the use of electronic waste for sustainable copper electrometallurgy. Hydrometallurgy 2022, 211, 105886. [Google Scholar] [CrossRef]
- Voigt, K.; Heubner, C.; Liebmann, T.; Matthey, B.; Weiser, M.; Schneider, M.; Michaelis, A. Electrodeposition of Versatile Nanostructured Sb/Sb2O3 Microcomposites: A Parameter Study. Adv. Mater. Interfaces 2020, 7, 2000004. [Google Scholar] [CrossRef]
- Heubner, C.; Voigt, K.; Lämmel, C.; Schneider, M.; Michaelis, A. Pattern formation during Sb/Sb2O3 electrodeposition. Appl. Surf. Sci. 2021, 563, 150206. [Google Scholar] [CrossRef]
- Voigt, K.; Heubner, C.; Schneider, M.; Michaelis, A. Formation mechanism of electrodeposited Sb/Sb2O3 micro-composites. Electrochim. Acta 2021, 366, 137430. [Google Scholar] [CrossRef]
- Pavlov, D.; Bojinov, M.; Laitinen, T.; Sundholm, G. Electrochemical behaviour of the antimony electrode in sulphuric acid solutions—I. Corrosion processes and anodic dissolution of antimony. Electrochim. Acta 1991, 36, 2081–2086. [Google Scholar] [CrossRef]
- Bojinov, M.; Pavlov, D. Anodic oxidation of antimony at high overpotentials–formation of a barrier layer and klebelsbergite. J. Electroanal. Chem. 1993, 346, 339–352. [Google Scholar] [CrossRef]
- Laitinen, L.; Revitzer, H.; Sundholm, G.; Vilhunen, J.K.; Pavlov, D.; Bojinov, M. Electrochemical behaviour of the antimony electrode in sulphuric acid solutions—III. Identification of corrosion products after long-term polarization. Electrochim. Acta 1991, 36, 2093–2102. [Google Scholar] [CrossRef]
- Gulevskii, V.A.; Antipov, V.I.; Vinogradov, L.V.; Kolmakov, A.G.; Kidalov, N.A.; Lazarev, E.M.; Mukhina, Y.E. Development of antimony-based impregnating alloys to produce electrographite frame composite materials. Russ. Metall. 2011, 2011, 78–84. [Google Scholar] [CrossRef]
- Gernon, M.D.; Wu, M.; Buszta, T.; Janney, P. Environmental benefits of methanesulfonic acid. Comparative properties and advantages. Green Chem. 1999, 1, 127–140. [Google Scholar] [CrossRef]
- Sknar, I.V.; Sknar, Y.E.; Savchuk, O.O.; Baskevich, A.S.; Kozhura, O.V.; Hrydnieva, T.V. Electrodeposition of copper from a methanesulphonate electrolyte. J. Chem. Technol. 2020, 28, 1–9. [Google Scholar] [CrossRef]
- Krastev, I.; Koper, M.T.M. Pattern formation during the electrodeposition of a silver-antimony alloy. Phys. A Stat. Mech. Appl. 1995, 213, 199–208. [Google Scholar] [CrossRef]
- Lin, Y.; Ning, P.; Cui, Y.; Zhang, C.; Xu, M.; Dong, P.; Zhou, Z.; Zhang, Y. Electrodeposition Behaviour of Antimony in H2SO4-NH4F-SbF3 Solutions. Int. J. Electrochem. Sci. 2019, 14, 4003–4019. [Google Scholar] [CrossRef]
- Guo, L.; Searson, P.C. On the influence of the nucleation overpotential on island growth in electrodeposition. Electrochim. Acta 2010, 55, 4086. [Google Scholar] [CrossRef]
- Nix, W.D.; Clemens, B.M. Crystallite coalescence: A mechanism for intrinsic tensile stresses in thin films. J. Mater. Res. 1999, 14, 3467–3473. [Google Scholar] [CrossRef]
- Tzaneva, B.R.; Kostov, V.S. Corrosion behaviour of heterogeneous antimony-copper layers in chloride media. Corros. Eng. Sci. Technol. 2023; in press. [Google Scholar] [CrossRef]
- Matkasymova, A.; Omurzak, E.; Sulaimankulova, S. Nanorods of Metallic Bismuth and Antimony by the Impulse Plasma in Liquid. J. Clust. Sci. 2009, 20, 153–158. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Li, X.; Xiao, Y.; Xu, G.-L.; Li, J.-T.; Sun, S.-G. Facile synthesis of hollow Cu2Sb@C core–shell nanoparticles as a superior anode material for lithium ion batteries. J. Mater. Chem. 2011, 21, 18517–18519. [Google Scholar] [CrossRef]
- Okamoto, H. Desk Handbook of Phase Diagrams for Binary Alloys, 2nd ed.; ASM International: Novelty, Ohio, USA, 2010; pp. 748–752. [Google Scholar]
- Laihonen, S.; Laitinen, T.; Sundholm, G.; Yli-Pentti, A. The anodic behaviour of Sb and Pb-Sb eutectic in sulphuric acid solutions. Electrochim. Acta 1990, 35, 229–238. [Google Scholar] [CrossRef]
- Mogoda, A.S.; Abd EI-Haleem, T.M. Electrochemical Behavior of Antimony in Sulfuric Acid and Sodium Sulfate Solutions Containing Potassium Dichromate. Corrosion 2003, 59, 3–10. [Google Scholar] [CrossRef]
- Mogoda, A.S.; Abd El-Haleem, T.M. Anodic oxide film formation on antimony and its currentless dissolution in sulphuric acid containing some monocarboxylic acids. Thin Solid Films 2003, 441, 6–12. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Brinić, S. EIS-in situ characterization of anodic films on antimony and lead–antimony alloys. J. Power Sources 2006, 157, 563–570. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F. Copper corrosion inhibition in O2-saturated H2SO4 solutions. Corros. Sci. 2010, 52, 1194–1204. [Google Scholar] [CrossRef]
- Thaçi, V.; Hoti, R.; Berisha, A.; Bogdanov, J. Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study. Open Chem. 2020, 18, 1412–1420. [Google Scholar] [CrossRef]
- Wikstrom, L.L.; Nobe, K. Potentiodynamic studies of antimony in acidic and alkaline solutions. Corrosion 1975, 31, 364–369. [Google Scholar] [CrossRef]
- Pérez, O.E.; Pérez, M.A.; Teijelo, M.L. Characterization of the anodic growth and dissolution of antimony oxide films. J. Electroanal. Chem. 2009, 632, 64–71. [Google Scholar] [CrossRef]
- Lins, V.F.C.; Soares, R.B.; Alvarenga, E.A. Corrosion behaviour of experimental copper–antimony–molybdenum carbon steels in industrial and marine atmospheres and in a sulphuric acid aqueous solution. Corros. Eng. Sci. Technol. 2017, 52, 397–403. [Google Scholar] [CrossRef]
- Ye, J.; Xia, G.; Zheng, Z.; Hu, C. Facile controlled synthesis of coral-like nanostructured Sb2O3@Sb anode materials for high performance sodium-ion batteries. Int. J. Hydrogen Energy 2020, 45, 9969–9978. [Google Scholar] [CrossRef]
- Pan, J.; Wang, N.; Zhou, Y.; Yang, X.; Zhou, W.; Qian, Y.; Yang, J. Simple synthesis of a porous Sb/Sb2O3 nanocomposite for a high-capacity anode material in Na-ion batteries. Nano Res. 2017, 10, 1794–1803. [Google Scholar] [CrossRef]
- Girginov, C.; Lilov, E.; Kozhukharov, S.; Lilova, V. Efficiency of the Galvanostatic Formation of Anodic Antimony Oxide in Oxalic Acid Solutions. Port. Electrochim. Acta 2022, 40, 89–98. [Google Scholar] [CrossRef]


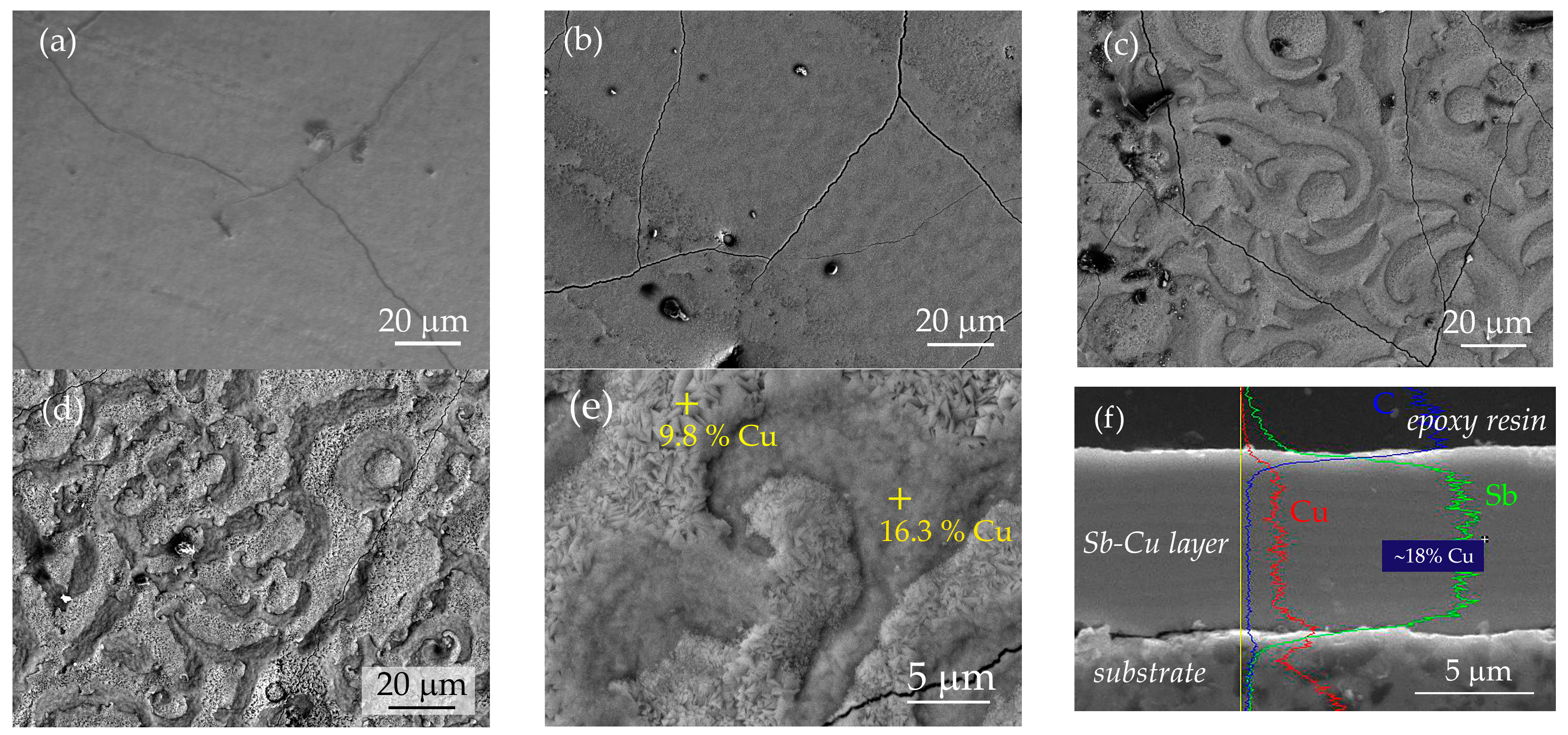
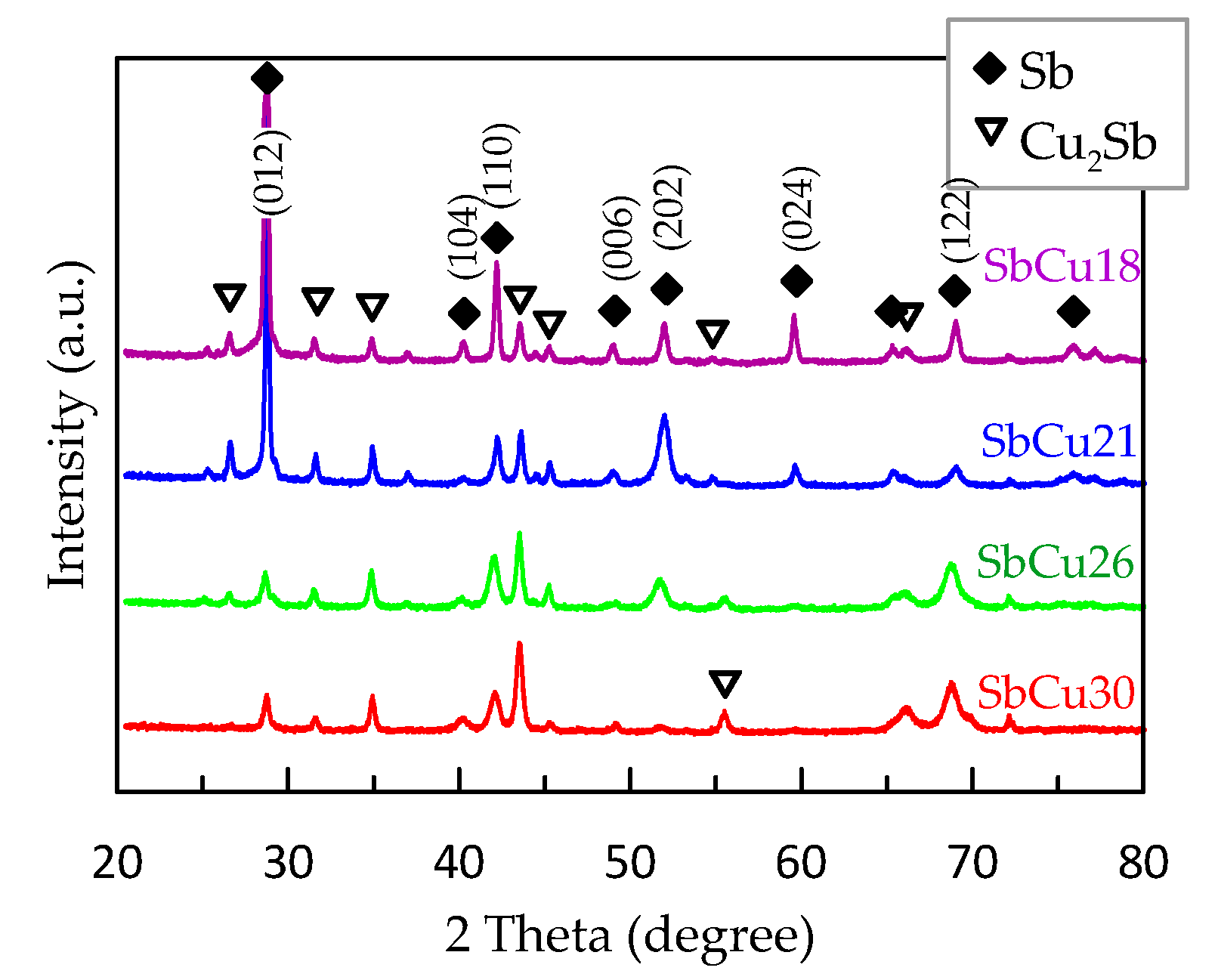
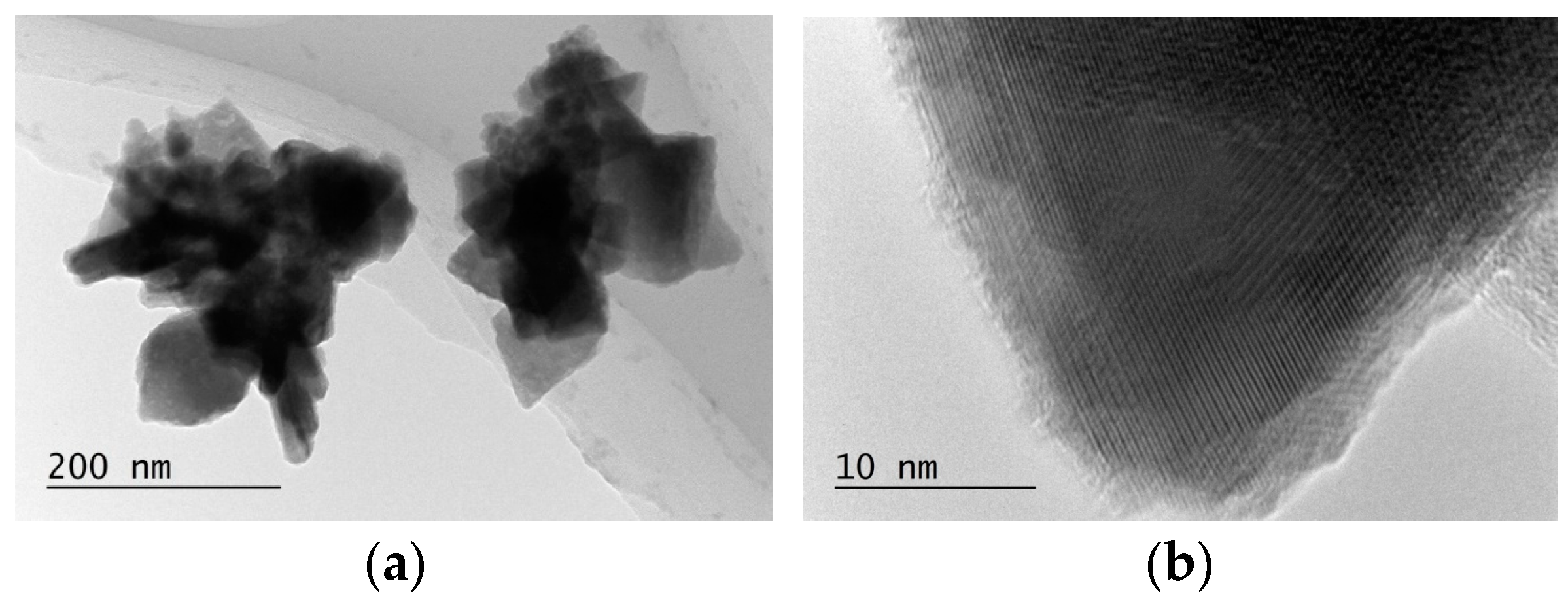
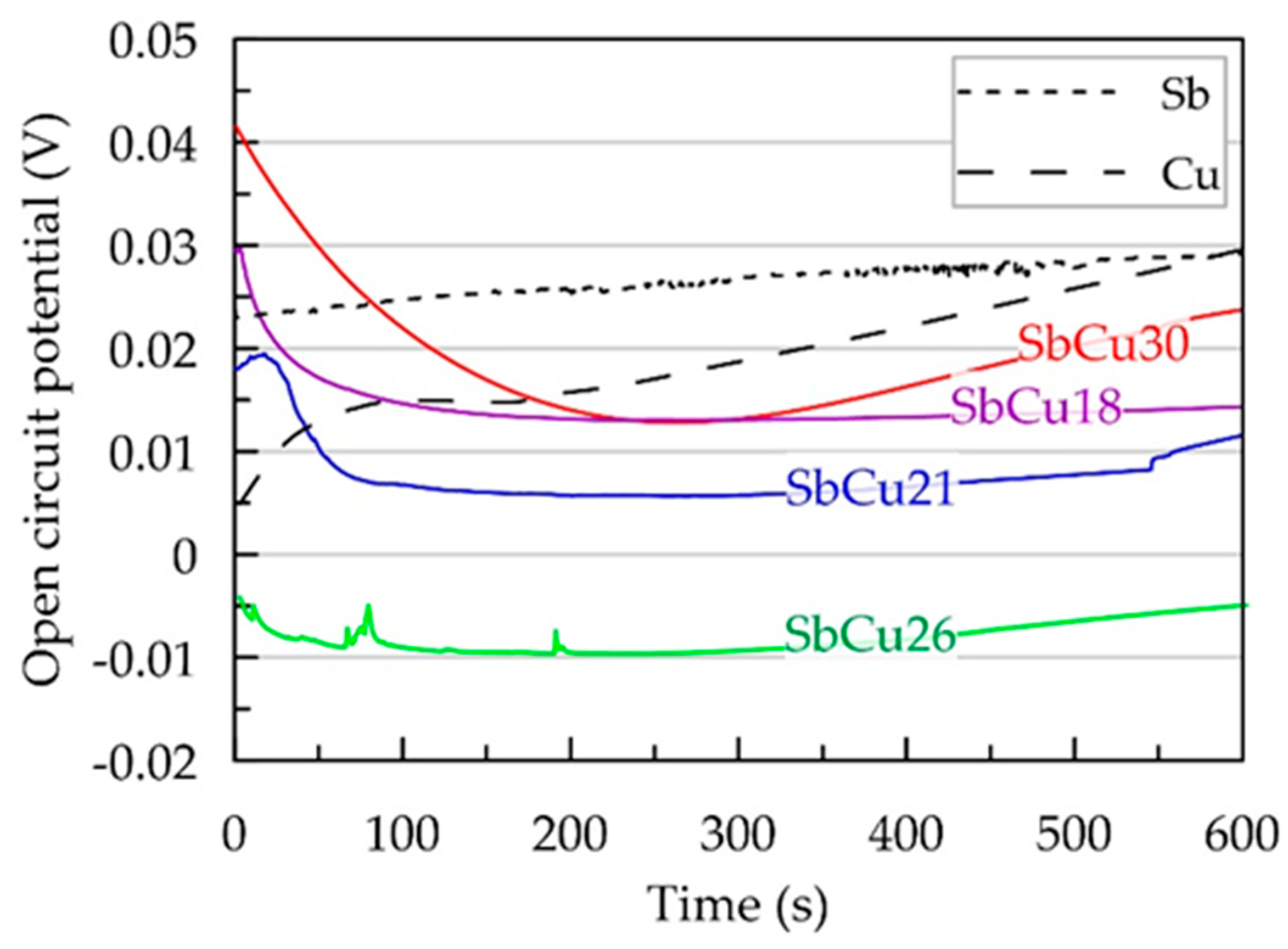


| Layer Abbreviation | SbCu30 | SbCu26 | SbCu21 | SbCu18 |
|---|---|---|---|---|
| Cathodic current densities, A dm−2 | 0.4 | 0.5 | 0.6 | 0.7 |
| Cu, wt.% (at.%) | 29.5 (44.5) | 25.8 (40.0) | 20.6 (33.2) | 17.9 (29.4) |
| Microhardness, kgf/mm2 | 201.3 | 204.56 | 197.21 | 214.28 |
| RZ, µm | 1.74 | 1.97 | 2.44 | 2.94 |
| Ra, µm | 0.364 | 0.338 | 0.418 | 0.506 |
| Coating | Sb Phase (nm) | Cu2Sb Phase (nm) |
|---|---|---|
| SbCu30 | 20.2 | 20.9 |
| SbCu26 | 25.4 | 31.2 |
| SbCu21 | 31.2 | 32.6 |
| SbCu18 | 37.6 | 33.8 |
| Sample | RS (Ω) | Rox (Ω) | Qox (×10−6 Ω−1 sn) | nox | Rct (kΩ) | Qdl (×10−6 Ω−1 sn) | ndl | χ2 |
|---|---|---|---|---|---|---|---|---|
| SbCu30 | 4.92 | 790 | 179 | 0.89 | 1.06 | 876 | 0.62 | 8.93 × 10−6 |
| SbCu26 | 1.63 | 310 | 359 | 0.94 | 0.299 | 1090 | 0.69 | 3.32 × 10−6 |
| SbCu21 | 2.89 | 14.8 | 246 | 0.97 | 0.392 | 746 | 0.51 | 2.13 × 10−7 |
| SbCu18 | 2.98 | 48.5 | 307 | 0.98 | 0.676 | 1140 | 0.50 | 3.69 × 10−7 |
| Cu | 4.77 | 26.3 | 24.1 | 0.92 | 1.22 | 195 | 0.49 | 9.21 × 10−6 |
| Sb | 5.53 | 7.32 | 124.4 | 0.70 | 1.68 | 72.4 | 0.80 | 3.10 × 10−4 |
| Sample | Ecorr, V vs. Ag/AgCl | Jcorr, µA cm−2 |
|---|---|---|
| SbCu30 | −0.008 ± 0.044 | 9.50 ± 2.2 |
| SbCu26 | −0.023 ± 0.018 | 25.2 ± 5.1 |
| SbCu21 | −0.012 ± 0.004 | 12.1 ± 1.5 |
| SbCu18 | −0.021 ± 0.011 | 23.2 ± 9.6 |
| Sb | −0.080 ± 0.007 | 23.0 ± 2.7 |
| Cu | 0.013 ± 0.005 | 5.44 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, V.; Tzaneva, B. Characterization and Electrochemical Investigation of Heterogeneous Sb-Cu Coatings. Coatings 2023, 13, 1540. https://doi.org/10.3390/coatings13091540
Kostov V, Tzaneva B. Characterization and Electrochemical Investigation of Heterogeneous Sb-Cu Coatings. Coatings. 2023; 13(9):1540. https://doi.org/10.3390/coatings13091540
Chicago/Turabian StyleKostov, Vasil, and Boriana Tzaneva. 2023. "Characterization and Electrochemical Investigation of Heterogeneous Sb-Cu Coatings" Coatings 13, no. 9: 1540. https://doi.org/10.3390/coatings13091540
APA StyleKostov, V., & Tzaneva, B. (2023). Characterization and Electrochemical Investigation of Heterogeneous Sb-Cu Coatings. Coatings, 13(9), 1540. https://doi.org/10.3390/coatings13091540









