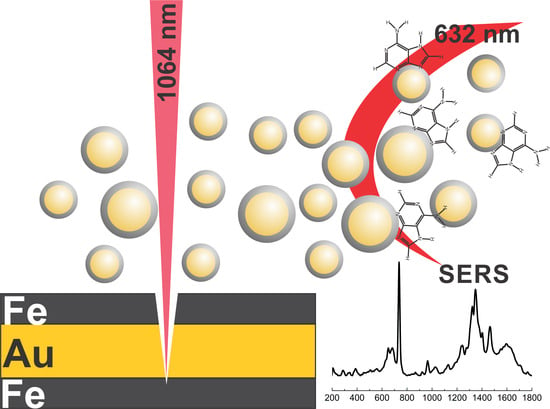
Magneto-Plasmonic Nanoparticles Generated by Laser Ablation of Layered Fe/Au and Fe/Au/Fe Composite Films for SERS Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Target Preparation
2.3. Laser Ablation Procedure
2.4. Sample Characterization and Preparation for SERS Measurements
2.5. Density Functional Theory Modelling
3. Results
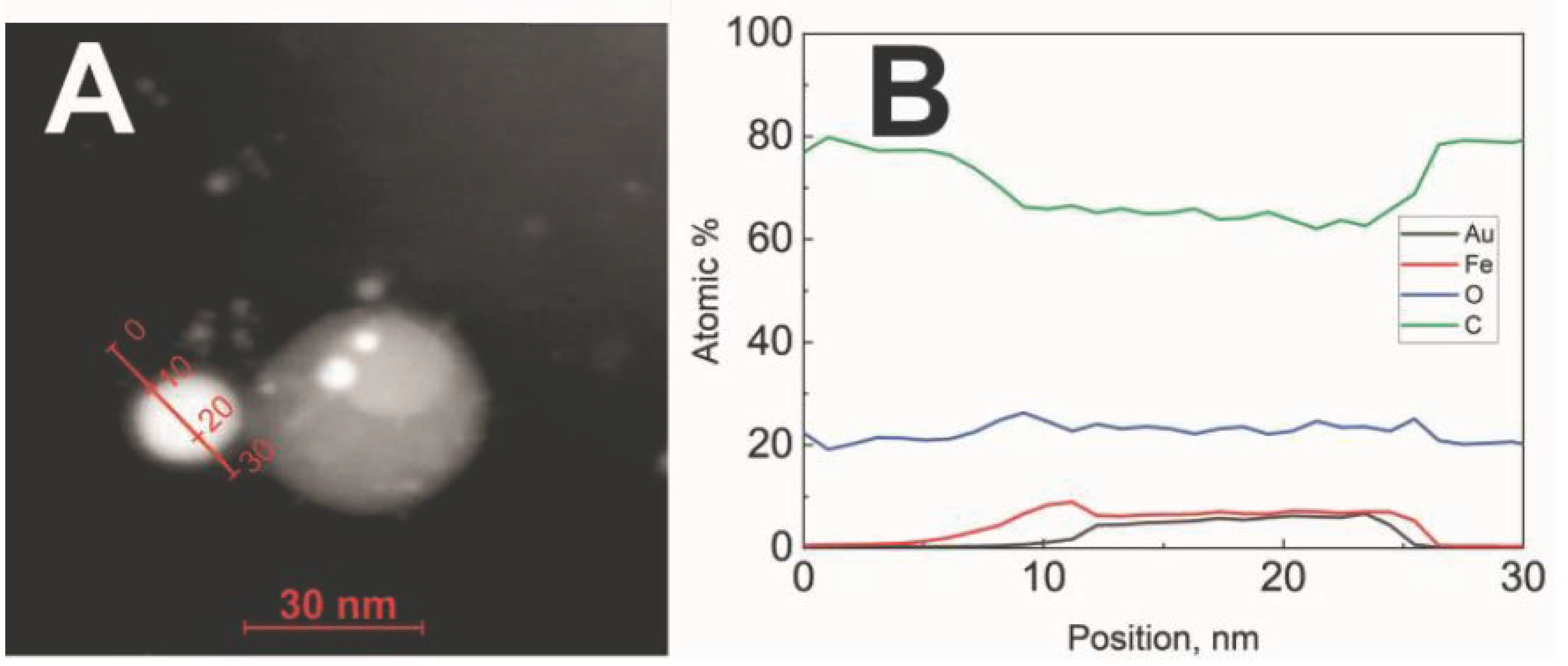
3.1. Structural and Magnetic Characterization of Magneto-Plasmonic Nanoparticles
3.2. UV-VIS Spectroscopic Analysis
3.3. SERS of Magneto-Plasmonic Nanoparticles
3.4. SERS of Adenine Adsorbed at Magneto-Plasmonic Nanoparticles
3.5. DFT Modelling of SERS Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, V.T.; Kim, J.; Tufa, L.T.; Oh, S.; Kwon, J.; Lee, J. Magnetoplasmonic Nanomaterials for Biosensing/Imaging and in Vitro/in Vivo Biousability. Anal. Chem. 2018, 90, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Xu, F.; Wang, L. A review of the preparation and application of magnetic nanoparticles for surface-enhanced Raman scattering. J. Mater. Sci. 2018, 53, 8677–8698. [Google Scholar] [CrossRef]
- Lim, J.; Majetich, S.A. Composite magnetic-plasmonic nanoparticles for biomedicine: Manipulation and imaging. Nano Today 2013, 8, 98–113. [Google Scholar] [CrossRef]
- Wang, C.; Meloni, M.M.; Wu, X.; Zhuo, M.; He, T.; Wang, J.; Wang, C.; Dong, P. Magnetic-plasmonic particles for SERS-based bacteria sensing: A review. AIP Adv. 2019, 9, 010701. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Materon, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. 2012, 6, 100163. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- He, Z.; Li, F.; Zuo, P.; Tian, H. Principles and Applications of Resonance Energy Transfer Involving Noble Metallic Nanoparticles. Materials 2023, 16, 3083. [Google Scholar] [CrossRef]
- Muntean, C.M.; Cuibus, D.; Boca, S.; Falamas, A.; Tosa, N.; Brezestean, I.A.; Bende, A.; Barbu-Tudoran, L.; Moldovan, R.; Bodoki, E.; et al. Gold vs. Silver Colloidal Nanoparticle Films for Optimized SERS Detection of Propranolol and Electrochemical-SERS Analyses. Biosensors 2023, 13, 530. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Martin, C.S.; Constantino, C.J.L. Gold conjugated-magnetite nanoparticles for magnetic concentration towards reproducibility and repeatability of SERS measurements. Colloids Surf. A Physicochem. Eng. 2023, 671, 131661. [Google Scholar] [CrossRef]
- Multari, C.; Miola, M.; Laviano, F.; Gerbaldo, R.; Pezzotti, G.; Debellis, D.; Verné, E. Magnetoplasmonic Nanoparticles for Photothermal Therapy. Nanotechnology 2019, 30, 255705. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.; Serrano Garcia, R.; Gun’ko, Y.K. Multimodal Magnetic-Plasmonic Nanoparticles for Biomedical Applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Raymond, A.; Yndart, A.; Kaushik, A.; Jayant, R.D.; Takemura, Y.; Cai, Y.; Toborek, M.; Nair, M. Development of Magneto-Plasmonic Nanoparticles for Multimodal Image-Guided Therapy to the Brain. Nanoscale 2017, 9, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, N.; Xu, L.; Wang, L.; Li, Z.; Ma, W.; Zhu, Y.; Xu, C.; Kotov, N.A. Multifunctional Magnetoplasmonic Nanoparticle Assemblies for Cancer Therapy and Diagnostics (Theranostics). Macromol. Rapid Commun. 2010, 31, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Urries, I.; Muñoz, C.; Gomez, L.; Marquina, C.; Sebastian, V.; Arruebo, M.; Santamaria, J. Magneto-Plasmonic Nanoparticles as Theranostic Platforms for Magnetic Resonance Imaging, Drug Delivery and NIR Hyperthermia Applications. Nanoscale 2014, 6, 9230–9240. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liang, M.; You, R.; Li, T.; Zhu, L. Femtosecond Laser Fabrication of Noble Metal Plasma Nanostructures and Its Application-A Mini Review. Front. Phys. 2022, 10, 918372. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Stankevičius, E.; Garliauskas, M.; Laurinavičius, L.; Trusovas, R.; Tarasenko, N.; Pauliukaitė, R. Engineering Electrochemical Sensors Using Nanosecond Laser Treatment of Thin Gold Film on ITO Glass. Electrochim. Acta 2019, 297, 511–522. [Google Scholar] [CrossRef]
- Stankevičius, E.; Ignatjev, I.; Petrikaitė, V.; Selskis, A.; Niaura, G. Gold Nanoparticles Generated Using the Nanosecond Laser Treatment of Multilayer Films and Their Use for SERS Applications. ACS Omega 2021, 6, 33889–33898. [Google Scholar] [CrossRef]
- Petrikaitė, V.; Skapas, M.; Stankevičius, E. Generation of gold and silver nanoparticles using laser ablation of thin bimetallic films and bulk targets in water. Opt. Mater. 2023, 137, 113535. [Google Scholar] [CrossRef]
- Verma, A.K.; Soni, R.K. Laser ablation synthesis of bimetallic gold-palladium core@shell nanoparticles for trace detection of explosives. Opt. Laser Technol. 2023, 163, 109429. [Google Scholar] [CrossRef]
- Ondieki, A.M.; Birech, Z.; Kaduki, K.A.; Mwangi, P.W.; Mwenze, N.M.; Juma, M.; Jeptoo, C.; Dlamini, M.S.; Maaza, M. Fabrication of surface-enhanced Raman spectroscopy substrates using silver nanoparticles produced by laser ablation in liquids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 296, 122694. [Google Scholar] [CrossRef] [PubMed]
- Rathod, J.; Byram, C.; Kanaka, R.K.; Bharati, M.S.S.; Banerjee, D.; Akkanaboina, M.; Soma, V.R. Hybrid Surface-Enhanced Raman Scattering Substrates for the Trace Detection of Ammonium Nitrate, Thiram, and Nile Blue. ACS Omega 2022, 7, 15969–15981. [Google Scholar] [CrossRef] [PubMed]
- Szymborski, T.; Stepanenko, Y.; Nicinski, K.; Piecyk, P.; Berus, S.M.; Adamczyk-Poplawska, M.; Kaminska, A. Ultrasensitive SERS platform made via femtosecond laser micromachining for biomedical applications. J. Mater. Res. Technol. 2021, 12, 1496–1507. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, T.H.; Nguyen, T.B. Silver nanostructure on ablated silicon wafer prepared via pulsed laser ablation for surface enhanced Raman spectroscopy. J. Raman Spectrosc. 2022, 53, 1039–1047. [Google Scholar] [CrossRef]
- Yan, Z.; Chrisey, D.B. Pulsed Laser Ablation in Liquid for Micro-/Nanostructure Generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in lasergenerated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef]
- Itina, T.E. On Nanoparticle Formation by Laser Ablation in Liquids. J. Phys. Chem. C 2011, 115, 5044–5048. [Google Scholar] [CrossRef]
- Zhu, X.P.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Jiang, W.; Niihara, K. Underwater Laser Ablation Approach to Fabricating Monodisperse Metallic Nanoparticles. Chem. Phys. Lett. 2006, 427, 127–131. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-Plasmonic Au-Fe Alloy Nanoparticles Designed for Multimodal SERS-MRI-CT Imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Tymoczko, A.; Kamp, M.; Prymak, O.; Rehbock, C.; Jakobi, J.; Schürmann, U.; Kienle, L.; Barcikowski, S. How the Crystal Structure and Phase Segregation of Au–Fe Alloy Nanoparticles Are Ruled by the Molar Fraction and Size. Nanoscale 2018, 10, 16434–16437. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Carraro, F.; Cattaruzza, E. Formation of Alloy Nanoparticles by Laser Ablation of Au/Fe Multilayer Films in Liquid Environment. J. Colloid Interface Sci. 2017, 489, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Tymoczko, A.; Kamp, M.; Rehbock, C.; Kienle, L.; Cattaruzza, E.; Barcikowski, S.; Amendola, V. One-step synthesis of Fe-Au core-shell magnetic-plasmonic nanoparticles driven by interface energy minimization. Nanoscale Horiz. 2019, 4, 1326–1332. [Google Scholar] [CrossRef]
- Byram, C.; Rathod, J.; Moram, S.S.B.; Mangababu, A.; Soma, V.R. Picosecond Laser-Ablated Nanoparticles Loaded Filter Paper for SERS-Based Trace Detection of Thiram, 1,3,5-Trinitroperhydro-1,3,5-triazine (RDX), and Nile Blue. Nanomaterials 2022, 12, 2150. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Jiang, L.; Li, X.; Tian, M.Y.; Yuan, Y.J.; Han, W.N.; Ma, L.; Hu, L.F.; He, Z.H.; Li, F. MoS2 core-shell nanoparticles prepared through liquid-phase ablation and light exfoliation of femtosecond laser for chemical sensing. Sci. China Technol. Sci. 2023, 66, 853–862. [Google Scholar] [CrossRef]
- Vendamani, V.S.; Beeram, R.; Soma, V.R. MoS2 nanosheets decorated plasmonic silicon nanowires as SERS substrates for ultra-sensitive multiple analyte detection. J. Alloys Compd. 2023, 959, 170573. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Hadi, I.H.; Jawad, M.F.; Alwan, A.M. Accurate Synthesis and Performance of SERS Sensing of Au/Ag Nanocomposites Site-Deposited on Apple Surfaces. Plasmonics 2023. [Google Scholar] [CrossRef]
- Qayyum, H.; Amin, S.; Ahmed, W.; Mohamed, T.; Rehman, Z.U.; Hussain, S. Laser-based two-step synthesis of Au-Ag alloy nanoparticles and their application for surface-enhanced Raman spectroscopy (SERS) based detection of rhodamine 6G and urea nitrate. J. Mol. Liq. 2022, 365, 120120. [Google Scholar] [CrossRef]
- Hammad, Q.K.; Ayyash, A.N.; Mutlak, F.A.H. Improving SERS substrates with Au/Ag-coated Si nanostructures generated by laser ablation synthesis in PVA. J. Opt. 2022, 52, 1528–1536. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Li, P.; Tian, Z.; Liang, C. Recent Advances in Surfactant-Free, Surface-Charged, and Defect-Rich Catalysts Developed by Laser Ablation and Processing in Liquids. ChemNanoMat 2017, 3, 512–533. [Google Scholar] [CrossRef]
- Nasiri, H.; Dorranian, D.; Sari, A.H. Green laser assisted gold-iron oxide nanocomposite production, Radiation Effects and Defects in Solids. Radiat. Eff. Defects Solids 2022, 177, 277–293. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Giorgetti, E. Spectroscopic and Microscopic Analyses of Fe3O4/Au Nanoparticles Obtained by Laser Ablation in Water. Nanomaterials 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Mikoliunaite, L.; Talaikis, M.; Michalowska, A.; Dobilas, J.; Stankevic, V.; Kudelski, A.; Niaura, G. Thermally stable magneto-plasmonic nanoparticles for SERS with tunable plasmon resonance. Nanomaterials 2022, 12, 2860. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.G. Determination of size and concentration of gold nanoparticles from extinction spectra. Anal. Chem. 2008, 80, 6620–6625. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent Advances in Laser Utilization in the Chemical Modification of Graphene Oxide and Its Applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Smith, M.W.; Dallmeyer, I.; Johnson, T.J.; Brauer, C.S.; McEwen, J.S.; Espinal, J.F.; Garcia-Perez, M. Structural Analysis of Char by Raman Spectroscopy: Improving Band Assignments through Computational Calculations from First Principles. Carbon 2016, 100, 678–692. [Google Scholar] [CrossRef]
- Vasquez, A.; Samolis, P.; Zeng, J.; Sander, M.Y. Micro-Structuring, Ablation, and Defect Generation in Graphene with Femtosecond Pulses. OSA Contin. 2019, 2, 2925. [Google Scholar] [CrossRef]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic nanoparticles of iron carbide, iron oxide, iron@iron oxide, and metal iron synthesized by laser ablation in organic solvents. J. Phys. Chem. C 2011, 115, 5140–5146. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman Spectroscopic Study of Magnetite (FeFe2O4): A New Assignment for the Vibrational Spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman investigation of iron oxide films produced by sol-gel synthesis. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C. Surface-enhanced Raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3236–3266. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; John Wiley & Sons: Chichester, UK, 2005. [Google Scholar]
- Shumskaya, A.; Korolkov, I.; Rogachev, A.; Ignatovich, Z.; Kozlovskiy, A.; Zdorovets, M.; Anisovich, M.; Bashouti, M.; Shalabny, A.; Busool, R.; et al. Synthesis of Ni@Au core-shell magnetic nanotubes for bioapplication and SERS detection. Colloids Surf. A. 2021, 626, 127077. [Google Scholar] [CrossRef]
- Li, Z.H.; Bai, J.H.; Zhang, X.; Lv, J.M.; Fan, C.S.; Zhao, Y.M.; Wu, Z.L.; Xu, H.J. Facile synthesis of Au nanoparticle-coated Fe3O4 magnetic composite nanospheres and their application in SERS detection of malachite green. Spectrochim. Acta A 2020, 241, 118532. [Google Scholar] [CrossRef]
- Wang, L.-P.; Huang, Y.-B.; Lai, Y.-H. Surface enhanced Raman scattering activity of dual-functional Fe3O4/Au composites. Appl. Surf. Sci. 2018, 435, 290–296. [Google Scholar] [CrossRef]
- Ye, M.; Wei, Z.; Hu, F.; Wang, J.; Ge, G.; Hu, Z.; Shao, M.; Lee, S.-T.; Liu, J. Fast assembling microarrays of superparamagnetic Fe3O4@Au nanoparticle clusters as reproducible substrates for surface-enhanced Raman scattering. Nanoscale 2015, 7, 13427–13437. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Lin, H.; Zhang, Z.; Liao, F.; Shao, M.; Lifshitz, Y.; Lee, S.-T. Smart liquid SERS substrates based on Fe3O4/Au nanoparticles with reversibly tunable enhancement factor for practical quantitative detection. Sci. Rep. 2014, 4, 7204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.V.P.; Rangarajan, N.; Sonia, B.; Deepika, P.; Rohman, N.; Narayana, C. Metal-coated magnetic nanoparticles for surface enhanced Raman scattering studies. Bull. Mater. Sci. 2011, 34, 207–216. [Google Scholar] [CrossRef]
- Kanitz, A.; Hoppius, J.S.; Sanz, M.M.; Maicas, M.; Ostendorf, A.; Gurevich, E.L. Synthesis of magnetic nanoparticles by ultrashot pulsed laser ablation of iron in different liquids. ChemPhysChem 2017, 18, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Kanitz, A.; Kalus, M.-R.; Gurevich, E.L.; Ostendorf, A. Review on experimental and theoretical investigations of the early stage, femtoseconds to microseconds processes during laser ablation in liquid-phase for the synthesis of colloidal nanoparticles. Plasma Sources Sci. Technol. 2019, 28, 103001. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Charron, G.; Cortés, E.; Kneipp, J.; de la Chapelle, M.L.; Langer, J.; Procházka, M.; Tran, V.; Schlücker, S. Towards reliable and quantitative surface-enhanced Raman scattering (SERS): From key parameters to good analytical practice. Angew. Chem. Int. Ed. 2020, 59, 5454–5462. [Google Scholar] [CrossRef] [PubMed]
- Niaura, G.; Malinauskas, A. Surface-enhanced Raman spectroscopy of ClO4– and SO42– anions adsorbed at a Cu electrode. J. Chem. Soc. Faraday Trans. 1998, 94, 2205–2211. [Google Scholar] [CrossRef]
- Kundu, J.; Neumann, O.; Janesko, B.G.; Zhang, D.; Lal, S.; Barhoumi, A.; Scuseria, G.E.; Halas, N.J. Adenine- and adenosine monophosphate (AMP)-gold binding interactions studied by surface-enhanced Raman and infrared spectroscopies. J. Phys. Chem. C 2009, 113, 14390–14397. [Google Scholar] [CrossRef]
- Russo, N.; Toscano, M.; Grand, A.; Jolibois, F. Protonation of thymine, cytosine, adenine, and guanine DNA nucleic acid bases: Theoretical investigation into the framework of density functional theory. J. Comput. Chem. 1998, 19, 989–1000. [Google Scholar] [CrossRef]
- Yao, G.; Zhai, Z.; Zhong, J.; Huang, Q. DFT and SERS Study of 15N Full-Labeled Adenine Adsorption on Silver and Gold Surfaces. J. Phys. Chem. C 2017, 121, 9869–9878. [Google Scholar] [CrossRef]
- Pagliai, M.; Caporali, S.; Muniz-Miranda, M.; Pratesi, G.; Schettino, V. SERS, XPS, and DFT study of adenine adsorption on silver and gold surfaces. J. Phys. Chem. Lett. 2012, 3, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Yang, H.-T.; Cui, L.; Wu, D.-Y.; Ren, B.; Tian, Z.-Q. Structural and charge sensitivity of surface-enhanced Raman spectroscopy of adenine on silver surface: A quantum chemical study. J. Phys. Chem. C 2013, 117, 23730–23737. [Google Scholar] [CrossRef]
- Alula, M.T.; Yang, J. Photochemical decoration of silver nanoparticles on magnetic microspheres as substrates for the detection of adenine by surface-enhanced Raman scattering. Anal. Chim. Acta 2014, 812, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Giese, B.; McNaughton, D. Surface-enhanced Raman spectroscopic and density functional theory study of adenine adsorption to silver surfaces. J. Phys. Chem. B 2002, 106, 101–112. [Google Scholar] [CrossRef]
- Lang, X.-F.; Yin, P.-G.; You, T.-T.; Jiang, L.; Guo, L. A DFT investigation of surface-enhanced Raman scattering of adenine and 2′-deoxyadenosine 5′-monophosphate on Ag20 nanoclusters. ChemPhysChem 2011, 12, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Gellini, C.; Deepak, F.L.; Muniz-Miranda, M.; Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Innocenti, C.; Sangregorio, C. Magneto-plasmonic colloidal nanoparticles obtained by laser ablation of nickel and silver targets in water. J. Phys. Chem. C 2017, 121, 3597–3606. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, C.; Wang, J.; Sun, Z.; Xiao, R.; Wang, S. Fe3O4@Ag magnetic nanoparticles for microRNA capture and duplex-specific nuclease signal amplification-based SERS detection in cancer cells. Biosens. Bioelectron. 2016, 79, 574–580. [Google Scholar] [CrossRef]

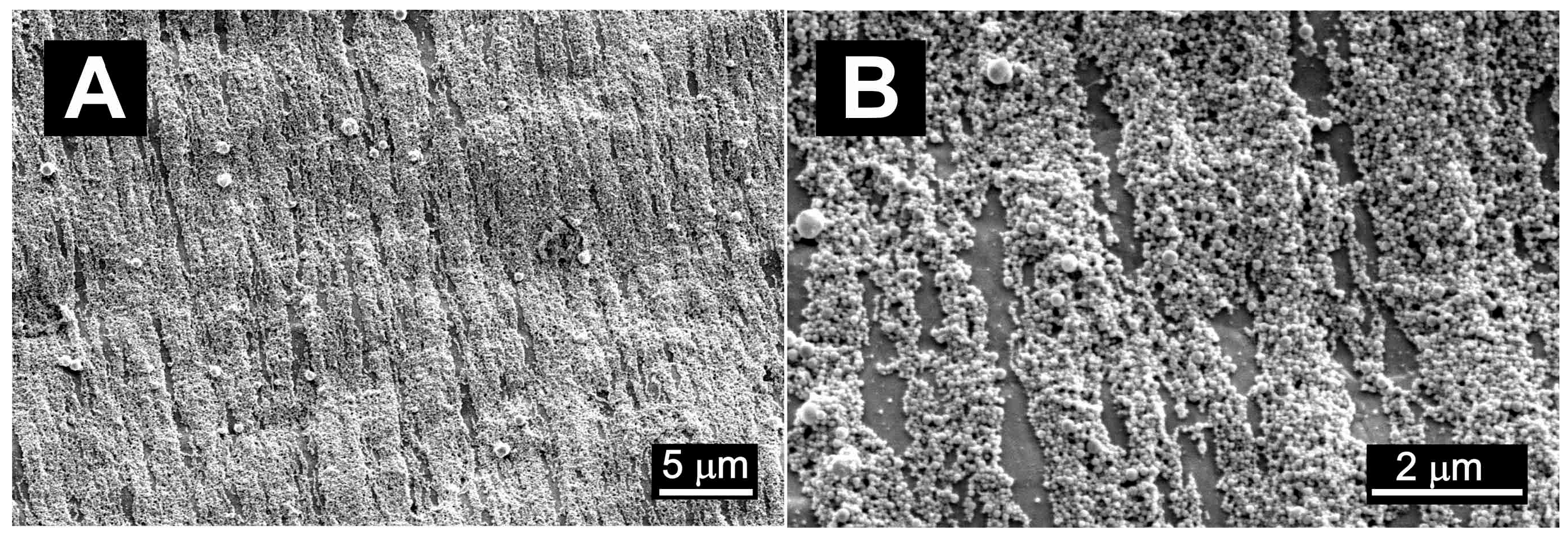
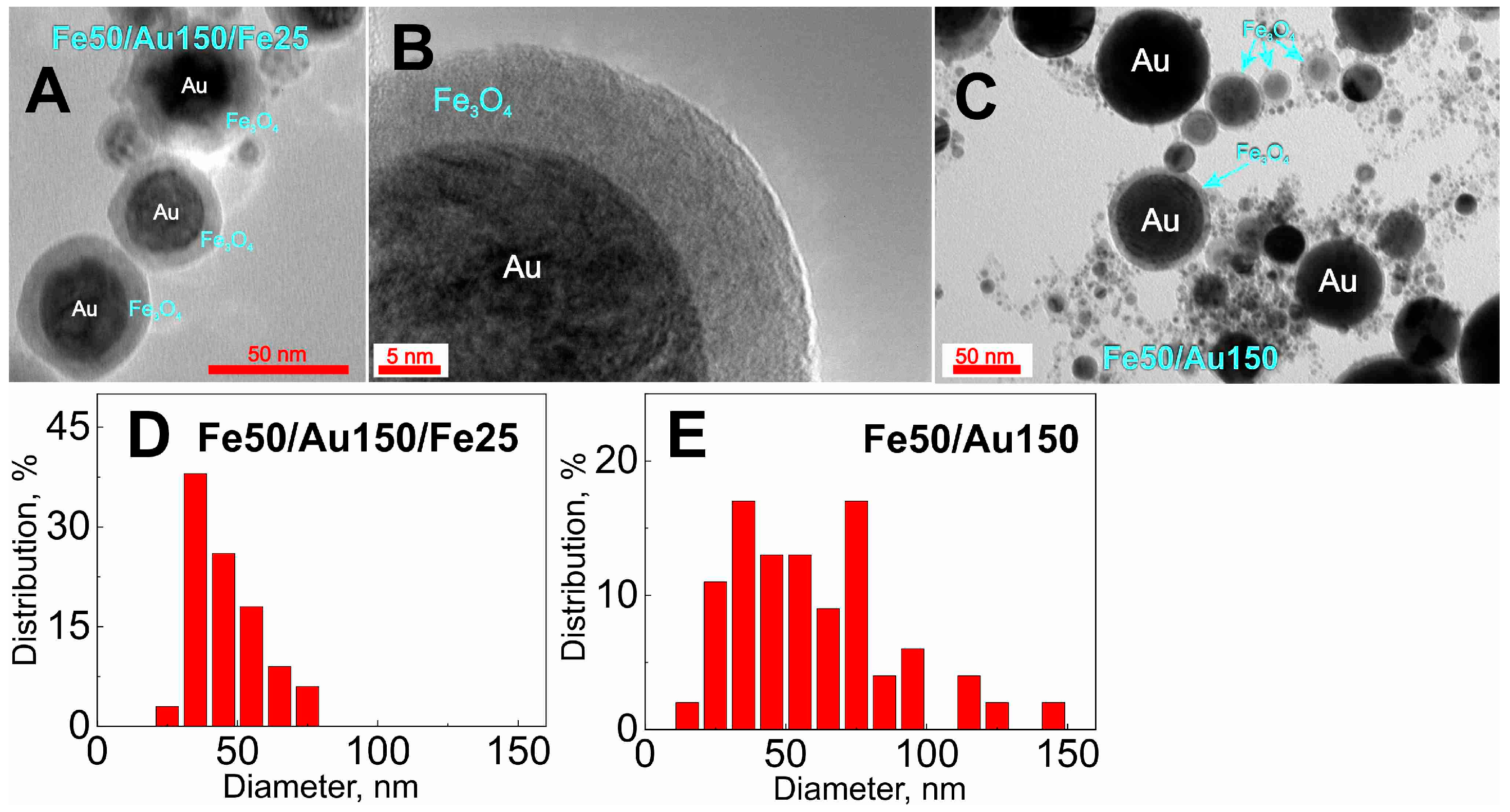

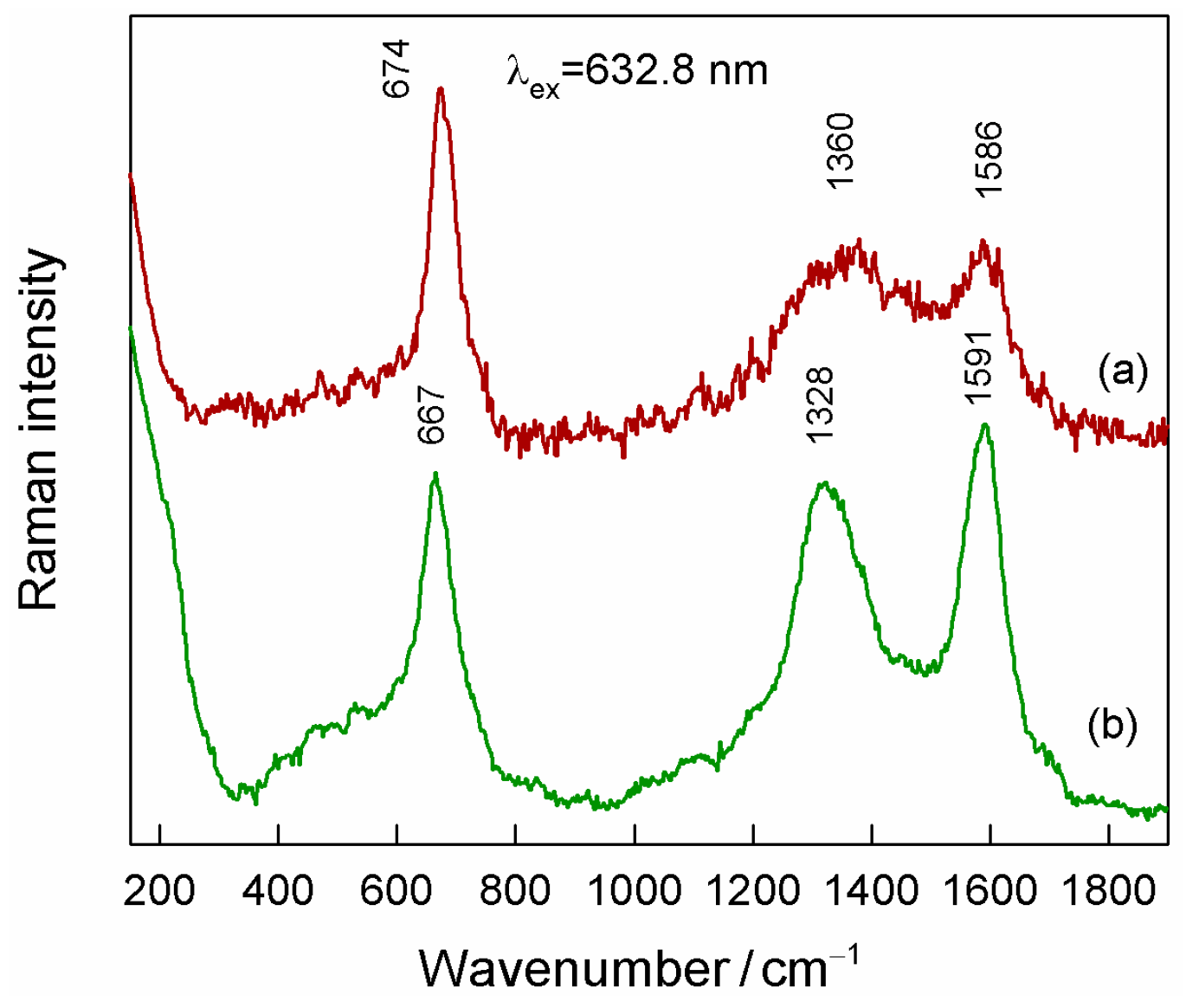



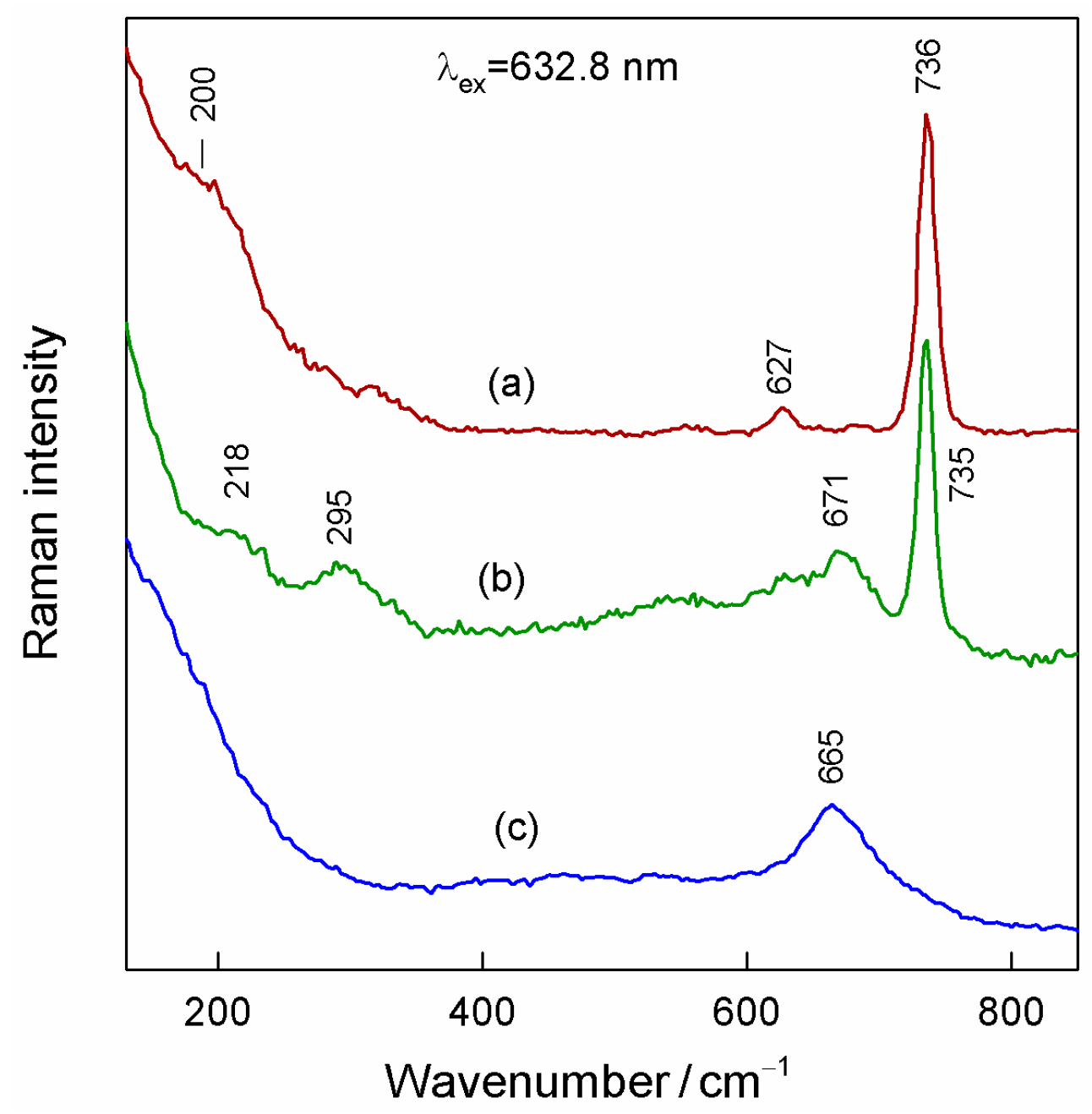
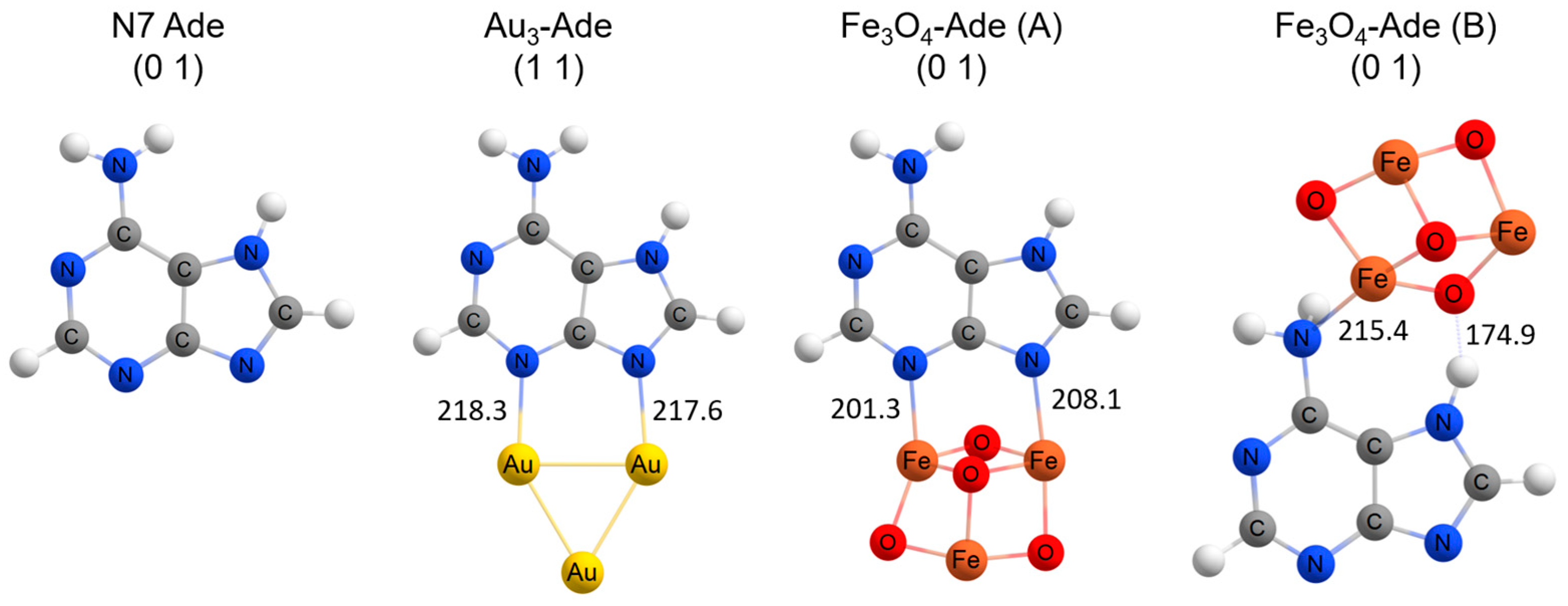
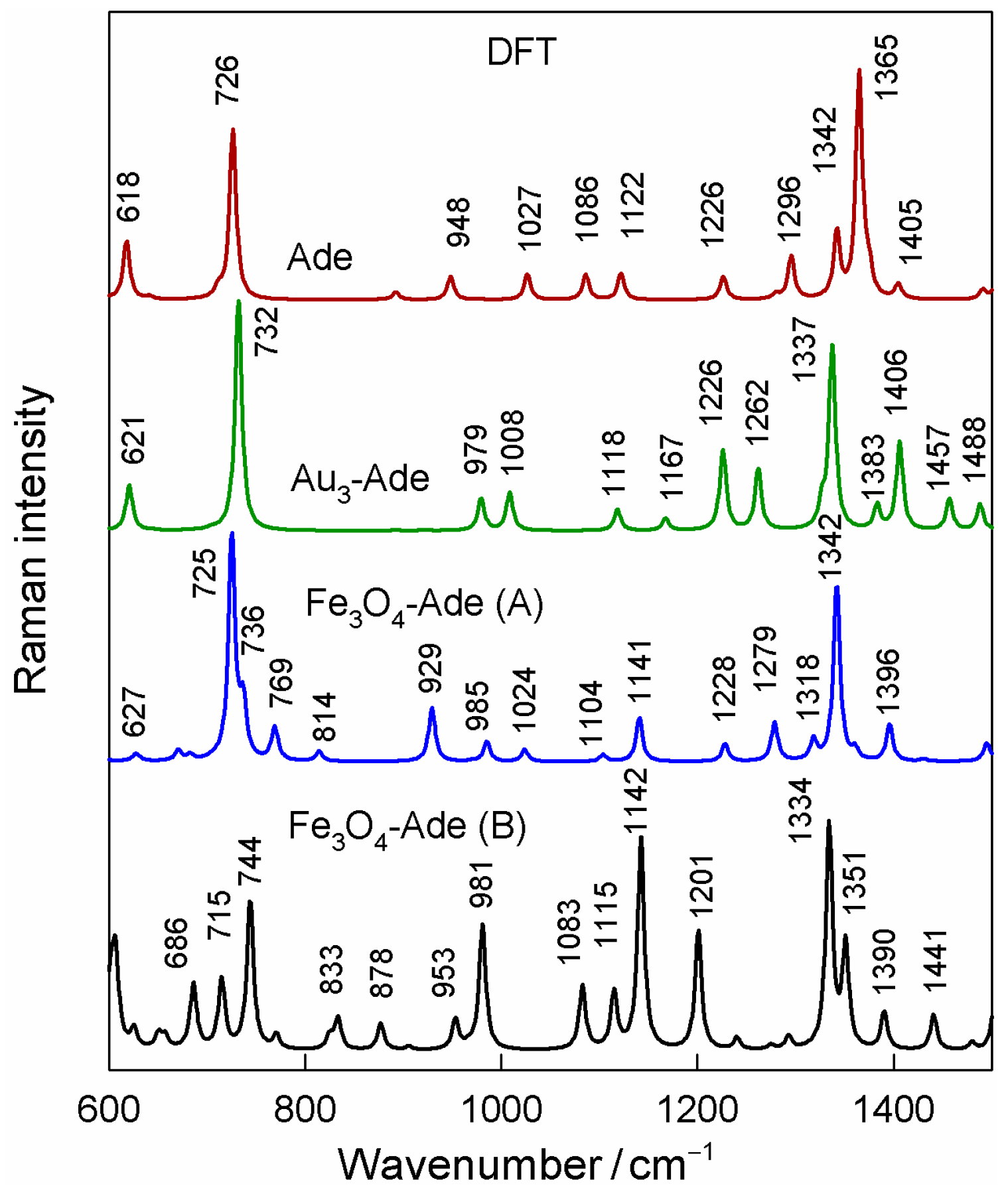
| Denotation | 1st Layer Fe Layer Thickness (nm) | 2nd Layer Au Layer Thickness (nm) | 3rd Layer Fe Layer Thickness (nm) |
|---|---|---|---|
| Fe50/Au150 | 50 | 150 | - |
| Fe100/Au150 | 100 | 150 | - |
| Fe50/Au150/Fe25 | 50 | 150 | 25 |
| Fe50/Au150/Fe50 | 50 | 150 | 50 |
| Solid State | Solution H2O (D2O) | SERS Au | SERS Au-Fe3O4 | Calc. Ade (N7H) | Calc. Au3-Ade(N7H) | Calc. Fe3O4-Ade(N7H) A | Calc. Fe3O4-Ade(N7H) B | Assignments |
|---|---|---|---|---|---|---|---|---|
| 1483 m | 1486 m (1476 m) | 1454 m | 1464 s | 1509 | 1488 | 1495 | 1503 | β(C2H), ν(N1-C6), ν(C2-N3), δ(NH2) |
| 1371 w | 1364 m (1378 w) | 1399 w | 1402 w | 1405 | 1383 | 1396 | 1390 | ν(C4-C5), β(CH2) |
| 1332 s | 1332 s (1329 vs) | 1342 m | 1348 | 1365 | 1337 | 1342 | 1334 | ν(C2-N3), ν(C8-N9), β(C2H) |
| 1248 m | 1251 m (1189 m) | 1236 | 1240 | 1226 | 1226 | 1228 | 1240 | ν(C2-N3), β(C8H), ν(C8-N9) |
| 941 m | 947 m (953 m) | 966 w | 965 m | 948 | 979 | 985 | 981 | δ(N7-C8-N9), ν(N1-C6), r(NH2) |
| 723 vs | 723 vs (707 vs) | 736 vs | 736 vs | 726 | 732 | 725, 736 | 715 | ring breathing, ν(Fe-O) |
| 622 m | 623 m (603 m) | 627 sh | 629 sh | 618 | 621 | 627 | 686 | ν(C5-C6), β(R1), β(R2) |
| 371 | ν(Fe-N10) | |||||||
| 295 m, br | 243, 263 | ν(Fe-N3), ν(Fe-N9), ν(Fe-O) | ||||||
| 218 w, br | 151, 208, 216 | ν(Fe-N3), ν(Fe-N9) | ||||||
| 200 m, br | 187 | ν(Au-N3), ν(Au-N9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikoliunaite, L.; Stankevičius, E.; Adomavičiūtė-Grabusovė, S.; Petrikaitė, V.; Trusovas, R.; Talaikis, M.; Skapas, M.; Zdaniauskienė, A.; Selskis, A.; Šablinskas, V.; et al. Magneto-Plasmonic Nanoparticles Generated by Laser Ablation of Layered Fe/Au and Fe/Au/Fe Composite Films for SERS Application. Coatings 2023, 13, 1523. https://doi.org/10.3390/coatings13091523
Mikoliunaite L, Stankevičius E, Adomavičiūtė-Grabusovė S, Petrikaitė V, Trusovas R, Talaikis M, Skapas M, Zdaniauskienė A, Selskis A, Šablinskas V, et al. Magneto-Plasmonic Nanoparticles Generated by Laser Ablation of Layered Fe/Au and Fe/Au/Fe Composite Films for SERS Application. Coatings. 2023; 13(9):1523. https://doi.org/10.3390/coatings13091523
Chicago/Turabian StyleMikoliunaite, Lina, Evaldas Stankevičius, Sonata Adomavičiūtė-Grabusovė, Vita Petrikaitė, Romualdas Trusovas, Martynas Talaikis, Martynas Skapas, Agnė Zdaniauskienė, Algirdas Selskis, Valdas Šablinskas, and et al. 2023. "Magneto-Plasmonic Nanoparticles Generated by Laser Ablation of Layered Fe/Au and Fe/Au/Fe Composite Films for SERS Application" Coatings 13, no. 9: 1523. https://doi.org/10.3390/coatings13091523
APA StyleMikoliunaite, L., Stankevičius, E., Adomavičiūtė-Grabusovė, S., Petrikaitė, V., Trusovas, R., Talaikis, M., Skapas, M., Zdaniauskienė, A., Selskis, A., Šablinskas, V., & Niaura, G. (2023). Magneto-Plasmonic Nanoparticles Generated by Laser Ablation of Layered Fe/Au and Fe/Au/Fe Composite Films for SERS Application. Coatings, 13(9), 1523. https://doi.org/10.3390/coatings13091523