Influence of Cu Substitution on the Properties of Hydroxyapatite Targets and Deposited Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
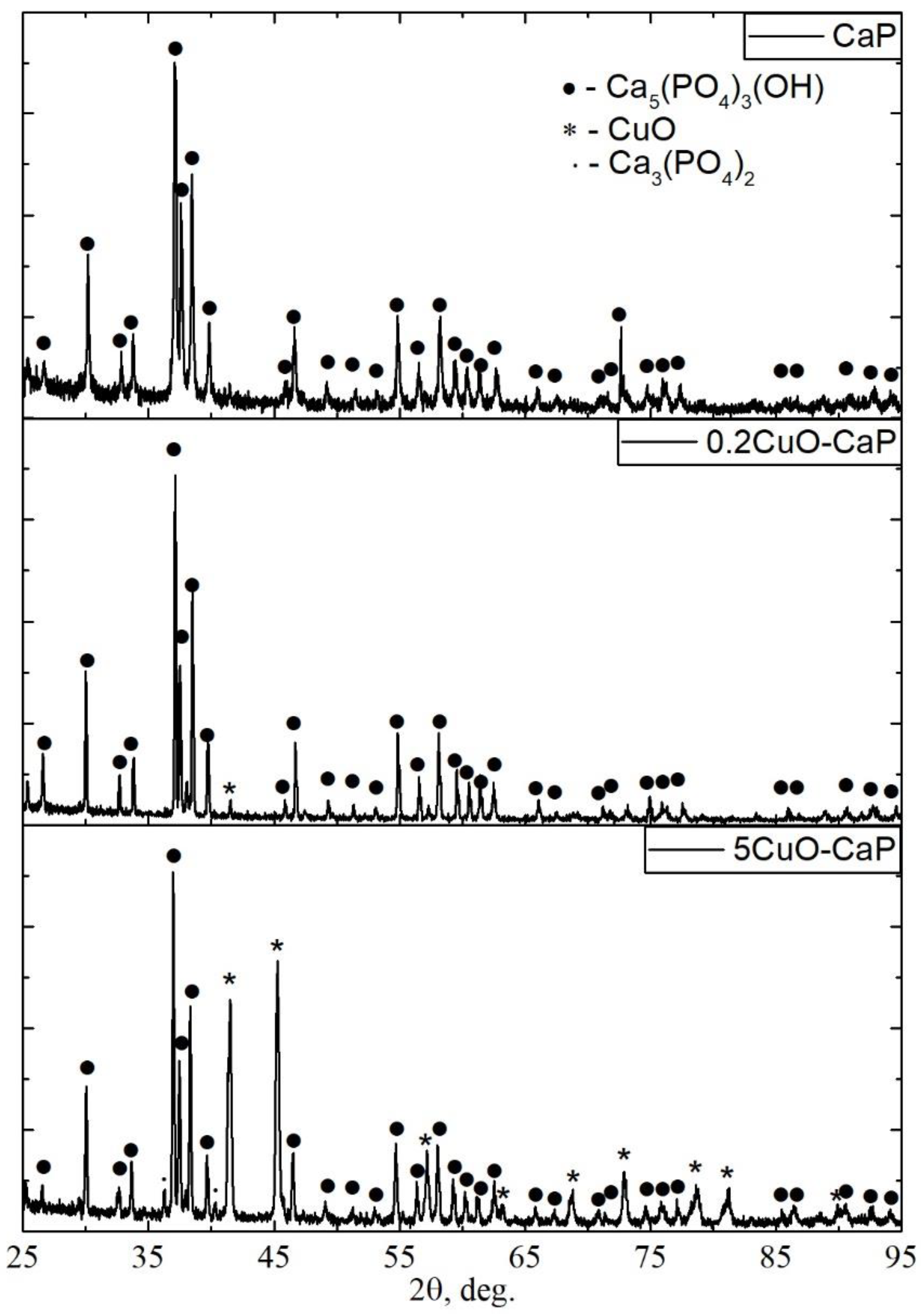
3.1. Characterization of Sintered Powders
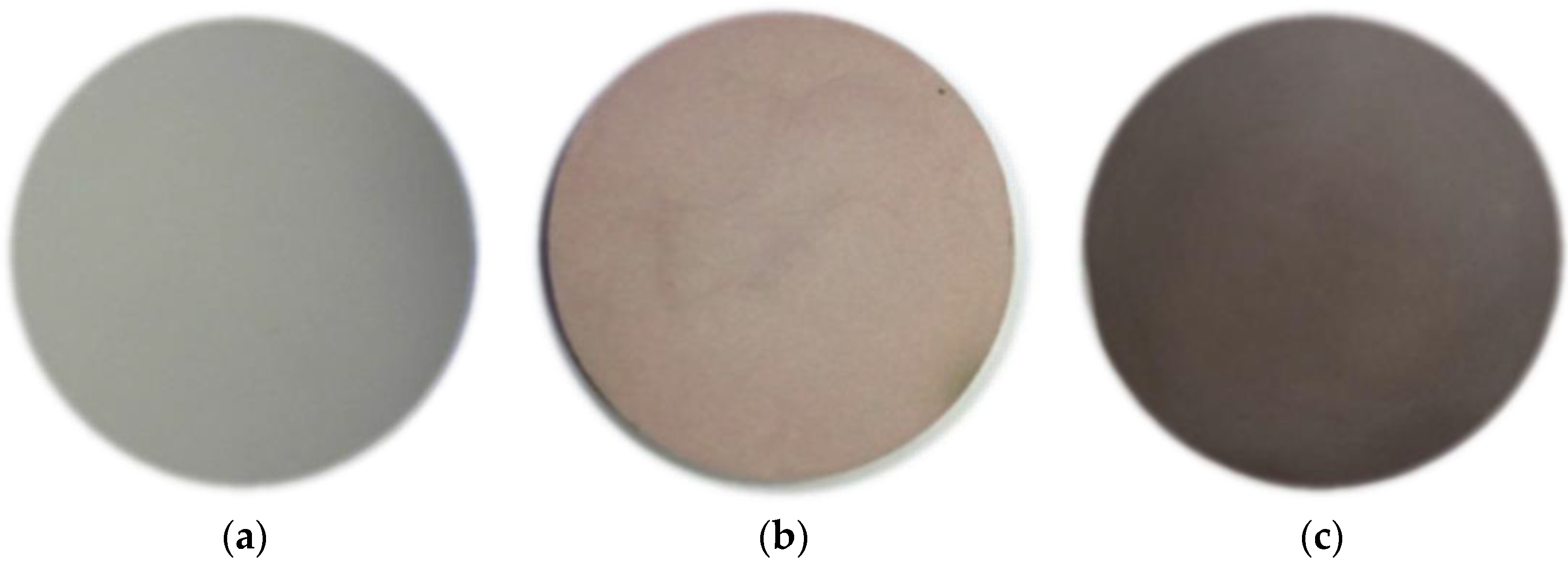
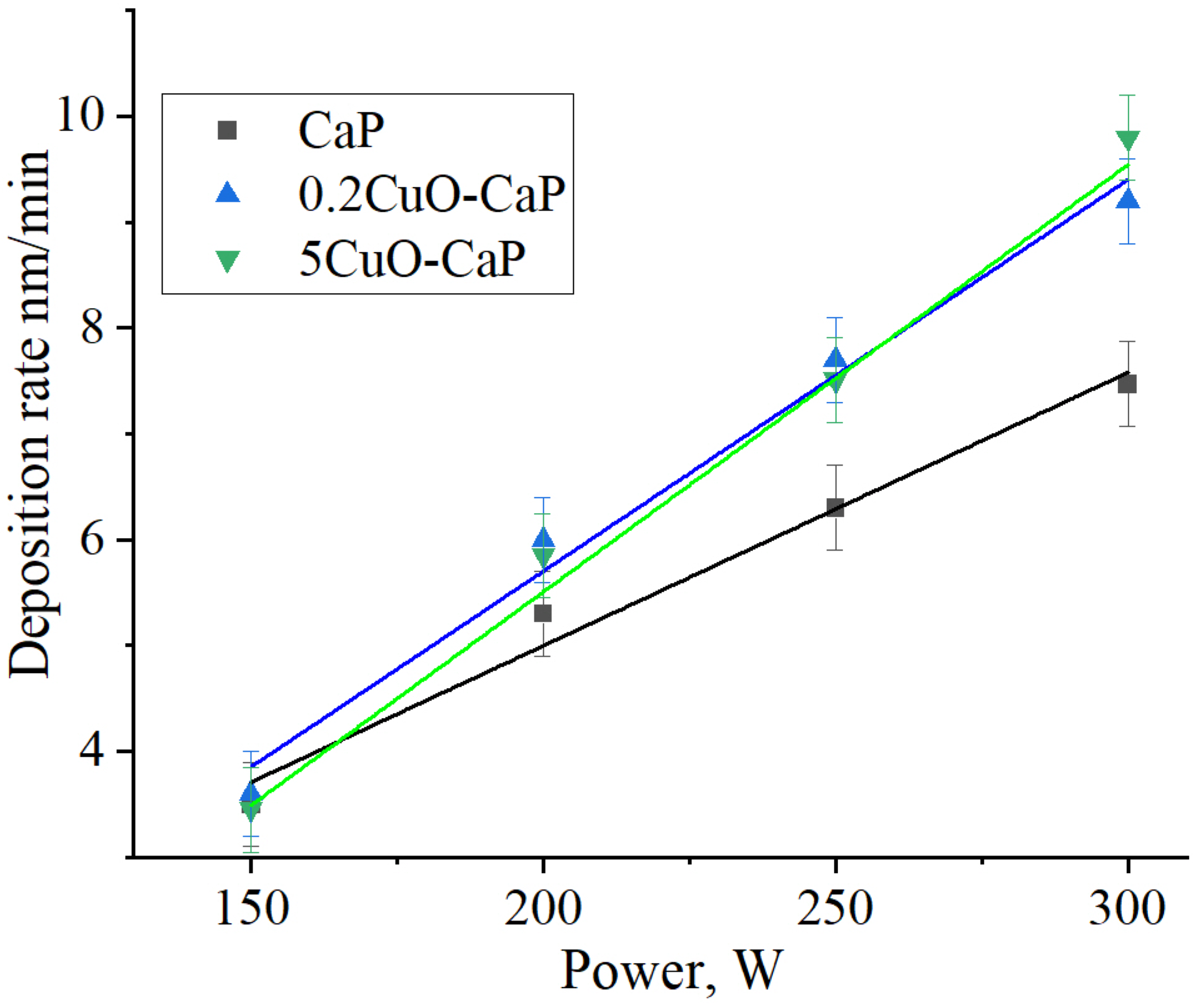
3.2. Sintered Targets for an RF Magnetron Sputtering
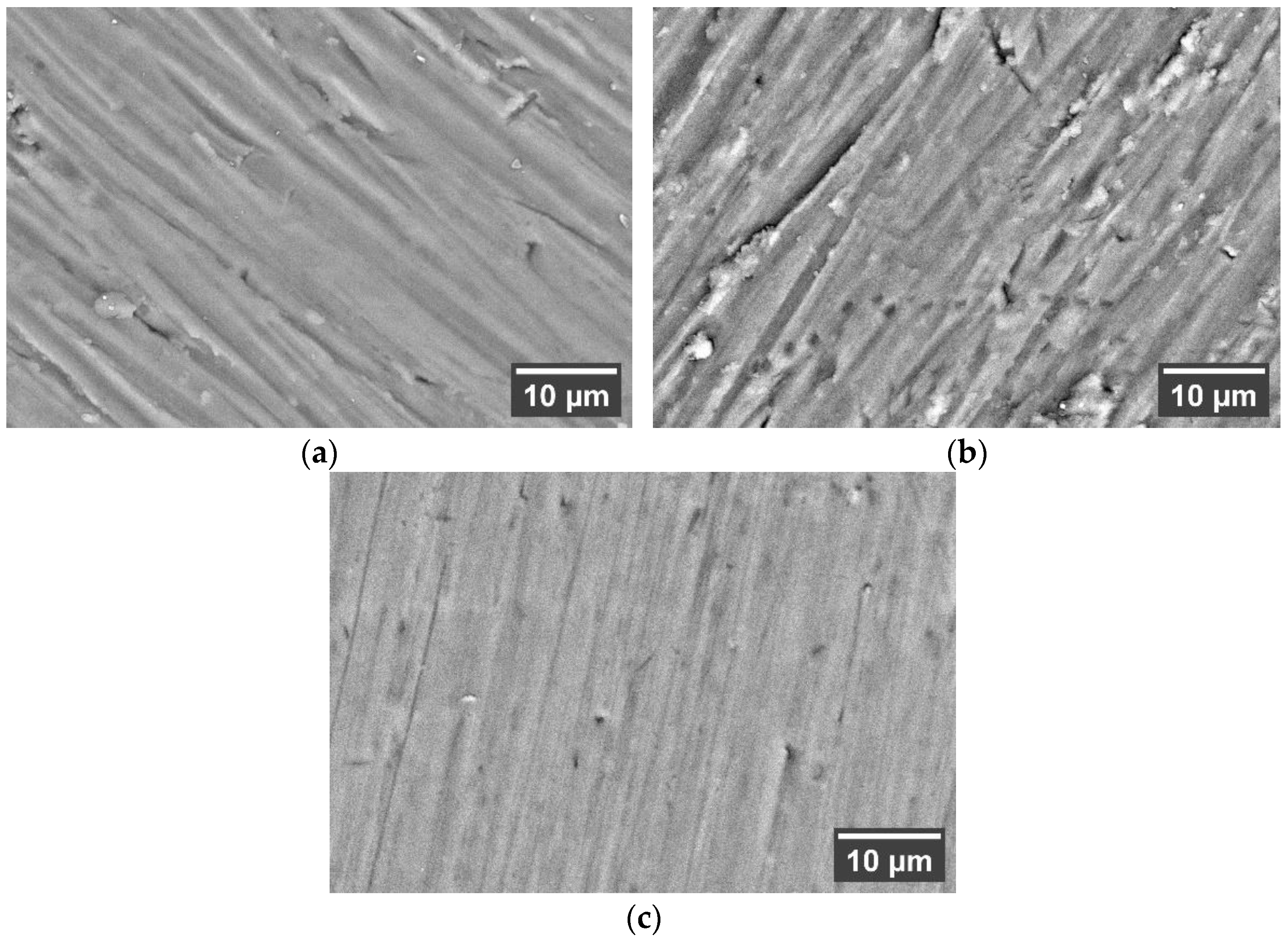
3.3. Analysis of Deposited Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Germaini, M.-M.; Belhabib, S.; Guessasma, S.; Deterre, R.; Corre, P.; Weiss, P. Additive Manufacturing of Biomaterials for Bone Tissue Engineering—A Critical Review of the State of the Art and New Concepts. Prog. Mater. Sci. 2022, 130, 100963. [Google Scholar] [CrossRef]
- Basu, B.; Gowtham, N.H.; Xiao, Y.; Kalidindi, S.R.; Leong, K.W. Biomaterialomics: Data Science-Driven Pathways to Develop Fourth-Generation Biomaterials. Acta Biomater. 2022, 143, 1–25. [Google Scholar] [CrossRef]
- Rial, R.; Liu, Z.; Messina, P.; Ruso, J.M. Role of Nanostructured Materials in Hard Tissue Engineering. Adv. Colloid Interface Sci. 2022, 304, 102682. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, M.L.; Dias, G.J.; Premadasa, K.M.G.P. A Novel Classification of Bone Graft Materials. J. Biomed. Mater. Res. 2022, 110, 1724–1749. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Parashar, V. Analytical Review on the Biocompatibility of Surface-Treated Ti-Alloys for Joint Replacement Applications. Expert Rev. Med. Devices 2022, 19, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Khimich, M.A.; Prosolov, K.A.; Mishurova, T.; Evsevleev, S.; Monforte, X.; Teuschl, A.H.; Slezak, P.; Ibragimov, E.A.; Saprykin, A.A.; Kovalevskaya, Z.G.; et al. Advances in Laser Additive Manufacturing of Ti-Nb Alloys: From Nanostructured Powders to Bulk Objects. Nanomaterials 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2022, 13, 69. [Google Scholar] [CrossRef]
- Kroczek, K.; Turek, P.; Mazur, D.; Szczygielski, J.; Filip, D.; Brodowski, R.; Balawender, K.; Przeszłowski, Ł.; Lewandowski, B.; Orkisz, S.; et al. Characterisation of Selected Materials in Medical Applications. Polymers 2022, 14, 1526. [Google Scholar] [CrossRef]
- Wang, R.; Ni, S.; Ma, L.; Li, M. Porous Construction and Surface Modification of Titanium-Based Materials for Osteogenesis: A Review. Front. Bioeng. Biotechnol. 2022, 10, 973297. [Google Scholar] [CrossRef]
- El-Saies, M.M.; Hassan, I.; El-Shazly, A.H.; El-Wakad, M.T. Titanium Foam for Dental Implant Applications: A Review. SVU-Int. J. Eng. Sci. Appl. 2023, 4, 107–112. [Google Scholar] [CrossRef]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Gao, P.; Han, S.; Kao, R.Y.T.; Wu, S.; Liu, X.; Qian, S.; Chu, P.K.; Cheung, K.M.C.; Yeung, K.W.K. A Tailored Positively-Charged Hydrophobic Surface Reduces the Risk of Implant Associated Infections. Acta Biomater. 2020, 114, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lisoń, J.; Taratuta, A.; Paszenda, Z.; Szindler, M.; Basiaga, M. Perspectives in Prevention of Biofilm for Medical Applications. Coatings 2022, 12, 197. [Google Scholar] [CrossRef]
- Nouri, A.; Rohani Shirvan, A.; Li, Y.; Wen, C. Surface Modification of Additively Manufactured Metallic Biomaterials with Active Antipathogenic Properties. Smart Mater. Manuf. 2023, 1, 100001. [Google Scholar] [CrossRef]
- Perrault, D.P.; Sharma, A.; Kim, J.F.; Gurtner, G.C.; Wan, D.C. Surgical Applications of Materials Engineered with Antimicrobial Properties. Bioengineering 2022, 9, 138. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Mitrichenko, D.V.; Prosolov, A.B.; Nikolaeva, O.O.; Lastovka, V.V.; Belyavskaya, O.A.; Chebodaeva, V.A.; Glukhov, I.A.; Litvinova, L.S.; Shupletsova, V.V.; et al. Zn-Doped CaP-Based Coatings on Ti–6Al–4V and Ti–6Al–7Nb Alloys Prepared by Magnetron Sputtering: Controllable Biodegradation, Bacteriostatic, and Osteogenic Activities. Coatings 2021, 11, 809. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Lastovka, V.V.; Belyavskaya, O.A.; Lychagin, D.V.; Schmidt, J.; Sharkeev, Y.P. Tailoring the Surface Morphology and the Crystallinity State of Cu- and Zn-Substituted Hydroxyapatites on Ti and Mg-Based Alloys. Materials 2020, 13, 4449. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Lastovka, V.V.; Khimich, M.A.; Chebodaeva, V.V.; Khlusov, I.A.; Sharkeev, Y.P. RF Magnetron Sputtering of Substituted Hydroxyapatite for Deposition of Biocoatings. Materials 2022, 15, 6828. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Mushtovatova, L.S.; Karpova, M.R.; Sheikin, V.V.; Litvinova, L.S.; Khlusov, I.A. Zn-, Cu- or Ag-Incorporated Micro-Arc Coatings on Titanium Alloys: Properties and Behavior in Synthetic Biological Media. Surf. Coat. Technol. 2019, 369, 52–68. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of Solid-Phase Mechanochemical Synthesis of Hydroxyapatite Doped by Copper and Zinc Ions. J. Solid State Chem. 2021, 296, 121973. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Prosanov, I.Y.; Vinokurova, O.B.; Ishchenko, A.V. Structure Formation of Zinc-Substituted Hydroxyapatite during Mechanochemical Synthesis. Inorg. Mater. 2020, 56, 402–408. [Google Scholar] [CrossRef]
- Khvostov, M.V.; Borisova, M.S.; Bulina, N.V.; Makarova, S.V.; Dumchenko, N.B.; Tolstikova, T.G.; Lyakhov, N.Z. The Influence of Zinc and Silicate Ions on Biological Properties of Hydroxyapatite Synthesized by a Mechanochemical Method. Ceram. Int. 2021, 47, 9495–9503. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Luginin, N.A.; Litvinova, L.S.; Fedorov, M.A.; Anisenya, I.I.; Mushtovatova, L.S.; Snetkov, A.A.; Bukharov, A.V.; Khlusov, I.A.; Sharkeev, Y.P. Antibacterial and Biocompatible Zn and Cu Containing CaP Magnetron Coatings for MgCa Alloy Functionalization. J. Mater. Res. Technol. 2023, 25, 2177–2203. [Google Scholar] [CrossRef]
- Akhtar, M.; Uzair, S.A.; Rizwan, M.; Ur Rehman, M.A. The Improvement in Surface Properties of Metallic Implant via Magnetron Sputtering: Recent Progress and Remaining Challenges. Front. Mater. 2022, 8, 747169. [Google Scholar] [CrossRef]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic Coatings on Metallic Implants: An Overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Ueda, K.; Narushima, T. Amorphous Calcium Phosphate Coatings. In Phosphate and Borate Bioactive Glasses; Obata, A., Brauer, D.S., Kasuga, T., Eds.; The Royal Society of Chemistry: London, UK, 2022; pp. 114–133. ISBN 978-1-83916-164-3. [Google Scholar]
- Drevet, R.; Fauré, J.; Benhayoune, H. Bioactive Calcium Phosphate Coatings for Bone Implant Applications: A Review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Ferreiro Balbuena, O.B.; Santos Paiva, L.F.; Ribeiro, A.A.; Monteiro, M.M.; Varella De Oliveira, M.; Pereira, L.C. Sintering Parameters Study of a Biphasic Calcium Phosphate Bioceramic Synthesized by Alcoholic Sol-Gel Technique. Ceram. Int. 2021, 47, 32979–32987. [Google Scholar] [CrossRef]
- Ayode Otitoju, T.; Ugochukwu Okoye, P.; Chen, G.; Li, Y.; Onyeka Okoye, M.; Li, S. Advanced Ceramic Components: Materials, Fabrication, and Applications. J. Ind. Eng. Chem. 2020, 85, 34–65. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzański, L.B.; Dobrzańska-Danikiewicz, A.D. Overview of Conventional Technologiesusing the Powders of Metals, Their Alloysand Ceramics in Industry 4.0 Stage. J. Achiev. Mater. Manuf. Eng. 2020, 2, 56–85. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Upadhyaya, G.S. Sintering of Copper-Alumina Composites through Blending and Mechanical Alloying Powder Metallurgy Routes. Mater. Des. 1995, 16, 41–45. [Google Scholar] [CrossRef]
- Rahaman, M.N. Ceramic Processing and Sintering. In Materials Engineering, 2nd ed.; M. Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-0988-4. [Google Scholar]
- Que, W.; Khor, K.A.; Xu, J.L.; Yu, L.G. Hydroxyapatite/Titania Nanocomposites Derived by Combining High-Energy Ball Milling with Spark Plasma Sintering Processes. J. Eur. Ceram. Soc. 2008, 28, 3083–3090. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C. Thermal Processing of Hydroxyapatite for Coating Production. J. Biomed. Mater. Res. 1998, 39, 580–587. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Prosanov, I.Y.; Dudina, D.V. Strontium and Silicate Co-Substituted Hydroxyapatite: Mechanochemical Synthesis and Structural Characterization. Mater. Sci. Eng. B 2020, 262, 114719. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B.; Gerasimov, K.B.; Prosanov, I.Y.; Kompankov, N.B.; Lapina, O.B.; Papulovskiy, E.S.; Ishchenko, A.V.; Makarova, S.V. Possibilities of Mechanochemical Synthesis of Apatites with Different Ca/P Ratios. Ceramics 2022, 5, 404–422. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B.; Prosanov, I.Y.; Dudina, D.V. Interaction of Calcium Phosphates with Calcium Oxide or Calcium Hydroxide during the “Soft” Mechanochemical Synthesis of Hydroxyapatite. Ceram. Int. 2019, 45, 16927–16933. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic Cell Responses and Antibacterial Efficacy of Cu/Zn Co-Substituted Hydroxyapatite Coatings on Pure Titanium Using Electrodeposition Method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Karthika, A. Biocompatible Iron and Copper Incorporated Nanohydroxyapatite Coating for Biomedical Implant Applications. Mater. Today Proc. 2022, 51, 1754–1759. [Google Scholar] [CrossRef]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-Magnetron Sputter Deposited Hydroxyapatite-Based Composite & Multilayer Coatings: A Systematic Review from Mechanical, Corrosion, and Biological Points of View. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted Hydroxyapatite Coatings of Bone Implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Wu, F.; Harper, B.J.; Crandon, L.E.; Harper, S.L. Assessment of Cu and CuO Nanoparticle Ecological Responses Using Laboratory Small-Scale Microcosms. Environ. Sci. Nano 2020, 7, 105–115. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, P.; Chakraborty, A.K.; Pradhan, S.K.; Meikap, A.K. Template-Free Growth of Copper-Doped Hydroxyapatite Nanowhiskers and Their Use as Uric Acid Electrochemical Sensor. Mater. Today Commun. 2022, 33, 104870. [Google Scholar] [CrossRef]
- Bulina, N.V.; Eremina, N.V.; Vinokurova, O.B.; Ishchenko, A.V.; Chaikina, M.V. Diffusion of Copper Ions in the Lattice of Substituted Hydroxyapatite during Heat Treatment. Materials 2022, 15, 5759. [Google Scholar] [CrossRef]
- Rouzé l’Alzit, F.; Bazin, T.; Cardinal, T.; Chung, U.-C.; Catros, S.; Bertrand, C.; Gaudon, M.; Vignoles, G. Powder Bed Laser Sintering of Copper-Doped Hydroxyapatite: Numerical and Experimental Parametric Analysis. Addit. Manuf. 2021, 46, 102044. [Google Scholar] [CrossRef]
- Khamaru, N.; Das, A.; Das, D.; Karmakar, A.; Chatterjee, S. Effect of Cu-Doping on the Dielectric Properties of MnV2O6 Compound. J. Magn. Magn. Mater. 2020, 512, 167044. [Google Scholar] [CrossRef]
- Liu, L.; Liu, T.; Liu, P.; Huang, Q.; Gui, H.; Lu, A. Crystallization, Spectroscopic and Dielectric Properties of CuO-Added Magnesium Aluminosilicate-Based Glasses. Ceram. Int. 2021, 47, 32666–32674. [Google Scholar] [CrossRef]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical Impedance Spectroscopy Study of the Passive Oxide Film on Titanium for Implant Application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]

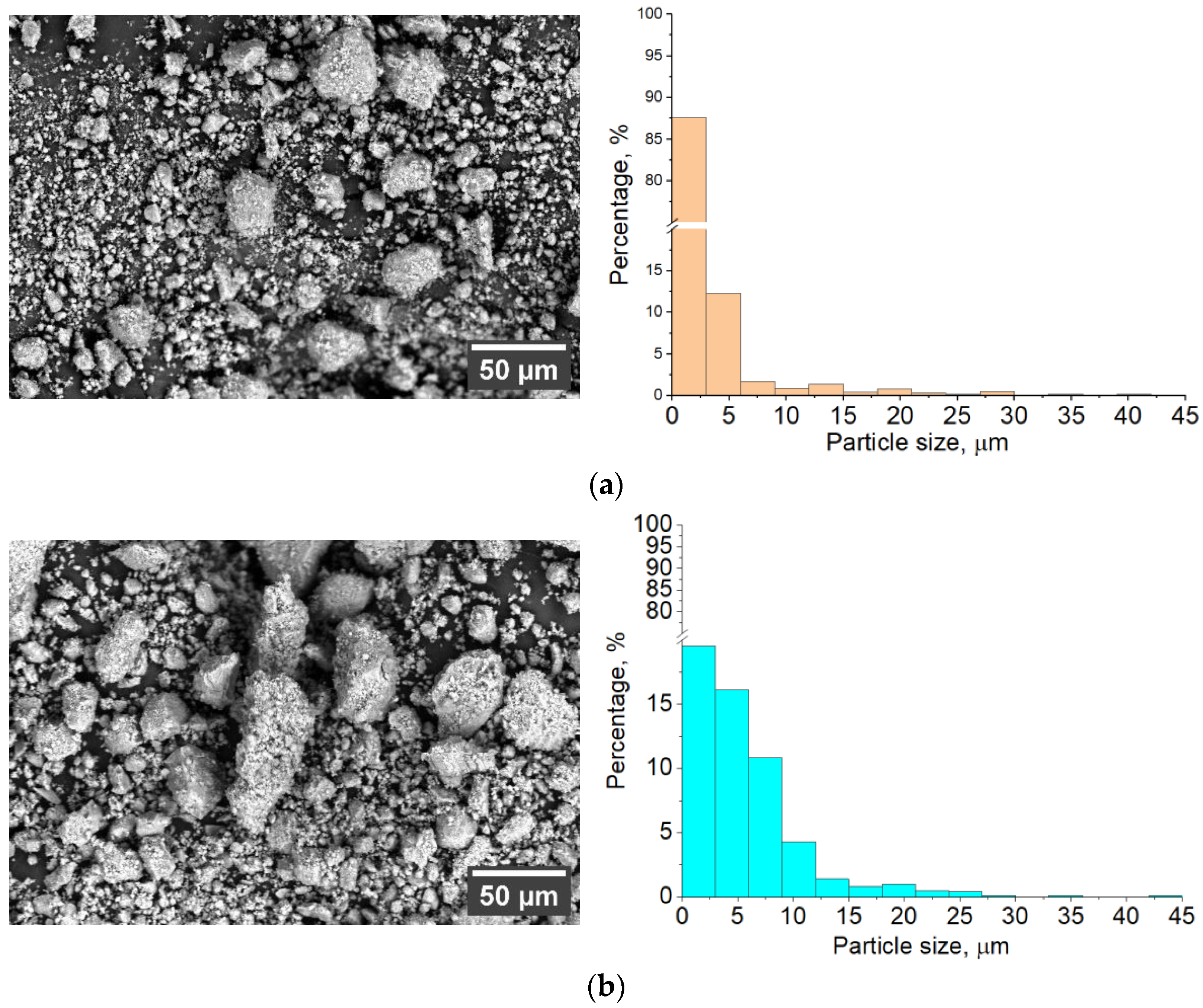
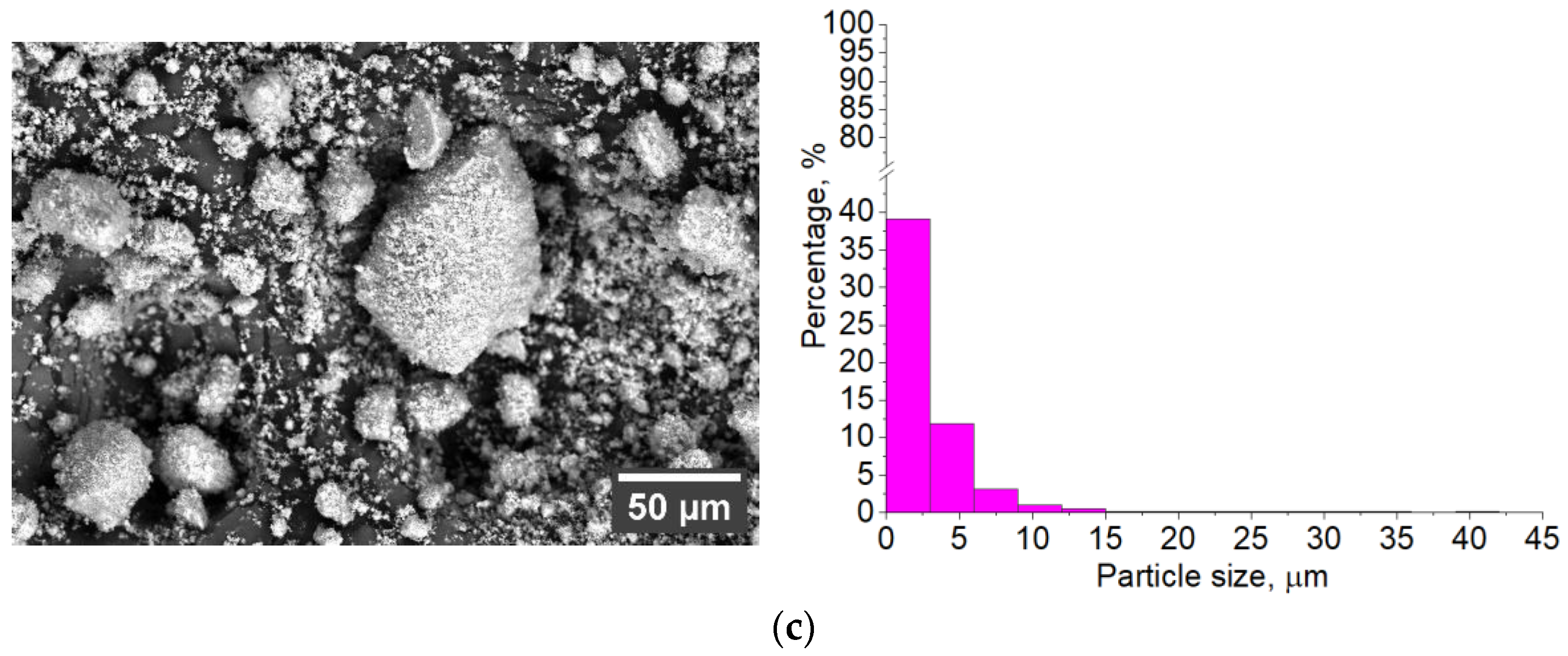
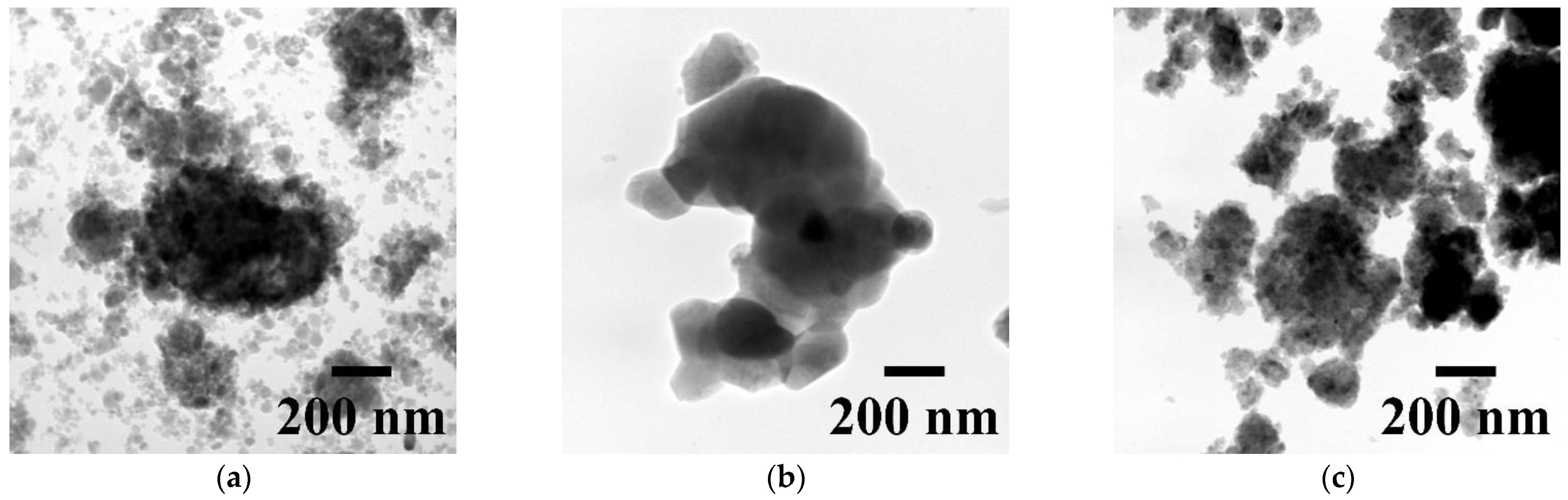
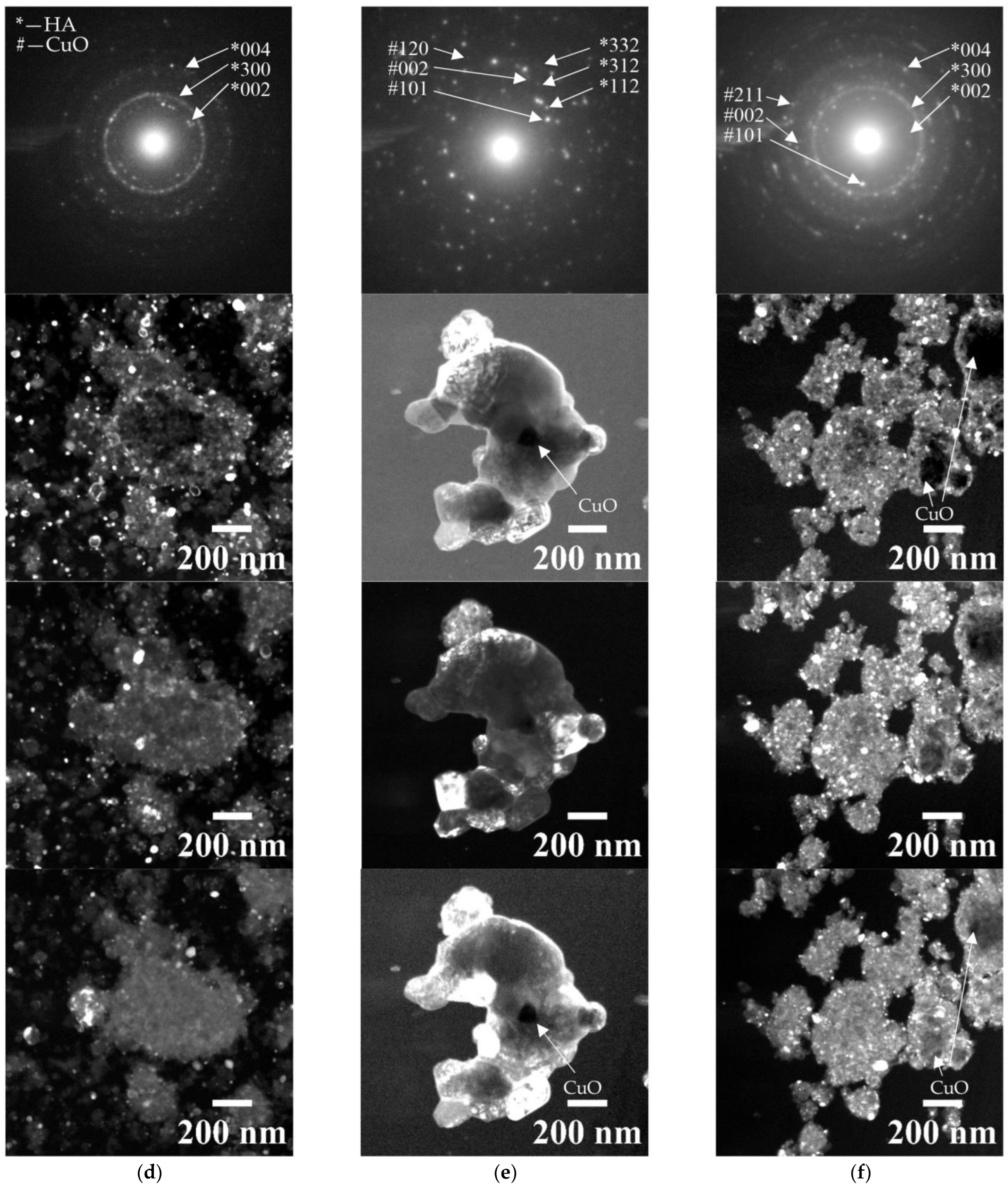

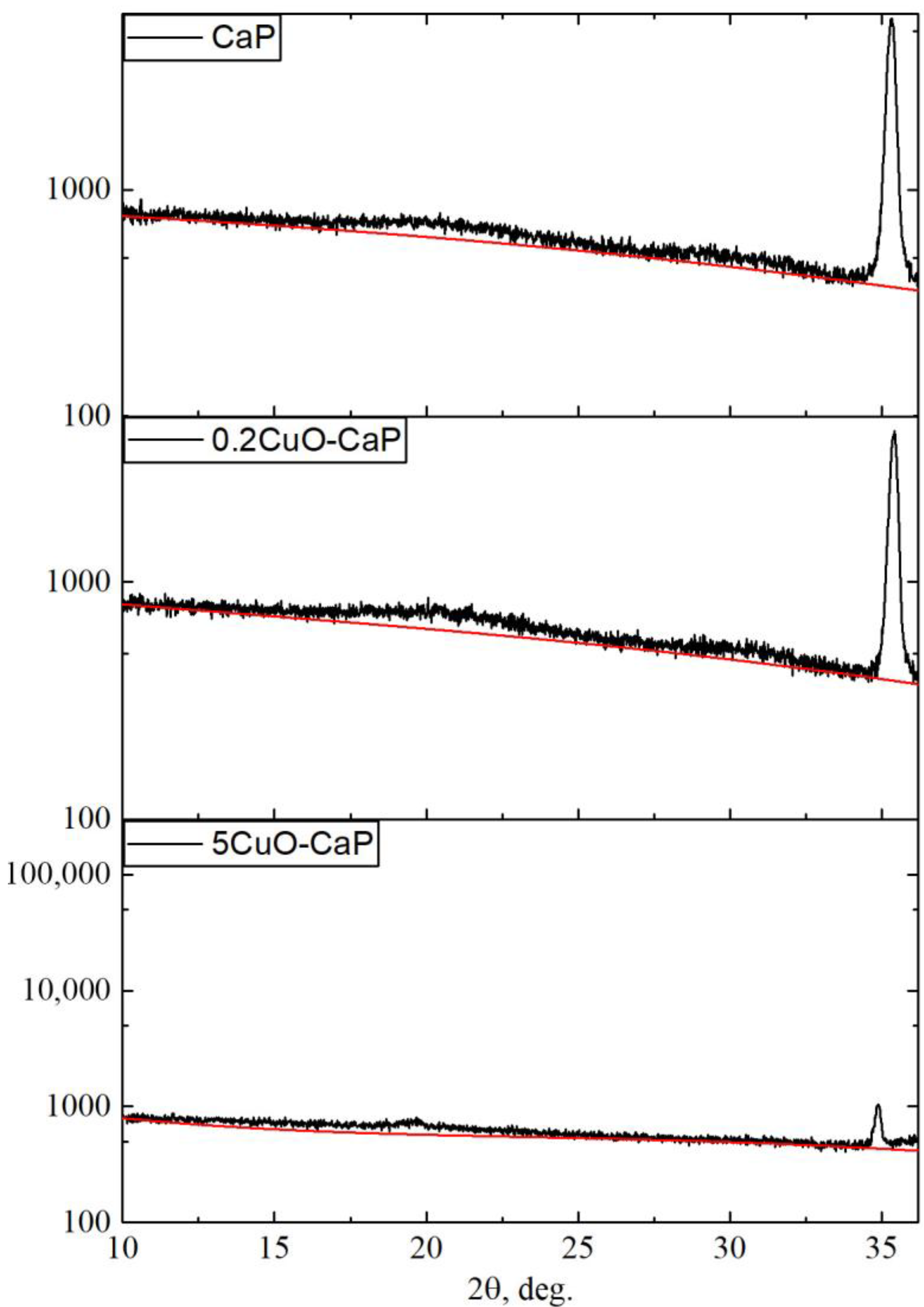
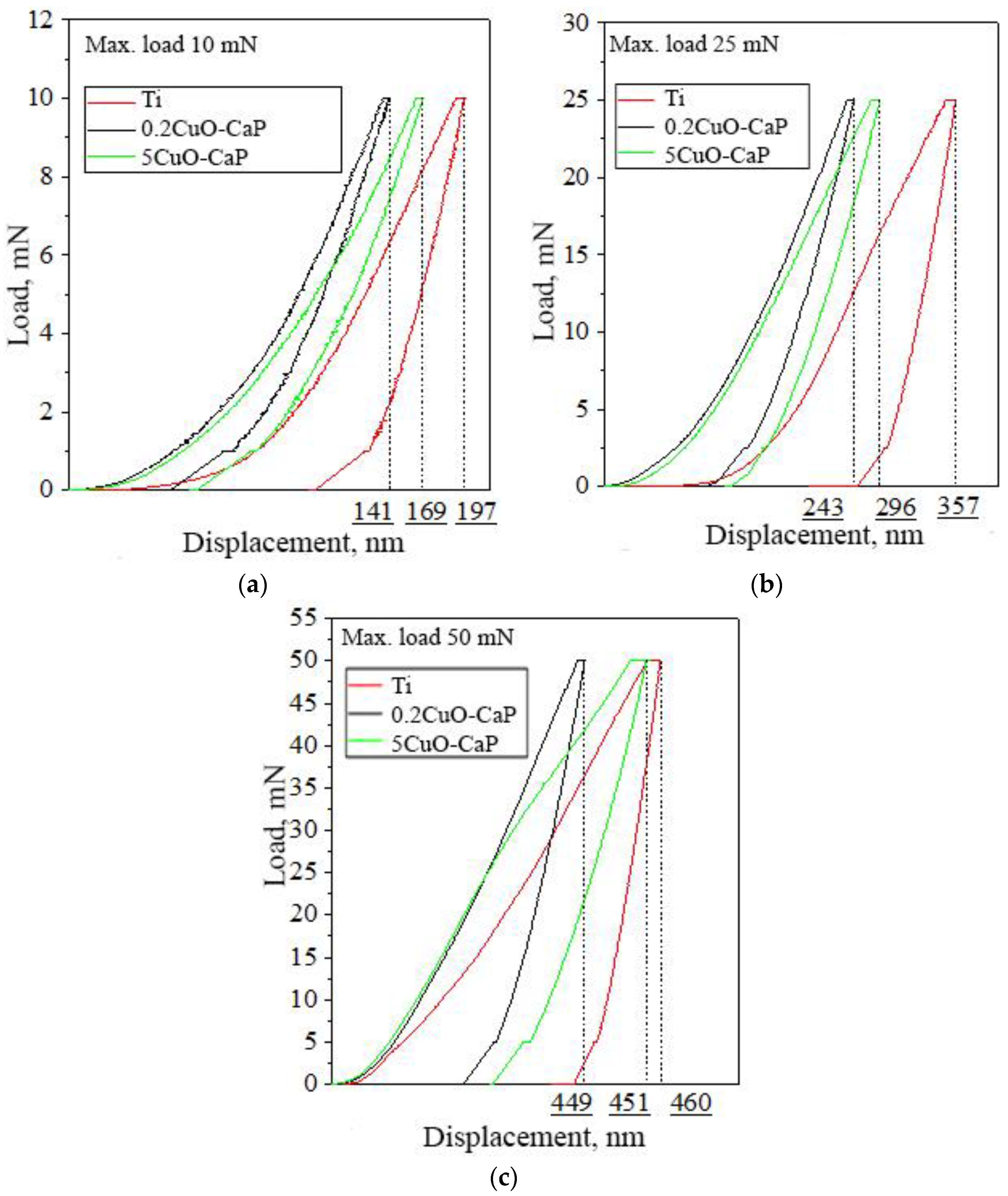



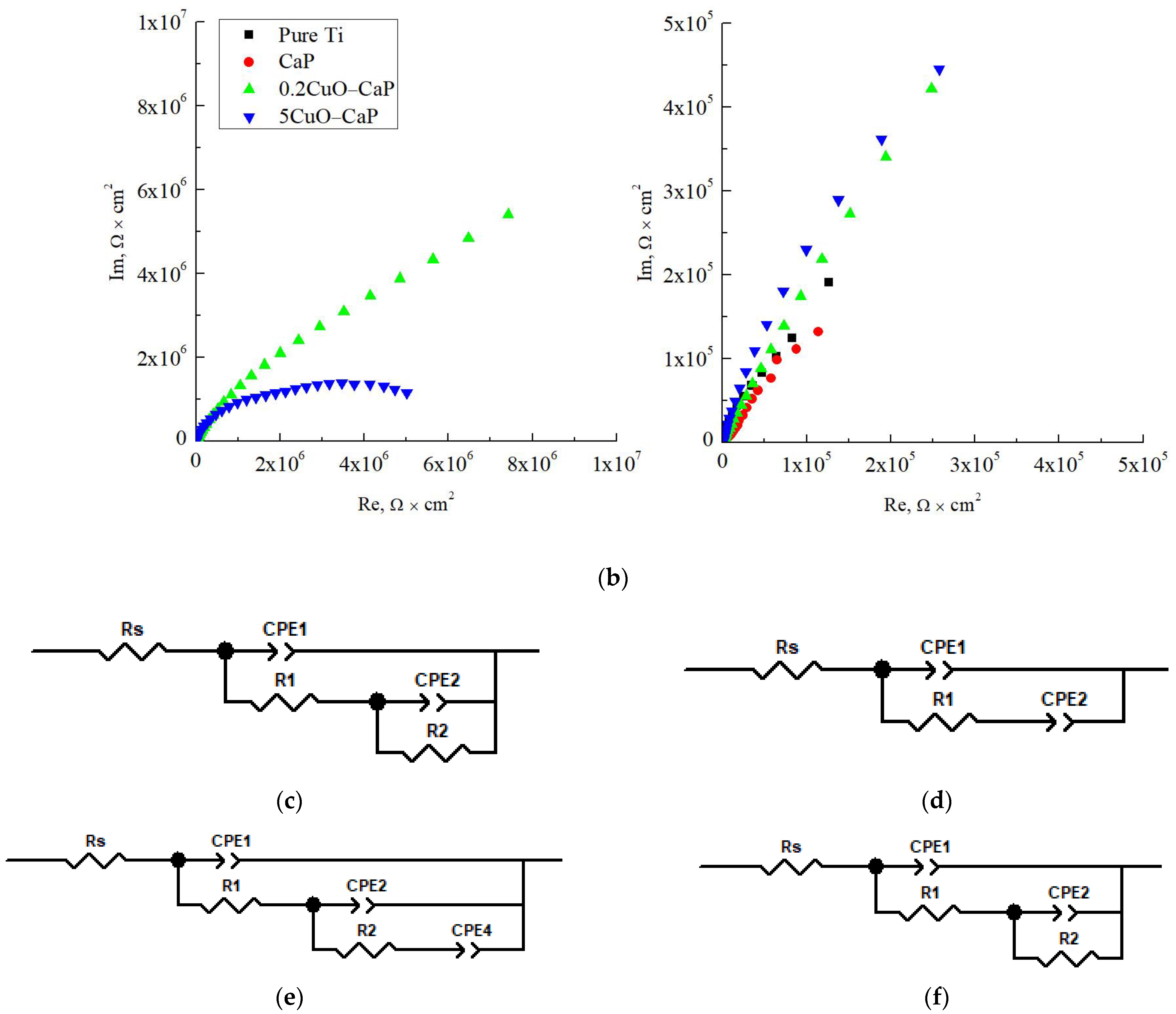
| Powder | Phase Concentration, Mas.% | a = b, Å | c, Å | CSR, nm |
|---|---|---|---|---|
| CaP | 100 HA | 9.4163 ± 0.0031 | 6.8763 ± 0.006 | 94 ± 20 |
| 0.2CuO-CaP | 100 HA | 9.4209 ± 0.0142 | 6.7664 ± 0.186 | 74 ± 14 |
| 5CuO-CaP | 64 HA/36 CuO | 9.4348 ± 0.0164 | 6.8858 ± 0.425 | 18 ± 5 |
| ICDD | 100 HA | 9.422 | 6.881 |
| Powder | Phase Concentration, Mas.% | a = b, Å | c, Å | CSR, nm |
|---|---|---|---|---|
| CaP | 100 HA | 9.4181 ± 0.0028 | 6.8765 ± 0.0027 | 93 ± 19 |
| 0.2CuO-CaP | 100 HA | 9.4158 ± 0.0013 | 6.8792 ± 0.0113 | 74 ± 15 |
| 5CuO-CaP | 57 HA/42 CuO | 9.4232 ± 0.0041 | 6.9038 ± 0.0036 | 57 ± 18 |
| ICDD | 100 HA | 9.422 | 6.881 |
| Sample | Eocp, V | Ecorr, V | Jcorr, nA/cm2 | βa, V/dec | βk, V/dec | Rp, MΩ/cm2 | |Z|f→0.01 Hz, MΩ × cm2 |
|---|---|---|---|---|---|---|---|
| Pure Ti | −0.03 ± 0.03 | −0.32 ± 0.03 | 60.2 ± 5.2 | 0.13 | 0.40 | 0.7 | 0.2 ± 0.1 |
| CaP | −0.32 ± 0.03 | −0.32 ± 0.03 | 3.9 ± 0.5 | 0.21 | 0.47 | 16.0 | 0.2 ± 0.1 |
| 0.2CuO-CaP | 0.22 ± 0.03 | −0.01 ± 0.03 | 16.3 ± 1.6 | 0.26 | 0.44 | 4.4 | 9.2 ± 0.8 |
| 5CuO-CaP | 0.07 ± 0.03 | −0.04 ± 0.03 | 6.4 ± 1.1 | 0.23 | 0.34 | 9.4 | 5.1 ± 0.3 |
| Sample | Pure Ti | CaP | 0.2CuO−CaP | 5CuO−CaP |
|---|---|---|---|---|
| Rs, Ω × cm2 | 140 | 20 | 80 | 100 |
| CPE1−T, F × cm−2 | 4.04 × 10−5 | 1.40 × 10−6 | 1.74 × 10−7 | 7.63 × 10−8 |
| CPE1−P | 0.89 | 0.57 | 0.76 | 0.81 |
| R1, Ω × cm2 | 2.65 × 105 | 1.63 × 103 | 2.53 × 105 | 1.88 × 106 |
| CPE2−T, F × cm−2 | 2.39 × 10−4 | 2.91 × 10−5 | 7.73 × 10−8 | 5.05 × 10−7 |
| CPE2−P | 1.44 | 0.58 | 0.66 | 0.53 |
| R2, Ω × cm2 | 1.36 × 105 | - | 2.72 × 106 | 5.09 × 106 |
| CPE4−T, F × cm−2 | - | - | 2.72 × 10−7 | - |
| CPE4−P | - | - | 0.35 | - |
| χ2 (KK) | 0.091 | 0.001 | 0.001 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosolov, K.; Lastovka, V.; Khimich, M.; Glukhov, I.; Kashin, A.; Luginin, N.; Sharkeev, Y. Influence of Cu Substitution on the Properties of Hydroxyapatite Targets and Deposited Coatings. Coatings 2023, 13, 1410. https://doi.org/10.3390/coatings13081410
Prosolov K, Lastovka V, Khimich M, Glukhov I, Kashin A, Luginin N, Sharkeev Y. Influence of Cu Substitution on the Properties of Hydroxyapatite Targets and Deposited Coatings. Coatings. 2023; 13(8):1410. https://doi.org/10.3390/coatings13081410
Chicago/Turabian StyleProsolov, Konstantin, Vladimir Lastovka, Margarita Khimich, Ivan Glukhov, Alexander Kashin, Nikita Luginin, and Yurii Sharkeev. 2023. "Influence of Cu Substitution on the Properties of Hydroxyapatite Targets and Deposited Coatings" Coatings 13, no. 8: 1410. https://doi.org/10.3390/coatings13081410
APA StyleProsolov, K., Lastovka, V., Khimich, M., Glukhov, I., Kashin, A., Luginin, N., & Sharkeev, Y. (2023). Influence of Cu Substitution on the Properties of Hydroxyapatite Targets and Deposited Coatings. Coatings, 13(8), 1410. https://doi.org/10.3390/coatings13081410







