Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings
Abstract
1. Introduction
- It is a non-line-of-sight technique, and the coating forms on any part of the catalytic surface placed in contact with the plating solution.
- No electric field participates in the reduction process, and a uniform, conformal and homogeneous coating can be obtained on any geometry, regardless of its shape.
- Nickel source: A soluble, hydrated and stable Ni2+ salt. The use of sulfate and acetate gives the same results in terms of coating quality, whereas using chloride negatively influences the corrosion resistance of the produced coatings [17]. Sulphate is the most used since it is generally cheaper than acetate.
- Complexing agent/buffer solution: The introduction of organic acids or their salts have a double function: first, they guarantee a buffer action that stabilizes the pH of the solution; second, the organic base can complex the nickel ions in solution and limit their reactivity, thus increasing bath stability. The ENP process is called autocatalytic because the substrate itself catalyzes the oxidation of the reducing agent and the reduction of the metal cations present in the solution [19]. The deposition reaction usually occurs along with the formation of byproducts that make the deposition process uncontrolled, and chemical stabilization is necessary to avoid the decomposition of the ENP plating bath. In most commercial ENP solutions, the extended service life of the bath is guaranteed by lead addition in ppm [20]. Despite this strategy being remarkably effective, the presence of Pb2+ in the solution causes health hazards and high costs the waste-solution disposal. The study of environmentally friendly alternatives to lead stabilizers is nowadays an important goal to improve ENP applicability and meet the strict environmental regulations towards a more sustainable development. This work presents the formulation, the study and the characterization of lead-free solutions capable of depositing medium phosphorus coatings and their optimization to increase the amount of co-deposited phosphorus to obtain HP coatings. Changes in the chemistry of the plating solution make it possible to manufacture Ni-P coatings with different P content in the alloy, according to the properties required for the specific applications. As an example, Table 1 reports different Ni2+/H2PO2− molar ratios and the consequent wt% of P in the matrix obtained from different recipes presented in a list of works from 1994 to 2020. It can be noted that a similar molar ratio of nickel sulfate and sodium hypophosphite does not have an obvious relation to the content of P inside the matrix. This is because the P quantity does not depend only on the Ni2+/H2PO2− ratio but on the whole system employed for the deposition, including temperature, stabilizers and complexing agents. Although all these parameters have been extensively studied, to the authors’ knowledge, there are currently no works in literature that systematically relate the concentrations of the various reagents to the properties of the coatings. The tailoring of the P content by the change in concentration of reagents in the deposition solution can be a desirable aspect to increase the flexibility and applicability of specific electroless Ni-P baths. Moreover, a comprehensive understanding of the cross-relations between reagents is necessary for a better comprehension of their effect on deposition and coating properties.
2. Materials and Methods
2.1. Surface Preparation
- Pre-cleaning: Performed to remove oils, lubricants or massive oxidation products that can hinder the deposition by reducing the exposed autocatalytic surface. The effect of soak cleaning and/or sandblasting was investigated. In particular, soak cleaning was carried out in a 1 M NaOH solution at 80 °C for 10 min.
- Sandblasting was used to remove the residuals from the soaked cleaning and to increase the roughness of the surface; it was performed by using corundum (mesh 80) as abrasive material.
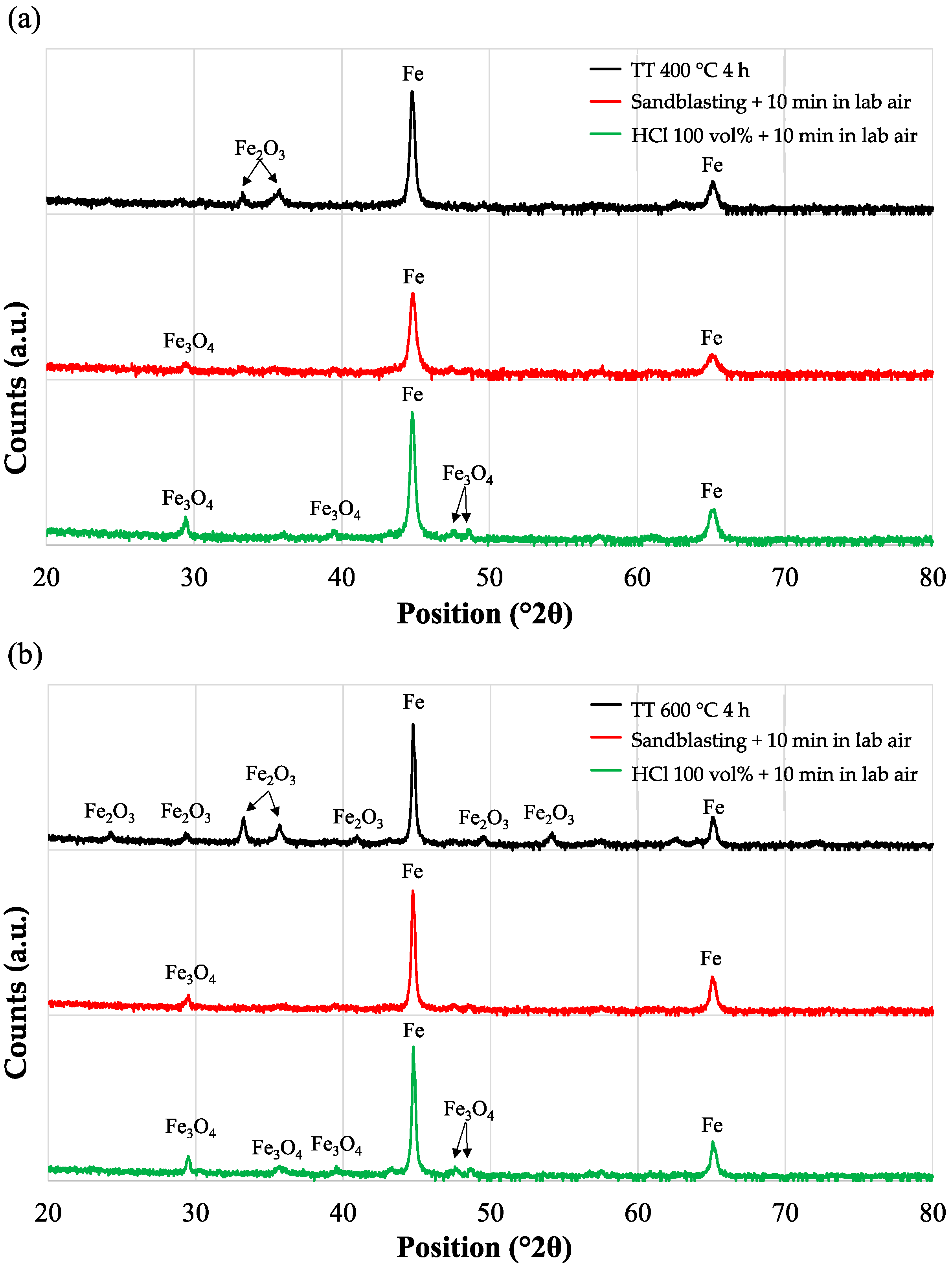
- Acid pickling: The effect of pickling with HCl 37% at different concentrations (30, 40, 50 and 100 vol.% of HCl 37 wt%) was studied to evaluate the best activation solution. The tests were carried out on samples in the as-received condition (AR). Heat treatments in the air at 400 °C and 600 °C for 4 h were carried out to obtain a thermally grown oxide layer and simulate a surface that is not intrinsically active to electroless deposition and evaluate the effectiveness of the different activation solutions. Acid pickling was performed by immersing specimens in 100 mL of acid solution at room temperature for 1 min.
- Water rinse: After every step of the activation procedure, samples were rinsed with deionized water (DW) in an ultrasonic bath for 1 min to remove every contaminant or residue.
2.2. Solution and Coating Preparation
2.3. Coating Characterization
3. Results
3.1. Surface Preparation
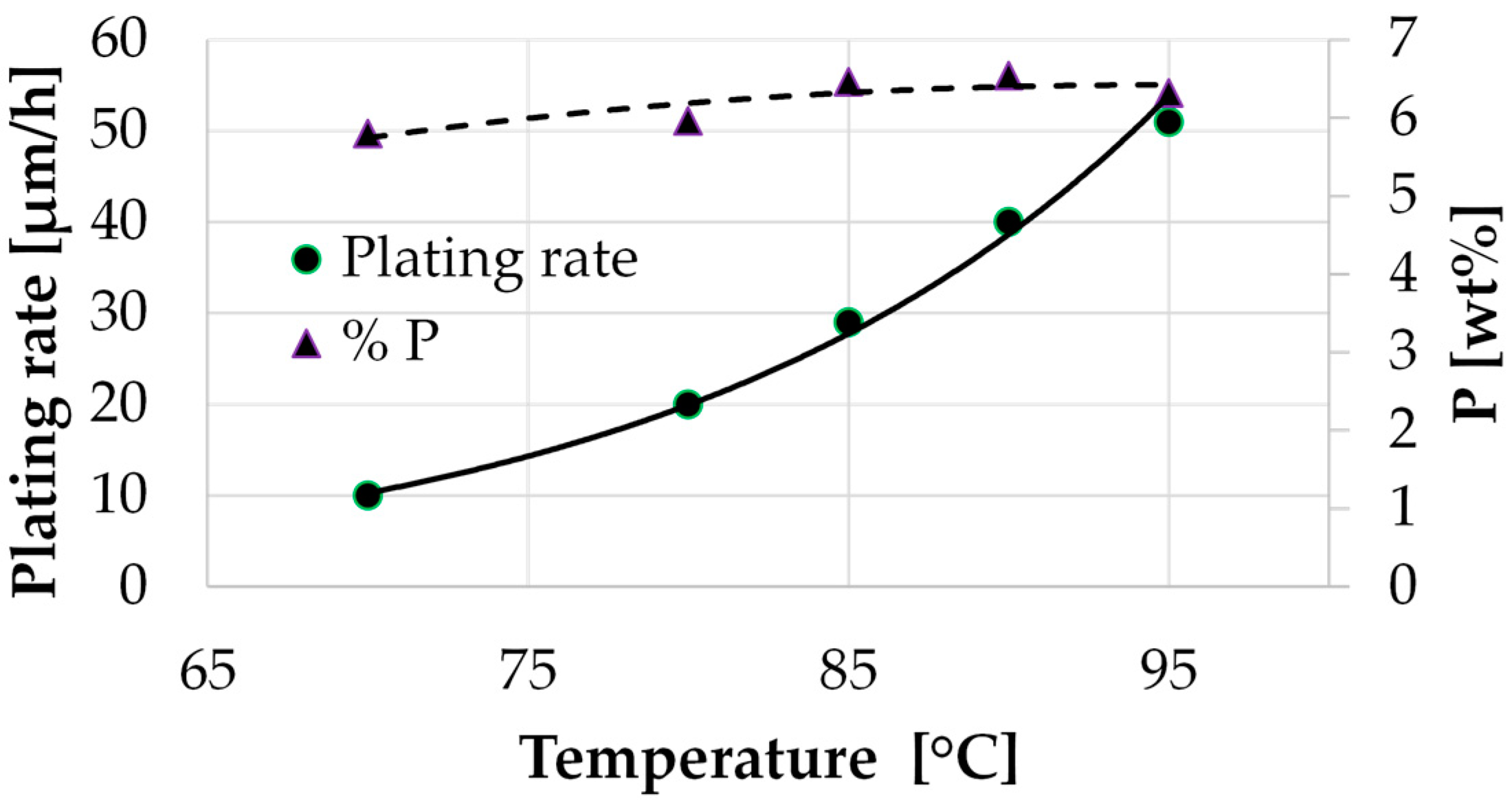
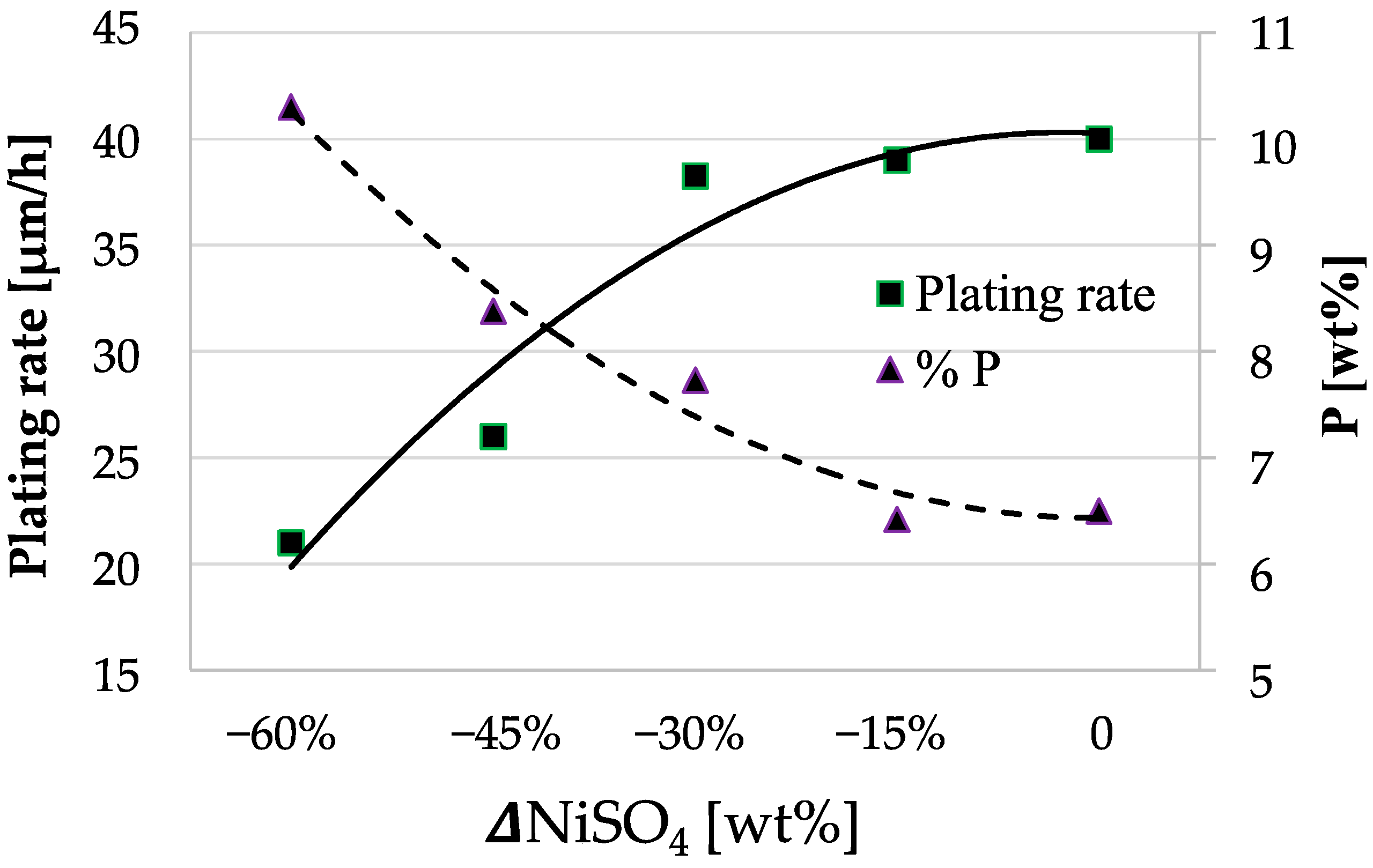
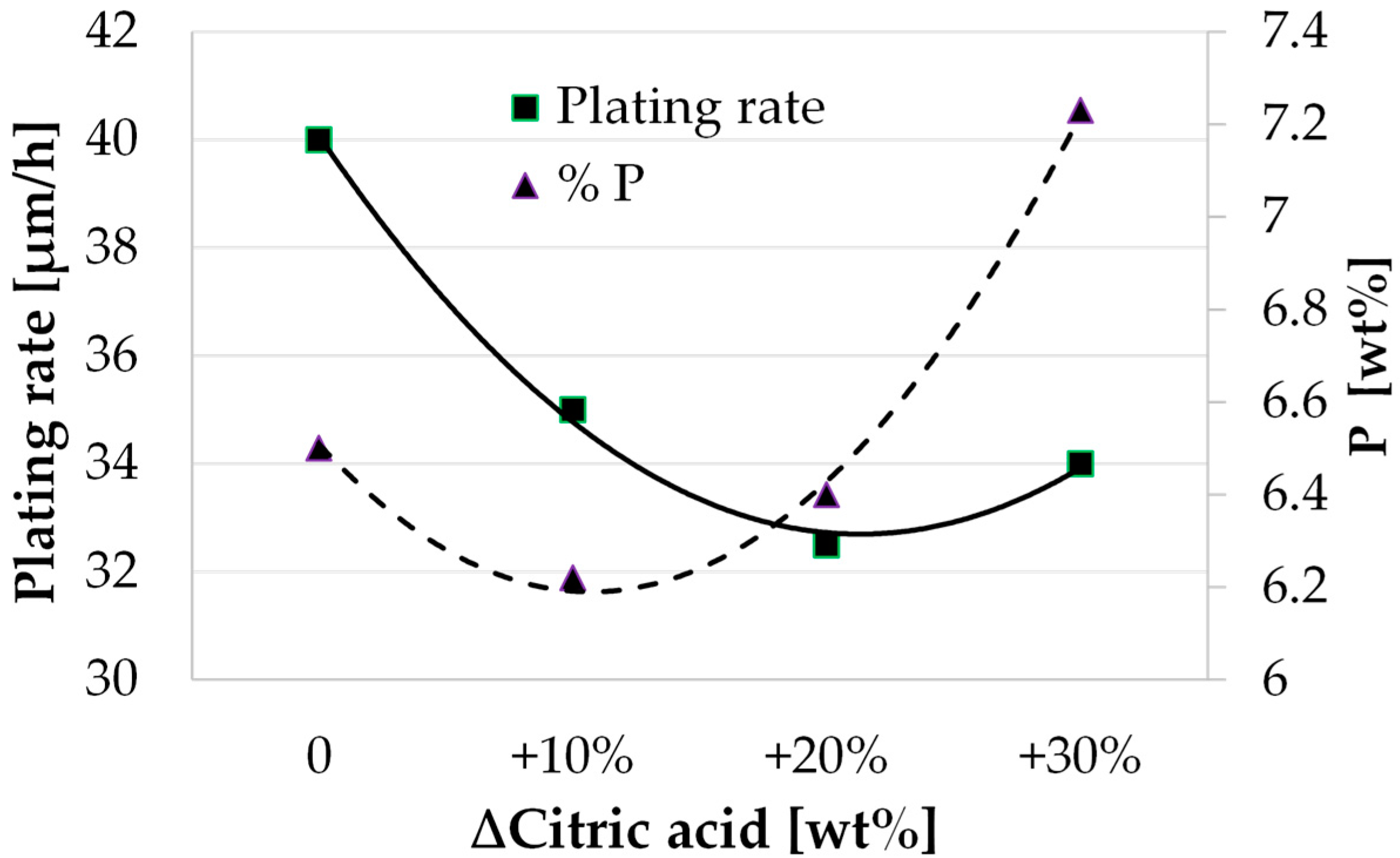
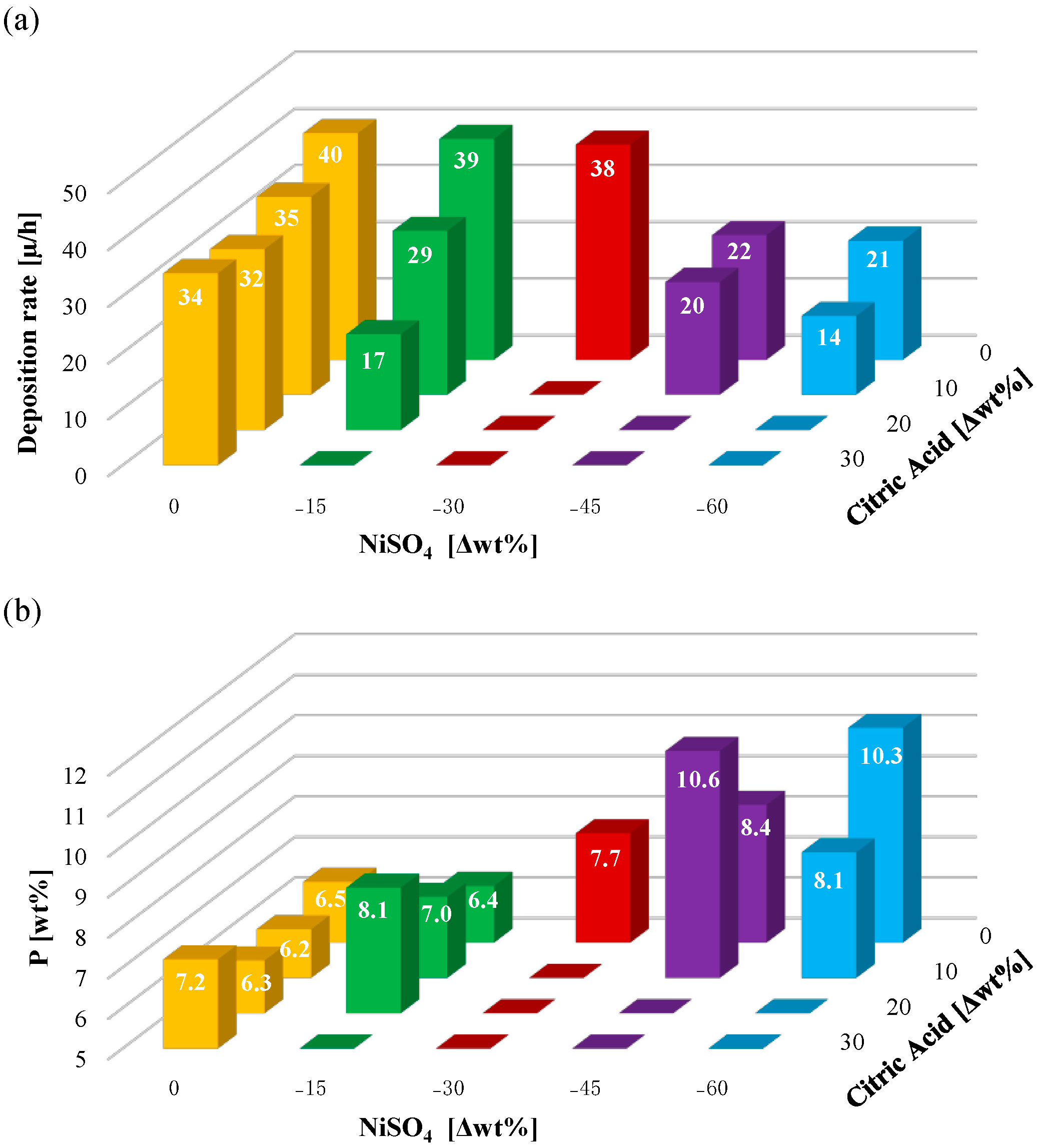
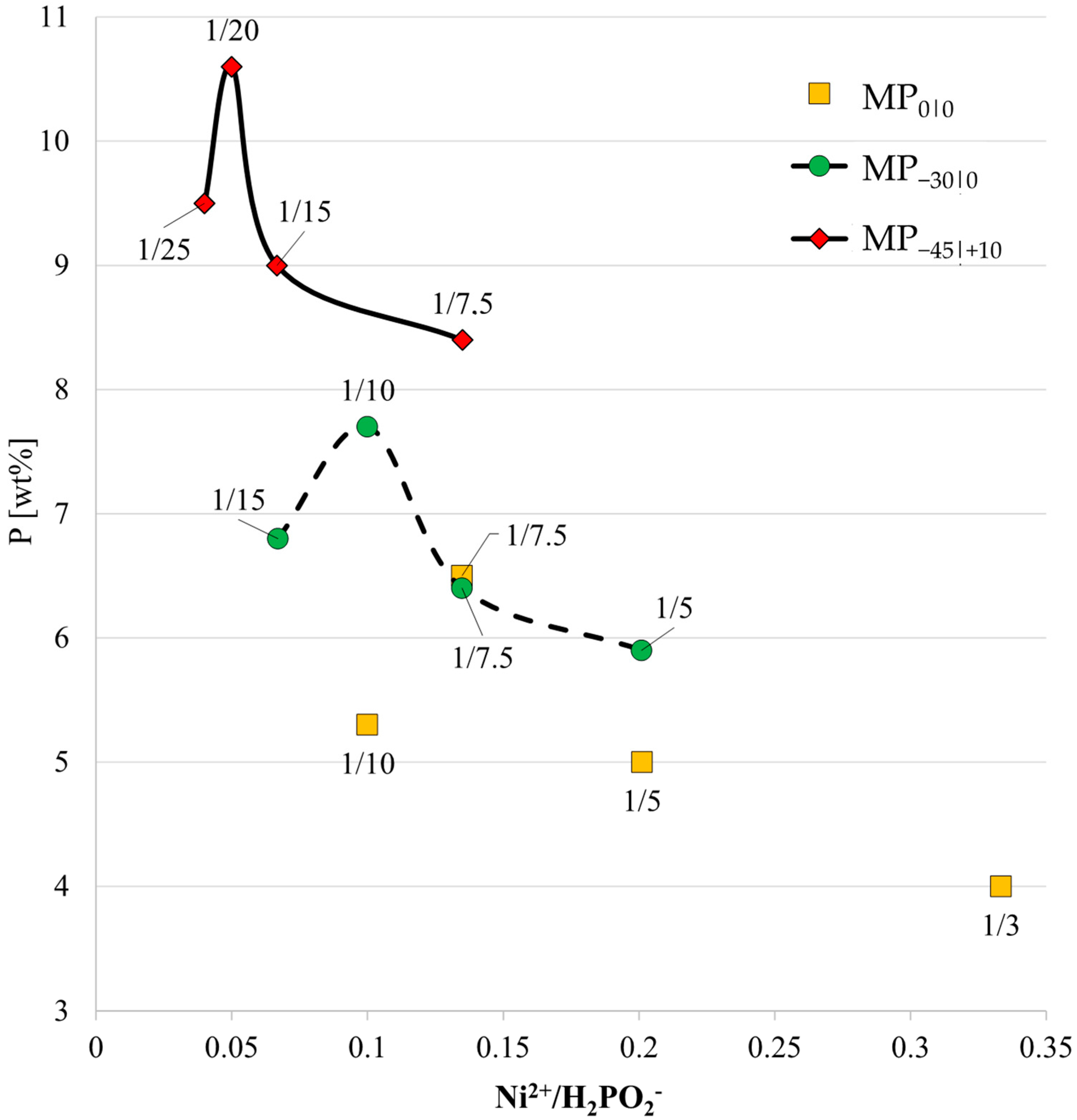
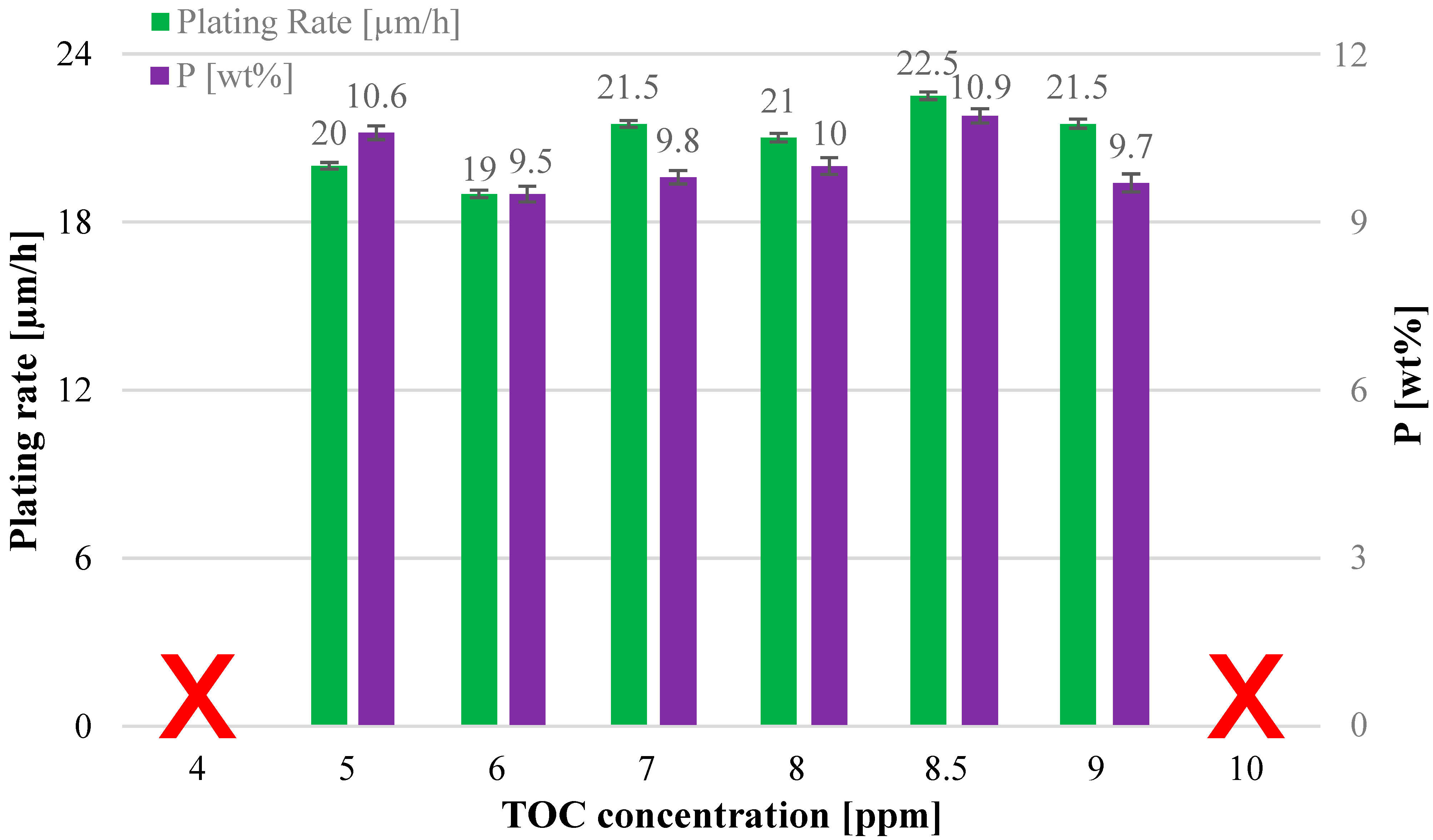
3.2. Study of the Solution
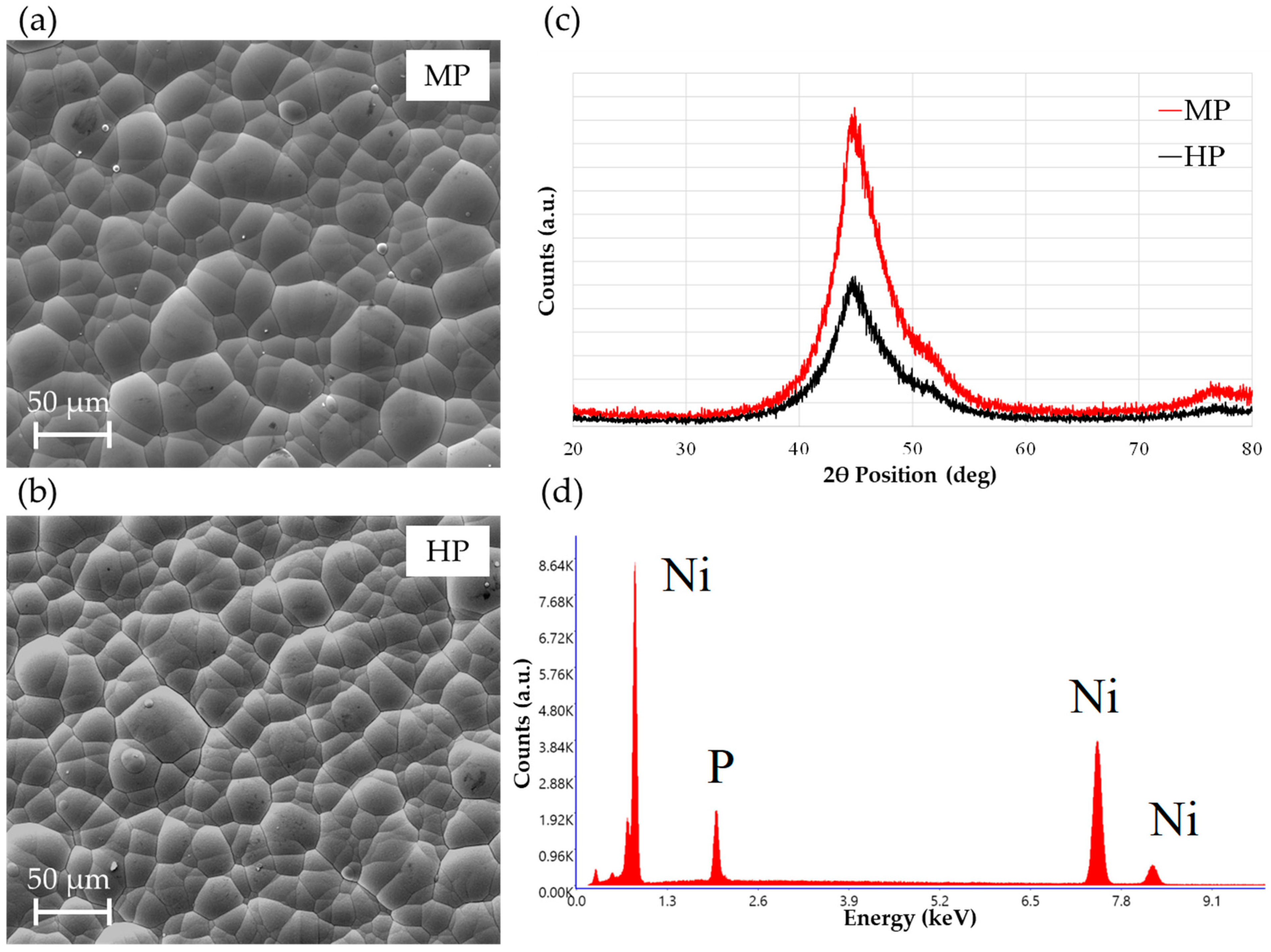
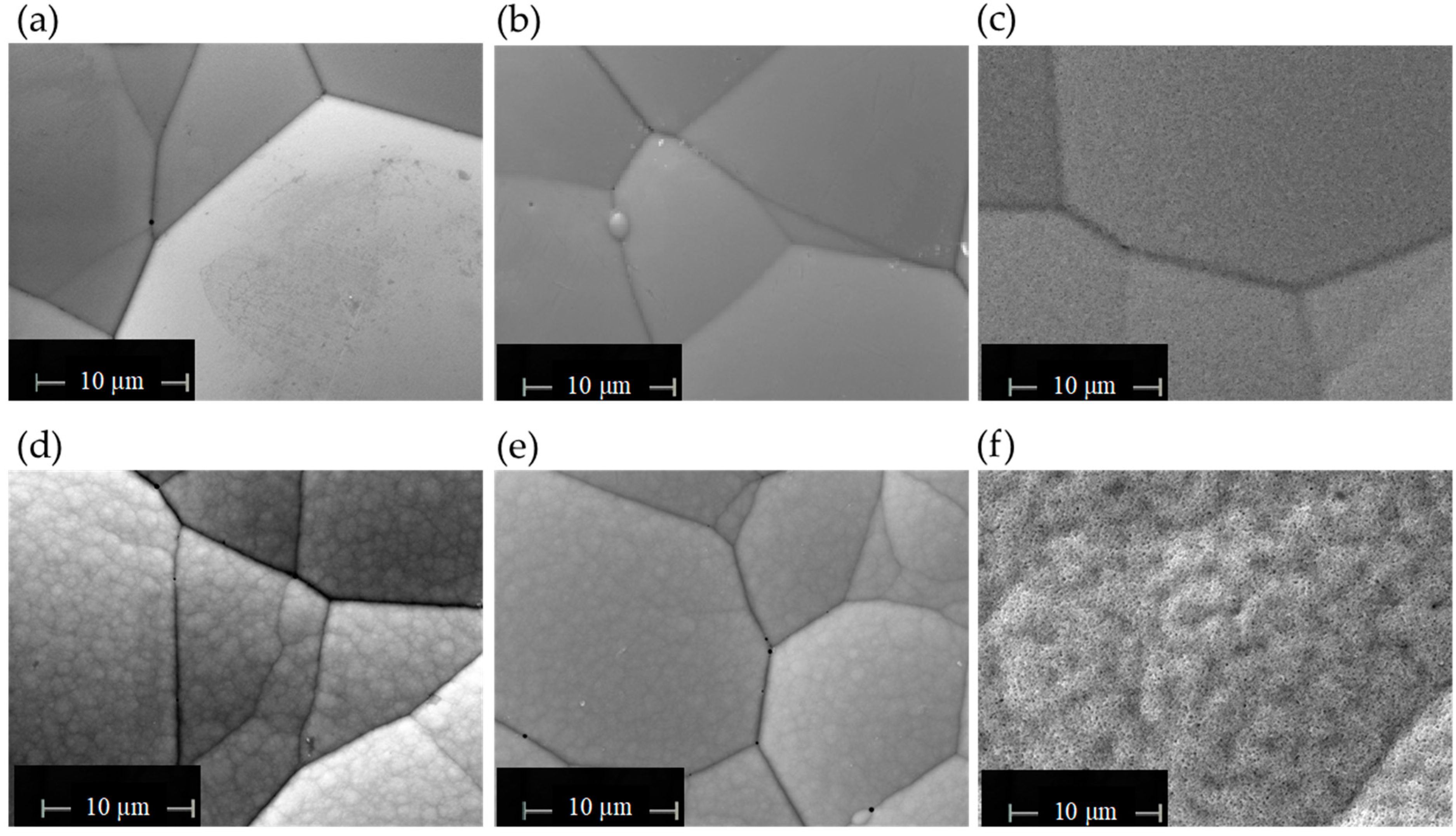
3.3. Coating Microstructure and Composition
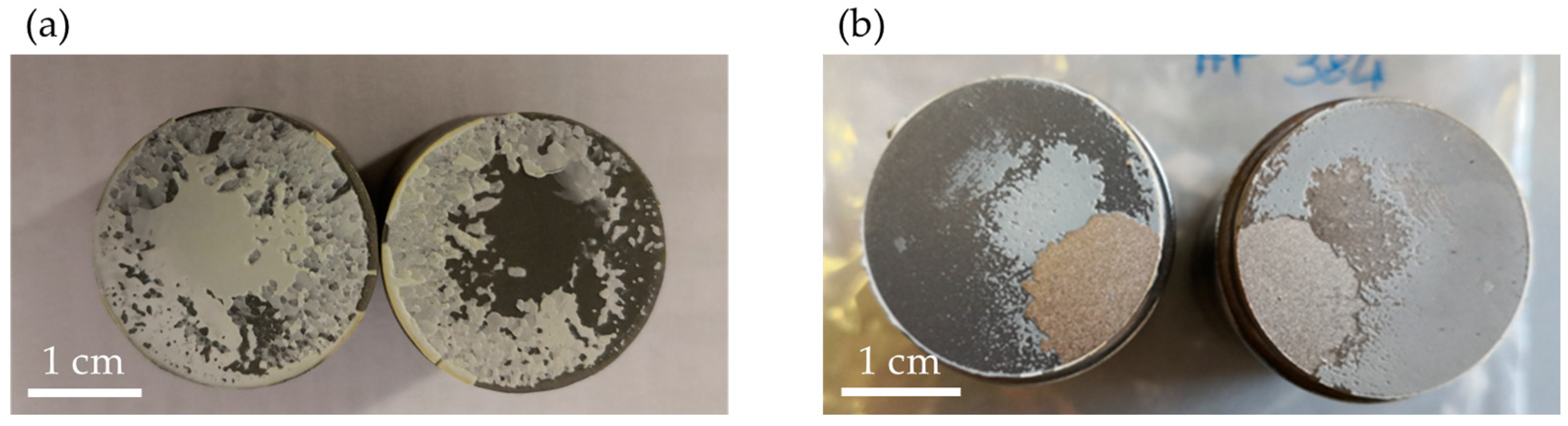
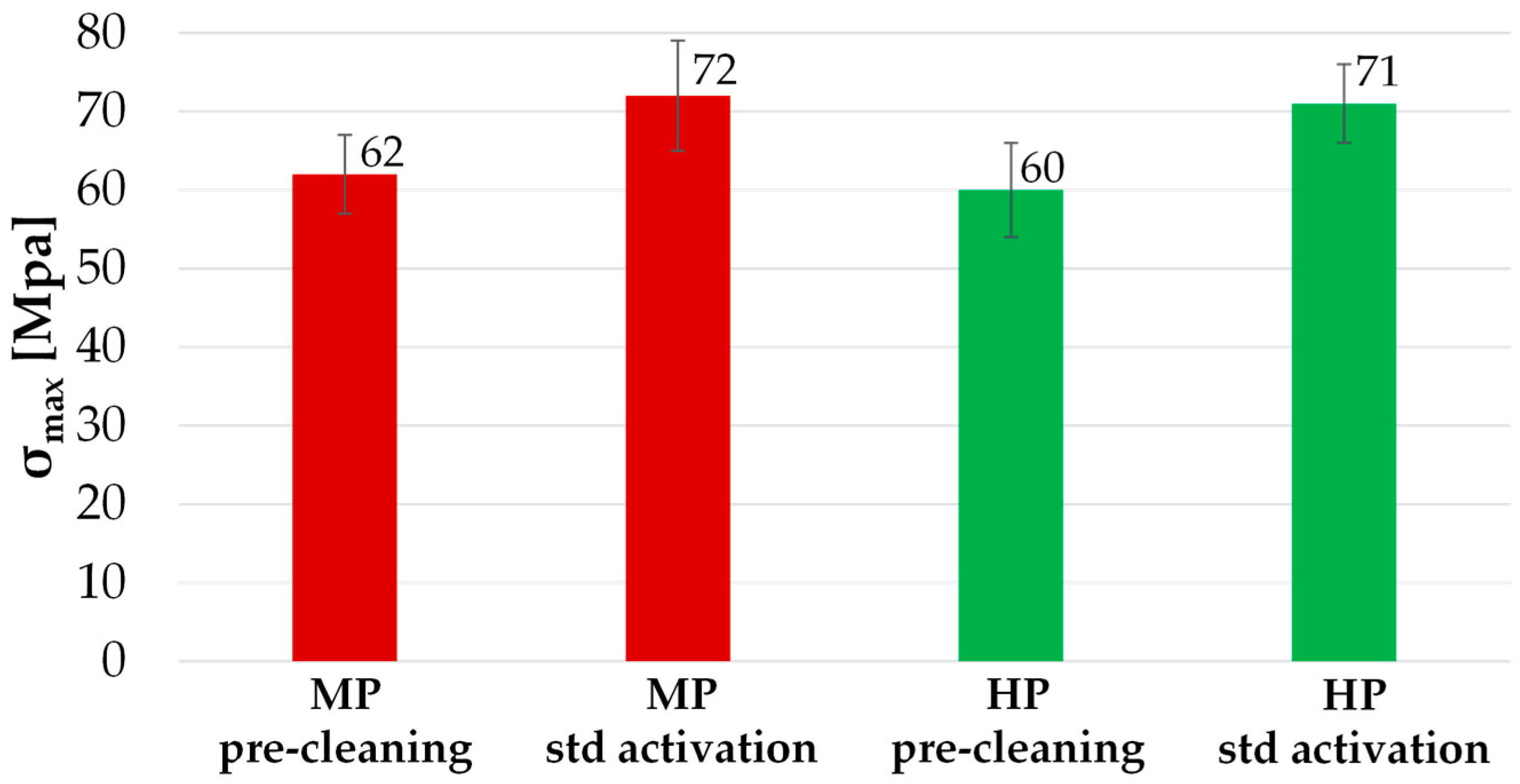
3.4. Pull Off-Test
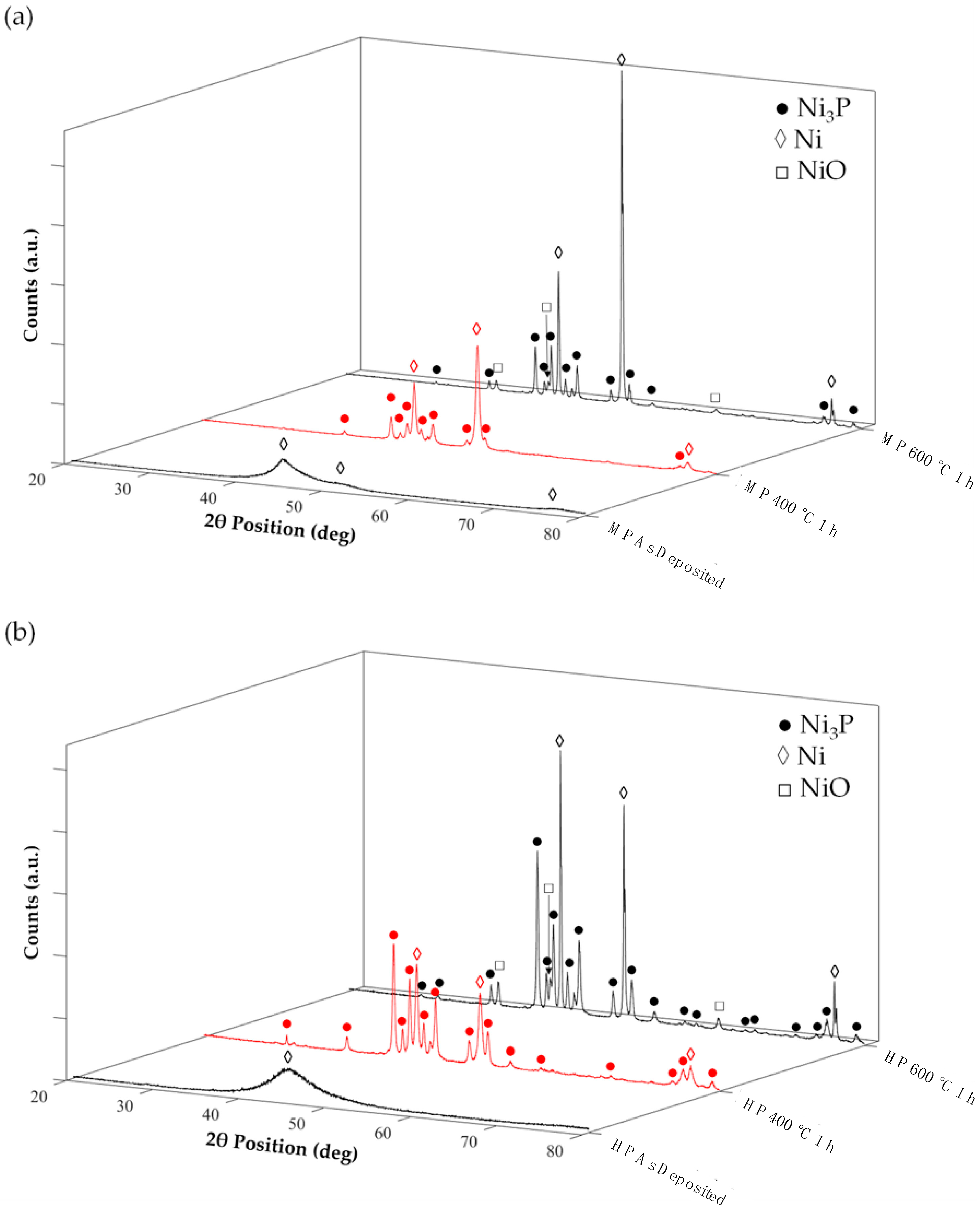
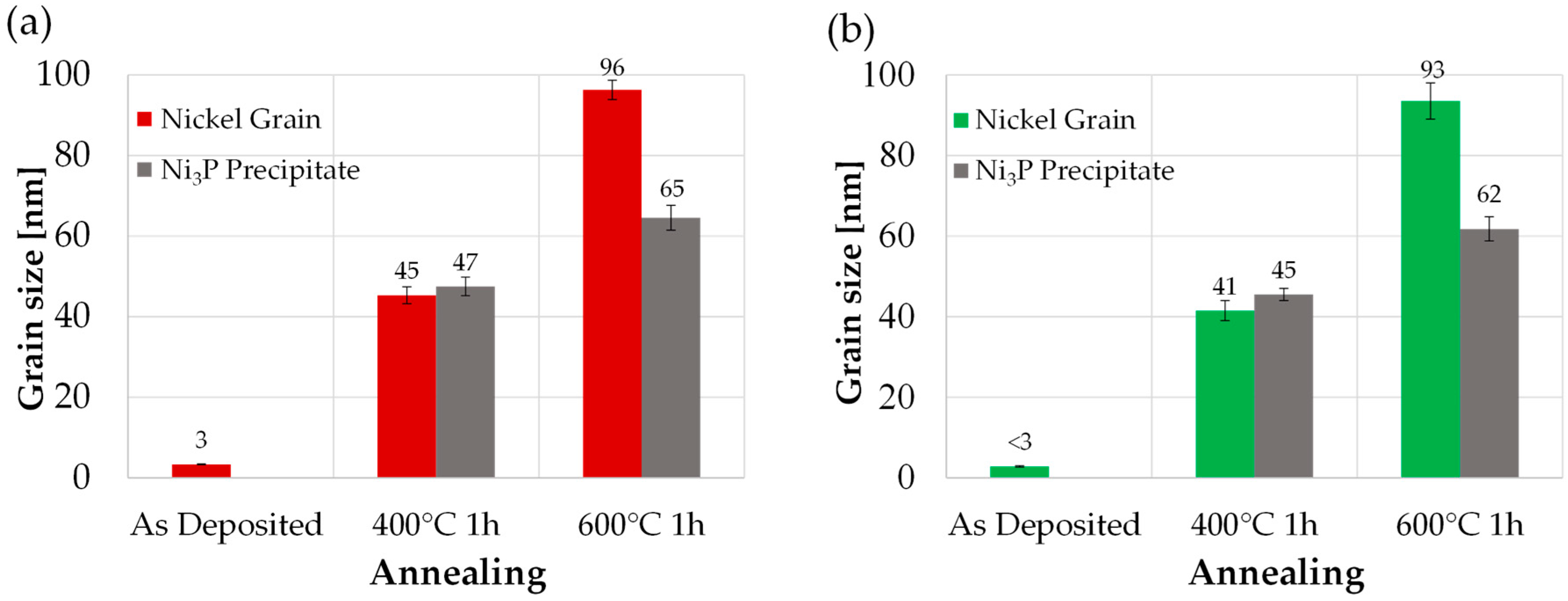
3.5. Heat Treatments
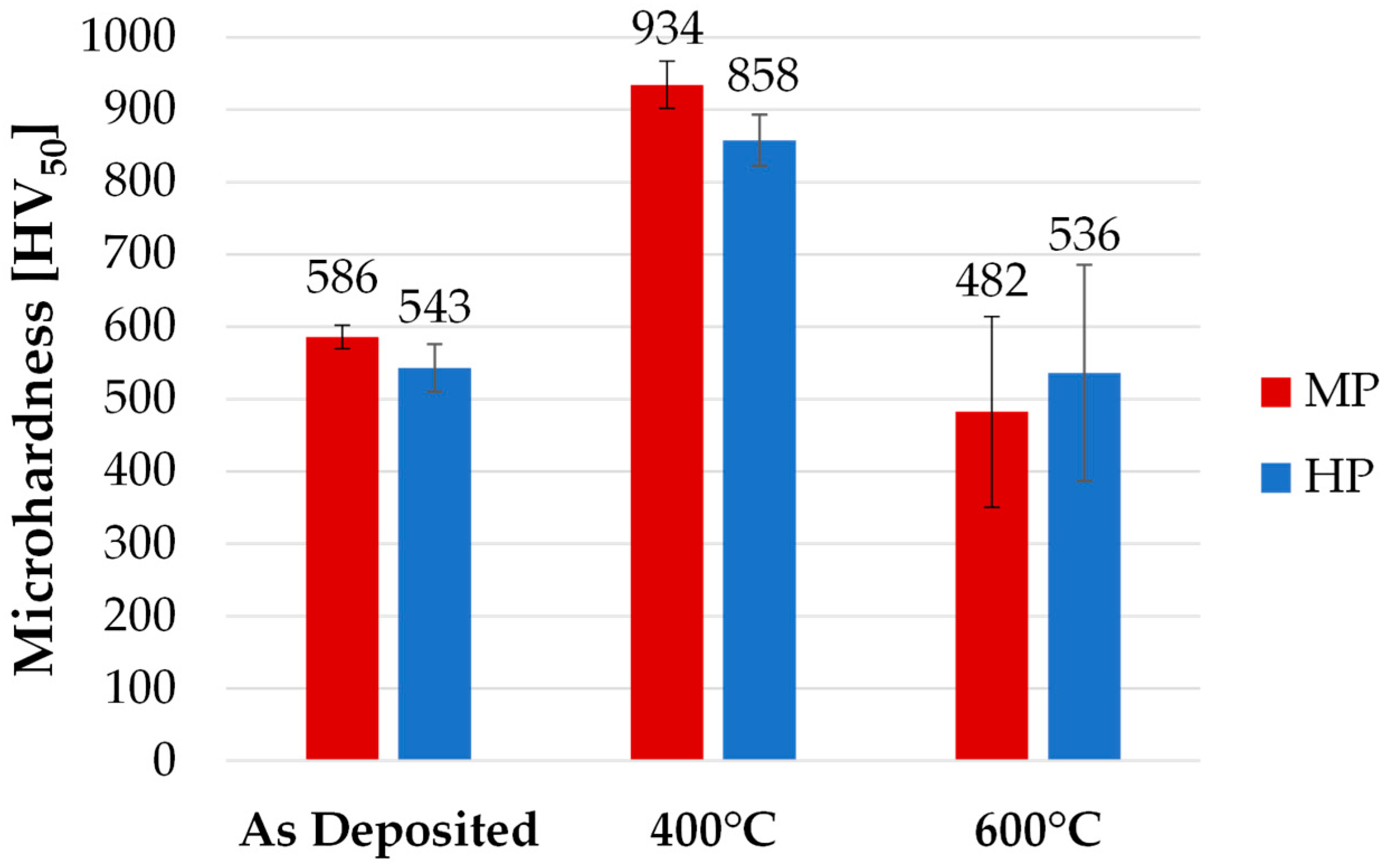
3.6. Vickers Hardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loto, C.A. Electroless Nickel Plating—A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of Electroless Nickel Coatings—A Review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Paglia, L.; Genova, V.; Conti, M.; Baiamonte, L.; Marra, F. The Effect of Composition and Heat Treatment on Microhardness of Ni-P and Ni-P-NanoZrO2 Coatings. Chem. Eng. Trans. 2023, 100, 433–438. [Google Scholar] [CrossRef]
- Genova, V.; Pedrizzetti, G.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Diffusion Aluminide Coating Modified via Electroless Nickel Plating for Ni-Based Superalloy Protection. Surf. Coat. Technol. 2022, 439, 128452. [Google Scholar] [CrossRef]
- Hari Krishnan, K.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An Overall Aspect of Electroless Ni-P Depositions-A Review Article. Metall. Mater. Trans. A 2006, 37A, 1917–1926. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Pure Thick Nickel Coating Obtained by Electroless Plating: Surface Characterization and Wetting Properties. Surf. Coat. Technol. 2019, 357, 595–603. [Google Scholar] [CrossRef]
- Singh, R. The Application of an Electrochemical Technique to Determine the Porosity of Electroless Nickel Coatings Produced in Hypophosphite Baths. Anti-Corros. Methods Mater. 2003, 50, 121–128. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni-P Alloy Coatings: A Review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Baskaran, I.; Krishnaveni, K.; Parthiban, S. Deposition of Electroless Ni-P Graded Coatings and Evaluation of Their Corrosion Resistance. Surf. Coat. Technol. 2006, 200, 3438–3445. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Kshetri, Y.K.; Chaudhary, B.; Kim, T.H.; Lee, S.W.; Kim, B.S.; Song, Y.; Kim, H.S.; Kim, H.H. Particle-Size-Dependent Anticorrosion Performance of the Si3 N4-Nanoparticle-Incorporated Electroless Ni-P Coating. Coatings 2022, 12, 9. [Google Scholar] [CrossRef]
- Gould, A.J.; Boden, P.J.; Harris, S.J. Phosphorus Distribution in Electroless Nickel Deposits. Surf. Technol. 1981, 12, 93–102. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallisation Kinetics and Phase Transformation Behaviour of Electroless Nickel-Phosphorus Deposits with High Phosphorus Content. J. Alloys Compd. 2002, 334, 192–199. [Google Scholar] [CrossRef]
- Buchtík, M.; Kosár, P.; Wasserbauer, J.; Tkacz, J.; Doležal, P. Characterization of Electroless Ni-P Coating Prepared on a Wrought ZE10 Magnesium Alloy. Coatings 2018, 8, 96. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V. Electroless Alloy/Composite Coatings: A Review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Abrantes, L.M.; Correia, J.P. On the Mechanism of Electroless Ni-P Plating. J. Electrochem. Soc. 1994, 141, 2356. [Google Scholar] [CrossRef]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990. [Google Scholar]
- Inam-Ul-Haque; Ahmad, S.; Khan, A. Electroless Nickel Plating on ABS Plastics from Nickel and Nickel Sulfate Baths. J. Chem. Soc. Pak. 2005, 27, 246–249. [Google Scholar]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Summary of Existing Models of the Ni-P Coating Electroless Deposition Process. Int. J. Chem. Kinet. 2013, 45, 755–762. [Google Scholar] [CrossRef]
- Genova, V.; Marini, D.; Valente, M.; Marra, F.; Pulci, G. Nanostructured Nickel Film Deposition on Carbon Fibers for Improving Reinforcement-Matrix Interface in Metal Matrix Composites. Chem. Eng. Trans. 2017, 60, 73–78. [Google Scholar] [CrossRef]
- Das, L.; Chin, D.-T. Effect of Bath Stabilizers on Electroless Nickel Deposition on Ferrous Substrates. Plat. Surf. Finish. 1996, 83, 55–61. [Google Scholar]
- Keping, H.; Fang, J.L. Stabilization Effect of Electroless Nickel Plating by Thiourea. Met. Finish. 1997, 95, 73–75. [Google Scholar] [CrossRef]
- Lin, K.-L.; Hwang, J.-W. Effect of Thiourea and Lead Acetate on the Deposition of Electroless Nickel. Mater. Chem. Phys. 2002, 76, 204–211. [Google Scholar] [CrossRef]
- Chen, B.H.; Hong, L.; Ma, Y.; Ko, T.M. Effects of Surfactants in an Electroless Nickel-Plating Bath on the Properties of Ni-P Alloy Deposits. Ind. Eng. Chem. Res. 2002, 41, 2668–2678. [Google Scholar] [CrossRef]
- Baskaran, I.; Sankara Narayanan, T.S.N.; Stephen, A. Effect of Accelerators and Stabilizers on the Formation and Characteristics of Electroless Ni-P Deposits. Mater. Chem. Phys. 2006, 99, 117–126. [Google Scholar] [CrossRef]
- Liu, H.P.; Li, N.; Bi, S.F.; Li, D.Y.; Zou, Z.L. Effect of Organic Additives on the Corrosion Resistance Properties of Electroless Nickel Deposits. Thin Solid Films 2008, 516, 1883–1889. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Modarres, H.; Abdouss, M. Study on Morphology and Corrosion Resistance of Electroless Ni-P Coatings. Surf. Eng. 2009, 25, 367–371. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Miao, N.; Jiang, J.; Zhang, Y.; Zhang, L.; Yuan, N.; Wang, Q.; Tang, L. Influence of Thiourea on Electroless Ni–P Films Deposited on Silicon Substrates. J. Mater. Sci. Mater. Electron. 2019, 30, 7717–7724. [Google Scholar] [CrossRef]
- Park, I.C.; Kim, S.J. Effect of Lead Nitrate Concentration on Electroless Nickel Plating Characteristics of Gray Cast Iron. Surf. Coat. Technol. 2019, 376, 2–7. [Google Scholar] [CrossRef]
- Lin, J.D.; Chou, C. Te the Influence of Phosphorus Content on the Microstructure and Specific Capacitance of Etched Electroless Ni-P Coatings. Surf. Coat. Technol. 2019, 368, 126–137. [Google Scholar] [CrossRef]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. Protective Coating on Mg AZ91D Alloy—The Effect of Electroless Nickel (EN) Bath Stabilizers on Corrosion Behaviour of Ni-P Deposit. Corros. Sci. 2007, 49, 1777–1798. [Google Scholar] [CrossRef]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. The Effects of Stabilizers on the Bath Stability of Electroless Ni Deposition and the Deposit. Appl. Surf. Sci. 2004, 229, 282–300. [Google Scholar] [CrossRef]
- Tulsi, S.S. Properties of Electroless Nickel. Trans. Inst. Met. Finish. 1986, 64, 73–76. [Google Scholar] [CrossRef]
- Staia, M.H.; Castillo, E.J.; Puchi, E.S.; Lewis, B.; Hintermann, H.E. Wear Performance and Mechanism of Electroless Ni-P Coating. Surf. Coat. Technol. 1996, 86, 598–602. [Google Scholar] [CrossRef]
- Apachitei, I.; Tichelaar, F.D.; Duszczyk, J.; Katgerman, L. The Effect of Heat Treatment on the Structure and Abrasive Wear Resistance of Autocatalytic NiP and NiP-SiC Coatings. Surf. Coat. Technol. 2002, 149, 263–278. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless Nickel, Alloy, Composite and Nano Coatings—A Critical Review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Tang, B.; Li, D.; Fu, F.; Xu, Y.; Yu, G.; Zhang, J. A Strategy for Cleaner Pickling: Effect, Mechanism, and Evaluation Method of a Complex-Inhibitor in Hydrochloric Acid Medium. Ind. Eng. Chem. Res. 2012, 51, 2615–2621. [Google Scholar] [CrossRef]
- Huang, Z.; Nguyen, T.T.; Zhou, Y.; Qi, G. A Low Temperature Electroless Nickel Plating Chemistry. Surf. Coat. Technol. 2019, 372, 160–165. [Google Scholar] [CrossRef]
- Salicio-Paz, A.; Ugarte, I.; Sort, J.; Pellicer, E.; García-Lecina, E. Full Optimization of an Electroless Nickel Solution: Boosting the Performance of Low-Phosphorous Coatings. Materials 2021, 14, 1501. [Google Scholar] [CrossRef]
- Huang, Y.S.; Cui, F.Z. Effect of Complexing Agent on the Morphology and Microstructure of Electroless Deposited Ni–P Alloy. Surf. Coat. Technol. 2007, 201, 5416–5418. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M.; Anderson, B.F.; Reeves, R.D. Chelation of Nickel (II) by Citrate. The Crystal Structure of a Nickel-Citrate. Inorganica Chim. Acta 1983, 78, 281–285. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, H.; Yu, D.; Sun, D. Effects of Complexing Agents on Acidic Electroless Nickel Deposition. Rare Met. 2010, 29, 401–406. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; ISBN 0-471-05542-5. [Google Scholar]
- Lin, Y.-M.; Yen, S.-C. Effects of Additives and Chelating Agents on Electroless Copper Plating. Appl. Surf. Sci. 2001, 178, 116–126. [Google Scholar] [CrossRef]
- Sun, R.; Yu, G.; Xie, Z.; Hu, B.; Zhang, J.; He, X.; Zhang, X. Influence of Hypophosphite on Efficiency and Coating Qualities of Electroless Ni-P Deposits on Magnesium Alloy AZ91D. Int. J. Electrochem. Sci. 2015, 10, 7893–7904. [Google Scholar] [CrossRef]
- Feldstein, N.; Amodio, P.R. Anionic Inhibition in Electroless Plating. J. Electrochem. Soc. 1970, 117, 1110. [Google Scholar] [CrossRef]
- Farber, B.; Cadel, E.; Menand, A.; Schmitz, G.; Kirchheim, R. Phosphorus Segregation in Nanocrystalline Ni-3.6 At% Alloy Investigated with the Tomographic Atom Probe (TAP). Acta Mater. 2000, 48, 789–796. [Google Scholar] [CrossRef]
- Elsener, B.; Crobu, M.; Scorciapino, M.A.; Rossi, A. Electroless Deposited Ni-P Alloys: Corrosion Resistance Mechanism. J. Appl. Electrochem. 2008, 38, 1053–1060. [Google Scholar] [CrossRef]
- Azumi, K.; Yugiri, T.; Kurihara, T.; Seo, M.; Habazaki, H.; Fujimoto, S. Direct Plating of Electroless Ni-P Layers on Sputter-Deposited Al-Ni Alloy Films. J. Electrochem. Soc. 2003, 150, C461. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Hardness Evolution of Electroless Nickel-Phosphorus Deposits with Thermal Processing. Surf. Coat. Technol. 2003, 168, 263–274. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Jarjoura, G.; Farhat, Z. Recent Advances in Electroless-Plated Ni-P and Its Composites for Erosion and Corrosion Applications: A Review. Emergent Mater. 2018, 1, 3–24. [Google Scholar] [CrossRef]
- Crobu, M.; Scorciapino, A.; Elsener, B.; Rossi, A. The Corrosion Resistance of Electroless Deposited Nano-Crystalline Ni-P Alloys. Electrochim. Acta 2008, 53, 3364–3370. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, Y.H.; Tang, Y.K.; Lin, E.J.; Lin, Y.X.; Wang, J.Y.; Zhuang, W.X.; Lee, C.H.; Chiu, C.Y.; Yeh, C.Y.; et al. Effect of Chemical Additives in the Plating Bath on Surface Corrosion Resistance of Ni(P). J. Electron. Mater. 2020, 49, 26–33. [Google Scholar] [CrossRef]
- Valli, J. A Review of Adhesion Test Methods for Thin Hard Coatings. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 3007–3014. [Google Scholar] [CrossRef]
- Correa, E.; Zuleta, A.A.; Sepúlveda, M.; Guerra, L.; Castaño, J.G.; Echeverría, F.; Liu, H.; Skeldon, P.; Thompson, G.E. Nickel-Boron Plating on Magnesium and AZ91D Alloy by a Chromium-Free Electroless Process. Surf. Coat. Technol. 2012, 206, 3088–3093. [Google Scholar] [CrossRef]
- Guo, C.Y.; Matinlinna, J.P.; Tin, A.; Tang, H. A Novel Effect of Sandblasting on Titanium Surface: Static Charge Generation. J. Adhes. Sci. Technol. 2012, 26, 2603–2613. [Google Scholar] [CrossRef]
- Huang, Y.S.; Zeng, X.T.; Annergren, I.; Liu, F.M. Development of Electroless NiP-PTFE-SiC Composite Coating. Surf. Coat. Technol. 2003, 167, 207–211. [Google Scholar] [CrossRef]
- Jiaqiang, G.; Lei, L.; Yating, W.; Bin, S.; Wenbin, H. Electroless Ni-P-SiC Composite Coatings with Superfine Particles. Surf. Coat. Technol. 2006, 200, 5836–5842. [Google Scholar] [CrossRef]
- Franco, M.; Sha, W.; Malinov, S.; Rajendran, R. Phase Composition, Microstructure and Microhardness of Electroless Nickel Composite Coating Co-Deposited with SiC on Cast Aluminium LM24 Alloy Substrate. Surf. Coat. Technol. 2013, 235, 755–763. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, H.; Zhu, L.; Qian, W.; Han, S.; Lin, H.; Wu, H. Effect of Heat Treatment on Structures and Mechanical Properties of Electroless Ni-P-GO Composite Coatings. RSC Adv. 2016, 6, 109001–109008. [Google Scholar] [CrossRef]
- Luo, H.; Leitch, M.; Behnamian, Y.; Ma, Y.; Zeng, H.; Luo, J.L. Development of Electroless Ni-P/Nano-WC Composite Coatings and Investigation on Its Properties. Surf. Coat. Technol. 2015, 277, 99–106. [Google Scholar] [CrossRef]
- Kundu, S.; Das, S.K.; Sahoo, P. Properties of Electroless Nickel at Elevated Temperature—A Review. In Proceedings of the Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 97, pp. 1698–1706. [Google Scholar]
- Buchtík, M.; Krystỳnová, M.; Másilko, J.; Wasserbauer, J. The Effect of Heat Treatment on Properties of Ni-P Coatings Deposited on a AZ91 Magnesium Alloy. Coatings 2019, 9, 461. [Google Scholar] [CrossRef]
- Hentschel, T.H.; Isheim, D.; Kirchheim, R.; Mu, F.; Ller, È.; Kreye, H. Nanocrystalline Ni-3.6 at.% P and Its Transformation Sequence Studied by Atom-Probe Field-Ion Microscopy. Acta Mater. 2000, 48, 933–941. [Google Scholar] [CrossRef]
- Boylan, K.I.; Ostrander, D.I.; Erb, U.I.; Palumbo, G.; Aust, K. An In-Situ TEM Study of the Thermal Stability of Nanocrystalline Ni-P. Scr. Metall. Mater. 1991, 25, 2711–2716. [Google Scholar] [CrossRef]
- Kalidindi, A.R.; Schuh, C.A. Phase Transitions in Stable Nanocrystalline Alloys. J. Mater. Res. 2017, 32, 1993–2002. [Google Scholar] [CrossRef]
- Buchtík, M.; Doskočil, L.; Brescher, R.; Doležal, P.; Másilko, J.; Wasserbauer, J. The Effect of Crystallization and Phase Transformation on the Mechanical and Electrochemical Corrosion Properties of Ni-p Coatings. Coatings 2021, 11, 447. [Google Scholar] [CrossRef]
- Apachitei, I.; Duszczyk, J. Autocatalytic Nickel Coatings on Aluminium with Improved Abrasive Wear Resistance. Surf. Coat. Technol. 2000, 132, 8998. [Google Scholar] [CrossRef]
- van Swygenhoven, H.; Caro, A. Plastic Behavior of Nanophase Ni: A Molecular Dynamics Computer Simulation. Appl. Phys. Lett. 1997, 71, 1652–1654. [Google Scholar] [CrossRef]
- Johnson, C.E.; Ogburn, F. Hardness of Heat Treated Electroless Nickel. Surf. Technol. 1976, 4, 161. [Google Scholar] [CrossRef]
- Franco, M.; Sha, W.; Aldic, G.; Malinov, S.; Çimenoğlu, H. Effect of Reinforcement and Heat Treatment on Elevated Temperature Sliding of Electroless Ni-P/SiC Composite Coatings. Tribol. Int. 2016, 97, 265–271. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, G. Friction and Wear of Electroless Ni-P-CS Composite Coating. Metals 2023, 13, 315. [Google Scholar] [CrossRef]
- Martin, J.W. Precipitation Hardening; Butterworth-Heinemann: Oxford, UK, 1998; ISBN 0750638850. [Google Scholar]



| Abrantes et al. (1994) [15] | Keping and Fang (1997) [21] | Lin and Hwang (2002) [22] | Chen et al. (2002) [23] | Cheong et al. (2004) [23] | Baskaran et al. (2005) [24] | Liu et al. (2008) [25] | Rahimi et al. (2009) [26] | Wu et al. (2019) [27] | Park and Kim (2019) [28] | Lin and Chou (2020) [29] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni2+/H2PO2− | 0.88 | 0.82 | 2.06 | 0.36 | 0.60 | 0.35 | 0.39 | 0.44 | 0.35 | 0.56 | 0.89 |
| wt% P | 12 | --- | 8 | 10 | 10 | 11 | 10.5 | 9 | 12 | 3–6 | 5–8 |
| Function | Name | Chemical Formula | MP (g/L) |
|---|---|---|---|
| Reducing agent | Sodium hypophosphite | NaH2PO2 | 70 |
| Buffer | Sodium acetate | C2H3NaO2 | 15 |
| Chelating Agent | Citric acid | C6H8O7 | 7 |
| Source of Nickel | Nickel sulfate | NiSO4 | 12 |
| Stabilizer * | Thio-organic compound (TOC) | R-CS | 5 (ppm) |
| Sample | HCl Concentration (%vol) | Result | Plating Rate (mg/cm2/h) |
|---|---|---|---|
| AR | (Pre-cleaning and 1 h air exposure, no activation procedure) | Not Activated | - |
| 30 | Activated | 8.60 | |
| 40 | Activated | 15.7 | |
| 50 | Activated | 14.0 | |
| 100 | Not activated | - | |
| HT 400 °C | (Pre-cleaning and 1 h air exposure, no activation procedure) | Not Activated | - |
| 30 | Activated | 13.8 | |
| 40 | Activated | 12.9 | |
| 50 | Activated | 15.1 | |
| 100 | Activated | 11.0 | |
| HT 600 °C | (Pre-cleaning and 1 h air exposure, no activation procedure) | Not Activated | - |
| 30 | Not activated | - | |
| 40 | Not activated | - | |
| 50 | Not Activated | - | |
| 100 | Activated | 9.80 |
| Treatment | Time |
|---|---|
| NaOH-1 M-80 °C | 10 min |
| Water rinsing | - |
| Sandblasting | - |
| Sonication in H2O | 5 min |
| HCl 50 %vol | 1 min |
| Water rinsing | - |
| Plating | - |
| Function | Name | Chemical Formula | HP (g/L) |
|---|---|---|---|
| Reducing agent | Sodium hypophosphite | NaH2PO2 | 51.5 |
| Buffer | Sodium acetate | C2H3NaO2 | 15.0 |
| Chelating Agent | Citric acid | C6H8O7 | 7.70 |
| Source of Nickel | Nickel sulfate | NiSO4 | 6.60 |
| Stabilizer * | Thio-organic compound (TOC) | R-CS | 8.50 (ppm) |
| Sample | wt% P | Heat Treatment | Activation |
|---|---|---|---|
| MPAC | 6.5 | - | HCl 50 vol.%, 1 min |
| MP400 | 6.5 | 400 °C, 1 h | HCl 50 vol.%, 1 min |
| MP600 | 6.5 | 600 °C, 1 h | HCl 50 vol.%, 1 min |
| HPAC | 10.9 | - | HCl 50 vol.%, 1 min |
| HP400 | 10.9 | 400 °C, 1 h | HCl 50 vol.%, 1 min |
| HP600 | 10.9 | 600 °C, 1 h | HCl 50 vol.%, 1 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genova, V.; Paglia, L.; Pulci, G.; Pedrizzetti, G.; Pranzetti, A.; Romanelli, M.; Marra, F. Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings. Coatings 2023, 13, 1490. https://doi.org/10.3390/coatings13091490
Genova V, Paglia L, Pulci G, Pedrizzetti G, Pranzetti A, Romanelli M, Marra F. Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings. Coatings. 2023; 13(9):1490. https://doi.org/10.3390/coatings13091490
Chicago/Turabian StyleGenova, Virgilio, Laura Paglia, Giovanni Pulci, Giulia Pedrizzetti, Alice Pranzetti, Marco Romanelli, and Francesco Marra. 2023. "Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings" Coatings 13, no. 9: 1490. https://doi.org/10.3390/coatings13091490
APA StyleGenova, V., Paglia, L., Pulci, G., Pedrizzetti, G., Pranzetti, A., Romanelli, M., & Marra, F. (2023). Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings. Coatings, 13(9), 1490. https://doi.org/10.3390/coatings13091490







