New Triple Metallic Carbonated Hydroxyapatite for Stone Surface Preservation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent advances in protective coatings for cultural heritage—An overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Hansen, E.; Doehne, E.; Fidler, J.; Larson, J.; Martin, B.; Matteini, M.; Rodriguez-Navarro, C.; Pardo, E.S.; Price, C.; de Tagle, A. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Stud. Conserv. 2003, 48, 13–25. [Google Scholar] [CrossRef]
- Price, C.A.; Doehne, E. Stone Conservation: An Overview of Current Research; Getty Conservation Institute Publisher: Los Angeles, CA, USA, 2011. [Google Scholar]
- Pinto, A.F.; Rodrigues, J.D. Stone consolidation: The role of treatment procedures. J. Cult. Herit. 2008, 9, 38–53. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Graziani, G. Brushing, poultice or immersion? The role of the application technique on the performance of a novel hydroxyapatite-based consolidating treatment for limestone. J. Cult. Herit. 2015, 16, 173–184. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Porcinai, S. Evaluation of the application conditions of artificial protection treatments on salt-laden limestones and marble. Constr. Build. Mater. 2011, 25, 2723–2732. [Google Scholar] [CrossRef]
- Pinto, A.P.F.; Rodrigues, J.D. Consolidation of carbonate stones: Influence of treatment procedures on the strengthening action of consolidants. J. Cult. Herit. 2012, 13, 154–166. [Google Scholar] [CrossRef]
- Rodrigues, A.; Sena da Fonseca, B.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.d.F. TEOS Nanocomposites for the Consolidation of Carbonate Stone: The Effect of Nano-HAp and Nano-SiO2 Modifiers. Materials 2022, 15, 981. [Google Scholar] [CrossRef]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, R.E.; Radu, G.-I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.-L. Ion-substituted carbonated hydroxyapatite coatings for model stone samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef]
- Rodrigues, A.; da Fonseca, B.S.; Pinto, A.F.; Piçarra, S.; Montemor, M. Synthesis and application of hydroxyapatite nanorods for improving properties of stone consolidants. Ceram. Int. 2022, 48, 14606–14617. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Herit. 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Ungureanu, D.N.; Angelescu, N.; Ion, R.M.; Stoian, E.V.; Rizescu, C.Z. Synthesis and Characterization of Hydroxyapatite Nanopowders by Chemical Precipitation; World Scientific and Engineering Academy and Society Press: Basking Ridge, NJ, USA, 2011. [Google Scholar]
- Cahyanto, A.; Maruta, M.; Tsuru, K.; Matsuya, S.; Ishikawa, K. Fabrication of bone cement that fully transforms to carbonate apatite. Dent. Mater. J. 2015, 34, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef]
- Zhu, Q.X.; Jiang, W.H.; Shao, C.; Bao, Y. Thermophysical and Mechanical Properties of Carbonated Hydroxyapatite. In Proceedings of the 7th China International Conference on High-Performance Ceramics (CICC 7), Xiamen, China, 4–7 November 2011; pp. 989–993. [Google Scholar]
- Khan, A.S.; Chaudhry, A.A. Handbook of Ionic Substituted Hydroxyapatites; Woodhead Publishing: Sawston, Cambridge, UK, 2019. [Google Scholar]
- Ren, F.Z.; Leng, Y.; Lu, X. Ab Initio Simulations on the Carbonated Apatite Structure. Key Eng. Mater. 2013, 529–530, 1–6. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Ion, R.M.; Iancu, L.; David, M.E.; Grigorescu, R.M.; Trica, B.; Somoghi, R.; Vasile, S.F.; Dulama, I.D.; Gheboianu, A.I.; Tincu, S. Multi-Analytical Characterization of Corvins’ Castle—Deserted Tower. Construction Materials and Conservation Tests. Heritage 2020, 3, 941–964. [Google Scholar] [CrossRef]
- Iancu, L.; Ion, R.-M.; Grigorescu, R.M.; David, M.E.; Ghiurea, M.; Vasilievici, G.; Stirbescu, R.M.; Dulama, I.D. Double substituted carbonated hydroxyapatite for stone consolidation. J. Sci. Arts 2020, 20, 713–730. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.; Li, Z.; Zhu, S.; Liang, Y.; Lu, W.W.; Yang, X. Synthesis, characterization and the formation mechanism of magnesium-and strontium-substituted hydroxyapatite. J. Mater. Chem. B 2015, 3, 3738–3746. [Google Scholar] [CrossRef]
- Garbo, C.; Locs, J.; D’Este, M.; Demazeau, G.; Mocanu, A.; Roman, C.; Horovitz, O.; Tomoaia-Cotisel, M. Advanced Mg, Zn, Sr, Si multi-substituted hydroxyapatites for bone regeneration. Int. J. Nanomed. 2020, 15, 1037–1058. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Krejsová, J.; Doleželová, M. Resistance of mortars with gypsum, lime and composite binders against molds. Acta Polytech. CTU Proc. 2019, 21, 16–20. [Google Scholar] [CrossRef]
- Middendorf, B. Physico-mechanical and microstructural characteristics of historic and restoration mortars based on gypsum: Current knowledge and perspective. Geol. Soc. Lond. Spec. Publ. 2002, 205, 165–176. [Google Scholar] [CrossRef]
- STAS 6200/12-73; Shaped Natural Stones for Construction. Methods of Physical, Mechanical and Mineralogical Tests. Determination of Water Absorption and Water Release. Romanian Standardization Association: Bucharest, Romania, 1973.
- ASTM C805/C805M; Standard Test Method for Rebound Number of Hardened Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- Drdácký, M.; Lesák, J.; Niedoba, K.; Valach, J. Peeling tests for assessing the cohesion and consolidation characteristics of mortar and render surfaces. Mater. Struct. 2015, 48, 1947–1963. [Google Scholar] [CrossRef]
- Becerra, J.; Ortiz, P.; Martín, J.M.; Zaderenko, A.P. Nanolimes doped with quantum dots for stone consolidation assessment. Constr. Build. Mater. 2019, 199, 581–593. [Google Scholar] [CrossRef]
- SR EN 12371:2010; Natural Stone Testing Methods. Determination of Frost Resistance. Romanian Standardization Association: Bucharest, Romania, 2010.
- ASTM D2244; Standard Practice for Calculation of Color Tolerances and Color Differences from Instrumentally Measured Color Coordinates. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; David, M.E.; Alexandrescu, E.; Ghiurea, M.; Stirbescu, R.M. Double-Substituted Carbonated Hydroxyapatites for Fir Wood Treatment. Sci. Bull. Valahia Univ.—Mater. Mech. 2022, 18, 15–22. [Google Scholar] [CrossRef]
- Syazwan, M.M.; Marliana, B.Y. The influence of simultaneous divalent cations (Mg2+, Co2+ and Sr2+) substitution on the physico-chemical properties of carbonated hydroxyapatite. Ceram. Int. 2019, 45, 14783–14788. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, M.; Cheung, W.; Guo, B.; Jia, D. Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103–110. [Google Scholar] [CrossRef]
- Zahra, G.; Nounah, A.; Belhorma, B.; Yahyaoui, A. Nanosized calcium-deficient carbonated hydroxyapatite synthesized by microwave activation. J. Mater. Environ. Sci. 2015, 6, 983–988. [Google Scholar]
- Kee, C.C.; Ismail, H.; Noor, A.F.M. Effect of synthesis technique and carbonate content on the crystallinity and morphology of carbonated hydroxyapatite. J. Mater. Sci. Technol. 2013, 29, 761–764. [Google Scholar] [CrossRef]
- Kweh, S.W.K.; Khor, K.A.; Cheang, P. The production and characterization of hydroxyapatite (HA) powders. J. Mater. Process. Technol. 1999, 89–90, 373–377. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Santos, M.H.; Oliveira, M.d.; Souza, L.P.d.F.; Mansur, H.S.; Vasconcelos, W.L. Synthesis control and characterization of hydroxyapatite prepared by wet precipitation process. Mater. Res. 2004, 7, 625–630. [Google Scholar] [CrossRef]
- Smičiklas, I.; Onjia, A.; Raičević, S. Experimental design approach in the synthesis of hydroxyapatite by neutralization method. Sep. Purif. Technol. 2005, 44, 97–102. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef]
- Matsunaga, K.; Murata, H.; Shitara, K. Theoretical calculations of the thermodynamic stability of ionic substitutions in hydroxyapatite under an aqueous solution environment. J. Phys. Condens. Matter 2010, 22, 384210. [Google Scholar] [CrossRef]
- Ezekiel, I.; Kasim, S.R.; Ismail, Y.M.B.; Noor, A.-F.M. Nanoemulsion synthesis of carbonated hydroxyapatite nanopowders: Effect of variant CO32−/PO43− molar ratios on phase, morphology, and bioactivity. Ceram. Int. 2018, 44, 13082–13089. [Google Scholar] [CrossRef]
- Dickens, B.; Schroeder, L.W.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem. 1974, 10, 232–248. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. Sect. B 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Vichi, L.; Sandri, M. Influence of synthesis and sintering parameters on the characteristics of carbonate apatite. Biomaterials 2004, 25, 1763–1770. [Google Scholar] [CrossRef]
- Le Gars Santoni, B.; Niggli, L.; Sblendorio, G.A.; Alexander, D.T.L.; Stähli, C.; Bowen, P.; Döbelin, N.; Bohner, M. Chemically pure β-tricalcium phosphate powders: Evidence of two crystal structures. J. Eur. Ceram. Soc. 2021, 41, 1683–1694. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Preti, L.; Mazzoni, E.; Iaquinta, M.R.; Martini, F.; Tognon, M.; Pugno, N.M.; Restivo, E.; Visai, L.; et al. Enhancement of the Biological and Mechanical Performances of Sintered Hydroxyapatite by Multiple Ions Doping. Front. Mater. 2020, 7, 224. [Google Scholar] [CrossRef]
- Gallo, M.; Le Gars Santoni, B.; Douillard, T.; Zhang, F.; Gremillard, L.; Dolder, S.; Hofstetter, W.; Meille, S.; Bohner, M.; Chevalier, J.; et al. Effect of grain orientation and magnesium doping on β-tricalcium phosphate resorption behavior. Acta Biomater. 2019, 89, 391–402. [Google Scholar] [CrossRef]
- Schroeder, L.W.; Dickens, B.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. II. Refinement of Mg-containing β-Ca3(PO4)2. J. Solid State Chem. 1977, 22, 253–262. [Google Scholar] [CrossRef]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium- and strontium-co-substituted hydroxyapatite: The effects of doped-ions on the structure and chemico-physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Noor, A.F.M.; Krishnasamy, S.; Alengaram, U.J. Effect of multi-ions doping on the properties of carbonated hydroxyapatite bioceramic. Ceram. Int. 2019, 45, 3473–3477. [Google Scholar] [CrossRef]
- Lowry, N.; Han, Y.; Meenan, B.; Boyd, A. Strontium and zinc co-substituted nanophase hydroxyapatite. Ceram. Int. 2017, 43, 12070–12078. [Google Scholar] [CrossRef]
- Lowry, N.; Brolly, M.; Han, Y.; McKillop, S.; Meenan, B.; Boyd, A. Synthesis and characterisation of nanophase hydroxyapatite co-substituted with strontium and zinc. Ceram. Int. 2018, 44, 7761–7770. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Preparation and characterisation of nanophase Sr, Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 106–114. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater 2009, 5, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.W.; Franzoni, E. Penetration depth and redistribution of an aqueous ammonium phosphate solution used for porous limestone consolidation by brushing and immersion. Constr. Build. Mater. 2017, 148, 571–578. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J. Stone Consolidation. Between Science and Practice. In Conserving Stone Heritage; Springer: Berlin/Heidelberg, Germany, 2022; pp. 101–135. [Google Scholar]
- Ion, R.-M.; Turcanu-Carutiu, D.; Fierascu, R.-C.; Fierascu, I. Chalk stone restoration with hydroxyapatite–based nanoparticles. Sci. Bull. Valahia Univ. Mater. Mech. 2014, 9, 16–19. [Google Scholar]
- Ion, R.-M.; Turcanu-Caruţiu, D.; Fierăscu, R.-C.; Fierăscu, I.; Bunghez, I.-R.; Ion, M.-L.; Teodorescu, S.; Vasilievici, G.; Rădiţoiu, V. Caoxite-hydroxyapatite composition as consolidating material for the chalk stone from Basarabi–Murfatlar churches ensemble. Appl. Surf. Sci. 2015, 358, 612–618. [Google Scholar] [CrossRef]
- Papatzani, S.; Dimitrakakis, E. A Review of the Assessment Tools for the Efficiency of Nanolime Calcareous Stone Consolidant Products for Historic Structures. Buildings 2019, 9, 235. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; La Russa, M. Consolidation of bio-calcarenite stone by treatment based on diammonium hydrogenphosphate and calcium hydroxide nanoparticles. Measurements 2018, 127, 396–405. [Google Scholar] [CrossRef]
- Ruffolo, S.; La Russa, M.; Aloise, P.; Belfiore, C.; Pezzino, A.; Crisci, G. Efficacy of nanolime in restoration procedures of salt weathered limestone rock. Appl. Phys. A 2013, 114, 753–758. [Google Scholar] [CrossRef]
- Martins, L.; Vasconcelos, G.; Lourenco, P.; Palha, C. Influence of the Freeze-Thaw Cycles on the Physical and Mechanical Properties of Granites. J. Mater. Civ. Eng. 2015, 28, 04015201. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison with ethyl silicate. Constr. Build. Mater. 2016, 102, 918–930. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol–gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Pesce, C.; Moretto, L.M.; Orsega, E.F.; Pesce, G.L.; Corradi, M.; Weber, J. Effectiveness and compatibility of a novel sustainable method for stone consolidation based on di-ammonium phosphate and calcium-based nanomaterials. Materials 2019, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G. Color fundamentals for digital imaging. In Digital Color Imaging Handbook; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–114. [Google Scholar]

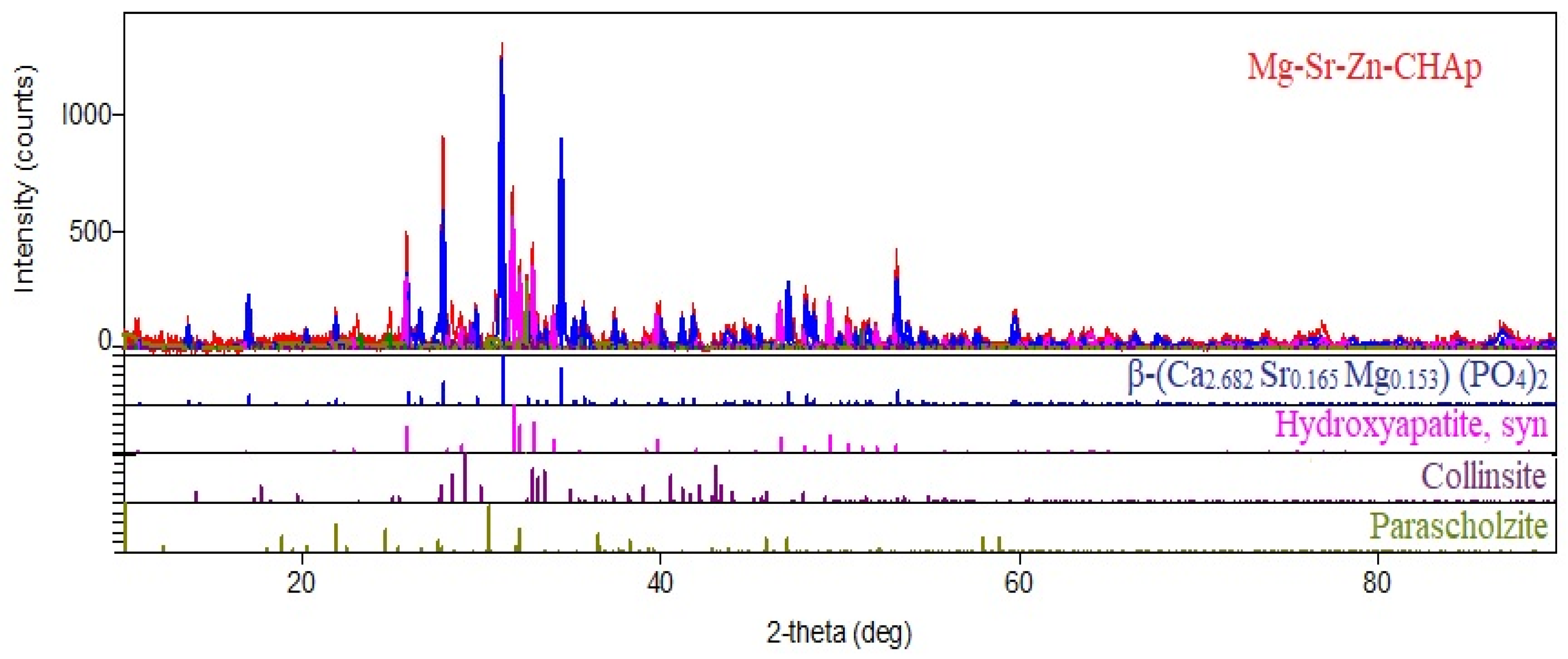
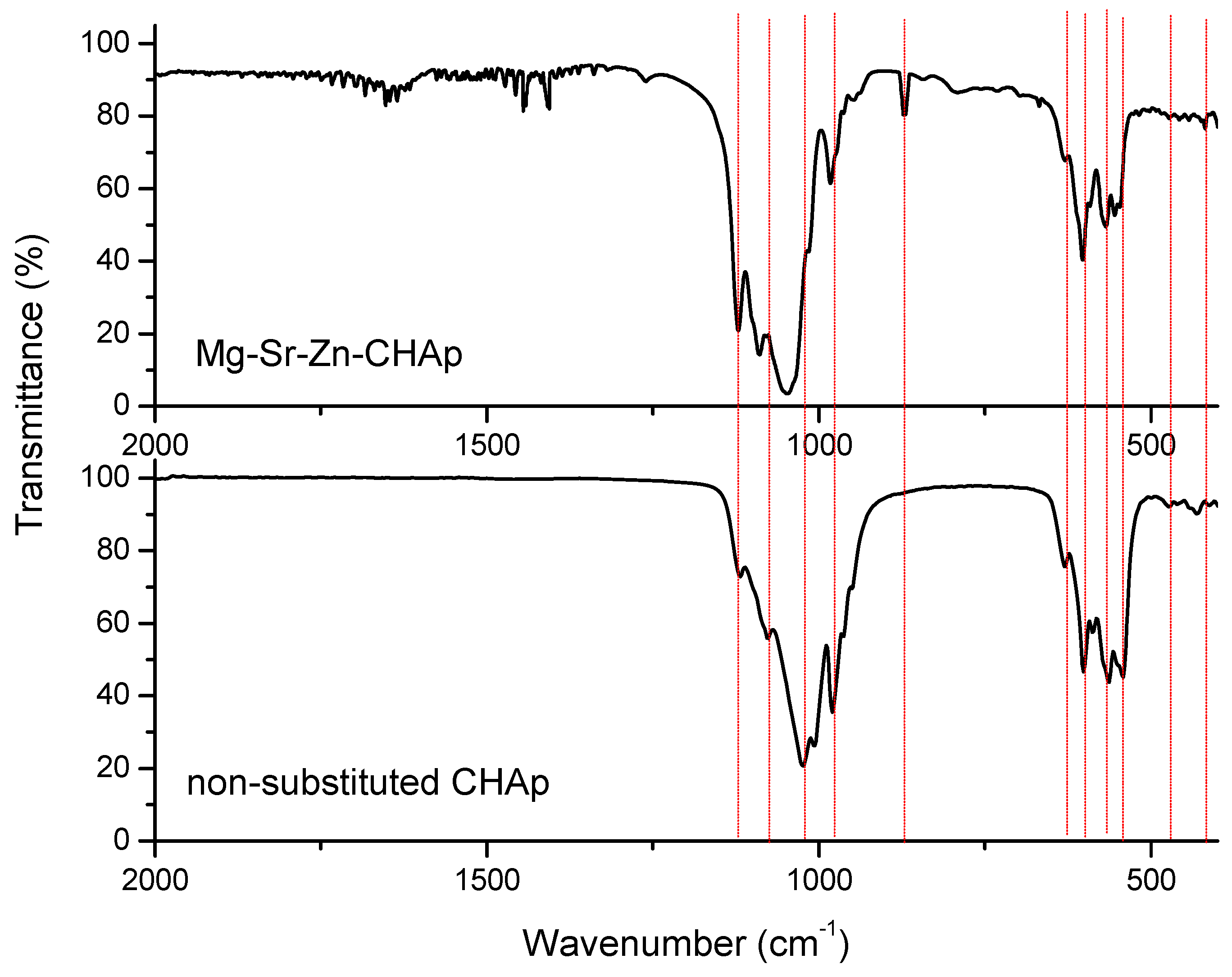
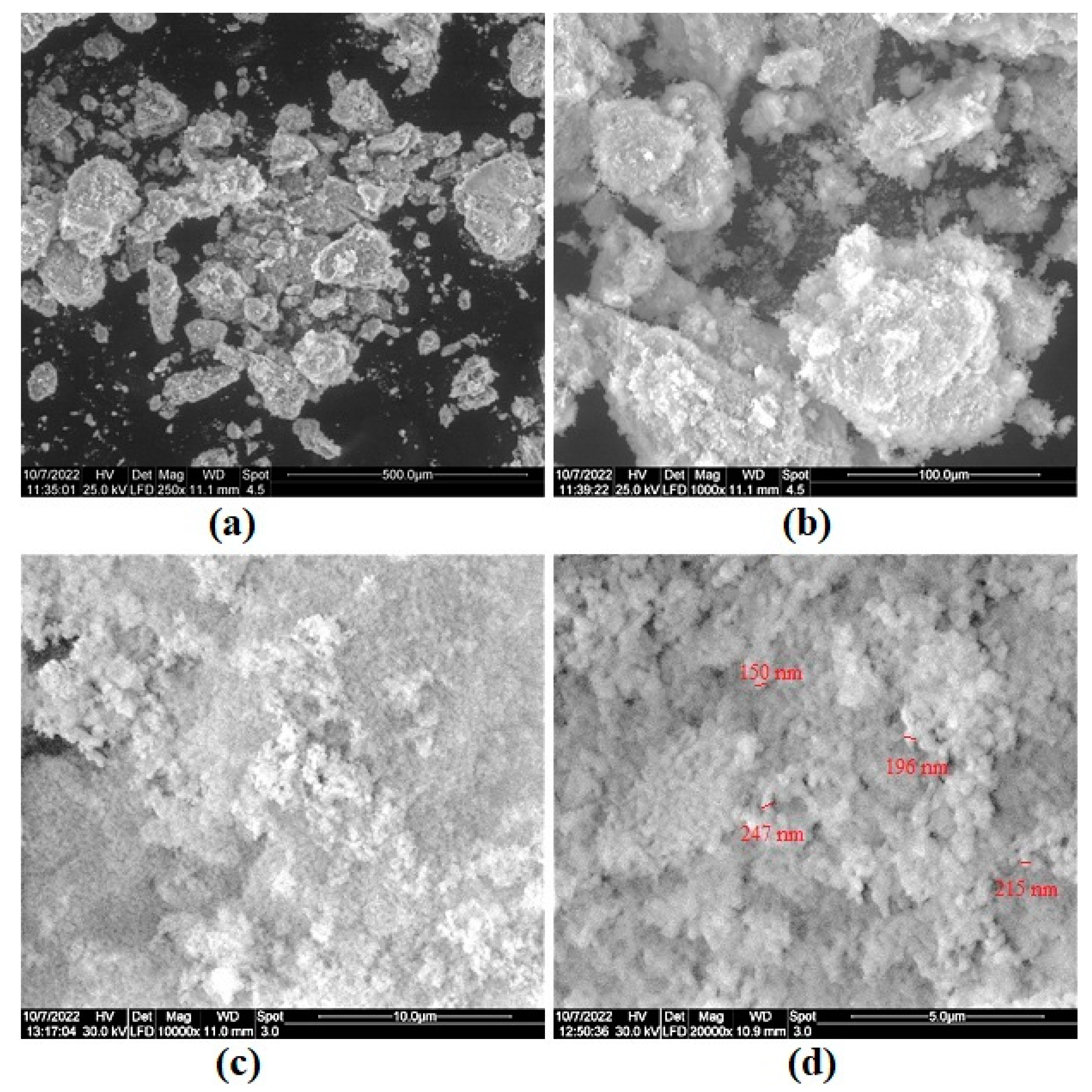



| Element | Mass % | Detection Limit | Element | Intensity | |||
|---|---|---|---|---|---|---|---|
| - | Mg-Sr-Zn-CHAp | CHAp | Mg-Sr-Zn-CHAp | CHAp | line | Mg-Sr-Zn-CHAp | CHAp |
| Mg | 0.3831 | - | 0.01665 | - | Mg-KA | 0.3626 | - |
| P | 18.1223 | 18.2630 | 0.02056 | 0.01957 | P-KA | 119.1271 | 130.2749 |
| Ca | 34.7967 | 35.8079 | 0.02689 | 0.02995 | Ca-KA | 122.7283 | 131.8156 |
| Zn | 1.2898 | - | 0.00829 | - | Zn-KA | 13.2132 | - |
| Sr | 1.7942 | - | 0.00473 | - | Sr-KA | 59.8188 | - |
| Si | - | 0.0136 | - | 0.00653 | Si-KA | - | 0.0399 |
| Na | - | 1.9411 | - | 0.04598 | Na-KA | - | 0.0399 |
| Identified Phase | Peaks/Miler Index | a, Å | b, Å | c, Å | Unit Cell, Å3 |
|---|---|---|---|---|---|
| β-(Ca2.682 Sr0.165 Mg0.153) (PO4)2 | 17.025 (1,1,0) 25.787 (1,0,10) 27.808 (2,1,4) 29.645 (3,0,0) 31.101 (0,2,10) 34.413 (2,2,0) | 10.424 | 10.424 | 37.19 | 3500 |
| Hydroxyapatite Ca10(PO4)6(OH)2 | 25.787 (0,0,2) 31.101 (2,1,1) 32.094 (1,1,2) 32.828 (3,0,0) 33.969 (2,0,2) 39.771 (1,3,0) | 9.421 | 9.421 | 6.9 | 576 |
| Collinsite Ca2Mg(PO4)2(H2O)2 | 17.025 (1,0,0) 25.787 (1,1,0) 28.310 (0,2,0) 28.780 (1,0,1) 41.783 (0,1,2) 44.890 (2,0,1) | 5.757 | 6.716 | 5.431 | 186 |
| Parascholzite CaZn2(PO4)2∙2H2O | 26.527 (0,2,1) 27.808 (5,1,0) 32.094 (1,1,2) 33.534 (5,1,1) 39.771 (2,2,2) 47.368 (2,4,0) | 17.82 | 7.92 | 6.36 | 881 |
| Sample | Material Detached, mg | Consolidation, % | |
|---|---|---|---|
| Untreated | 7.11 | - | |
| CHAp Brushing | 0.25 g/L | 3.33 | 53.16 |
| Mg-Sr-Zn-CHAp Spraying | 0.1 g/L | 3.45 | 51.48 |
| 0.25 g/L | 3.36 | 52.74 | |
| 0.5 g/L | 3.75 | 47.26 | |
| Mg-Sr-Zn-CHAp Brushing | 0.1 g/L | 3.03 | 57.38 |
| 0.25 g/L | 2.92 | 58.93 | |
| 0.5 g/L | 2.98 | 58.09 | |
| Sample | Aspect before Test | Aspect after Test | Gelivity Coefficient, % |
|---|---|---|---|
| Untreated |  |  | 9.56 |
| Mg-Sr-Zn-CHAp Spraying (0.25g/L) |  |  | 6.89 |
| Mg-Sr-Zn-CHAp Brushing (0.25g/L) |  |  | 6.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, L.; Grigorescu, R.M.; Ion, R.-M.; David, M.E.; Predoana, L.; Gheboianu, A.I.; Alexandrescu, E. New Triple Metallic Carbonated Hydroxyapatite for Stone Surface Preservation. Coatings 2023, 13, 1469. https://doi.org/10.3390/coatings13081469
Iancu L, Grigorescu RM, Ion R-M, David ME, Predoana L, Gheboianu AI, Alexandrescu E. New Triple Metallic Carbonated Hydroxyapatite for Stone Surface Preservation. Coatings. 2023; 13(8):1469. https://doi.org/10.3390/coatings13081469
Chicago/Turabian StyleIancu, Lorena, Ramona Marina Grigorescu, Rodica-Mariana Ion, Madalina Elena David, Luminita Predoana, Anca Irina Gheboianu, and Elvira Alexandrescu. 2023. "New Triple Metallic Carbonated Hydroxyapatite for Stone Surface Preservation" Coatings 13, no. 8: 1469. https://doi.org/10.3390/coatings13081469
APA StyleIancu, L., Grigorescu, R. M., Ion, R.-M., David, M. E., Predoana, L., Gheboianu, A. I., & Alexandrescu, E. (2023). New Triple Metallic Carbonated Hydroxyapatite for Stone Surface Preservation. Coatings, 13(8), 1469. https://doi.org/10.3390/coatings13081469








