Abstract
Natural remedies derived from plants have a long history of usage in the treatment of a wide variety of severe diseases. This study aims to develop a Capparis spinosa (C. spinosa) oil nanoemulgel and evaluate its antimicrobial, anticancer, and anti-inflammatory effects. C. spinosa oil was developed into a nanoemulsion using a self-nanoemulsifying method with Span 80 and Tween 80 as emulsifying agents. Carbopol hydrogel was mixed with the nanoemulsion to form nanoemulgel. After this, we tested the particle size, polydispersity index (PDI), rheology, antimicrobial, cytotoxic, and anti-inflammatory activities. The nanoemulsion formulation that has a PDI of 0.159 and a particle size of 119.87 nm is considered to be the optimum formulation. The C. spinosa oil nanoemulgel gave results similar to its nanoemulsion, where it had a PDI lower than 0.2, droplet size below 200 nm, and zeta potential less than −35. Also, it had a pseudoplastic rheological behavior. The C. spinosa oil nanoemulgel showed a significant effect on Methicillin-Resistant Staphylococcus Aureus (MRSA) and Klebsiella pneumoniae (K. pneumonia) (ATCC 13883) with zone inhibition diameters of 33 ± 1.9 mm and 30 ± 1.4 mm, respectively, as well as significant activities on the MCF-7, HepG2, and HeLa cancer cell lines with IC50 values of 194.98, 91.2, and 251.18 µg/mL, respectively, which were better than those of the original oil. Regarding its anti-inflammatory effect, C. spinosa oil had a positive impact on both COX-1 and COX-2 but was more selective for COX-1. Consequently, simple nanotechnology techniques provide a promising step forward in the development of pharmacological dosage forms.
1. Introduction
Herbalism, also known as “folk medicine,” is a traditional practice that employs botanicals and plant extracts [1]. Since ancient times, people all over the world have been searching for medications in nature to treat their illnesses [2]. The initial utilization of medicinal plants was instinctual, as is the case with animals [3]. Currently, herbs are used to treat chronic and acute conditions, as well as a variety of maladies and problems, including cardiovascular disease, prostate issues, melancholy, inflammation, and to boost the immune system [4]. Today, many “conventional” medical practitioners do not hesitate to recommend botanicals, herbal products, or complementary and alternative medicine (CAM) therapy for the effective treatment of certain diseases [5]. Herbal therapy utilizes seeds, berries, roots, foliage, fruits, bark, blossoms, and even the entire plant [6].
One of the most commonly used medicinal plants in the world for the treatment of numerous ailments is C. spinosa, which is included in the family Capparidaceae [7]. Its aerial parts (roots and seeds) offer medicinal benefits [8]. Studies have demonstrated that it possesses antioxidant, antibacterial, anti-inflammatory, and anticancer properties [9]. These effects are based on the phytochemical examination of the plant, as the C. spinosa plant contains substantial amounts of phenol [10]. Generally, C. spinosa boasts a diverse array of bioactive agents, including terpenoids, alkaloids, steroids, flavonoids, and tocopherols. These compounds contribute to its potential health benefits and have been identified through thorough chemical analysis previously [10,11]. Additionally, it contains numerous components that are beneficial to health, including rutin, stigmasterol, quercetin, carotenoids, and tocopherols [12,13]. Its oil has been widely used for cancer, bacterial and fungal infections, and inflammation [14], as it contains cancer-causing methyl isothiocyanate, isopropyl isothiocyanate, and sec-butyl isothiocyanates [15].
Natural bio-compounds have been studied for their potential use in the treatment of a variety of diseases, which may contribute towards future drug development [16]. Cancer is considered the second-leading cause of death in the world and is defined as the abnormal growth and spread of cells. As a result, treatment procedures for each type of cancer are complicated [17]. Pharmaceutical societies primarily seek anti-cancer drugs with higher efficacy, fewer side effects, and lower costs [18]. C. spinosa extract has a lot of antitumor effects from the quercetin in it. It might be a good natural drug for treating cancer [19]. A study conducted on the extracted materials from the leaves and flower buds of C. spinosa showed that the plant has strong antioxidant activity as it contains tocopherols, carotenoids, and vitamin C [20]. Zhang et al. (2018) examined the antimicrobial activity of different extracts (aqueous, methanol, ethanol, and ethyl acetate) from C. spinosa fruits and roots. They revealed that the aqueous extract from the roots exhibited an inhibitory effect against different species of microorganisms [10]. It inhibits the growth of many types of bacteria, such as S. aureus, K. pneumoniae, and E. coli, and fungi, such as C. albicans and others [21]. In addition to that, Etemadi et al. (2022) studied the pain-relieving properties of C. spinosa roots in rat models of osteoarthritis and rheumatoid arthritis. This study supports the traditional use of C. spinosa roots to treat various types of pain in humans [22].
C. spinosa oil was studied by Bilusic et al. [23], who concluded that the extract showed significant antitumor effects and could be considered an ideal natural drug for cancer therapy by inhibiting cell growth, nuclear factor kappa-light-chain-enhancer of activated B cell activation, apoptosis, and the cell cycle in a human colon carcinoma cell line. Methyl, isopropyl, and sec-butyl isothiocyanates are carcinogens that can be found in this product [15]. The present work aimed to develop a C. spinosa oil nanoemulgel, which is a new approach to the enhancement of anticancer, antimicrobial, and anti-inflammatory activities. Therefore, this research explored the synthesis of a nanoemulgel containing C. spinosa oil and evaluated its antibacterial, anti-inflammatory, and anticancer properties.
2. Materials and Methods
2.1. Materials
To prepare the culture media, we used Mueller and Hinton agar (manufactured by the Becton, Dickinson, and Sparks Co. in Le Pont-de-Claix, France) and C. spinosa roots (collected from the mountains in various parts of Palestine). The carboxyvinyl polymer (Carbopol 940) was purchased from CBC Co., Ltd., Tokyo, Japan, and the dimethyl sulfoxide (DMSO) was purchased from Riedel De Haen, Germany. An analysis of the plant’s roots was performed at An-Najah National University in the Pharmacy Department of the Medical and Health Sciences Faculty.
2.2. C. spinosa Oil Extraction
Extensive extraction was performed by combining 100 g of powdered C. spinosa roots with 400 mL of 50% ethanol and 200 mL of n-hexane in a container with triple-distilled water, resulting in a total volume of 900 mL. The liquid was put into a mixer and set to 200 revolutions per minute, and it was mixed for 72 h at room temperature. Then, a Buchner funnel and filtration suction flask were used to filter the solution. We used a separatory funnel to separate the aqueous phase from the organic phase in the filtrate we produced. The bottom layer represented the watery phase, whereas the top layer represented the oilier organic phase. In order to remove all traces of organic solvents from the organic phase, we ran it through a rotary evaporator at 40 °C for an hour before storing the resulting oil at room temperature. In 125 mL of 50% ethanol and 50 mL of hexane in triple-distilled water, the residual solid material was extracted once more [24].
The percentage yield (%) was calculated by the following equation:
where M denotes the extracted oil mass (g) and Bm is the starting plant biomass (g).
2.3. Preparation of C. spinosa Oil Nanoemulgel
C. spinosa oil nanoemulsion formulation was prepared first, and then nanoemulgel was prepared by incorporating C. spinosa oil nanoemulsion with Carbopol 940 hydrogel.
2.3.1. Preparation of C. spinosa Oil Nanoemulsion
The self-nanoemulsifying method was used to transform the extracted C. spinosa oil into a nanoemulsion, and the relative amounts of surfactant, co-surfactant (Tween 80 and Span 80), and C. spinosa oil were varied to draw a ternary phase diagram. These three substances were present in the formulations, although their relative amounts varied. The mixture was then gently agitated with the vortex mixer for 3 min to ensure uniformity at room temperature. Polydispersity index (PDI) and Droplet size were used to determine the best formulation. Each formulation was tested by first being self-emulsified in distilled water with gentle agitation and then having its droplet size, polydispersity index (PDI), and overall appearance evaluated [25].
2.3.2. Droplet Size and Polydispersity Index Analysis of C. spinosa Oil Nanoemulsion
A master size analyzer (Brookhaven Instruments, Nano Brook Omni, New York, NY, USA) was used to measure the droplet size and PDI of the C. spinosa oil nanoemulsion. Before using the measuring procedure, the C. spinosa oil nanoemulsion was self-emulsified in distilled water. [26].
2.3.3. Preparation of Carbopol 940 Hydrogel
The hydrogel was made by adding Carbopol 940 to water at a concentration of 4% and shaking the liquid constantly until it became homogenous. This process was repeated until the mixture was completely smooth. The resulting mixture was left for a period of twenty-four hours in order to finish the gelation process.
2.3.4. Formulation of C. spinosa Oil Nanoemulgel
The C. spinosa oil nanoemulgel formulations were obtained by adding C. spinosa oil nanoemulsion to the Carbopol hydrogel at different concentrations (0.4, 0.6, and 0.8%). The formulations were pretty well combined until homogeneity was achieved. Then, the droplet size, polydispersity, and zeta potential were measured using a master size analyzer (Brookhaven Instruments, Nano Brook Omni, New York, NY, USA).
2.3.5. Physical Characterization of C. spinosa Oil Nanoemulgel
Many physical properties, such as homogeneity, phase separation, visual appearance, spreadability, and consistency, were visually examined while preparing the nanoemulgel. A pH meter (CG 820, Schott Gerate GmbH, Hofheim, Germany) was used accordingly to measure the pH levels.
2.3.6. Analysis of the C. spinosa Oil Nanoemulgel Zeta Potential
We used the zeta potential technique to determine the particle dispersion stability and surface charge using the NanoBrook Omni. The zeta potential value was tested three times, and the average was determined and graphed by plotting the zeta potential vs Carbopol concentrations. [27].
2.3.7. Rheological Measurement of C. spinosa Oil Nanoemulgel
Nanoemulgel formulations of C. spinosa oil were produced with thickening agents in different concentrations (0.4, 0.6, and 0.8%) of Carbopol 940, and their rheological behavior was evaluated using a 7s-size spindle. At a temperature of 25 °C, a viscometer (Brookfield DVI, Middleboro, MA, USA) with a shear rate range of 0–100 rpm was used. Triple measurements were performed for accuracy. Viscosity was determined by multiplying the sample density by the obtained value [26].
2.4. Antimicrobial Evaluation of C. spinosa Oil and Its Nanoemulgel
2.4.1. Antibacterial and Antifungal
Six different bacteria were employed in the antibacterial test, all of which can be found in the ATCC: MRSA, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, and Proteus mirabilis. Moreover, for the antifungal evaluation, Candida albicans was used accordingly.
2.4.2. Culture Media
The culture media consisted of Mueller Hinton agar (manufactured in France by the Becton, Dickinson, and Sparks Co., Sparks, MD, USA), to which 17.5 g of acid hydrolysate of casein, 1.5 g of starch, 2 g of beef extract, and 17 g of agar were added per litter of sterile water. After the ingredients were well combined, they were heated to boiling while being gently stirred in order to dissolve. The mixture was then autoclaved for 20 min at 121 °C. Before being placed onto clean Petri plates, the agar was dyed. A smooth surface was utilized so that the height and width would be consistent throughout. Finally, the agar was kept at 4–8 °C.
Agar diffusion was used to evaluate the antimicrobial and antifungal properties. The agar plates were prepared by punching four holes, each 6 mm in diameter (labeled A, B, C, and D). Hole A only received DMSO, while oil extracted from C. spinosa was inserted into hole B. Nanoemulgel containing oil from C. spinosa was used to fill hole C, whereas emulgel without C. spinosa oil served as a control for hole D. For the antibacterial evaluation, the plates were kept for 24 h in the incubator at 37 °C, as well as for the antifungal evaluation at 25 °C. In order to determine the antibacterial and antifungal activities, the width of the inhibitory zone had to be measured [25].
2.5. Cytotoxicity Evaluation of C. spinosa Oil and Its Nanoemulgel
Human MCF-7 breast cancer cells, HepG2 human hepatocellular carcinoma cells, and HeLa human cervical epithelioid carcinoma cells were cultured in RPMI 1640 medium (Biological Industries, Cromwell, CT, USA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine. Cell cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
For the experimental setup, cells were seeded in triplicate at a density of approximately 1000 cells per well in 96-well plates with 100 µL of their respective culture media. After a 24 h incubation period, the culture medium was replaced with fresh medium containing different concentrations of C. spinosa oil (200, 400, 600, 800, and 1000 µg/mL). The cells were then incubated for an additional 72 h. To assess the potential anti-proliferative effects, the Cell Titer 96® Aqueous One Solution Cell Proliferation (MTS) Assay (Promega Corporation, Madison, WI, USA) was employed following the manufacturer’s guidelines. After treatment, each well received 20 µL of MTS solution per 100 µL of medium and was subsequently incubated for 2 h at 37 °C. The absorbance was measured at 490 nm to determine the impact of the plant oil on cell proliferation [28].
2.6. COX Enzyme Evaluation of C. spinosa Oil and Its Nanoemulgel
Employing a COX inhibitor screening assay kit (Item No. 460104) developed by Cayman Chemical Manufacturer (USA), we determined whether or not C. spinosa oil might inhibit the formation of prostaglandin H2 (PGH2) from arachidonic acid (AA) by bovine COX-1 and human recombinant COX-2. To determine the 50% inhibitory concentration (IC50), two concentrations (50 and 300 µg/mL) of each compound (C. spinosa oil and its nanoemulgel) were tested in a triplicate experiment. Utilizing the generated multiple regression best-fit line, we conducted calculations to determine the extent of inhibition exhibited by the sample plant. This was achieved by referencing a standard curve consisting of eight different doses of prostaglandin, along with a non-specific binding sample and a maximal binding sample, in accordance with the instructions provided in the assay kit manual. The IC50 was determined by using the percentage inhibition at both concentrations [29].
3. Results
3.1. Yield of C. spinosa Root Extraction
For extraction, about 1500 g of C. spinosa roots was prepared, and 6 g of C. spinosa oil was collected from these roots with a yield of 0.4%.
3.2. Droplet Size and PDI Analysis of C. spinosa Oil Nanoemulsion Formulations
Ternary phase diagrams were constructed using varied concentrations of Span 80, Tween 80, and C. spinosa oil to find the optimal formulation. As a result, a nanoemulsion with a PDI of less than 0.3 and a droplet size of less than 200 nm was created (Figure 1). To compare the selected formulations that showed droplet sizes below 200 nm, we used the optimum nanoemulsion formulation (formulation 2), which comprised 28% Tween 80, 12% Span 80, and 60% C. spinosa oil and had a PDI of 0.159 ± 0.09 and a droplet size of 119.87 ± 3.78 nm (Table 1).
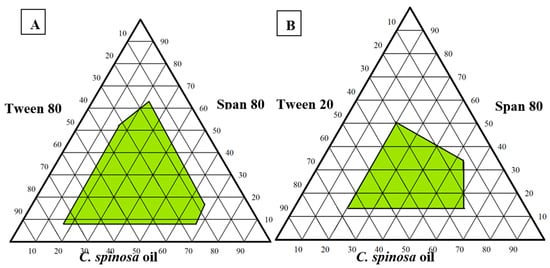
Figure 1.
Pseudo-ternary phase diagrams of C. spinosa oil nanoemulsion: (A) Composed of oil, Tween 80, and Spam 80; (B) composed of oil, Tween 20, and Span 80.

Table 1.
The selected formulations of C. spinosa oil nanoemulsion.
3.3. C. spinosa Oil Nanoemulgel Formulations
A nanoemulgel containing C. spinosa oil was prepared by using different concentrations of Carbopol 940 (0.4, 0.6, and 0.8% w/w%). Carbopol was utilized as the gelling agent because of its edematous qualities. Tween 80 and Span 80 were employed as surfactant and co-surfactant, respectively, in the preparation of the nanoemulsion formulation, which was then mixed with distilled water and the Carbopol 940 hydrogel using the self-emulsification approach to create the nanoemulgel. The nanoemulgel formulation’s droplet size, viscosity, and size distribution were measured.
3.4. Influence of Different Carbopol Concentrations on Droplet Size and PDI of C. spinosa Oil Nanoemulgel
The results showed that the mean droplet size was submicron and that its size distribution was narrow and had a low PDI. To determine the best formulation of C. spinosa oil nanoemulgel, which has the smallest particle size and PDI, three different concentrations (0.4%, 0.6%, and 0.8%) of Carbopol 940 were used to make the nanoemulsion. Nanoemulgels were prepared using varying doses of Carbopol and compared to the first ideal nanoemulsion for variations in PDI and particle size (Figure 2).
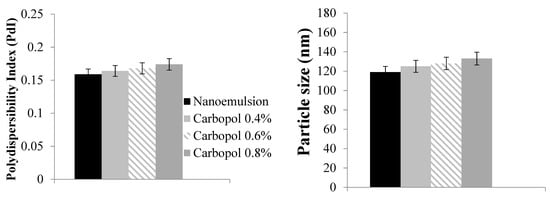
Figure 2.
Droplet size and polydispersibility index (PDI) of C. spinosa oil nanoemulgel with different Carbopol concentrations.
3.5. Sensorial Property Analysis and Physical Characterization of C. spinosa Oil Nanoemulgel
The nanoemulgel made from C. spinosa oil should be spreadable and simple to use. The more Carbopol we included in the mixture, the less spreadable it became and the more cumbersome it was to handle. Therefore, we opted for 0.4% Carbopol rather than 0.6% or 0.8% because of its lower concentration. In terms of spreadability, there was little to no noticeable variation between the various concentrations. The nanoemulgel had a pH of 6. The ideal formulation has a low emulsification time (less than 30 s), high spreadability, and a transparent color.
3.6. Zeta Potential Measurement of C. spinosa Oil Nanoemulgel
Figure 3 demonstrates that the zeta potential of each and every C. spinosa oil nanoemulgel formulation was lower than −35.
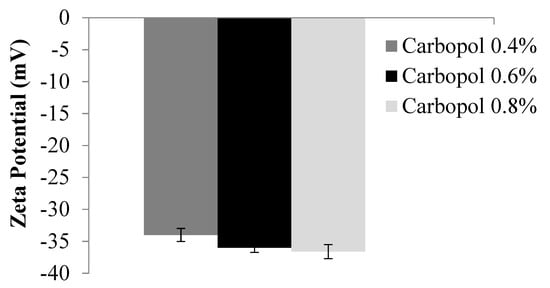
Figure 3.
Zeta potential of C. spinosa oil nanoemulgel formulations.
3.7. The Rheological Behavior of C. spinosa Oil Nanoemulgel Formulations
The rheological characterization analyzes the flow characteristics of semisolid pharmaceutical preparations in order to identify the quality of these products and how well they function. The results of an investigation into the rheology of the C. spinosa oil nanoemulgel formulations are presented in Figure 4. Since a reduction in viscosity occurs whenever there is a rise in shear rate, the rheology of these compositions exhibits a pseudoplastic behavior.
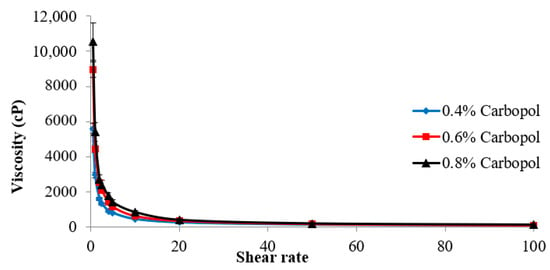
Figure 4.
Rheological behavior of C. spinosa oil nanoemulgel formulations.
3.8. Antibacterial Activity of C. spinosa Oil and Its Nanoemulgel
The antibacterial investigations involving C. spinosa oil and its nanoemulgel against a range of gram-positive and gram-negative bacterial strains exhibited outcomes different from those observed with control-positive antibiotics and antifungal agents like ampicillin and fluconazole. According to the zone inhibition diameter (in millimeters), it was observed that the oil has an impact on K. pneumoniae that is greater than that of the positive control drug (ampicillin), with a zone inhibition diameter of 25 ± 1.7 mm, while the nanoemulgel has a better effect than oil on MRSA and K. pneumoniae compared with ampicillin, with a zone inhibition diameter of 33 ± 1.9 mm and 30 ± 1.4 mm, respectively. Although the oil has a lower effect than the positive control drug (fluconazole) against C. albicans, the nanoemulgel has a good effect with a zone inhibition diameter of 17 ± 0.5 mm (as shown in Table 2 below).

Table 2.
Antimicrobial activity of C. spinosa oil and C. spinosa oil nanoemulgel compared with ampicillin and fluconazole antibiotics.
3.9. Cytotoxic Activity of C. spinosa Oil and Its Nanoemulgel
Within this investigation, we explored the potential anticancer properties of C. spinosa oil and its nanoemulgel formulation across three distinct cancer cell lines: HeLa, HepG2, and MCF-7 cells. HeLa cells, sourced from cervical cancer specimens, are notably associated with being a prominent contributor to fatal cancer instances among women. Key factors contributing to this specific cancer type encompass smoking, oral contraceptive use, and infection by the human papillomavirus (HPV) [30]. The MCF-7 cell line, which is derived from breast cancer, is considered one of the three most common cancers worldwide. Many risk factors for this kind of cancer exist, including estrogen hormones and family history [31]. HepG2 cells originate from hepatocellular carcinoma, a significant health concern characterized by its severity. Viral hepatitis and alcohol are considered important risk factors for hepatocellular carcinoma [32].
After conducting cytotoxic tests, we acquired some intriguing results, which are depicted in Figure 5. These results illustrate the connection between the concentration of C. spinosa oil and its nanoemulgel, which is then plotted against the percentage of cancer cell growth that is inhibited. There was an impact of the oil and its nanoemulgel on these cancer cells since the suppression of their growth increased as the concentrations of the oil and its nanoemulgel increased. This indicates that the oil and its nanoemulgel have an effect on these cancer cells.
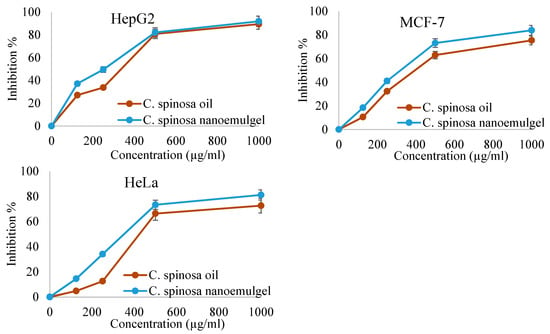
Figure 5.
Cytotoxic effects of C. spinosa oil and C. spinosa oil nanoemulgel.
Figure 6 and Table 3 provide an explanation of the IC50 values for the oil and its nanoemulgel derived from C. spinosa in relation to the three different kinds of cancer cells. Their effects on cancer cells are reduced in proportion to the increase in the IC50. HepG2 cells were judged to be the most impacted by the oil and its nanoemulgel, with IC50 values of 144.54 µg/mL and 91.2 µg/mL, respectively. MCF-7 cells were influenced by the oil and its nanoemulgel with IC50 values of 436.51 µg/mL and 194.98 µg/mL, respectively. However, Hela cells were the least impacted by the oil and its nanoemulgel, with an IC50 of 831.71 µg/mL and 251.18 µg/mL, respectively. This was determined by measuring the concentration of the drug in the medium. In spite of the fact that both C. spinosa oil and its nanoemulgel were shown to have a negligible impact on cancer cells, the results indicated that the nanoemulgel was more effective than the oil.
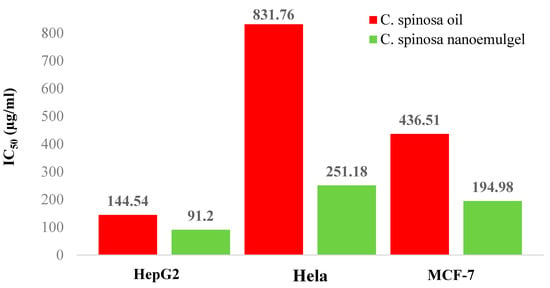
Figure 6.
The IC50 values (µg/mL) of C. spinosa oil and C. spinosa oil nanoemulgel against different cancer cell lines.

Table 3.
The IC50 values (µg/mL) of C. spinosa oil and C. spinosa oil nanoemulgel against different cancer cell lines.
3.10. Anti-Inflammatory Activity of C. spinosa Oil and Its Nanoemulgel
In this study, we tested the anti-inflammatory effects of C. spinosa oil and its nanoemulgel by evaluating the ability of C. spinosa oil to inhibit the conversion of arachidonic acid to prostaglandin H2 by cyclooxygenase enzymes (COX-1 and COX-2) at two concentrations, 50 µg and 300 µg. It was found that C. spinosa oil has inhibitory activity on both COX-1 and COX-2 (Figure 7). However, C. spinosa oil nanoemulgel showed a significant improvement in COX-2 inhibition, as shown in Figure 8.
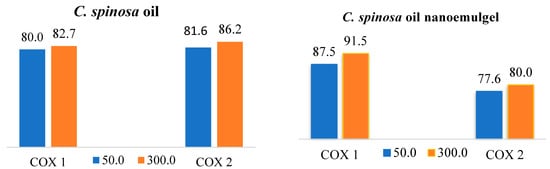
Figure 7.
Cyclooxygenase percentage of inhibition activity against COX-1 and COX-2 of C. spinosa oil and its nanoemulgel.
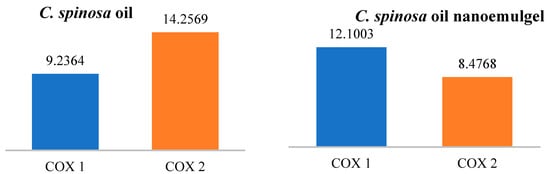
Figure 8.
The IC50 values (µg/mL) of C. spinosa oil and its nanoemulgel.
The figures below explain the percentage inhibition and IC50 of C. spinosa oil and its nanoemulgel on COX-1 and COX-2. When the IC50 increases, the anti-inflammatory effects decrease. C. spinosa oil inhibited both COX-1 and COX-2 enzymes at both concentrations (50 and 300 µg), with the IC50 for COX-1 being 9.2364 µg/mL and the IC50 for COX-2 being 14.2596 µg/mL. On the other hand, the C. spinosa oil nanoemulgel showed a significant improvement in COX-2 enzyme inhibition with an IC50 of 8.4768 µg/mL. There was no significant improvement in COX-1 enzyme inhibition.
4. Discussion
Plant oils have been used in traditional human medicine for many years [33]. Plant oils contain active components that have antioxidant, antimicrobial, antiviral, and anti-inflammatory activities [34]. Since oils have hydrophobic characteristics, a nanoemulgel was developed to improve the delivery of oils and increase their bioavailability [35]. Nanoemulsions and hydrogels are the main components of nanoemulgels [36]. Nanoemulsions are nanosized emulsions composed of two immiscible fluids and an emulsifying agent that aids in their combination and formation of a single phase [37]. Carbopol 940 was used in various concentrations. We chose the lowest concentration of Carbopol 940, which is 0.4%, to improve the bioavailability and spreadability. The selection was based on the results of nanoemulgel formulations (rheological behavior of droplet size, PDI, and zeta potential).
Carbopol 940 is used as a thickening agent because it has gel-forming and swelling properties in addition to its ability to increase viscosity [38]. The C. spinosa oil nanoemulgel’s rheological behavior was pseudoplastic, which means that as shear rate increases, viscosity decreases [39]. The magnitude of the nanoemulgel’s zeta potential determines its stability. The large negative and positive values of the zeta potential cause repulsion between particles, resulting in a stable dispersion. Otherwise, when the zeta potential is low, the dispersion is unstable, which means there is no force keeping the particles apart. In general, the line separating the stability of the dispersions is 30 mV or −30 mV, with dispersions higher than 30 mV and lower than −30 mV considered stable systems [40]. As shown in the results, the optimum nanoemulgel has a value of -35 mV because of the non-ionic surfactants added to the formulation, which coat the system around the surface, assisting in its stabilization [41].
Sungpud et al. (2020) incorporated mangostin extract into a nanoemulgel using mixed surfactants (Tween and Span) that was able to produce surfactant mixtures with hydrophilic–lipophilic balances (HLBs) of 12.6 and 15.1, respectively. This blend of surfactants was capable of producing small particle sizes (typically 18–62 nm) and a low zeta potential of −39 to −54.5 mV [42]. These results support the findings obtained in our study, as we used a mixture of Tween and Span to produce a nanoemulsion with a small droplet size, low PDI, and low zeta potential.
The PDI is important in evaluating the stability of the nanoemulgel formulation because it represents the size distribution of a population within a given sample. When the PDI is high, the particles in the formulation become less homogeneous [43]. In this study, the C. spinosa oil nanoemulgel formulations had a PDI < 0.2, demonstrating a narrow and uniform globule size distribution. The optimum formulation had a PDI value of 0.159 and a droplet size of around 119.87 nm. The selection of the appropriate surfactant is critical to the development of an appropriate nanoemulgel formulation [44]. Span 80 and Tween 80 (non-ionic surfactants), with HLP values of 4.3 and 15, respectively, were selected for C. spinosa oil nanoemulgel formulation because of their low critical micelle concentration, their ability to form uniform and superior droplets that aid in the rapid absorption and release of the nanoemulgel, their low toxicity compared to others, and their low risk of irritation [45]. The particle size and zeta potential were affected by the emulsifier type, concentration, pH, and ionic strength, so the low particle size and low PDI were particularly due to the high solubility and high emulsification ability of Tween 80 [46]. After the preparation of formulations that contained different concentrations of oil, surfactant, and co-surfactant, the particle size was tested, and the ternary phase diagram was plotted to demonstrate the best formulation possible with a droplet size of less than 200 nm. Tween 80 and Span 80 are non-ionic surfactants that represent 28.67% and 12.29%, respectively, of our formulation. Several studies have shown that as surfactant concentration increases, droplet size decreases. Smaller droplets require a higher surfactant concentration to be stabilized due to their larger surface area [47]. Infectious diseases are among the most frequently reported diseases. Many plants are used in traditional medicine to treat various diseases [48]. In this study, we evaluated the antimicrobial activity of C. spinosa oil and its nanoemulgel. The nanoemulgel has a high zone of inhibition as compared with that of C. spinosa oil. Bacterial inhibition occurs due to the greater concentration of nanoemulgel compared to oil and because the particle size is very small and the surface area is large, which improves the nanoemulgel’s interaction with bacteria [43]. Eid et al. (2019) showed that the incorporation of fusidic acid (FA) and sodium fusidate (SF) into a nanoemulgel improves their antimicrobial activity as a result of the increased contact between the nanoemulgel and bacteria [26].
A previous study in 2021 showed that the anticancer activity of safrole nanoemulgel was improved compared with that of safrole oil [28]. In the current study, the anticancer activity of C. spinosa oil and its nanoemulgel was evaluated against three types of cells: HeLa, MCF-7, and HepG2. The nanoemulgel showed better results compared with the crude oil because it has a small particle size (119.87 nm), which improves its penetration and interaction with cancer cells.
C. spinosa oil has been used in traditional medicine to treat inflammatory disorders such as rheumatism [19]. A study in 2016 examined the effect of C. spinosa root extracts on rat articular pain and found that C. spinosa extracts relieved pain associated with rheumatoid arthritis and osteoarthritis after a single administration [49]. Rahimi et al., 2020, recently showed that extracts of the aerial parts of C. spinosa significantly and dose-dependently reduced the level of inflammation after the evaluation of the anti-inflammatory and immunomodulatory effects of C. spinosa both in vivo and in vitro [50]. In our study, C. spinosa oil inhibited both COX-1 and COX-2, but it was more selective against COX-1. The nanoemulgel formulation performed similarly to the oil on COX-1, but with better results and a significant improvement in COX-2. This improvement in the effect could be due to the nano-sized oil droplets in the nanoemulgel.
5. Conclusions
The present study evaluated the antimicrobial, anticancer, and anti-inflammatory effects of C. spinosa oil and its nanoemulgel compared with control medications. The crude oil showed anticancer activity against three different cancer cell lines (HeLa, MCF-7, and HepG2) and antimicrobial activity against all the tested microorganisms except E. coli, and these activities were improved when the oil was prepared in nanoemulgel form. A nanoemulsion consisting of C. spinosa oil, Span 80, and Tween 80 was prepared using the self-nanoemulsifying technique and then incorporated with a 0.4% concentration of Carpobol 940 as the hydrogel material to form a nanoemulgel, which improved its penetration through the skin due to the small droplet size, and this improvement was evident when comparing the performance of the original oil vs the nanoemulgel on bacterial strains and cancer cell lines, where the activities were markedly enhanced. The nanoemulgel had a pH of 6 and high spreadability; it also had good rheological and physical characteristics. According to the results we obtained, nanotechnology techniques will be a promising step in the preparation of pharmaceutical dosage forms.
Author Contributions
Conceptualization, methodology, A.M.E. and M.H.; validation, A.M.E., M.A. and M.H.; formal analysis, A.M.E. and M.A.; investigation, A.M.E., M.A. and M.H.; data curation, A.M.E.; writing—original draft preparation, A.M.E., M.A., S.N., M.D., M.M. and M.H.; writing—review and editing, A.M.E. and M.H.; visualization, A.M.E., M.A., S.N., M.D., M.M. and M.H.; supervision, A.M.E. and M.H.; project administration, A.M.E. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank An-Najah National University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ang, L.; Lee, H.W.; Kim, A.; Lee, J.A.; Zhang, J.; Lee, M.S. Herbal medicine for treatment of children diagnosed with COVID-19: A review of guidelines. Complement. Ther. Clin. Pract. 2020, 39, 101174. [Google Scholar] [CrossRef] [PubMed]
- Gunjan, M.; Naing, T.W.; Saini, R.S.; Ahmad, A.; Naidu, J.R.; Kumar, I. Marketing trends & future prospects of herbal medicine in the treatment of various disease. World J. Pharm. Res. 2015, 4, 132–155. [Google Scholar]
- Furman, B.L.; Candasamy, M.; Bhattamisra, S.K.; Veettil, S.K. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol. 2020, 247, 112264. [Google Scholar] [CrossRef]
- Jain, S.; Buttar, H.S.; Chintameneni, M.; Kaur, G. Prevention of cardiovascular diseases with anti-inflammatory and anti-oxidant nutraceuticals and herbal products: An overview of pre-clinical and clinical studies. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Krug, K.; Kraus, K.I.; Herrmann, K.; Joos, S. Complementary and alternative medicine (CAM) as part of primary health care in Germany–comparison of patients consulting general practitioners and CAM practitioners: A cross-sectional study. BMC Complement. Altern. Med. 2016, 16, 409. [Google Scholar] [CrossRef] [PubMed]
- Upasani, S.V.; Beldar, V.G.; Tatiya, A.U.; Upasani, M.S.; Surana, S.J.; Patil, D.S. Ethnomedicinal plants used for snakebite in India: A brief overview. Integr. Med. Res. 2017, 6, 114–130. [Google Scholar] [CrossRef]
- Vahid, H.; Rakhshandeh, H.; Ghorbani, A. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharmacother. 2017, 92, 293–302. [Google Scholar] [CrossRef]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Ahmad, I.; Alamri, A.; Anwar, S.; Alamri, A.S.; Locatelli, M.; Tartaglia, A.; Mahomoodally, M.F.; et al. Investigation into the biological properties, secondary metabolites composition, and toxicity of aerial and root parts of Capparis spinosa L.: An important medicinal food plant. Food Chem. Toxicol. 2021, 155, 112404. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, T.; Wang, C. Capparis spinosa L. as a potential source of nutrition and its health benefits in foods: A comprehensive review of its phytochemistry, bioactivities, safety, and application. Food Chem. 2023, 409, 135258. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F. Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef]
- Tir, M.; Feriani, A.; Labidi, A.; Mufti, A.; Saadaoui, E.; Nasri, N.; Khaldi, A.; El Cafsi, M.; Tlili, N. Protective effects of phytochemicals of Capparis spinosa seeds with cisplatin and CCl4 toxicity in mice. Food Biosci. 2019, 28, 42–48. [Google Scholar] [CrossRef]
- Tlili, N.; Khaldi, A.; Triki, S.; Munné-Bosch, S. Phenolic compounds and vitamin antioxidants of caper (Capparis spinosa). Plant Foods Hum. Nutr. 2010, 65, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Tlili, N.; Elfalleh, W.; Saadaoui, E.; Khaldi, A.; Triki, S.; Nasri, N. The caper (Capparis L.): Ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia 2011, 82, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Bakrim, W.B.; Drissi, B.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Caper (Capparis spinosa L.): An updated review on its phytochemistry, nutritional value, traditional uses, and therapeutic potential. Front. Pharmacol. 2022, 13, 878749. [Google Scholar] [CrossRef]
- Alkhaibari, A.M.; Alanazi, A.D. Chemical composition and insecticidal, antiplasmodial, and anti-leishmanial activity of Capparis spinosa essential oil and its main constituents. Evid. -Based Complement. Altern. Med. 2022, 2022, 6371274. [Google Scholar] [CrossRef]
- Hamidi, M.; Kozani, P.S.; Kozani, P.S.; Pierre, G.; Michaud, P.; Delattre, C. Marine bacteria versus microalgae: Who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar. Drugs 2019, 18, 28. [Google Scholar] [CrossRef]
- Sadoughi, F.; Kazemy, Z.; Hamedan, F.; Owji, L.; Rahmanikatigari, M.; Azadboni, T.T. Artificial intelligence methods for the diagnosis of breast cancer by image processing: A review. Breast Cancer Targets Ther. 2018, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.-S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Rahnavard, R.; Razavi, N. A review on the medical effects of Capparis spinosa L. Adv. Herb. Med. 2017, 3, 44–53. [Google Scholar]
- Al Badri, S.; Al Janabi, N. Estimation of the antioxidant activity of local Capparis spanosa leaves. Iraqi J. Agric. Sci. 2018, 49, 64–70. [Google Scholar]
- Al-Snafi, A.E. Medicinal plants with antimicrobial activities (part 2): Plant based review. Sch. Acad. J. Pharm. 2016, 5, 208–239. [Google Scholar] [CrossRef]
- Etemadi, E.; Fazilati, M.; Karimi, A.; Nazem, H.-A. Protective effect of hydroalcoholic extract of Capparis spinosa L. root on inflammatory factors of rheumatoid arthritis in rats. J. Isfahan Med. Sch. 2022, 40, 307–317. [Google Scholar]
- Kulisic-Bilusic, T.; Schmöller, I.; Schnäbele, K.; Siracusa, L.; Ruberto, G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.). Food Chem. 2012, 132, 261–267. [Google Scholar] [CrossRef]
- Eid, A.M.; Jaradat, N.A.; Elmarzugi, N.A.; Alkowni, R.; Hussen, F.; Ayyash, L.A.; Sawafta, M.; Danaa, H. Anti-microbial and free radical scavenging activities of nigella sativa colloidal-emulgel. Lett. Drug Des. Discov. 2019, 16, 408–416. [Google Scholar] [CrossRef]
- Eid, A.M.; Jaradat, N.A.; Al-Masri, M.; Issa, L.; Zubidat, F.; Asrawi, H.; Ahmad, S. Development and antimicrobial evaluation of Eruca sativa oil nanoemulgel with determination of the oil antioxidant, sun protection factor and elastase inhibition. Curr. Pharm. Biotechnol. 2020, 21, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Istateyeh, I.; Salhi, N.; Istateyeh, T. Antibacterial activity of Fusidic acid and sodium Fusidate nanoparticles incorporated in pine oil nanoemulgel. Int. J. Nanomed. 2019, 14, 9411. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.M.; Cekić, N.D.; Savić, M.M.; Isailović, T.M.; Ranđelović, D.V.; Marković, B.D.; Savić, S.R.; Stamenić, T.T.; Daniels, R.; Savić, S.D. Parenteral nanoemulsions as promising carriers for brain delivery of risperidone: Design, characterization and in vivo pharmacokinetic evaluation. Int. J. Pharm. 2015, 493, 40–54. [Google Scholar] [CrossRef]
- Eid, A.M.; Hawash, M. Biological evaluation of Safrole oil and Safrole oil Nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complement. Med. Ther. 2021, 21, 159. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Hameedi, S.; Mousa, A. Design, synthesis and biological evaluation of novel benzodioxole derivatives as COX inhibitors and cytotoxic agents. BMC Chem. 2020, 14, 54. [Google Scholar] [CrossRef]
- Fitzpatrick, M.B.; Dube Mandishora, R.S.; Katzenstein, D.A.; McCarty, K.; Weber, J.; Sahoo, M.K.; Manasa, J.; Chirenje, Z.M.; Pinsky, B.A. hrHPV prevalence and type distribution in rural Zimbabwe: A community-based self-collection study using near-point-of-care GeneXpert HPV testing. Int. J. Infect. Dis. 2019, 82, 21–29. [Google Scholar] [CrossRef]
- Hernández-Silva, C.D.; Villegas-Pineda, J.C.; Pereira-Suárez, A.L. Expression and role of the G protein-coupled estrogen receptor (GPR30/GPER) in the development and immune response in female reproductive cancers. Front. Endocrinol. 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Abass, S.A.; Mohamed, A.A.; Hamid, D.M. Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed. Pharmacother. 2018, 107, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Steflitsch, W. Aromatherapy—from traditional and scientific evidence into clinical practice. Dtsch. Med. Wochenschr. 2017, 142, 1936–1942. [Google Scholar]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, B.K.; Dubey, N.K. Encapsulation of essential oils-a booster to enhance their bio-efficacy as botanical preservatives. J. Sci. Res. 2020, 64, 175–178. [Google Scholar] [CrossRef]
- Ahmad, J.; Gautam, A.; Komath, S.; Bano, M.; Garg, A.; Jain, K. Topical nano-emulgel for skin disorders: Formulation approach and characterization. Recent Pat. Anti-Infect. Drug Discov. 2019, 14, 36–48. [Google Scholar] [CrossRef]
- Jadhav, R.P.; Koli, V.W.; Kamble, A.B.; Bhutkar, M.A. A review on nanoemulsion. Asian J. Res. Pharm. Sci. 2020, 10, 103–108. [Google Scholar] [CrossRef]
- Ranch, K.; Patel, H.; Chavda, L.; Koli, A.; Maulvi, F.; Parikh, R.K. Development of in situ ophthalmic gel of dexamethasone sodium phosphate and chloramphenicol: A viable alternative to conventional eye drops. J. Appl. Pharm. Sci. 2017, 7, 101–108. [Google Scholar]
- Bouhoute, M.; Nakajima, M.; Isoda, H. Design of nanoemulgel using Argania spinosa microfibrillated cellulose and natural emulsifiers foreseeing melanogenesis enhancement. Carbohydr. Polym. 2021, 274, 118632. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Drenckhan, W.; Salonen, A.; Rodrigues, J.A.; Iniguez-Palomares, R.; Rio, E.; Langevin, D. On the long-term stability of foams stabilised by mixtures of nano-particles and oppositely charged short chain surfactants. J. Soft Matter 2012, 8, 11085–11097. [Google Scholar] [CrossRef]
- Salim, N.; Basri, M.; Rahman, M.A.; Abdullah, D.; Basri, H.; Salleh, A. Phase behaviour, formation and characterization of palm-based esters nanoemulsion formulation containing ibuprofen. J. Nanomed. Nanotechnol. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Chaijan, M.; Sae Yoon, A. Techno-biofunctionality of mangostin extract-loaded virgin coconut oil nanoemulsion and nanoemulgel. PLoS ONE 2020, 15, e0227979. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Issa, L.; Al-Kharouf, O.; Jaber, R.; Hreash, F. Development of coriandrum sativum oil nanoemulgel and evaluation of its antimicrobial and anticancer activity. BioMed Res. Int. 2021, 2021, 5247816. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Rosly, M.B.; Jusoh, N.; Othman, N.; Rahman, H.A.; Sulaiman, R.N.R.; Noah, N.F.M. Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chem. Eng. Process.-Process Intensif. 2020, 148, 107790. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, Z.; Du, X.; Zhang, F.; Liu, Y.; Bai, X.; Sun, G. Impact of surfactants on nanoemulsions based on fractionated coconut oil: Emulsification stability and in vitro digestion. J. Oleo Sci. 2020, 69, 227–239. [Google Scholar] [CrossRef]
- Politova, N.I.; Tcholakova, S.; Tsibranska, S.; Denkov, N.D.; Muelheims, K. Coalescence stability of water-in-oil drops: Effects of drop size and surfactant concentration. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 32–39. [Google Scholar] [CrossRef]
- Siddique, H.; Pendry, B.; Rashid, M.A.; Rahman, M.M. Medicinal plants used to treat infectious diseases in the central part and a northern district of Bangladesh–An ethnopharmacological perception. J. Herb. Med. 2021, 29, 100484. [Google Scholar] [CrossRef]
- Maresca, M.; Micheli, L.; Di Cesare Mannelli, L.; Tenci, B.; Innocenti, M.; Khatib, M.; Mulinacci, N.; Ghelardini, C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016, 193, 456–465. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Rajabian, A.; Rajabi, H.; Vosough, E.M.; Mirkarimi, H.R.; Hasanpour, M.; Iranshahi, M.; Rakhshandeh, H.; Askari, V.R. The effects of hydro-ethanolic extract of Capparis spinosa (C. spinosa) on lipopolysaccharide (LPS)-induced inflammation and cognitive impairment: Evidence from in vivo and in vitro studies. J. Ethnopharmacol. 2020, 256, 112706. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).