A Review of Paper-Based Sensors for Gas, Ion, and Biological Detection
Abstract
1. Introduction
2. Background
2.1. Performance Factors
2.2. Properties
3. Paper as Sensor Substrate
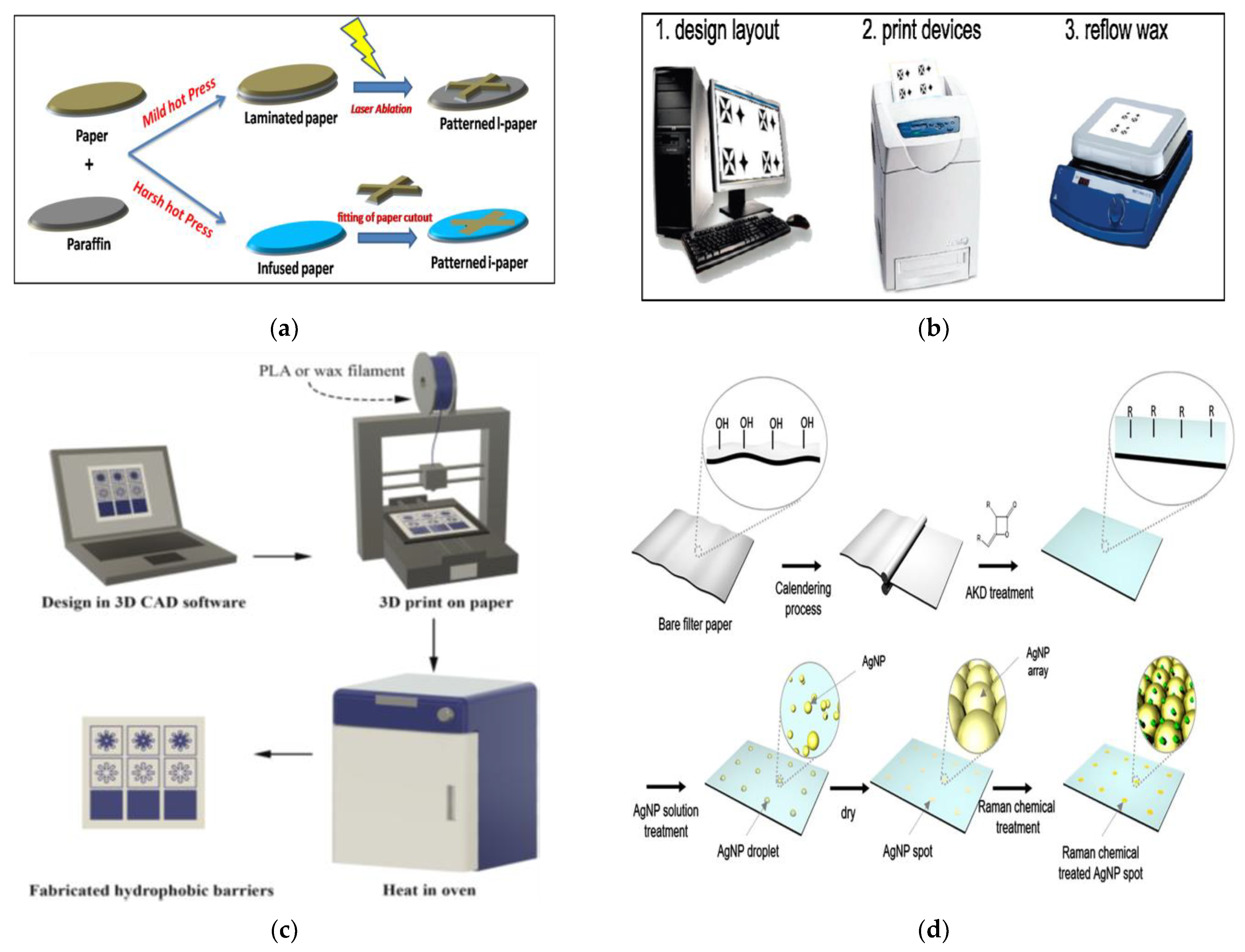
4. Fabrication

5. Paper-Based Detection Methods
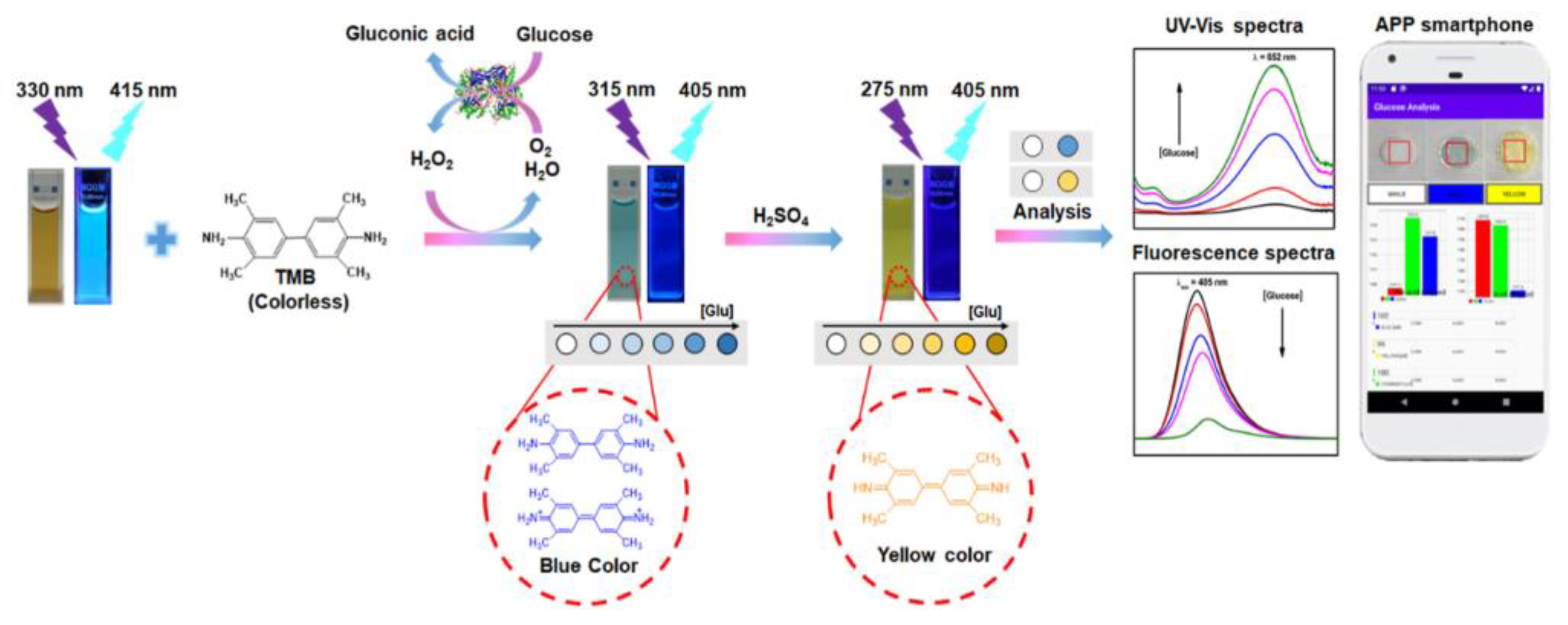
5.1. Optical Detection
5.2. Electrochemical Detection
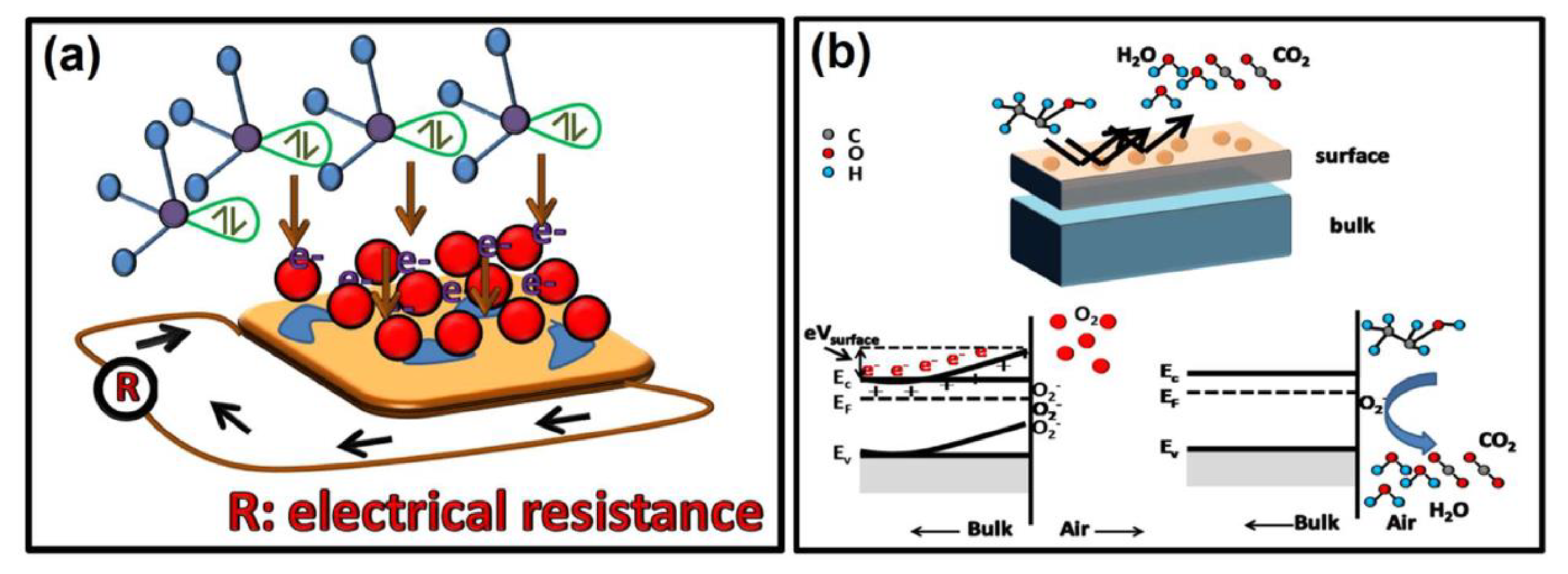
5.3. Chemiresistive Detection
6. Paper-Based Sensors to Detect Gas
7. Paper-Based Sensors to Detect Ions
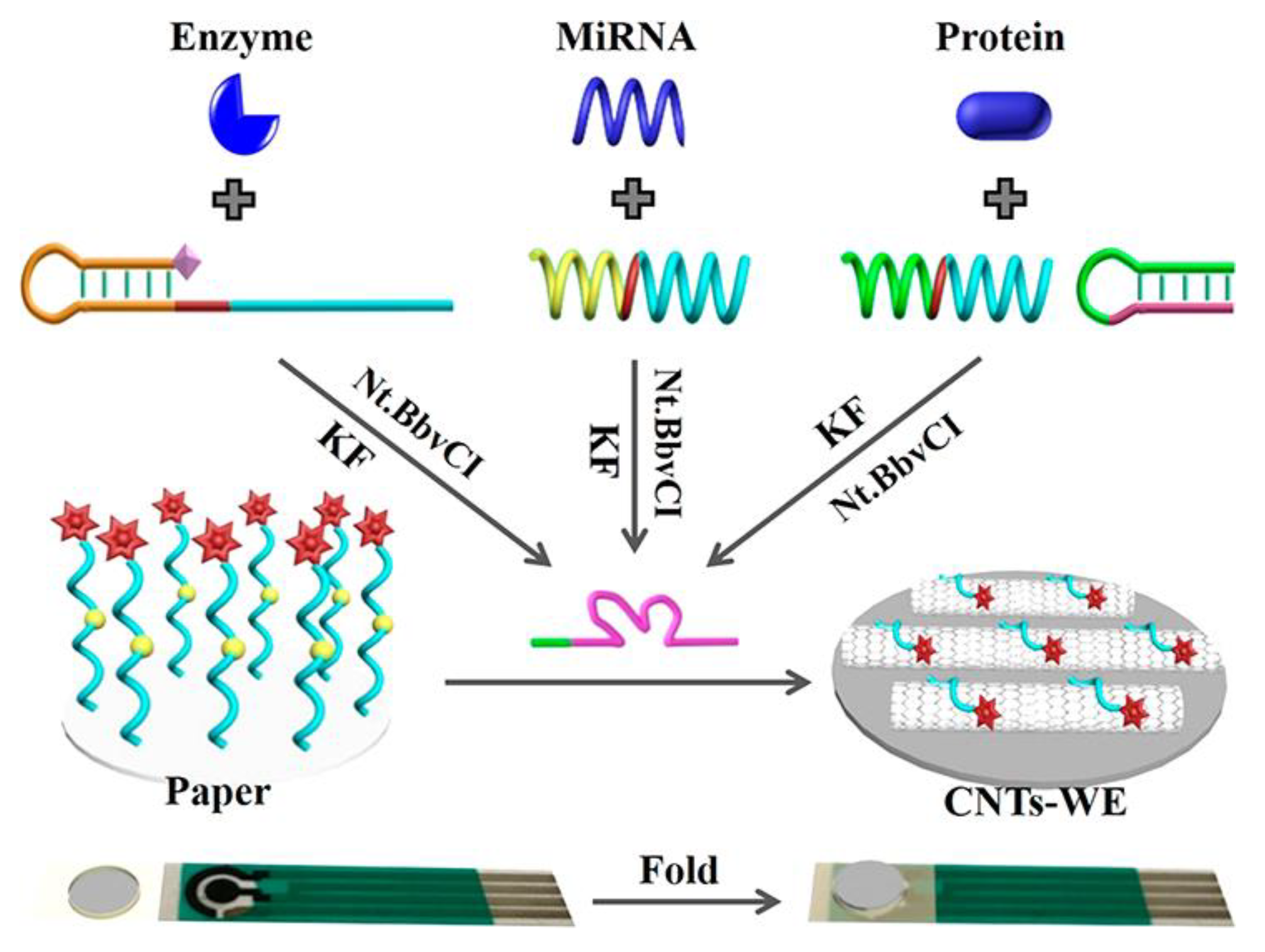
8. Paper-Based Sensors for Biological Detection
9. Challenges and Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, S. Toxic Gases. In Toxicology Cases for the Clinical and Forensic Laboratory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 387–396. ISBN 9780128158463. [Google Scholar]
- Srinivasan, P.; Ezhilan, M.; Kulandaisamy, A.J.; Babu, K.J.; Rayappan, J.B.B. Room Temperature Chemiresistive Gas Sensors: Challenges and Strategies—A Mini Review. J. Mater. Sci. Mater. Electron. 2019, 30, 15825–15847. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [PubMed]
- Nigam, A.; Sharma, N.; Tripathy, S.; Kumar, M. Physical Development of Semiconductor Based Heavy Metal Ion Sensors for Water Analysis: A Review. Sens. Actuators A Phys. 2021, 330, 112879. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent Advances in Energy-Saving Chemiresistive Gas Sensors: A Review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Chailapakul, O.; Siangproh, W.; Jampasa, S.; Chaiyo, S.; Teengam, P.; Yakoh, A.; Pinyorospathum, C. Paper-based sensors for the application of biological compound detection. In Comprehensive Analytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 89, pp. 31–62. [Google Scholar]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-Oxide-Semiconductor Based Gas Sensors: Screening, Preparation, and Integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-Based Sensors: A Review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Kundu, S.; Nag, A.; Afsarimanesh, N.; Sapra, S.; Mukhopadhyay, S.C.; Han, T. Silicon-Based Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 2908. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Kang, Y. Paper-based sensors and microfluidic chips. In Encyclopedia of Microfluidics and Nanofluidics; Springer: New York, NY, USA, 2015. [Google Scholar]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- Mandrić Radivojević, V.; Rupčić, S.; Srnović, M.; Benšić, G. Measuring the Dielectric Constant of Paper Using a Parallel Plate Capacitor. Int. J. Electr. Comput. Eng. Syst. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, R.L.; Nahirniak, S. Review: Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Loock, H.P.; Wentzell, P.D. Detection Limits of Chemical Sensors: Applications and Misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.-C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ Choice—Critical Review—A Critical Review of Solid State Gas Sensors. J. Electrochem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Kadu, A.; Matsumoto, K.; Uno, S.; Badhulika, S. Ultra-Low Cost, Smart Sensor Based on Pyrite FeS2 on Cellulose Paperfor the Determination of Vital Plant Hormone Methyl Jasmonate. Eng. Res. Express 2020, 2, 025020. [Google Scholar] [CrossRef]
- Pozuelo, M.; Blondeau, P.; Novell, M.; Andrade, F.J.; Rius, F.X. Paper-Based Chemiresistor for Detection of Ultralow Concentrations of Protein. Biosens. Bioelectron. 2013, 49, 462–465. [Google Scholar] [CrossRef]
- Honeycutt, W.T.; Ley, M.T.; Materer, N.F. Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors 2019, 19, 3157. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.; Bhat, T.N.; Bhati, V.S.; Dolmanan, S. Bin MPA-GSH Functionalized AlGaN/GaN Sensor for Cadmium Ion Detection. IEEE Sens. J. 2019, 19, 2863–2870. [Google Scholar] [CrossRef]
- Lavín, Á.; de Vicente, J.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaría, B.; Maigler, M.V.; Hernández, A.L.; Ramírez, Y. On the Determination of Uncertainty and Limit of Detection in Label-Free Biosensors. Sensors 2018, 18, 2038. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Jeong, B.; Lee Editor, H. Paper-Based Medical Diagnostic Devices as a Part of Bioanalysis-Advanced Materials, Methods, and Devices; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Fu, H.; Liu, X. Experimental Comparison of Surface Chemistries for Biomolecule Immobilization on Paper-Based Microfluidic Devices. J. Micromech. Microeng. 2019, 29, 124003. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Simonenko, N.P.; Simonenko, E.P.; Sysoev, V.V.; Brinzari, V. Paper-Based Humidity Sensors as Promising Flexible Devices, State of the Art, Part 2: Humidity-Sensor Performances. Nanomaterials 2023, 13, 1381. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Nassar, J.M.; Hussain, M.M. Paper as a Substrate and an Active Material in Paper Electronics. ACS Appl. Electron. Mater. 2021, 3, 30–52. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Review of Recent Inkjet-Printed Capacitive Tactile Sensors. Sensors 2017, 17, 2593. [Google Scholar] [CrossRef]
- Eom, S.; Lim, S. Stretchable Complementary Split Ring Resonator (CSRR)-Based Radio Frequency (RF) Sensor for Strain Direction and Level Detection. Sensors 2016, 16, 1667. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.W.; Kubota, L.T. Sensing Approaches on Paper-Based Devices: A Review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Guo, Z. A Paper-Making Transformation: From Cellulose-Based Superwetting Paper to Biomimetic Multifunctional Inorganic Paper. J. Mater. Chem. A Mater. 2020, 8, 20238–20259. [Google Scholar] [CrossRef]
- Gabrielli, V.; Frasconi, M. Cellulose-Based Functional Materials for Sensing. Chemosensors 2022, 10, 352. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef]
- Natarajan, S.; Jayaraj, J.; Prazeres, D.M.F. A Cellulose Paper-Based Fluorescent Lateral Flow Immunoassay for the Quantitative Detection of Cardiac Troponin I. Biosensors 2021, 11, 49. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, J.; Huang, Y.; Théato, P.; Huang, Q.; Chen, T. Self-Diffusion Driven Ultrafast Detection of Ppm-Level Nitroaromatic Pollutants in Aqueous Media Using a Hydrophilic Fluorescent Paper Sensor. ACS Appl. Mater. Interfaces 2017, 9, 23884–23893. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; de la Calle, I.; Romero, V.; Bendicho, C. Detection of Gases and Organic Vapors by Cellulose-Based Sensors. Anal. Bioanal. Chem. 2023, 415, 4039–4060. [Google Scholar] [CrossRef]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Donato, N.; Saitta, G.; Bonavita, A.; Rizzo, G.; Neri, G. Flexible Ethanol Sensors on Glossy Paper Substrates Operating at Room Temperature. Sens. Actuators B Chem. 2010, 145, 488–494. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I.; Pena-Pereira, F.; de la Calle, I.; Romero, V. Paper-Based Analytical Devices for Colorimetric and Luminescent Detection of Mercury in Waters: An Overview. Sensors 2021, 21, 7571. [Google Scholar] [CrossRef]
- Yao, Z.; Coatsworth, P.; Shi, X.; Zhi, J.; Hu, L.; Yan, R.; Güder, F.; Yu, H.-D. Paper-Based Sensors for Diagnostics, Human Activity Monitoring, Food Safety and Environmental Detection. Sens. Diagn. 2022, 1, 312–342. [Google Scholar] [CrossRef]
- Shen, L.L.; Zhang, G.R.; Etzold, B.J.M. Paper-Based Microfluidics for Electrochemical Applications. ChemElectroChem 2020, 7, 10–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible Electronics Based on Micro/Nanostructured Paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. -Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A Low-Cost, Simple, and Rapid Fabrication Method for Paper-Based Microfluidics Using Wax Screen-Printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, Y.S. Fabrication and applications of paper-based microfluidics. In Diagnostic Devices with Microfluidics; Taylor and Francis: Abingdon-on-Thames, UK, 2017; pp. 45–64. [Google Scholar]
- Määttänen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A Low-Cost Paper-Based Inkjet-Printed Platform for Electrochemical Analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [Google Scholar] [CrossRef]
- Jarujamrus, P.; Meelapsom, R.; Naksen, P.; Ditcharoen, N.; Anutrasakda, W.; Siripinyanond, A.; Amatatongchai, M.; Supasorn, S. Screen-Printed Microfluidic Paper-Based Analytical Device (ΜPAD) as a Barcode Sensor for Magnesium Detection Using Rubber Latex Waste as a Novel Hydrophobic Reagent. Anal. Chim. Acta 2019, 1082, 66–77. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-Based Microfluidics: Simplified Fabrication and Assay Methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Suvanasuthi, R.; Chimnaronk, S.; Promptmas, C. 3D Printed Hydrophobic Barriers in a Paper-Based Biosensor for Point-of-Care Detection of Dengue Virus Serotypes. Talanta 2022, 237, 122962. [Google Scholar] [CrossRef] [PubMed]
- Ogundare, S.A.; van Zyl, W.E. A Review of Cellulose-Based Substrates for SERS: Fundamentals, Design Principles, Applications. Cellulose 2019, 26, 6489–6528. [Google Scholar] [CrossRef]
- Lee, M.; Oh, K.; Choi, H.K.; Lee, S.G.; Youn, H.J.; Lee, H.L.; Jeong, D.H. Subnanomolar Sensitivity of Filter Paper-Based SERS Sensor for Pesticide Detection by Hydrophobicity Change of Paper Surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ji, Y.; Li, H.; Wang, Z.; Shi, L.; Zhang, S.; Wang, T.; Gong, Z. Recent Advances of Environmental Pollutants Detection via Paper-Based Sensing Strategy. Luminescence 2021, 36, 1818–1836. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-Based Sensors for Rapid Important Biomarkers Detection. Biosens. Bioelectron. X 2022, 12, 100246. [Google Scholar] [CrossRef]
- Kummari, S.; Panicker, L.R.; Rao Bommi, J.; Karingula, S.; Sunil Kumar, V.; Mahato, K.; Goud, K.Y. Trends in Paper-Based Sensing Devices for Clinical and Environmental Monitoring. Biosensors 2023, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.L.T.; Nguyen, P.L.; Jana, J.; Choi, W.M.; Chung, J.S.; Hur, S.H. Simple Paper-Based Colorimetric and Fluorescent Glucose Sensor Using N-Doped Carbon Dots and Metal Oxide Hybrid Structures. Anal. Chim. Acta 2021, 1147, 187–198. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Gao, X.; Ge, L.; Sun, X.; Li, F. A Universal Paper-Based Electrochemical Sensor for Zero-Background Assay of Diverse Biomarkers. ACS Appl. Mater. Interfaces 2019, 11, 15381–15388. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.W.; Tang, K.T. Towards a Chemiresistive Sensor-Integrated Electronic Nose: A Review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Das, S.; Saha, D.; Mondal, S. Material Surface-Analyte Interactions with Similar Energy Rates Vary as Univariate Quadratic Function of Topological Polar Surface Area of Analytes. arXiv 2021, arXiv:2104.14867. [Google Scholar]
- Koga, H.; Nagashima, K.; Huang, Y.; Zhang, G.; Wang, C.; Takahashi, T.; Inoue, A.; Yan, H.; Kanai, M.; He, Y.; et al. Paper-Based Disposable Molecular Sensor Constructed from Oxide Nanowires, Cellulose Nanofibers, and Pencil-Drawn Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 15044–15050. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Li, M.; Luo, J.; Zhang, B.; Liu, J.; Hu, Z. PbS Nanowires-on-Paper Sensors for Room-Temperature Gas Detection. IEEE Sens. J. 2019, 19, 846–851. [Google Scholar] [CrossRef]
- Kumar, A.; Zhao, Y.; Abraham, S.R.; Thundat, T.; Swihart, M.T. Pd Alloy Nanosheet Inks for Inkjet-Printable H 2 Sensors on Paper. Adv. Mater. Interfaces 2022, 2200363, 1–9. [Google Scholar] [CrossRef]
- Shobin, L.R.; Manivannan, S. Sensors and Actuators B: Chemical Carbon Nanotubes on Paper: Flexible and Disposable Chemiresistors. Sens. Actuators B Chem. 2015, 220, 1178–1185. [Google Scholar] [CrossRef]
- Han, J.; Kim, B.; Li, J.; Meyyappan, M. RSC Advances a Carbon Nanotube Based Ammonia Sensor on Cellulose Paper. R. Soc. Chem. 2014, 4, 549–553. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Z.; Hu, J.; Cao, Y.; Guo, J.; Long, M. Sensors and Actuators B: Chemical Flexible Chemiresistive Sensor of Polyaniline Coated Fi Lter Paper Prepared by Spraying for Fast and Non-Contact Detection of Nitroaromatic Explosives. Sens. Actuators B Chem. 2020, 304, 127233. [Google Scholar] [CrossRef]
- Ye, X.; Ge, L.; Jiang, T.; Guo, H.; Chen, B.; Liu, C.; Hayashi, K. Fully Inkjet-Printed Chemiresistive Sensor Array Based on Molecularly Imprinted Sol—Gel Active Materials. Sensors 2022, 7, 1819–1828. [Google Scholar] [CrossRef]
- Hamouma, O.; Kaur, N.; Oukil, D.; Mahajan, A.; Electrochimie, L.; Énergétique, D.V.; Mira, U.A. Paper Strips Coated with Polypyrrole-Wrapped Carbon Nanotube Composites for Chemi-Resistive Gas Sensing. Synth. Met. 2019, 258, 116223. [Google Scholar] [CrossRef]
- Lim, G.; Bae, S.; Kim, Y.; Seung, K.; Cho, H.; Jae, Y.; Lee, H.; Kim, S.; Kim, S.; Chung, H.; et al. Sensors and Actuators: B. Chemical Boron Nitride/Carbon Nanotube Composite Paper for Self-Activated Chemiresistive Detection. Sens. Actuators B Chem. 2022, 355, 131273. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, P.; Wang, D.; Luo, Y.; Li, M.; Lee, H.; Gerhardt, R.A. Sensors and Actuators B: Chemical A Novel Paper-Based Flexible Ammonia Gas Sensor via Silver and SWNT-PABS Inkjet Printing. Sens. Actuators B Chem. 2014, 197, 308–313. [Google Scholar] [CrossRef]
- Huang, S.J.; Immanuel, P.N.; Yen, Y.K.; Yen, C.L.; Tseng, C.E.; Lin, G.T.; Lin, C.K.; Huang, Z.X. Tungsten Disulfide Nanotube-Modified Conductive Paper-Based Chemiresistive Sensor for the Application in Volatile Organic Compounds’ Detection. Sensors 2021, 21, 6121. [Google Scholar] [CrossRef]
- Noah, N.M. Design and Synthesis of Nanostructured Materials for Sensor Applications. J. Nanomater. 2020, 2020, 8855321. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Goncalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal Oxide Nanostructures for Sensor Applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef]
- Ram, C.; Jitender. A Review—Nanosensors and Promising Materials. JETIR J. Emerg. Technol. Innov. Res. 2022, 9, g347–g352. [Google Scholar]
- Zhang, W.; Zhu, S.; Luque, R.; Han, S.; Hu, L.; Xu, G. Recent Development of Carbon Electrode Materials and Their Bioanalytical and Environmental Applications. Chem. Soc. Rev. 2016, 45, 715–752. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Saucedo, N.M.; Mulchandani, A. Carbon Nanomaterial-Based Electrochemical Biosensors for Label-Free Sensing of Environmental Pollutants. Chemosphere 2016, 143, 85–98. [Google Scholar] [CrossRef]
- Young, S.-J.; Lin, Z.-D. Sensing Performance of Carbon Dioxide Gas Sensors with Carbon Nanotubes on Plastic Substrate. ECS J. Solid State Sci. Technol. 2017, 6, M72–M74. [Google Scholar] [CrossRef]
- Yang, J.; Dou, B.; Yuan, R.; Xiang, Y. Proximity Binding and Metal Ion-Dependent DNAzyme Cyclic Amplification-Integrated Aptasensor for Label-Free and Sensitive Electrochemical Detection of Thrombin. Anal. Chem. 2016, 88, 8218–8223. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, W.; Xiang, H.; Wu, H.; Li, Z.; Zhou, H.; Huang, W. Paper-Based Flexible Strain and Pressure Sensor with Enhanced Mechanical Strength and Super-Hydrophobicity That Can Work under Water. J. Mater. Chem. C Mater. 2022, 10, 3908–3918. [Google Scholar] [CrossRef]
- Xiang, H.; Li, Z.; Wang, W.; Wu, H.; Zhou, H.; Ni, Y.; Liu, H. Enabling a Paper-Based Flexible Sensor to Work under Water with Exceptional Long-Term Durability through Biomimetic Reassembling of Nanomaterials from Natural Wood. ACS Sustain. Chem. Eng. 2023, 11, 8667–8674. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hsu, C.H.; Lu, B.J.; Lin, P.Y.; Ho, M.L. Determination of Nitrite Ions in Environment Analysis with a Paper-Based Microfluidic Device. Dalton Trans. 2018, 47, 14799–14807. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Reza, M.; Izadyar, M. Analytica Chimica Acta a Simple Paper-Based Aptasensor for Ultrasensitive Detection of Lead (II) Ion. Anal. Chim. Acta 2019, 1071, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yan-qi, L.I.; Liang, F. Progress in Paper-Based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457. [Google Scholar] [CrossRef]
- Machhindra, L.A.; Yen, Y. A Highly Sensitive Electrochemical Sensor for Cd 2 + Detection Based on Prussian Blue-PEDOT-Loaded Laser-Scribed Graphene-Modified Glassy Carbon Electrode. Chemosensors 2022, 10, 209. [Google Scholar] [CrossRef]
- Rostampour, M.; Bailey, B.; Autrey, C.; Ferrer, K.; Vantoorenburg, B.; Patel, P.K.; Calvo-Marzal, P.; Chumbimuni-Torres, K.Y. Single-Step Integration of Poly(3-Octylthiophene) and Single-Walled Carbon Nanotubes for Highly Reproducible Paper-Based Ion-Selective Electrodes. Anal. Chem. 2021, 93, 1271–1276. [Google Scholar] [CrossRef]
- Ho, M.L.; Liu, W.F.; Tseng, H.Y.; Yeh, Y.T.; Tseng, W.T.; Chou, Y.Y.; Huang, X.R.; Hsu, H.C.; Ho, L.I.; Pan, S.W. Quantitative Determination of Leukocyte Esterase with a Paper-Based Device. RSC Adv. 2020, 10, 27042–27049. [Google Scholar] [CrossRef]
- Leelasree, T.; Selamneni, V.; Akshaya, T.; Sahatiya, P.; Aggarwal, H. MOF Based Flexible, Low-Cost Chemiresistive Device as a Respiration Sensor for Sleep Apnea Diagnosis. J. Mater. Chem. B 2020, 8, 10182–10189. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Journal of Industrial and Engineering Chemistry Enzyme Immobilized Multi-Walled Carbon Nanotubes on Paper-Based Biosensor Fabricated via Mask-Less Hydrophilic and Hydrophobic Microchannels for Cholesterol Detection. J. Ind. Eng. Chem. 2022, 113, 401–410. [Google Scholar] [CrossRef]
- Giaretta, J.E.; Oveissi, F.; Dehghani, F.; Naficy, S. Paper-Based, Chemiresistive Sensor for Hydrogen Peroxide Detection. Adv. Mater. Technol. 2021, 2001148, 1–8. [Google Scholar] [CrossRef]
- Shen, Y.; Tran, T.T.; Modha, S.; Tsutsui, H.; Mulchandani, A. A Paper-Based Chemiresistive Biosensor Employing Single-Walled Carbon Nanotubes for Low-Cost, Point-of-Care Detection. Biosens. Bioelectron. 2019, 130, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Mazzaracchio, V.; Bagheri, N.; Chiara, F.; Fiore, L.; Moscone, D.; Roggero, S.; Arduini, F. A Smart Paper-Based Electrochemical Sensor for Reliable Detection of Iron Ions in Serum. Anal. Bioanal. Chem. 2023, 415, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Edgar, J.M.; Majumdar, S.; Briggs, J.S.; Patterson, S.V.; Tan, M.X.; Kudlacek, S.T.; Schneider, C.A.; Weiss, G.A.; Penner, R.M. Virus-Enabled Biosensor for Human Serum Albumin. Anal. Chem. 2017, 89, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.Z.; Chen, C.J.; Settu, K.; Lin, Y.F.; Chen, C.L.; Liu, J.T. Screen-Printed Carbon Electrode-Based Electrochemical Immunosensor for Rapid Detection of Microalbuminuria. Biosens. Bioelectron. 2016, 77, 1175–1182. [Google Scholar] [CrossRef]
- Caratelli, V.; Di Meo, E.; Colozza, N.; Fabiani, L.; Fiore, L.; Moscone, D.; Arduini, F. Nanomaterials and Paper-Based Electrochemical Devices: Merging Strategies for Fostering Sustainable Detection of Biomarkers. J. Mater. Chem. B 2022, 10, 9021–9039. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoçi, A. Paper-Based Nanobiosensors for Diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Ozer, T.; Mcmahon, C.; Henry, C.S. Annual Review of Analytical Chemistry Advances in Paper-Based Analytical Devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic Self-Assembly Enabled Flexible Paper-Based Humidity Sensor with High Sensitivity and Superior Durability. Chem. Eng. J. 2021, 404, 127105. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.D.; Guppy-Coles, K.; Nihal, S.; Langguth, D.; Sonar, P.; Ayoko, G.A.; Punyadeera, C.; Izake, E.L. A Paper-Based Optical Sensor for the Screening of Viruses through the Cysteine Residues of Their Surface Proteins: A Proof of Concept on the Detection of Coronavirus Infection. Talanta 2022, 248, 123630. [Google Scholar] [CrossRef]
- Hui, Q.; Pan, Y.; Yang, Z. Paper-Based Devices for Rapid Diagnostics and Testing Sewage for Early Warning of COVID-19 Outbreak. Case Stud. Chem. Environ. Eng. 2020, 2, 100064. [Google Scholar] [CrossRef]
- Chowdury, M.A.; Khalid, F. Application of Microfluidic Paper-Based Analytical Device (ΜPAD) to Detect COVID-19 in Energy Deprived Countries. Int. J. Energy Res. 2021, 45, 18275–18280. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.H. Current Advances in Paper-Based Biosensor Technologies for Rapid COVID-19 Diagnosis. Biochip. J. 2022, 16, 376–396. [Google Scholar] [CrossRef]
- Faizul Zaki, M.; Chen, P.C.; Yeh, Y.C.; Lin, P.H.; Xu, M.Y. Engineering a Monolithic 3D Paper-Based Analytical Device (ΜPAD) by Stereolithography 3D Printing and Sequential Digital Masks for Efficient 3D Mixing and Dopamine Detection. Sens. Actuators A Phys. 2022, 347, 113991. [Google Scholar] [CrossRef]
- Chen, Y.; Owyeung, R.E.; Sonkusale, S.R. Analytica Chimica Acta Combined Optical and Electronic Paper-Nose for Detection of Volatile Gases. Anal. Chim. Acta 2018, 1034, 128–136. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Aydindogan, E.; Guler Celik, E.; Timur, S. Paper-Based Analytical Methods for Smartphone Sensing with Functional Nanoparticles: Bridges from Smart Surfaces to Global Health. Anal. Chem. 2018, 90, 12325–12333. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Zhou, M.; Han, Y.; Zhang, M.; Gao, Z.; Liu, Z.; Chen, P.; Du, W.; Zhang, X.; et al. Smartphone-Based Platforms Implementing Microfluidic Detection with Image-Based Artificial Intelligence. Nat. Commun. 2023, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Ai, J.; Wang, N.; Pu, Q. Recent Progress of Smartphone-Assisted Microfluidic Sensors for Point of Care Testing. TrAC -Trends Anal. Chem. 2022, 157, 116792. [Google Scholar] [CrossRef]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-Based Multiplexed Biosensing Tools for Health Monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef]
- Lan, Y.; He, B.; Tan, C.S.; Ming, D. Applications of Smartphone-Based Aptasensor for Diverse Targets Detection. Biosensors 2022, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Lamaoui, A.; Amine, A. Paper-Based Optical Sensors Paired with Smartphones for Biomedical Analysis. J. Pharm. Biomed. Anal. 2023, 225. [Google Scholar] [CrossRef] [PubMed]

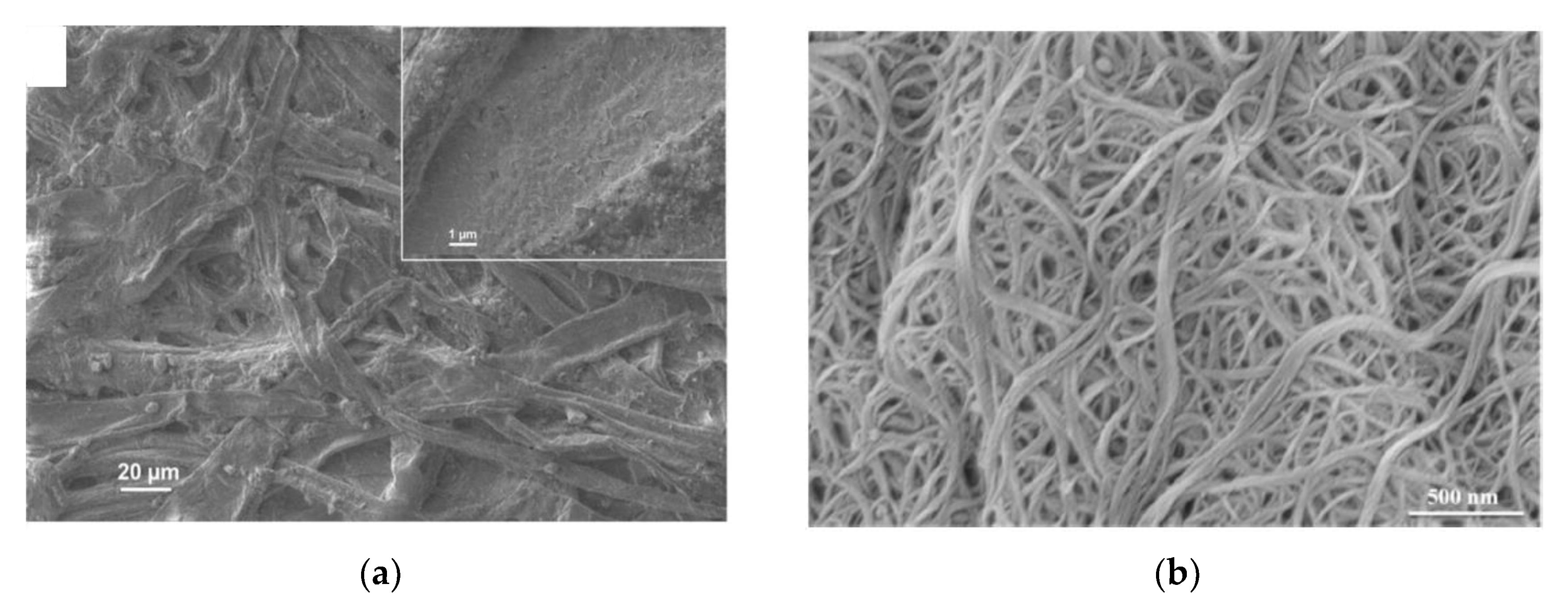
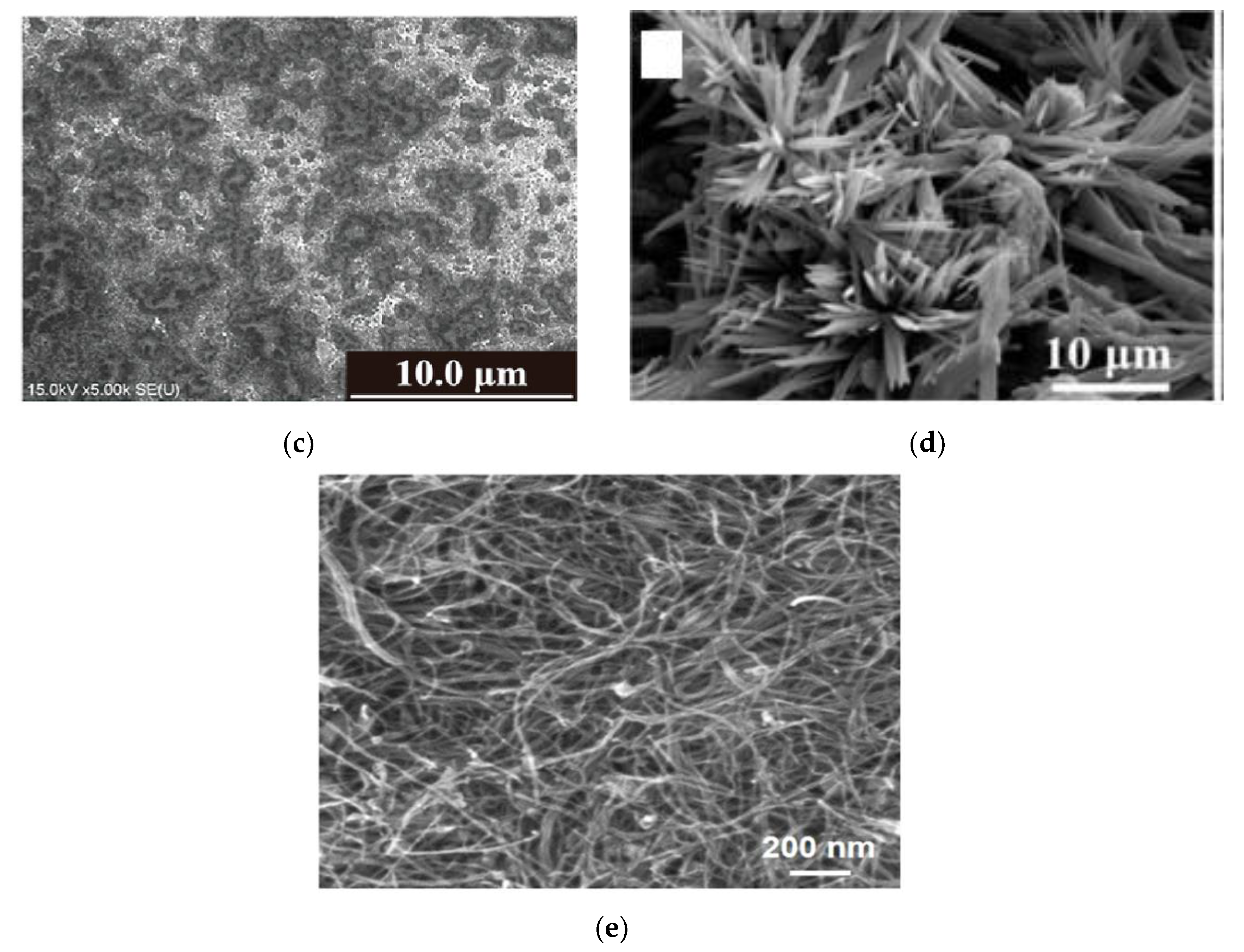

| Property | Paper | Glass | Silicon | PDMS |
|---|---|---|---|---|
| Surface Profile | Medium | Very Low | Very Low | Very Low |
| Flexibility | Yes | No | No | Yes |
| Physical Structure | Fibrous | Solid | Solid | Solid, Gas-Permeable |
| Surface to Volume Ratio | High | Low | Low | Low |
| Fluid Flow | Capillary Action | Forced | Forced | Forced |
| Biodegradability | Yes | No | No | To Some Extent |
| High-throughput Fabrication | Yes | Yes | Yes | No |
| Cost | Low | Medium | High | Medium |
| Sensing Material | Type of Sensing Material | Type of Paper | Contact/Detect/Analyte | Morphology and Chemical Bonding | Result | Reference |
|---|---|---|---|---|---|---|
| ZnO | Metal Oxide | Cellulose Paper | NO2 | Morphology:
| The paper-based sensor detected NO2 at 98 ppm with a sensitivity of 9 (Equation (1)), and the detection was about 3.9 ppm. | [57] |
| PbS | Inorganic Material | Cellulose Paper | NO2 | Morphology:
| The sensitivity of the PbS NWs sensor to detect NO2 was 17.5 (Equation (2)) with a response time of about 3 s and a recovery time of about 148 s at a concentration of 50 ppm. | [58] |
| PdMoY | Inorganic Material | Cellulose Paper | H2 | Morphology:
| The sensitivity of the paper-based PdMoY NS sensor was about 18.7% (Equation (4)). | [59] |
| SWCNT | Carbon Based Material | Filter Paper | NH3 | Morphology:
| At 62.5 ppm NH3, the paper-based sensor exhibited fast response (30 s) and recovery (30 s) characteristics, and it was able to detect concentrations as low as 80 ppb. | [60] |
| CNT | Carbon Based Material | Cellulose Paper | NH3 | Morphology: - Chemical Bonding: -
| The minimum detection limit of the CNT-on-paper sensor was found to be 5 ppm. | [61] |
| PCFP | Conductive Polymer | Filter Paper | TNT, 2, 4-DNT, PA | Morphology:
|
| [62] |
| KB/MISG | Conductive Polymer | Glossy Paper | VOAs | Morphology: MISG has high porosity with mesoporous structure. Chemical Bonding: - | The LOD was about 0.018 ppm. | [63] |
| Pap@CNT-NH2@PPy (minophenyl-modified multiwalled carbon nanotubes) | Composite | Cellulose Paper | NH3 | Morphology: The surface of the cellulosic fibers was covered in aggregated nanostructures. Chemical Bonding: - |
| [64] |
| BNCNT | Composite | Filter Paper | NO2 | Morphology: BNCNT papers have porous and fibrous structure Chemical Bonding: - | The sensitivity of this paper-based sensor was 16.5% with LOD about 3.41 ppb (0.00341 ppm). | [65] |
| SWCNT-PABS | Composite | Glossy Paper | NH3 | Morphology: - Chemical Bonding: - | The sensitivity of this paper-based sensor was 201% (Equation (4)). | [66] |
| Graphene– PEDOT:PSS/WS2 NT | Composite | Filter Paper | Butanol | Morphology: The nanotube has a bundle structure Chemical Bonding: - | The detection limit was 44.92 ppm, with a response time of 205 s and a recovery time of 20 s | [67] |
| Sensing Material | Type of Sensing Material | Type of Paper | Contact/Detect/Analyte | Morphology and Chemical Bonding | Result | Reference |
|---|---|---|---|---|---|---|
| Pyrite FeS2 | Inorganic Material | Cellulose Paper | MeJa | Morphology:
| The sensor had a detection limit of 0.68 mM and showed good sensitivity of 12.24 ± 14% mM−1 (Equation (6)) in the 1–2.5 mM range of MeJa. | [17] |
| Ag-PAD | Inorganic Material | Cellulose Paper | NO2 | Morphology:
| The LOD was 8.5 × 10−11 M. | [78] |
| GO | Carbon-Based Material | Chromatography paper | Pb2+ | Morphology: - Chemical Bonding: Hydrogen Bonding | The LOD was 0.5 pM. | [79] |
| C15H11N3O | Carbon-Based Material | Array Paper | Hg2+, Cd2+, Pb2+, Ni2+, Cu2+, Zn2+, and Co2+ | Morphology: - Chemical Bonding: - | The LOD was 50 µm. | [81] |
| Graphene and PEDOT: PSS | Composite | Filter Paper | Cl− | Morphology:
| The paper-based sensor was able to detect chlorine in a linear range from 0.1 to 500 ppm, with a detection limit of 0.18 ppm. | [81] |
| POT and SWCNT | Composite | Filter Paper | K+ and Na+ | Morphology: - Chemical Bonding: - | The LOD were 7.3 ± 0.4 × 10−7 (K+) and 1.1 ± 0.1 × 10−6 M (Na+) | [82] |
| Sensing Material | Type of Sensing Material | Type of Paper | Contact/Detect/Analyte | Morphology and Chemical Bonding | Result | Reference |
|---|---|---|---|---|---|---|
| PE/DAS/Ag | Inorganic Materials | Cellulose Paper | Urinary Leukocyte Esterase | Morphology: Nanoparticles and NWs Chemical Bonding: - | The LOD was 1.91 (×5.1 U mg−1 mL−1, S/N = 3). | [83] |
| MoS2 | Inorganic Materials | Cellulose Paper | Human Breath | Morphology: Porous Chemical Bonding: - | The sensor’s response time was 0.38 s. | [84] |
| MWCNTs | Carbon based material | Filter Paper | Cholesterol | Morphology: Cellulose Microfiber Chemical Bonding: - | The LOD was 3.2 nM. | [85] |
| SWCNTs and anti-human immunoglobulin G (antiHIgG). SWCNTs | Carbon based material | Filter Paper | Human Immunoglobulin G (HIgG) | Morphology: - Chemical Bonding: - | The sensitivity for the range 0–6.3 pM was −1.737 ± 0.85 nA/pmols L−1 (Equation (6)) | [18] |
| Horseradish Peroxidase (HRP)—PEDOT:PSS | Conductive Polymer | Filter Paper | Hydrogen peroxide (H2O2) | Morphology: - Chemical Bonding: Carbons Double bonding | The LOD was 61.3 × 10−9 M. | [86] |
| PCA and SWNTs | Composite | Filter Paper | Human Serum albumin (HSA) | Morphology: Porous Structure Chemical Bonding: - | The LOD was 1 pM, and the sensitivity was 9.44% (Equation (6)). | [87], |
| CB-AuNPs nanocomposite | Composite | Cellulose Paper | Iron in Blood Serum | Morphology: - Chemical Bonding: - | The LOD was 0.05 mg/L. | [88] |
| Sensing Material | Detection Method |
|---|---|
| PE/DAS/Ag | Optical |
| MoS2 | Chemiresistive |
| MWCNTs | Electrochemical |
| SWCNTs) and anti-human immunoglobulin G (antiHIgG). SWCNTs | Electrochemical |
| Horseradish Peroxidase (HRP)/(PEDOT:PSS) | Chemiresistive |
| PCA and SWNTs | Electrochemical |
| CB-AuNPs nanocomposite | Electrochemical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Immanuel, P.N.; Huang, S.-J.; Adityawardhana, Y.; Yen, Y.-K. A Review of Paper-Based Sensors for Gas, Ion, and Biological Detection. Coatings 2023, 13, 1326. https://doi.org/10.3390/coatings13081326
Immanuel PN, Huang S-J, Adityawardhana Y, Yen Y-K. A Review of Paper-Based Sensors for Gas, Ion, and Biological Detection. Coatings. 2023; 13(8):1326. https://doi.org/10.3390/coatings13081326
Chicago/Turabian StyleImmanuel, Phillip Nathaniel, Song-Jeng Huang, Yudhistira Adityawardhana, and Yi-Kuang Yen. 2023. "A Review of Paper-Based Sensors for Gas, Ion, and Biological Detection" Coatings 13, no. 8: 1326. https://doi.org/10.3390/coatings13081326
APA StyleImmanuel, P. N., Huang, S.-J., Adityawardhana, Y., & Yen, Y.-K. (2023). A Review of Paper-Based Sensors for Gas, Ion, and Biological Detection. Coatings, 13(8), 1326. https://doi.org/10.3390/coatings13081326









