Abstract
In this paper, Zr(Hf)xCN coatings were deposited onto 316L stainless steel (316L SS) substrates at 400 °C using the radio-frequency (R.F.) magnetron sputtering technique with Hf-Zr-C composite target materials. The influence of Hf element content on microstructure, mechanical properties, and corrosion resistance and platelet interaction with the surface of Zr(Hf)xCN coatings were then investigated. The results indicate that the microstructure, mechanical properties, and corrosion resistance of coatings depend on their chemical composition. Corrosion resistance and platelet interaction with the surface could be improved by adding Hf to the Zr2CN. By optimizing the Hf-Zr-C ratio, Zr(Hf)xCN coatings with excellent corrosion resistance were obtained. The ZrHfCN-3 coating with a Hf element content of 7.33% possesses excellent mechanical properties and good corrosion resistance. The result of the platelet interaction with the surface confirms that Zr(Hf)xCN coatings have better platelet interaction with the surface than 316L SS.
1. Introduction
Over the past decade, 316L stainless steel has been widely used in the fields of orthopedic implants, dental implants, and cardiovascular stents due to its suitable mechanical properties, excellent corrosion resistance, and cost effectiveness [1,2,3]. However, its poor pitting-resistance property imposes limitations on its application. Furthermore, some researchers have discovered that 316L SS releases nickel (Ni), chromium (Cr), and molybdenum (Mo) ions that can cause local immunoreactions and inflammatory response or stimulate platelet activation, which in turn may induce intimal hyperplasia and in-stent restenosis [4,5,6]. The surface modification of this material is a hot research topic in the fields of material science and engineering because the surface is the main portion to interact with the environment. Therefore, the study of surface modification methods is necessary to improve the surface performance, including better corrosion resistance, mechanical properties, and surface compatibility of 316L SS.
Transition metal carbonitride coatings have attracted wide attention because of their excellent biomaterial characteristics, such as their mechanical properties, corrosion resistance, and hemocompatibility [7,8]. Among the large transition metal carbonitrides family of materials, ZrCN coatings have been proven to have the better comprehensive performance. Thus, a good combination of mechanical properties and anti-corrosion properties has made ZrCN coating widely used for coated cemented carbide and as a high-temperature protection coating [9,10,11,12,13].
In previous studies, different compounds based on ZrCN with added titanium (Ti), niobium (Nb), and/or hafnium (Hf) as alloying elements were investigated due to their good corrosion resistance and their tendency to be inert in the human body [14]. The ZrHfCN coating exhibited better hardness and corrosion resistance than other coatings under dry sliding conditions [15]. However, the improvement in ZrHfCN coatings with regard to corrosion resistance and platelet interaction with the surface of 316L SS have rarely been studied. In the present study, the magnetron sputtering method was used to prepare Zr(Hf)xCN coatings on the surface of 316L SS, and their microstructure, mechanical properties, corrosion resistance, and surface compatibility were systematically investigated.
2. Experimental Section
2.1. Preparation of Zr(Hf)xCN Coatings
In this study, 316L SS (Baosteel Group Co., Ltd., Shanghai, China) plate samples (20 mm × 10 mm × 1.5 mm) were selected as the substrate. First, 316L SS was polished using water-resistant silicon carbide sandpaper with 1000, 1200, 1500, 2000, and 3000 grits. Next, the sample was mechanically polished using an emulsion of carborundum granules (0.05 μm) to obtain a smooth surface. Each sample was sequentially cleaned in acetone and distilled water via ultrasonication to remove organic substances. Further, Zr(Hf)xCN coating was fabricated by magnetron sputtering (Sky Technology Development Co., Ltd., Chinese Academy of Science, Shengyang, China, JGP450-PECVD200) with a base pressure of 2.0 × 10−5 Pa on 316L SS. The experimental parameters are as follows: sputtering pressure, N2/Ar ratio, negative bias voltage, and substrate temperature of 0.35 Pa, 2:18, −100 V, and 400 °C, respectively. Moreover, the RF magnetron sputtering power and the distance between the substrate to the target were 120 W and 70 mm, respectively. The Hf–Zr–C composite target was sputter cleaned for 10 min under an Ar–N2 mixture atmosphere in order to first remove the surface impurities. The Zr(Hf)xCN coating was prepared at different Hf element content and the deposition duration was 120 min.
2.2. Characterization
The crystalline phases of Zr(Hf)xCN coating were characterized via X-ray diffraction (XRD, X′Pert Pro, PANalytical, Eindhoven, The Netherlands) using a diffractometer with CuKα radiation and operating at 40 kV and 40 mA. The XRD patterns were collected in the 2θ range from 20° to 80°. The phase composition, elemental compositions, and chemical bond states of samples were investigated using transmission electron microscopy (TEM, JEM-2100, JEOL Ltd., Tokyo, Japan) and X-ray photoelectron spectroscopy (XPS, K-alpha, Thermo Scientific, Waltham, MA, USA) [16], respectively. Bright field imaging and an operating voltage of 220 kV were used as the test conditions for TEM. The preparation process of the TEM sample is as follows: First, the uncoated layer of the sample was polished to ensure that the thickness of the sample was in the range of 80–100 μm. Second, the uncoated layer of the sample polished to 80–100 μm was pitted with Gatan’s pitting instrument to reduce the thickness of the sample to 20 μm. Finally, the 20 μm thick sample was thinned until the appearance of the thin region. In this study, a GATAN-600 type ion thinning instrument (GATAN, Pleasanton, CA, USA) was used to thin the sample at angles of 10°, 8°, and 6° under the operating voltage of 5 kV and current of 2 mA. The test conditions of XPS are as follows: the test voltage was 14 kV, the power was 250 W, and the vacuum degree of the cavity was less than 10−3 Pa. In order to avoid errors caused by surface impurities during XPS, Ar ions were bombarded on the sample surface prior to the test to avoid interference from impurities. The surface roughness and section thickness of the Zr(Hf)xCN coating were separately observed via atomic force microscopy (AFM, AJ-IIIa, Aijian Nanotechnology Inc., Shanghai, China) and scanning electron microscopy (SEM, Sirion200, FEI, Hillsboro, OR, USA) at a voltage of 15 kV. The average thickness of the sample was obtained from three samples; five tests were performed for each sample, and the errors were calculated as the standard deviation.
A nanoindentor (CSM Instruments, Peseux, Switzerland), friction tester (UMT-2), and scratch test instrument were used to measure the nano-mechanical properties, friction coefficient, and adhesion strength of the Zr(Hf)xCN coating, respectively. During scratching, acoustic emission signal intensity was continuously monitored to determine the critical load (Lc) in order to evaluate the adhesive strength, and the results were then verified via SEM. Three samples were used in the adhesion test and average value and standard deviation were then calculated.
The electrochemical workstation (CHI660E, ChenHua, Shanghai, China) was used to test the corrosion resistance of the Zr(Hf)xCN coating. Potentiodynamic polarization experiments were conducted in phosphate-buffered saline (PBS), which was mainly used to evaluate the drug release profile of the drug-eluting stents. The chemical composition of PBS consisted of a water solution containing NaCl (8 g·L−1), KCl (0.2 g·L−1), Na2HPO4 (1.44 g·L−1), and KH2PO4 (0.24 g·L−1) at the pH of 7.4. The sample, a platinum electrode, and a saturated calomel electrode (SCE) were used as the working electrode, counter-electrode, and reference electrode, respectively. Furthermore, the temperature of the polarization cell was maintained at 37 ± 1 °C using a thermostatic water bath to simulate the human body temperature. During the potentiodynamic sweep experiments, the samples were first immersed in the electrolyte for 120 min to stabilize the open circuit potential (OCP), and then the potentiodynamic polarization curves of the test samples were measured from −0.5 V (vs. SCE) to 0.5 V (vs. SCE) with a scan rate of 1 mV·s−1. Five samples from each group were investigated. The polarization curves were obtained for each sample and the corrosion current densities and corrosion potentials of various samples were determined from the potentiodynamic polarization curves via the Tafel extrapolation method. The mean value and standard deviation of the results were also calculated.
The platelet interaction with the surface was characterized via a platelet adhesion experiment and the performance was judged by the platelet adhesion quantity and morphology on the surfaces of uncoated 316L SS and Zr(Hf)xCN-coated 316L SS. The experimental steps of the platelet adhesion process are as follows: First, the entire blood (laboratory rabbit, blood obtained from Tianjin Bainong Laboratory Animal Breeding Technology Co. Ltd., Tianjin, China) was centrifuged for 30 min at a constant velocity to obtain platelet-rich plasma, and then the platelet-rich plasma was attached to the sample surface hatching at 37 °C for one hour. Next, the adhered platelets were fixed using glutaraldehyde for 30 min and the non-adherent platelets were washed with PBS solution. Before observation using SEM, the samples were dried with CO2 and plated with gold. To obtain the statistical results, three samples were used and ten different areas were randomly selected for each sample [16].
3. Results and Discussion
3.1. Elemental Composition and Chemical Bonds
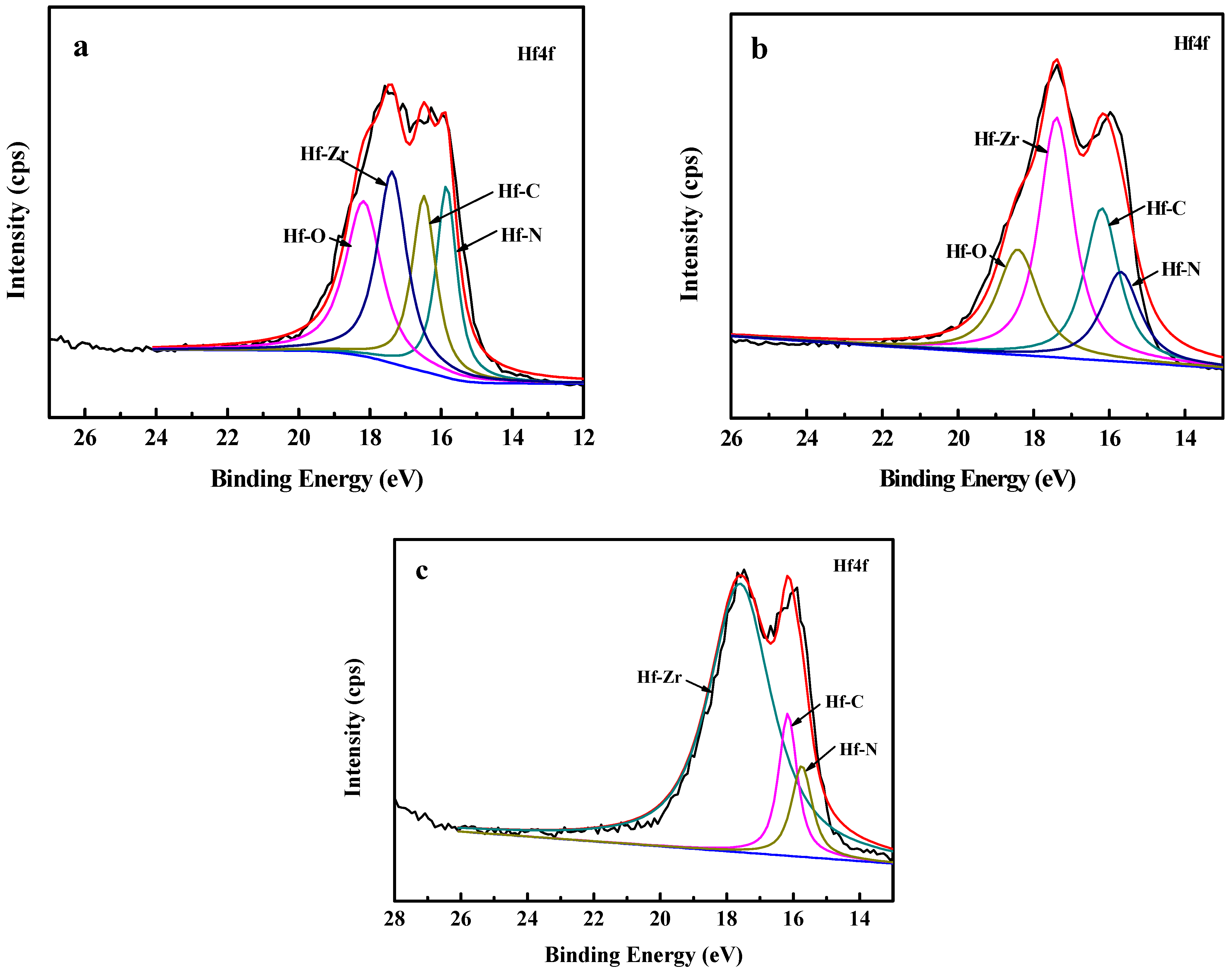
The elemental composition and chemical bonds of Zr(Hf)xCN coatings, in particular the Hf bonds, were investigated via XPS. The XPS spectra of Hf4f for the Zr(Hf)xCN coating are shown in Figure 1. Figure 1a,b exhibit the Hf4f peaks at 15.19, 16.54, 17.6, and 18.19 eV corresponding to Hf–N bonding, Hf–C bonding, Hf–Zr bonding, and Hf–O bonding, respectively. With the increase in the content of Hf element to 7.33% (Figure 1c), the Hf4f peak at 18.19 eV disappeared and the area of Hf–Zr peak at 17.6 eV increased. The content of Zr element decreased with the increase in Hf content. However, the content of C element and N element exhibited a slight change, and are clearly presented in Table 1. Notably, similar results were reported in studies in the literature [17,18].
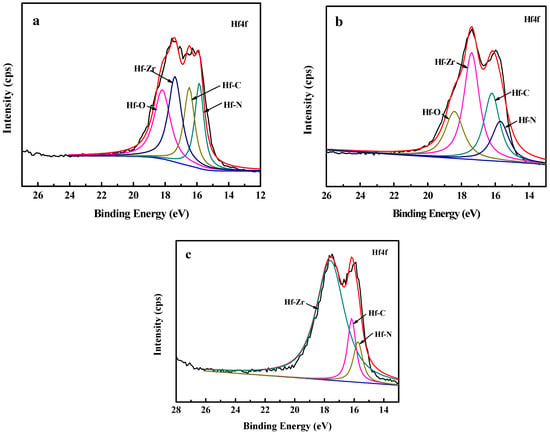
Figure 1.
XPS of Hf4f spectrum of the Zr(Hf)xCN coatings. (a) ZrHfCN−1, (b) ZrHfCN−2, (c) ZrHfCN−3.

Table 1.
Percentage composition of Zr(Hf)xCN coatings.
According to the literature, the ratio of non-metals to metals and the ratio of N/C elements in the quaternary inert nitrogen carbide coating exhibit an impact on the surface roughness, blood compatibility, and corrosion resistance of the coating. Therefore, the elemental compositions, (N + C)/(Zr + Hf) and C/N atomic concentration ratios were determined via XPS and the results are listed in Table 1. Clearly, the N/C ratio remained almost constant at 0.6–0.7, and the significant differences between the non-metal/metal ratios for the coatings were measured: almost 1 (1.78) for ZrCN and higher than 2 (2.20, 2.26, and 2.30) for (Zr,Hf)CN−1, (Zr,Hf)CN−2, and (Zr,Hf)CN−3, respectively.
3.2. Microstructure Analysis
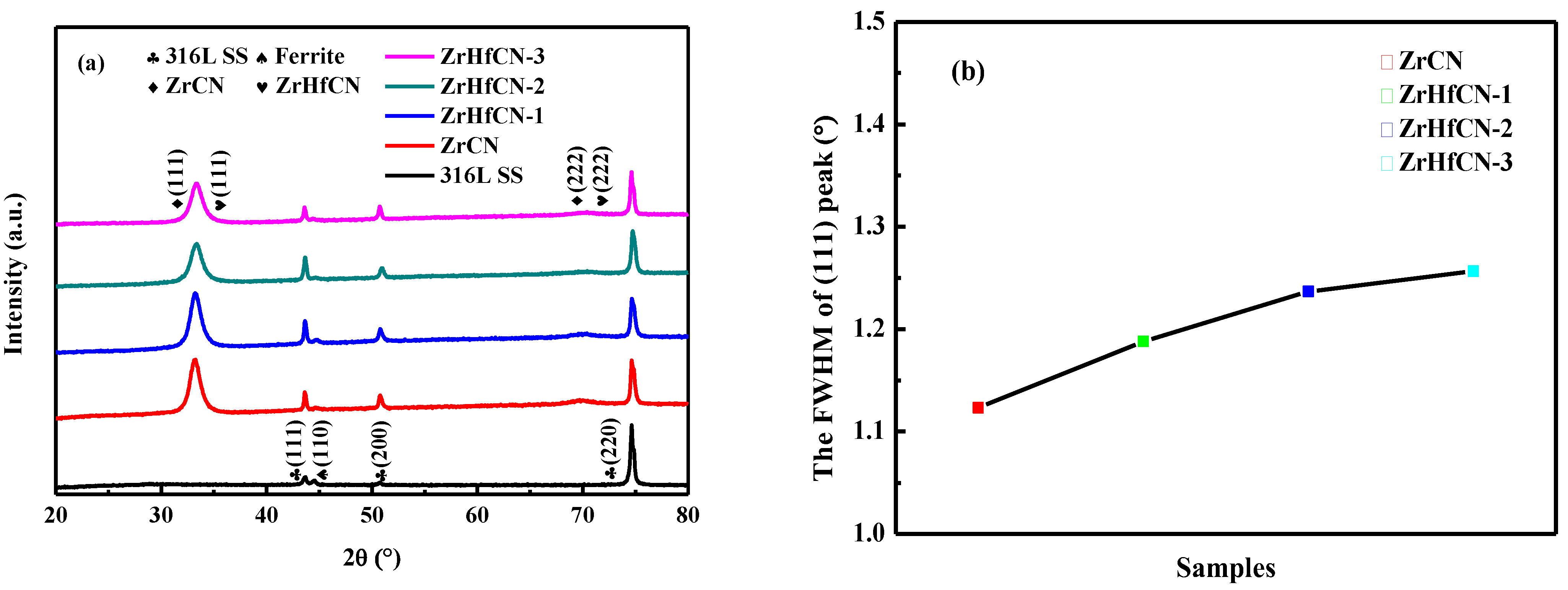
The XRD patterns of Zr(Hf)xCN coatings and the full-width at half maximum (FWHM) of the (111) diffraction peak are illustrated in Figure 2. Figure 2a clearly shows that the 316L SS mainly comprised the austenite phase (JCPDS No: 01-071-4649). Furthermore, the ferrite (110) diffraction peak was also observed in the 316L SS substrates. Clearly, two features were indicated by the Bragg diffraction peaks at 2θ between 33.26° and 70.43°, which were identified as the (111) and (222) diffraction peaks, respectively, of the (Zr,Hf)CN phase (JCPDS No: 01-089-7444). In addition, with the increase in Hf element content, the angle of the (111) diffraction peak became larger and the half width trend broadened, and the corresponding results are illustrated in Figure 2a,b. This result first indicates that the grain sizes of the Zr(Hf)xCN coatings decreased with increasing Hf element content. Then, the angle of the (111) diffraction peak became larger, which demonstrates that the Hf caused the lattice distortion of the Zr2CN phase. The lattice distortion of the ZrCN phase possibly contributed to the improvement in mechanical properties of the coatings.
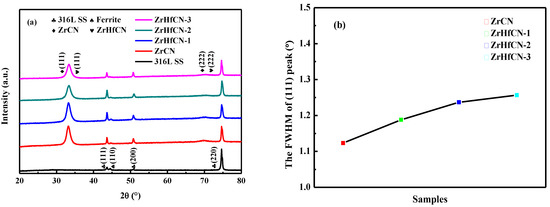
Figure 2.
XRD pattern and FWHM of (111) peak of 316L SS and Zr(Hf)xCN coatings. (a) XRD pattern (b) image of FWHM of (111) peak.
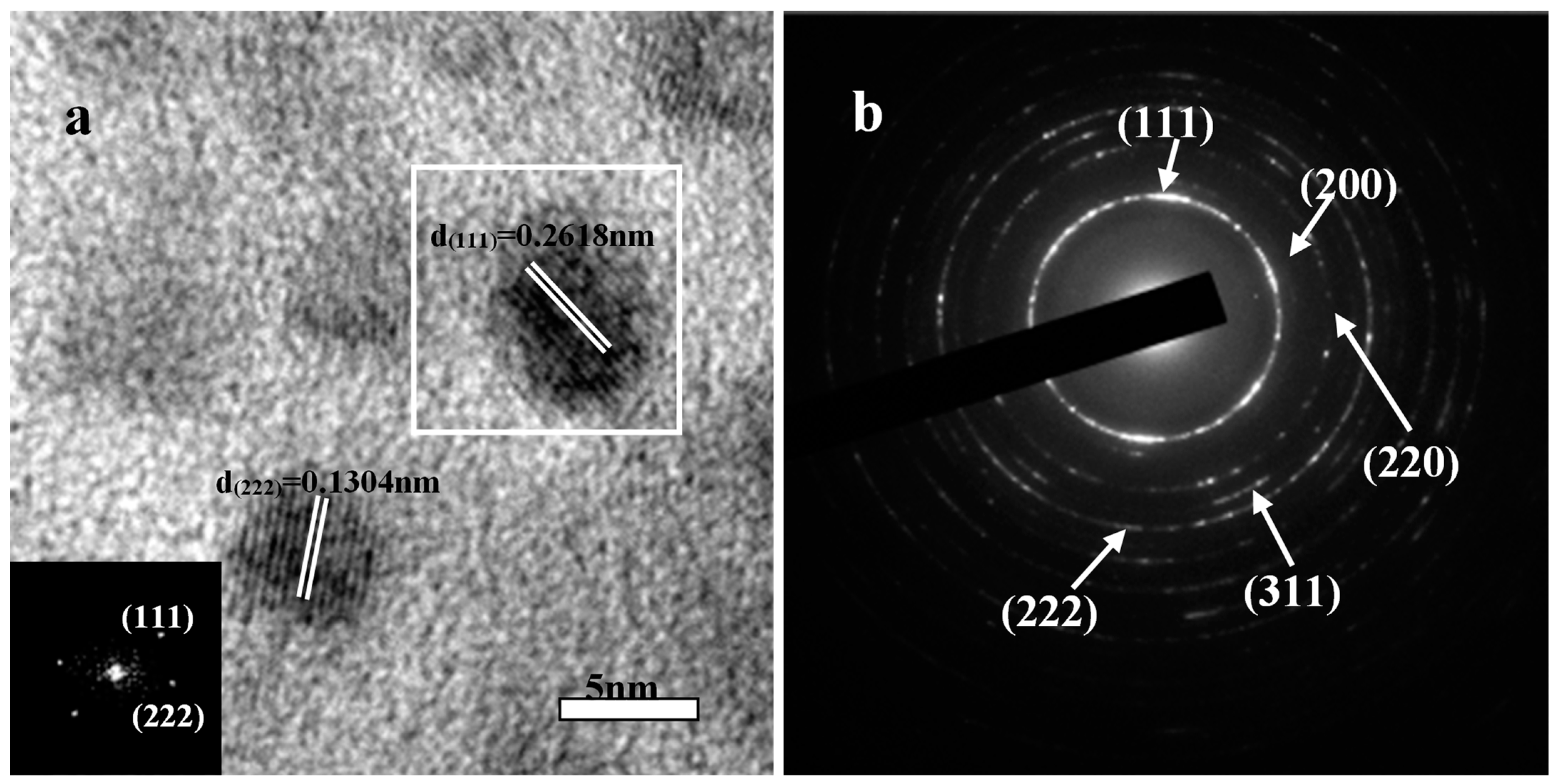
Further details of the microstructure of the ZrHfCN-3 coating were revealed by the HRTEM as shown in Figure 3, and the inset image is the corresponding fast Fourier transformation (FFT) spectrum. Typically, nano-scale (Zr,Hf)CN grains with diameters of 5 nm were found to be surrounded by amorphous matrix. Moreover, both (111) and (222) lattices fringed with lattice spacings of about 0.2618 nm and 0.1304 nm, respectively, were frequently observed. The same region of FFT pattern was indexed into diffraction spots, indicating the single-crystalline nature of the nanoparticles. A selected area electron diffraction (SAED) image (Figure 3b) reveals the crystalline nature of the sample, which was also confirmed by XRD results (Figure 3). The well-defined peaks in the XRD pattern of the Zr(Hf)xCN coatings indicates the high extent of crystallinity for Zr(Hf)xCN coatings with a face-centered cubic structure.
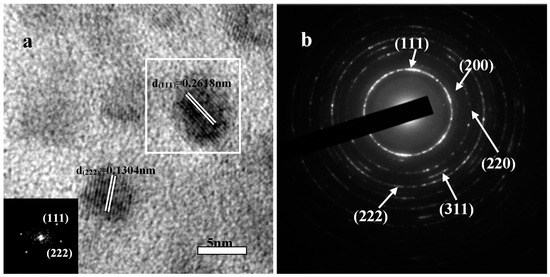
Figure 3.
Representative TEM micrographs of ZrHfCN−3 coating. (a) HRTEM image and crystal lattice; (b) the SAED pattern.
3.3. Morphology Analysis
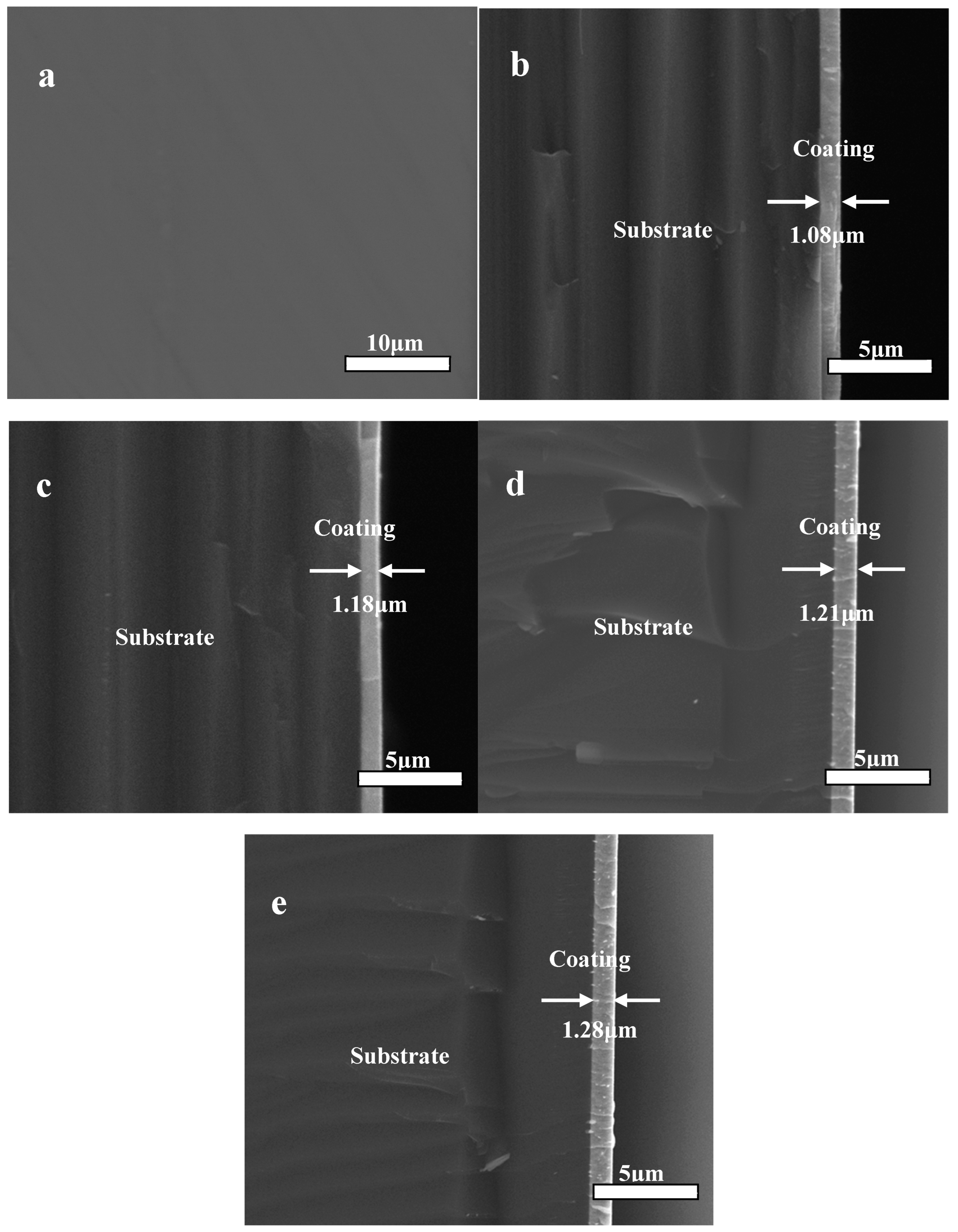
Figure 4 presents the surface appearance image and cross-section shape images of the Zr(Hf)xCN coatings deposited with different Hf element content. A dense, pore-free Zr(Hf)xCN coating with a thickness of approximately 1.08 μm to 1.28 μm is clearly observed from the section SEM image (Figure 4), and its deposition rate is listed in Table 2. The thickness of the Zr(Hf)xCN coatings deposited with various Hf element content are presented in Table 2. It was concluded from Table 2 that the Hf element content had a relatively low impact on the coating thickness [19].
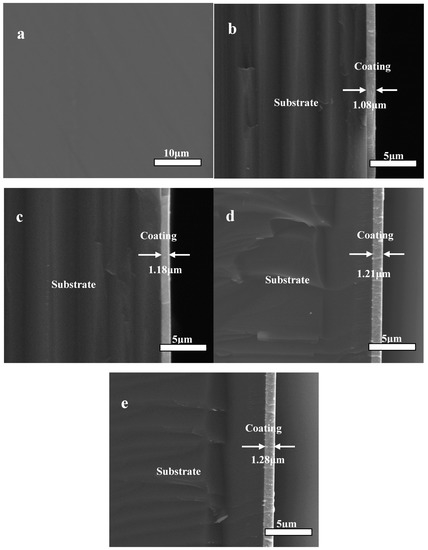
Figure 4.
SEM of surface appearance images (a) and cross-section images of Zr(Hf)xCN coatings, (b) ZrCN, (c) ZrHfCN−1, (d) ZrHfCN−2, and (e) ZrHfCN−3.

Table 2.
Thickness, deposition rate, and surface roughness of Zr(Hf)xCN coatings.
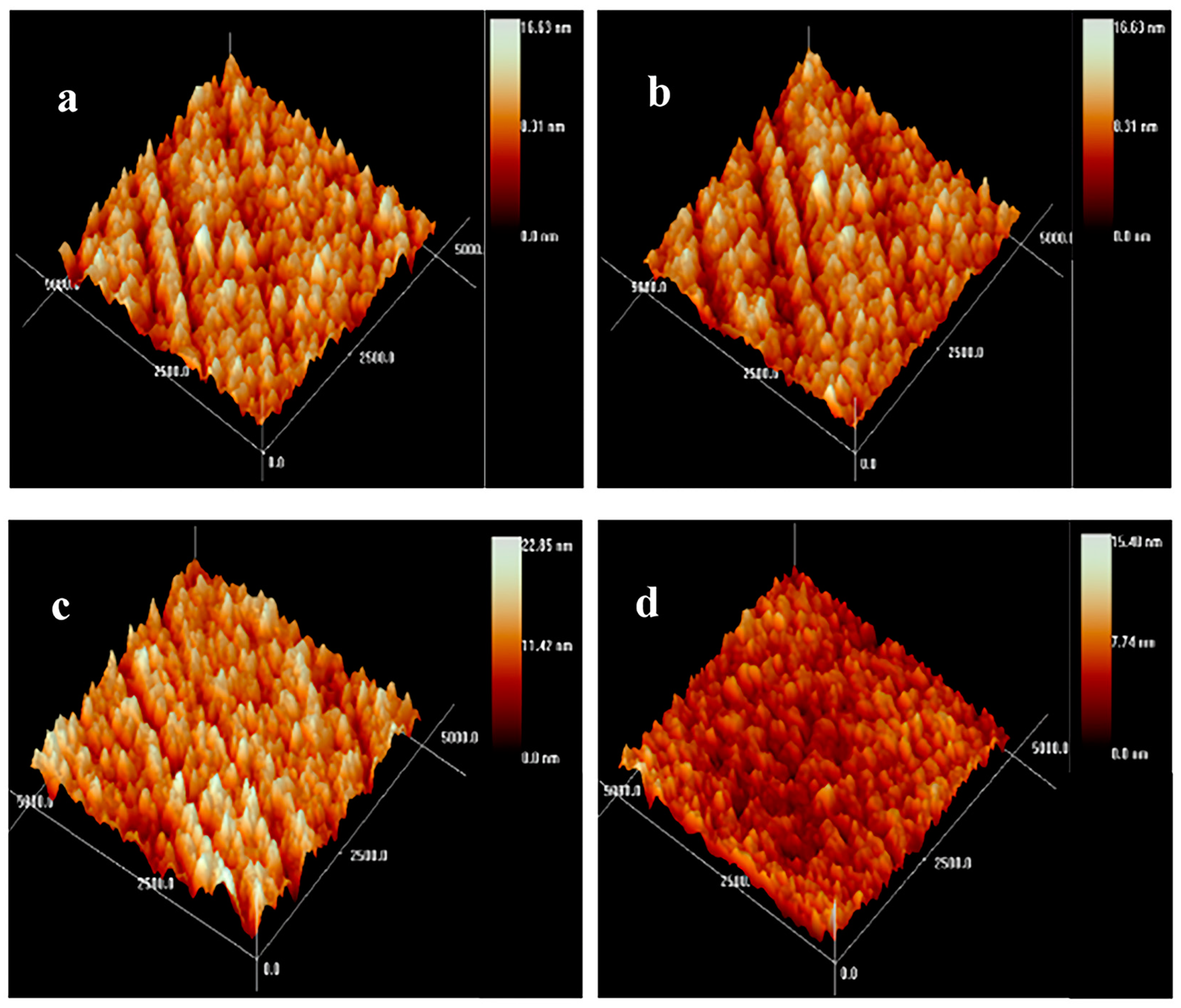
Noteworthily, the surface morphology and roughness markedly affect the hemocompatibility. The root-mean-square (RMS) values of the Zr(Hf)xCN coatings were determined via AFM. The AFM images with the test area exceeding 5 μm × 5 μm are shown in Figure 5 and the RMS value results are listed in Table 2. Figure 5 demonstrates that the surface of the deposited Zr(Hf)xCN coatings with different Hf content present brightness without any particle assembling. This result indicates that the coating deposited at the Ts of 400 °C shows a smooth surface. In fact, the surface morphology, the crystal structure, and the diffusion mechanism exhibit a combined effect on the RMS roughness of the coatings. At higher temperatures, the diffusion mechanism played a major role, which resulted in an increase in atomic diffusion and subsequent decrease in RMS values. Furthermore, Hf, with its smaller radius, was embedded in the Zr2CN crystal lattice, which contributed to the decrease in surface roughness [20]. Table 2 presents that the RMS values of Zr(Hf)CxN coatings decreased slightly with the increase in Hf element content.
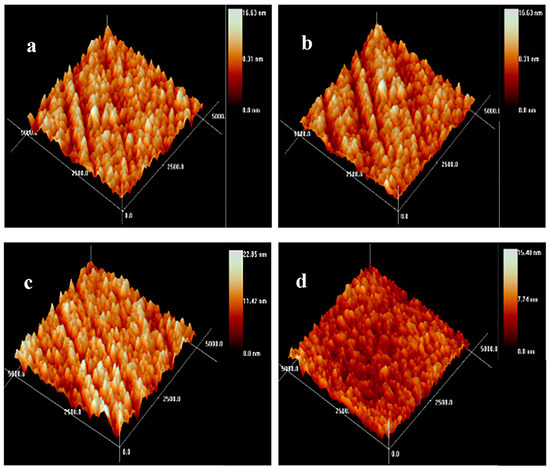
Figure 5.
AFM topography images of Zr(Hf)xCN coatings. (a) ZrCN, (b) ZrHfCN−1, (c) ZrHfCN−2, and (d) ZrHfCN−3.
3.4. Mechanical Properties
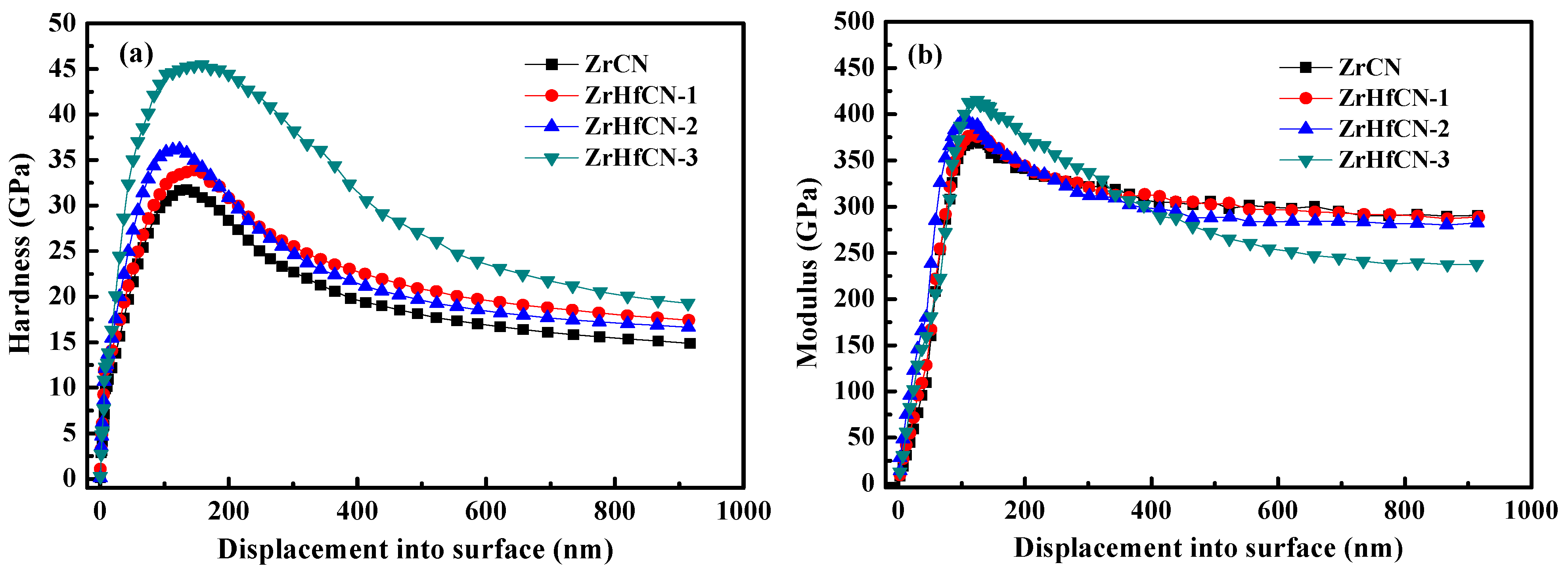
The service life of coatings relies on the hardness and Young’s modulus, which are extremely important material properties [21]. The results of hardness and Young’s modulus of Zr(Hf)xCN coatings with a maximum indentation depth of 1000 nm are shown in Figure 6a,b. Clearly, the hardness and Young’s modulus first increased and then decreased with the increase in indentation depth due to the fact that the lower hardness and Young’s modulus of 316L SS exhibited an impact on coatings. These results are in good agreement with the results reported by Lofaj et al. [22]. The hardness and Young’s modulus of all Zr(Hf)xCN coatings as a function of Hf element content are listed in Table 3. The hardness and Young’s modulus of the Zr(Hf)xCN coatings increased from 31.03 and 359.58 to 41.94 and 381.28 GPa, respectively, with the increase in the Hf element content from 0 to 7.33%. This result may be attributed to the internal stress and dense structure of the coatings. Table 1 presents the XPS results, indicating that the increase in Hf element content is inconsistent with the decrease in Zr element content, which indicates that Hf enters the lattice of Zr2CN to form an interstitial solid solution, besides replacing Zr atoms after entering the lattice. On the one hand, this can produce a solid solution strengthening effect. On the other hand, owing to the larger atomic radius of Hf compared to Zr, lattice expansion occurs, which results in increased internal stress. The combined action of the two is helpful to improve the mechanical properties of the coating [23,24].
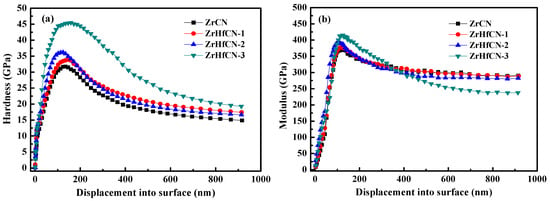
Figure 6.
Hardness and Young’s modulus changed with the displacement of Zr(Hf)xCN coatings. (a) Hardness changed with the displacement, (b) Young’s modulus changed with the displacement.

Table 3.
Hardness, Young’s modulus, and critical load of Zr(Hf)xCN coatings.
The H/E or H3/E2 ratios relate to the durability and plastic deformation resistance of the coatings, respectively, which provide close correlation with wear resistance [25]. This plays an important role in measuring the strength of the coatings on the local dynamic loads. The values of the H/E and H3/E2 ratios listed in Table 3 indicate that the Zr(Hf)xCN coatings deposited with 7.33% Hf element content show the highest H/E and H3/E2 values, which are 0.1099 and 0.5075 GPa, respectively, thus exhibiting potential application prospects.
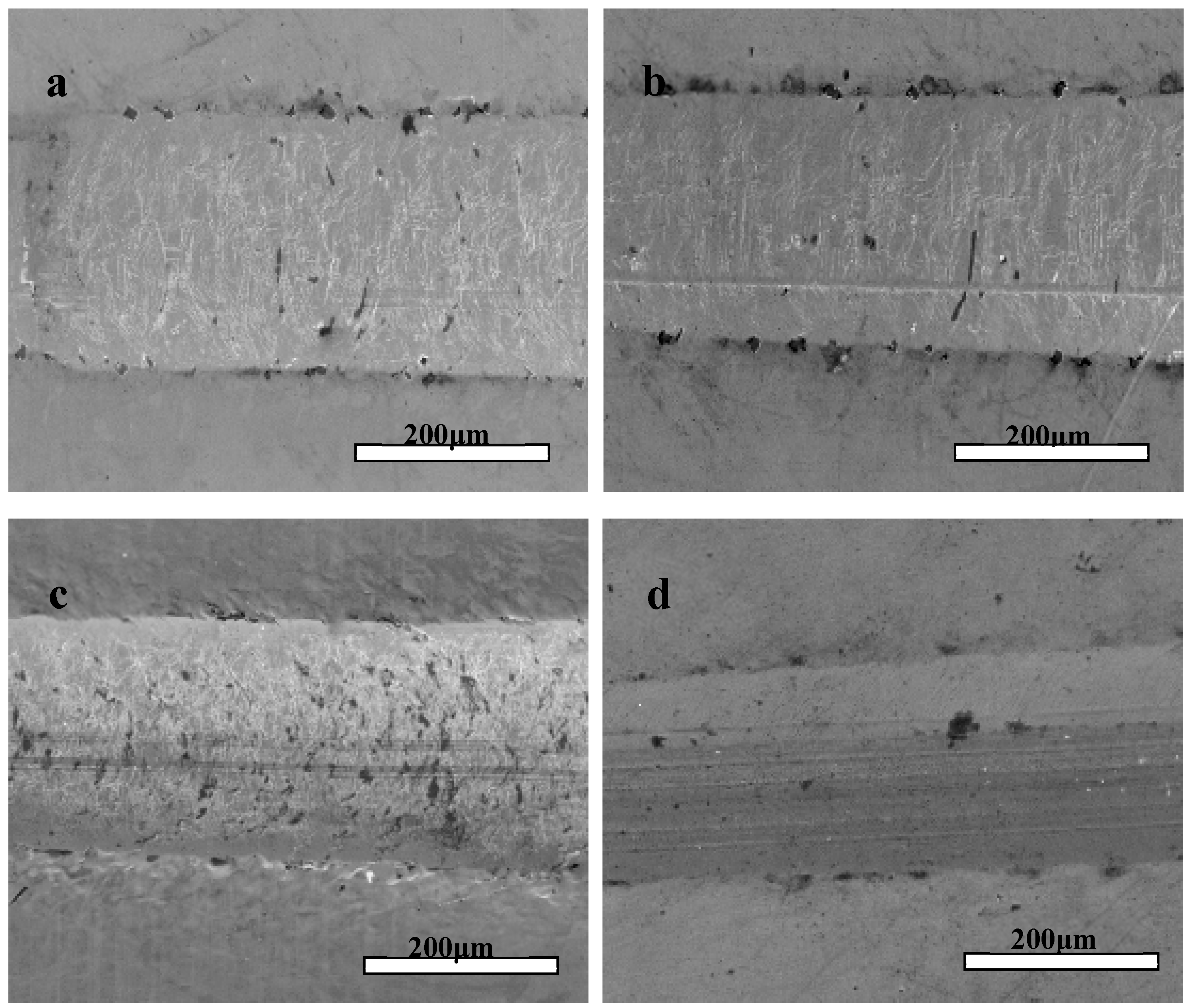
The micro-scratch test was used to characterize the adhesion strength and Lc values of the Zr(Hf)xCN coatings, and the corresponding results are listed in Table 3. The Lc values of the Zr(Hf)xCN coatings with different Hf content were 42, 43.1, 44.8, and 45.7 N. Consequently, Hf element content exhibits a certain impact on the adhesion strength of Zr(Hf)xCN coatings. Figure 7 shows SEM images of micro-scratched Zr(Hf)xCN coatings with different Hf content. The images exhibit cleaner and smoother scratch tracks, indicating the formation of a strong coating–substrate adhesion. For the Zr(Hf)xCN coating deposited at 400 °C, such a good coating–substrate adhesion is not only relevant to adatom mobility, but is also related to atom and solid interactions on the surface of the active coating. The mobility of adatoms improved at higher Ts, and impinging particles with additional energy contributed to the formation of the closed packed structure under the condition of near thermodynamic equilibrium. Moreover, higher Ts contributed to the reduction in defects and the good order in the grain growth along certain directions, resulting in the increase of Lc.
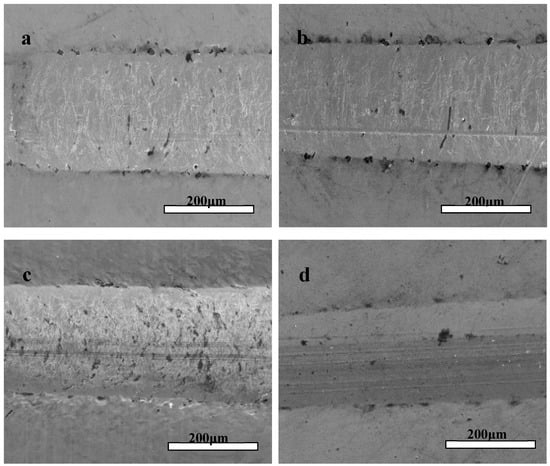
Figure 7.
Typical scratch channel images of Zr(Hf)xCN coatings. (a) ZrCN, (b) ZrHfCN−1, (c) ZrHfCN−2, and (d) ZrHfCN−3.
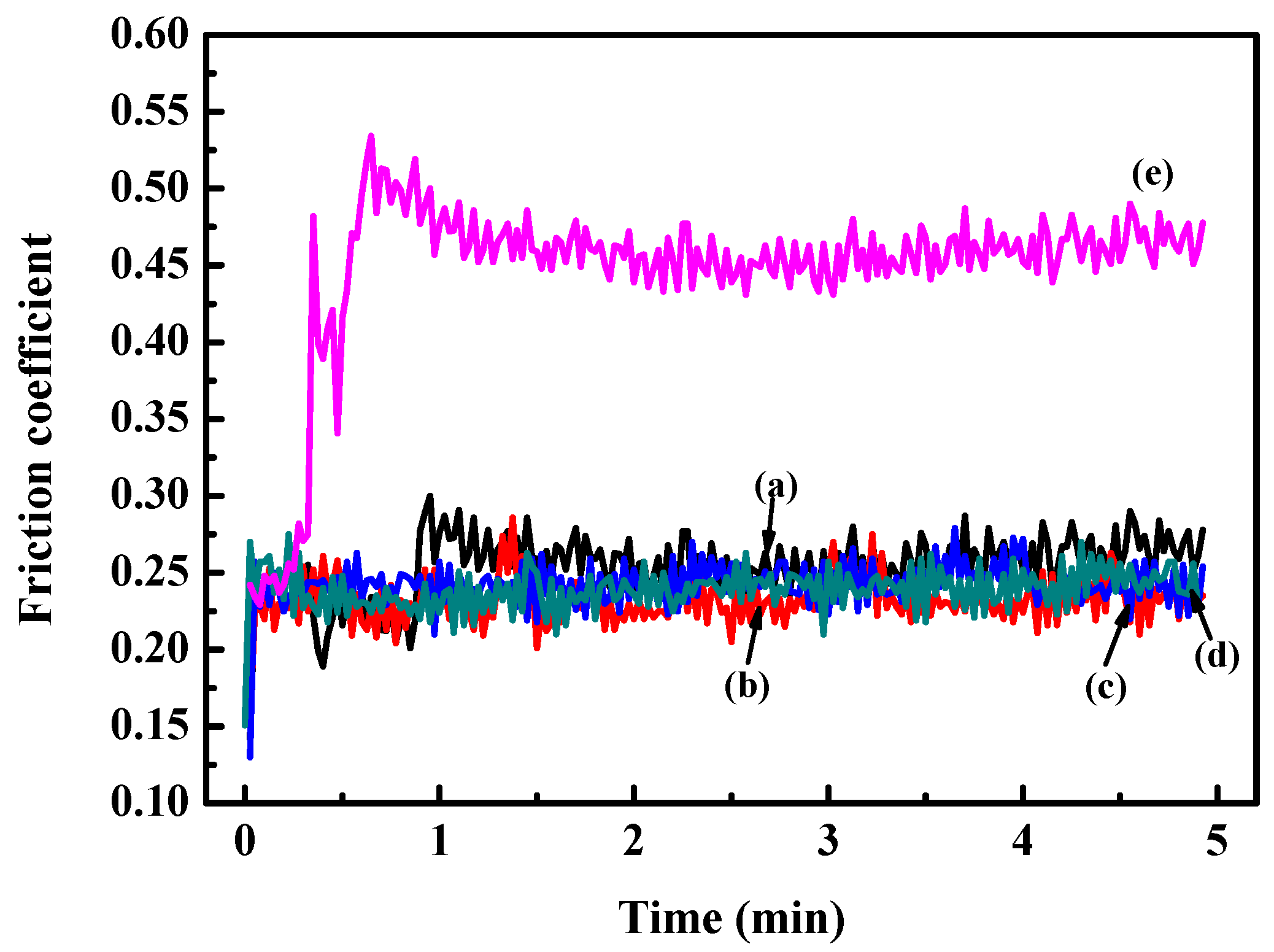
In order to reveal the relationship between coefficient of friction (COF) and Hf content, the tribological behavior was characterized. The results of COF for the Zr(Hf)xCN coatings and uncoated 316L SS are shown in Figure 8. The COF values show an obvious decline compared to substrate 316L SS due to increasing H/E and H3/E2 ratios [26,27]. Moreover, the results indicate that the wear behavior was relying on the H/E and H3/E2 ratios, providing significant information concerning the plastic and elastic deformation. This result may be attributed to internal stress and dense structure.
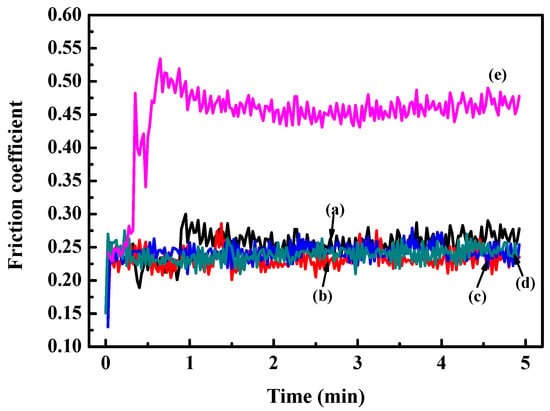
Figure 8.
Friction coefficient curves of 316L SS and Zr(Hf)xCN coatings. (a) ZrCN, (b) ZrHfCN−1, (c) ZrHfCN−2, (d) ZrHfCN−3, and (e) 316L SS.
3.5. Corrosion Resistance
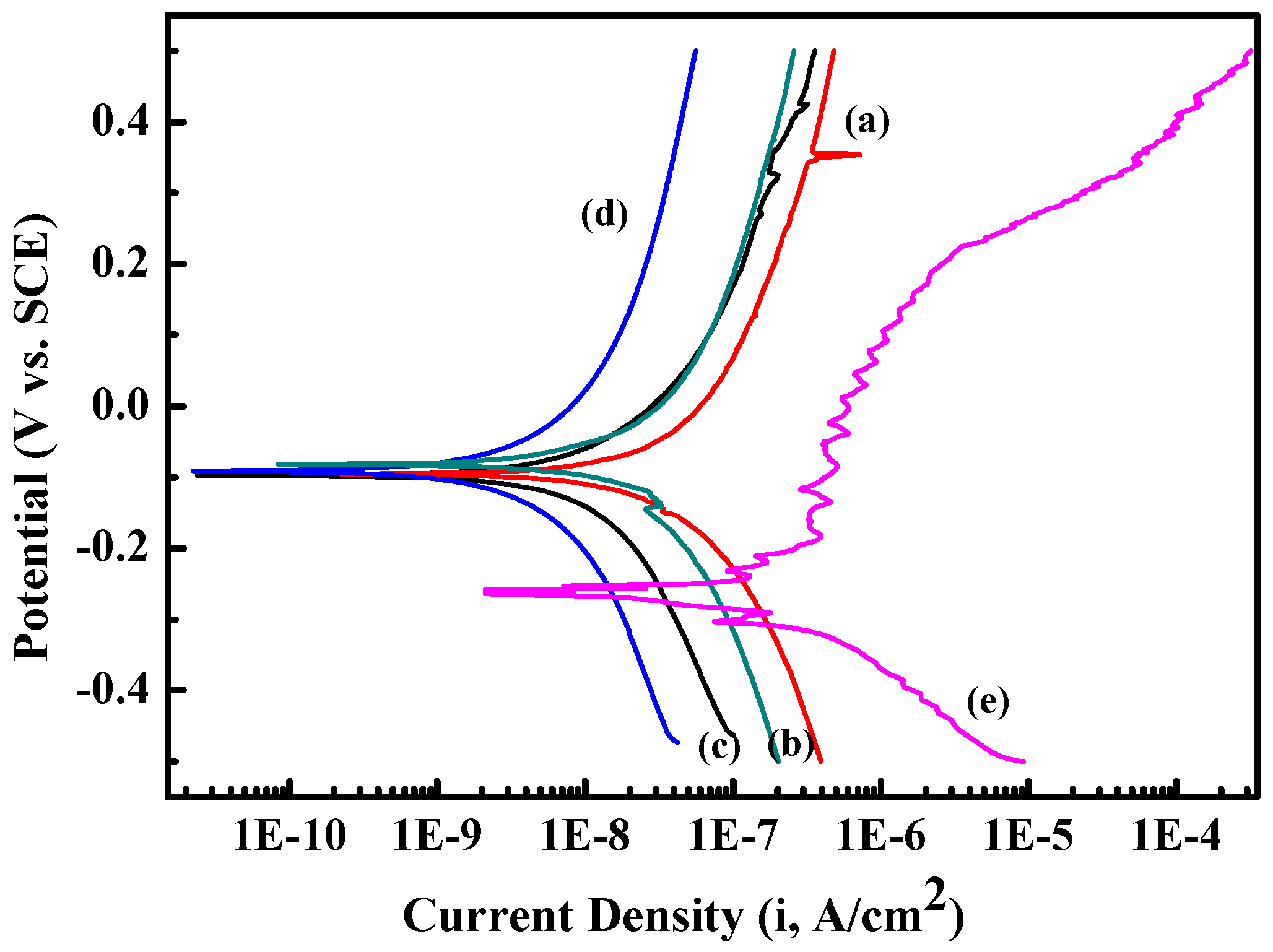
The potentiodynamic polarization curve and electrochemical impedance spectroscopy were used to determine the corrosion resistance of Zr(Hf)xCN coatings and uncoated 316L SS. The current density (Icoor) and the corrosion potential (Ecoor) were obtained from the polarization curves via the Tafel extrapolation method, and both the positive and negative sides of the curve were considered [28,29]. The porosity results are presented in Table 4. Figure 9 illustrates that Zr(Hf)xCN coatings possess better corrosion resistance compared to 316L SS because of the lower Icoor and higher Ecoor, which indicates that the coating protects the substrate from corrosion more efficiently [30,31]. In addition, with the increase in Hf content from 0% to 7.33%, the porosity values of the Zr(Hf)xCN coatings gradually decreased from 0.97 to 0.022, respectively. This was possibly attributed to increasing non-metal/metal ratios, as the coating formed a dense structure with low surface roughness; it acted as an effective inhibitor of the corrosive solution effect. Furthermore, the XPS test results reveal that the increase in Hf element in the coating is greater than the decrease in Zr element, which indicates that Hf enters the gap in the Zr2CN phase lattice, besides replacing Zr element in the coating after entering the coating lattice. The radius of the Hf atom is larger than that of the Zr atom; therefore, the lattice expands, which eventually results in the formation of denser coating.

Table 4.
Corrosion parameters in PBS for 316L SS and Zr(Hf)xCN coatings.
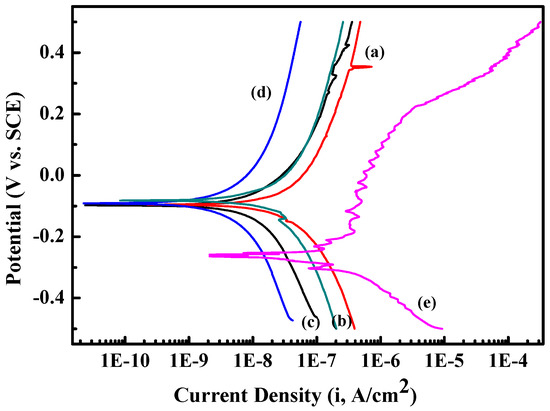
Figure 9.
Potentiodynamic polarization curve of 316L SS and Zr(Hf)xCN coatings. (a) ZrCN, (b) ZrHfCN−1, (c) ZrHfCN−2, (d) ZrHfCN−3, and (e) 316L SS.
3.6. Surface Compatibility
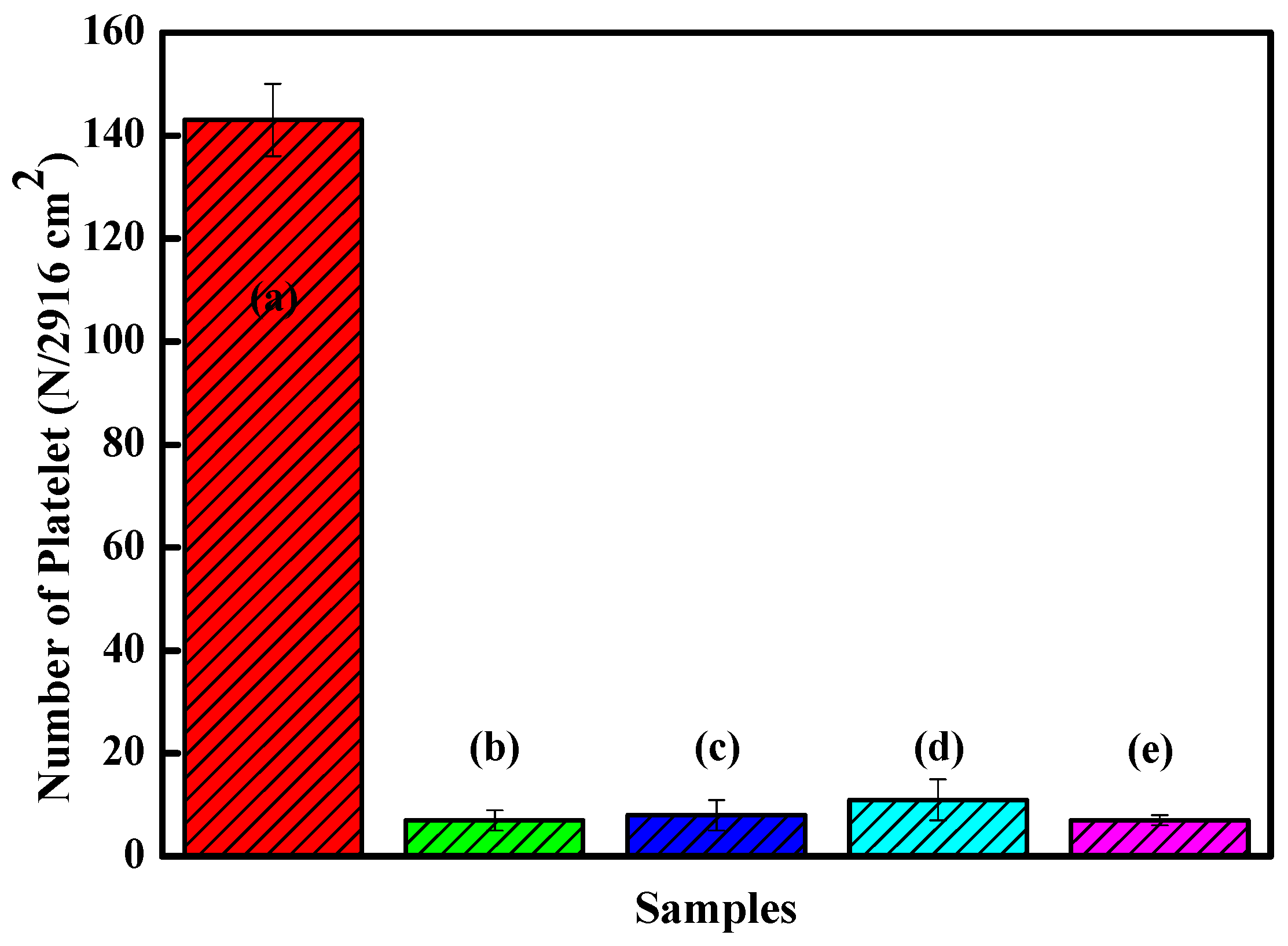
Figure 10 shows the platelet counts on the Zr(Hf)xCN coatings and uncoated 316L SS. The results show that more platelets adhered to 316L SS than to Zr(Hf)xCN coatings. For Zr(Hf)xCN coatings, the number of adhered platelets decreased with increasing Hf content. Based on the number of adhered platelets, the results of statistical analysis indicate that the platelet interaction with the surface of 316L SS improved effectively due to the deposition of Zr(Hf)xCN coatings. Moreover, the increase in Hf content was favorable for the improvement of surface compatibility. This is attributed to the fact that with the increase in Hf element content, the ratio of non-metal to metal increases, and a high ratio is favorable for the blood compatibility of the material [13]. Furthermore, with the addition of Hf element, the surface roughness of the coating gradually decreases. According to our previous research [9,27], low surface roughness is beneficial to improve surface compatibility and corrosion resistance.
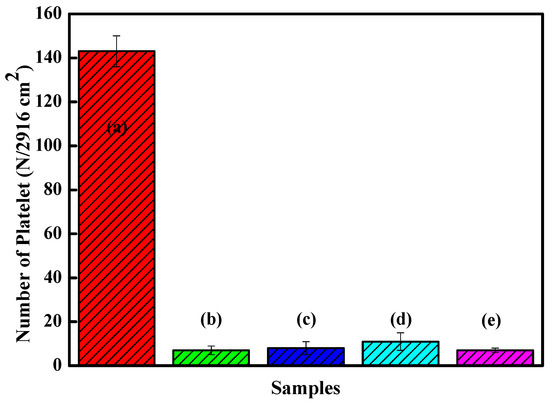
Figure 10.
Statistic analysis of the adhered platelet numbers of 316L SS and Zr(Hf)xCN coatings. (a) 316L SS, (b) ZrCN, (c) ZrHfCN−1, (d) ZrHfCN−2, and (e) ZrHfCN−3.
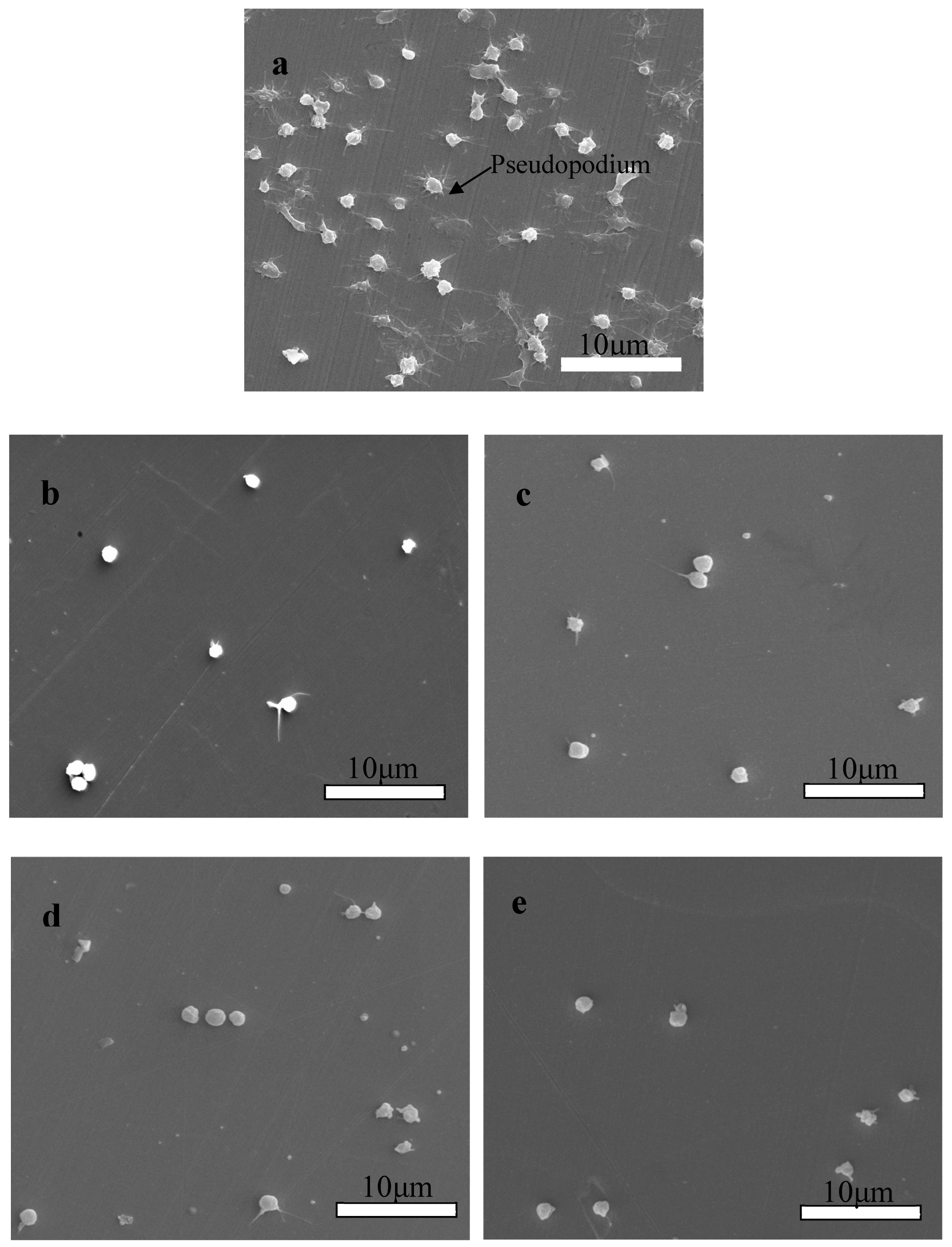
SEM was used to characterize the morphology of the platelets adhered to Zr(Hf)xCN-coated 316L SS and uncoated 316L SS. Figure 11a illustrates that numerous platelets adhered to and aggregated on the surface of 316L SS, and anomalous pseudopodia extended outwards. Figure 11b–e show the morphological characteristics of the platelets adhered to deposited Zr(Hf)xCN coatings containing different Hf content. Figure 11e exhibits that the number of adhered platelets declined and no anomalous pseudopodia extended outwards. It indicates that the platelet interaction with the surface of Zr(Hf)xCN coating is better than that with the surface of 316L SS. When platelets contact the uncoated 316L SS, due to the poor blood compatibility of 316L SS, the platelets are stimulated and activated to extend pseudopodia, resulting in local platelet aggregation and thrombosis. The surface contact properties and roughness of coatings with different Hf content were improved, which inhibited platelet activation. In addition, the smooth surface inhibited the local adhesion of platelets.
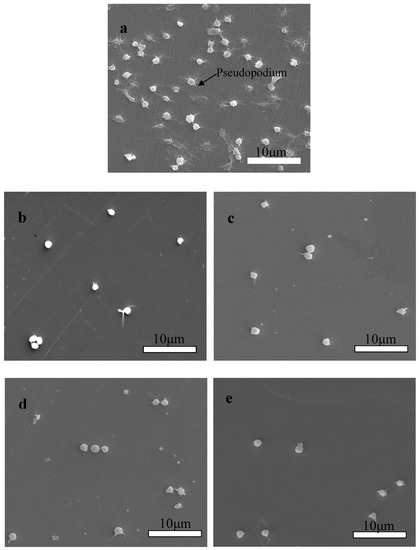
Figure 11.
SEM morphologies of platelets adhered on the surface of 316L SS and Zr(Hf)xCN coatings. (a) 316L SS, (b) ZrCN, (c) ZrHfCN−1, (d) ZrHfCN−2, and (e) ZrHfCN−3.
4. Conclusions
The microstructure, mechanical properties, corrosion resistance, and surface compatibility of Zr(Hf)xCN coatings with different Hf element content were investigated. The results are as follows:
(1) Zr(Hf)xCN coatings with thicknesses in the range of 1.08 to 1.28 μm were obtained for a deposition time of 120 min and the AFM results show that the RMS values decreased slightly as the Hf element content increased. The results of XRD and TEM reveal that Hf embedded into Zr2CN crystal grain caused the lattice distortion and that the grain size was approximately 5 nm.
(2) The results of the mechanical properties test revealed that Hf has a important impact on the mechanical properties of Zr(Hf)xCN coatings and the maximum mechanical properties were associated with the formation of Hf-Zr2CN nanocrystalline when the Hf element content reached 7.33%. In the meantime, the COF and adhesion strength reached the best results.
(3) The results of electrochemical measurements demonstrate that the Zr(Hf)xCN coatings could significantly improve the corrosion resistance of 316L SS, and the optimal level was reached when Hf element content was 7.33%.
(4) The platelet adhesion tests indicate that the Zr(Hf)xCN coatings present better surface compatibility compared to 316L SS.
In conclusion, Zr(Hf)xCN coatings with different Hf element contents could be a promising solution for improving the characteristics of 316L SS used in biomedical applications due to their favorable mechanical properties, excellent corrosion resistance, and good surface compatibility.
Author Contributions
Data curation, D.P., C.-Y.D., H.-S.Y. and Z.-N.H.; Writing—original draft, J.-Y.Z. and D.H.; Writing—review and editing, L.W., M.-H.D., B.Z. and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2022 Science and technology progress plan project of Chongqing Gear Box. Co LTD (ky2022-04) and the National Key Research and Development Program of China (2020YFB2010104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, J.B.; Kim, Y.K.; Park, J.B.; Bronzino, J.D. (Eds.) Biomaterials Principles and Applications; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–20. [Google Scholar]
- Guo, D.; Chen, J.; Wen, L.; Wang, P.; Xu, S.; Cheng, J.; Wen, X.; Wang, S.; Huang, C.; Pi, P. A superhydrophobic polyacrylate film with good durability fabricated via spray coating. J. Mater. Sci. 2018, 53, 15390–15400. [Google Scholar] [CrossRef]
- Richards, B.T.; Zhao, H.; Wadley, H.N.G. Structure, composition, and defect control during plasma spray deposition of ytterbium silicate coatings. J. Mater. Sci. 2015, 50, 7939–7957. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.J.; Zhao, Z.H. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Design 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Parsapour, A.; Khorasani, S.N.; Fathi, M.H. Effect of surface treatment and metallic coating on corrosion behavior and biocompatibility of surgical 316L stainless steel implant. J. Mater. Sci. Technol. 2012, 28, 125–131. [Google Scholar] [CrossRef]
- Penttila, S.; Toivonen, A.; Li, H.; Zheng, W.; Novotny, R. Effect of surface modification on the corrosion resistance of austenitic stainless steel 316L in supercritical water conditions. J. Supercrit. Fluids 2013, 81, 157–163. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Zhou, Z.; Li, L.K.-Y.; Yan, J. Electrochemical performance of TiCN coatings with low carbon concentration in simulated body fluid. Surf. Coat. Technol. 2014, 253, 199–204. [Google Scholar] [CrossRef]
- Gilewicz, A.; Chmielewska, P.; Murzynski, D.; Dobruchowska, E.; Warcholinski, B. Corrosion resistance of CrN and CrCN/CrN coatins deposited using cathodic arc evaporation in Ring’s and Hank’s solutions. Surf. Coat. Technol. 2016, 299, 7–14. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Ding, M.; Zheng, H.; Zhang, H.; Zhang, B.; Li, X.; Wu, G. Surface modification of biomedical AISI 316L stainless steel with zirconium carbonitride coatings. Appl. Surf. Sci. 2015, 340, 113–119. [Google Scholar] [CrossRef]
- Xie, Z.-H.; Shan, S. Nanocontainers-enhanced self-healing Ni coating for corrosion protection of Mg alloy. J. Mater. Sci. 2018, 53, 3744–3755. [Google Scholar] [CrossRef]
- Silva, E.; de Figueriredo, M.R.; Franz, R.; Galindo, R.E.; Palacio, C.; Espinosa, A.; Calderon, V.S.; Mitterer, C.; Carvalho, S. Structure-property relations in ZrCN coatings for tribological applications. Surf. Coat. Technol. 2010, 205, 2134–2141. [Google Scholar] [CrossRef]
- Larijani, M.M.; Zanjanbar, M.B.; Majdabadi, A. The effect of carbon fraction in Zr(C,N) films on the nano-structure properties and hardness. J. Alloys Compd. 2010, 492, 735–738. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Braic, V.; Balaceanu, M.; Titorencu, I.; Braic, M.; Parau, A.C. Corrosion resistance, mechanical properties and biocompatibility of Hf-containing ZrCN coatings. Thin Solid Film. 2013, 538, 48–55. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Vladescu, A.; Zoita, C.N.; Braic, V. Study of (Zr,Ti)CN, (Zr,Hf)CN and (Zr,Nb)CN films prepared by reactive magnetron sputtering. Thin Solid Film. 2011, 519, 4092–4096. [Google Scholar] [CrossRef]
- Negrila, C.-C.; Lazarescu, M.F.; Logofatu, C.; Cotirlan, C.; Ghita, R.V.; Frumosu, F.; Trupina, L. XPS Analysis of AuGeNi/Cleaved GaAs (110) interface. J. Cryst. Growth 2008, 310, 1576. [Google Scholar] [CrossRef]
- Zhao, J.P.; Chen, Y.; Tu, M. Improving blood-compatibility via surface heparin-immobilization based on a liquid crystalline matrix. Mater. Sci. Eng. C 2016, 58, 133–141. [Google Scholar] [CrossRef]
- Ferreri, I.; Henriques, M.; De Hosson, J.T.M.; Cavaleiro, A.; Carvalho, S. Nano-galvanic coupling for enhanced Af= release in ZrCN-Ag films: Antibacterial application. Surf. Coat. Technol. 2016, 298, 1–6. [Google Scholar]
- Zhou, F.Z.; Fu, K.H.; Liao, B.; Yu, J.; Yang, C.; Zhang, X. Effect of carbon content on nanostructural, mechanical and electrochemical characteristics of self-organized nc-ZrCN/a-CNx nanocomposite films. Appl. Surf. Sci. 2015, 327, 350–357. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Chen, H.; Li, J.; Zhou, S.; Xue, Q. Influences of bias voltage on the microstructures and tribological performances of Cr-C-N coatings in seawater. Surf. Coat. Technol. 2015, 270, 305–313. [Google Scholar] [CrossRef]
- Sowjanya, V.; Bangera, K.V.; Shivakumar, G.K. Effect of substrate temperature and film thickness on the thermoelectric properties of In2Te3 thin films. J. Alloys Compd. 2017, 714, 224–229. [Google Scholar] [CrossRef]
- Braic, V.; Braic, M.; Balaceanu, M.; Vladescu, A.; Zoita, C.; Titorencu, I.; Jinga, V. (Zr, Ti)CN coatings as potential candidates for biomedical applications. Surf. Coat. Technol. 2011, 206, 604–609. [Google Scholar] [CrossRef]
- Lofaj, F.; Hviščová, P.; Zubko, P.; Kabátová, M. Mechanical and tribological properties of the high target utilization sputtering W-C coatings on different substrate. Int. J. Refract. Met. Hard Mater. 2017, 80, 305–314. [Google Scholar] [CrossRef]
- Tétard, F.; Djemia, P.; Angleraud, B.; Mubumbila, N.; Tessier, P.Y. Surface and bulk characterization of CNx thin film made by r.f. magnetron sputtering. Surf. Coat. Technol. 2002, 151–152, 184–188. [Google Scholar] [CrossRef]
- Vitelaru, C.; Balaceanu, M.; Parau, A.; Luculescu, C.R.; Vladescu, A. Investigation of nanostructured TiSiC-Zr and TiSiC-Cr hard coatings for industrial applications. Surf. Coat. Technol. 2014, 251, 21–28. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Luo, P.; Chen, W.Z.; Pan, S.R.; Chen, D.H. Hemocompatibility of ZnO thin films prepared by filtered cathodic vacuum are deposition. Vacuum 2013, 89, 220–224. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Ha, P.C.T.; Mckenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Biocompatibility of calcium and phospuorus doped diamond-like films synthesized by plasma immersion ion implantation and deposition. Diam. Relat. Mater. 2006, 15, 893–897. [Google Scholar] [CrossRef]
- Viteri, V.S.; Barandika, G.; Peremarch, C.P.J. Development of Ti-C-N coatings with improved tribological behavior and antibacterial properties. J. Mech. Hehav. Biomed. 2016, 55, 75–86. [Google Scholar] [CrossRef]
- Zhang, P.R.; Liu, Z.Q. Enhancing surface integrity and corrosion resistance of laser cladded Cr-Ni alloys by hard turning and low plasticity burnishing. Appl. Surf. Sci. 2017, 409, 169–178. [Google Scholar] [CrossRef]
- Li, W.; Ming-hui, D.; Hong-sen, Z.; Bin, Z. Study on HfCxN1-x coatings deposited on biomedical AISI 316L by radio-frequency magnetron sputtering. J. Alloys Comp. 2018, 730, 219–227. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Ebrahimi, S.; Yarmand, B. Highly corrosion protection properties of plasma electrolytic oxidized titanium usiong rGO nanosheets. Appl. Surf. Sci. 2019, 486, 153–165. [Google Scholar] [CrossRef]
- Hou, X.L.; Ren, Q.Y.; Yang, Y.; Cao, X.L.; Hu, J.; Zhang, C.; Deng, H.; Yu, D.; Li, K.; Lan, W. Effect of temperature on the electrochemical pitting corrosion behavior of 316L stainless steel in chloride-containing MDEA solution. J. Net. Gas. Sci. Eng. 2021, 86, 103718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).