Refined Grain Enhancing Lithium-Ion Diffusion of LiFePO4 via Air Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of FePO4 and LiFePO4/C
2.3. Characterization of FePO4 and LiFePO4/C
2.4. Electrochemical Measurement
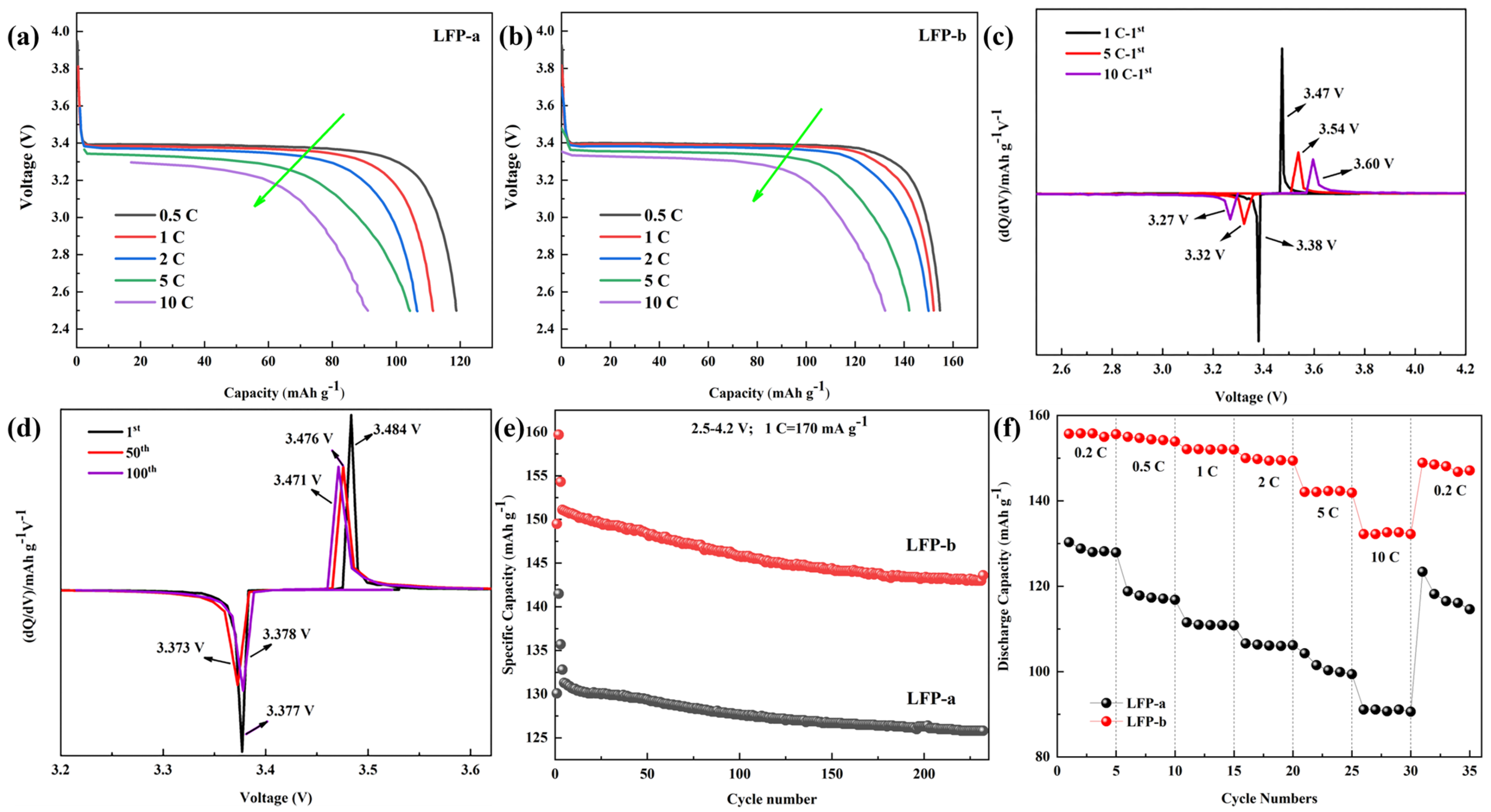
3. Results
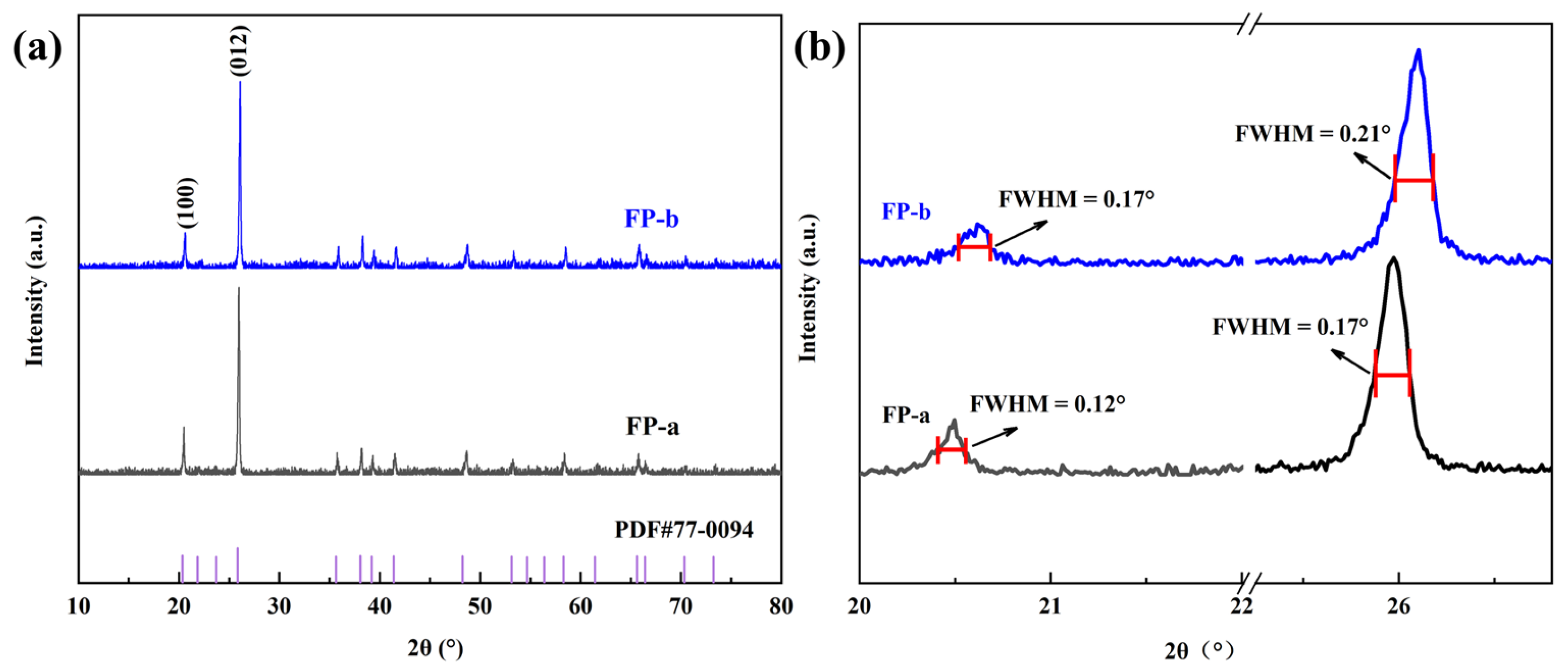
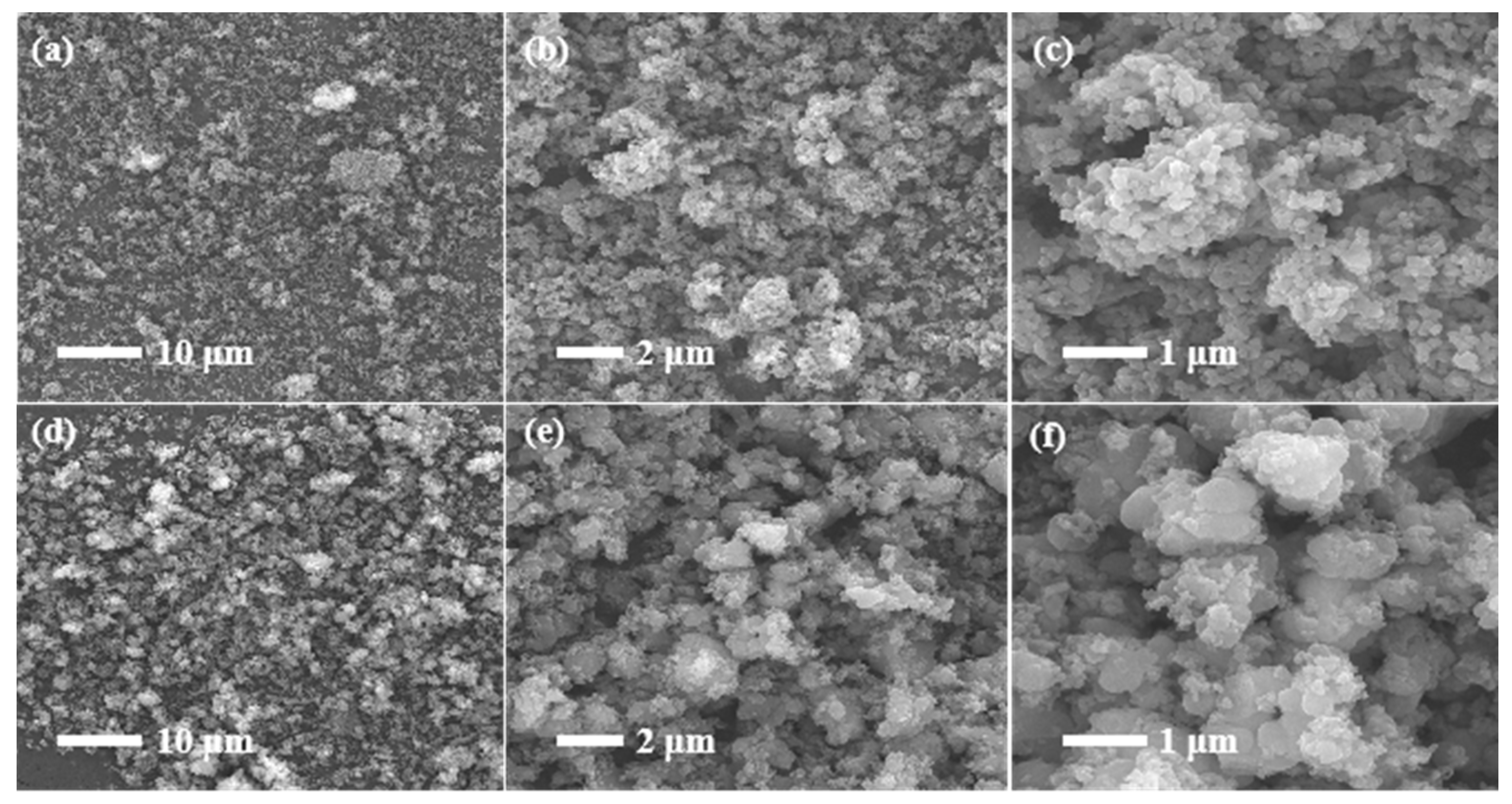
3.1. Characterization of Precursor FePO4
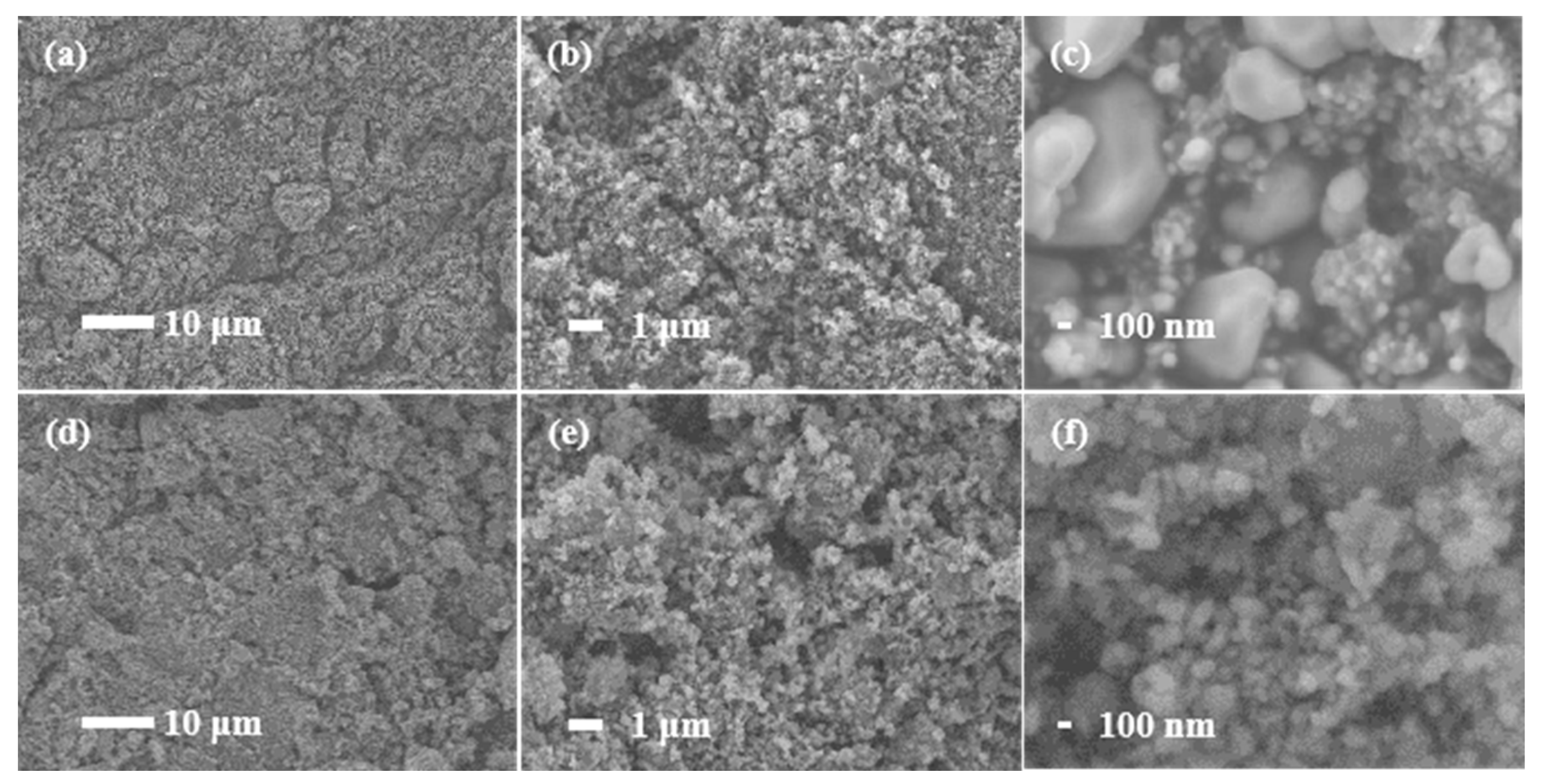
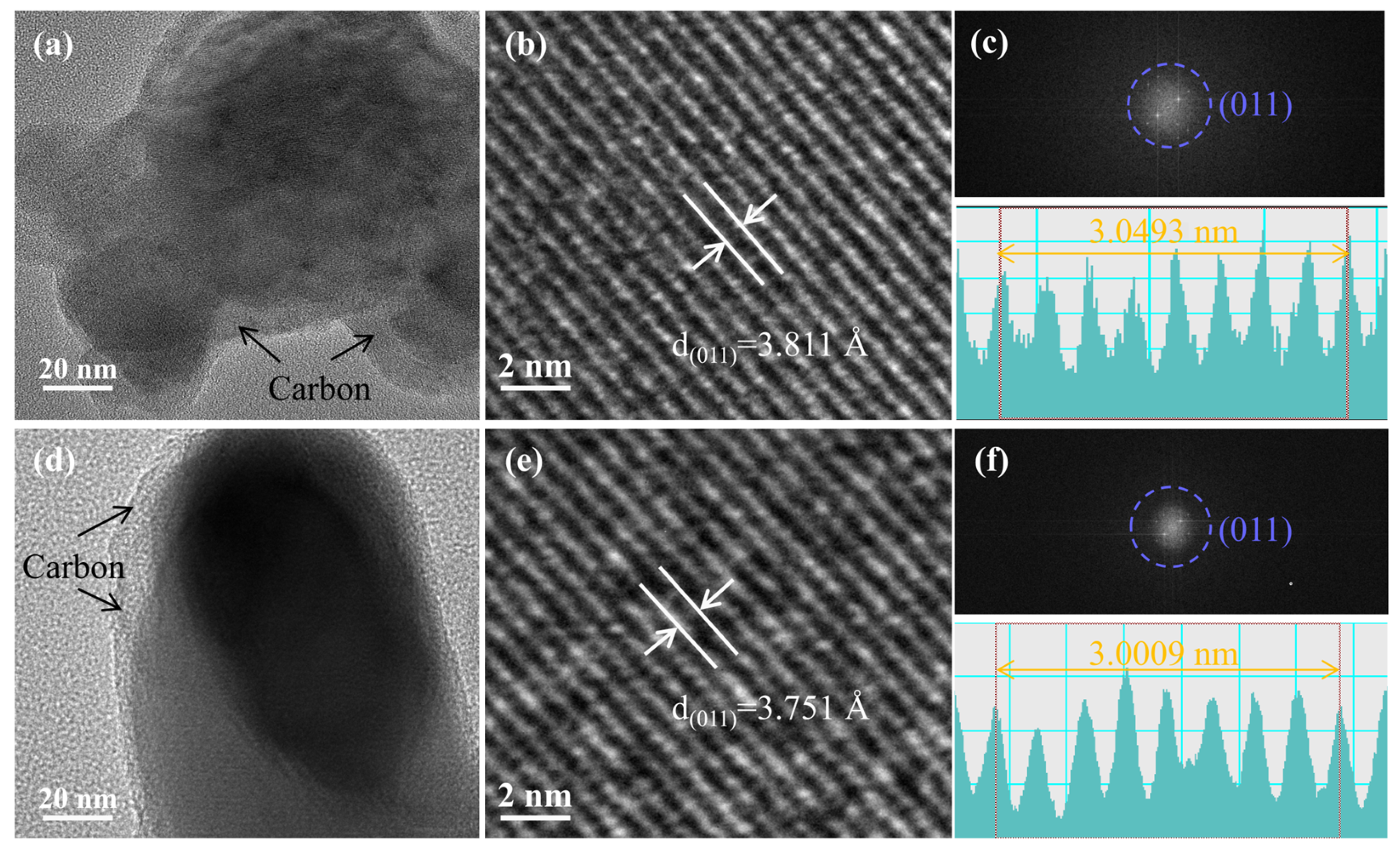
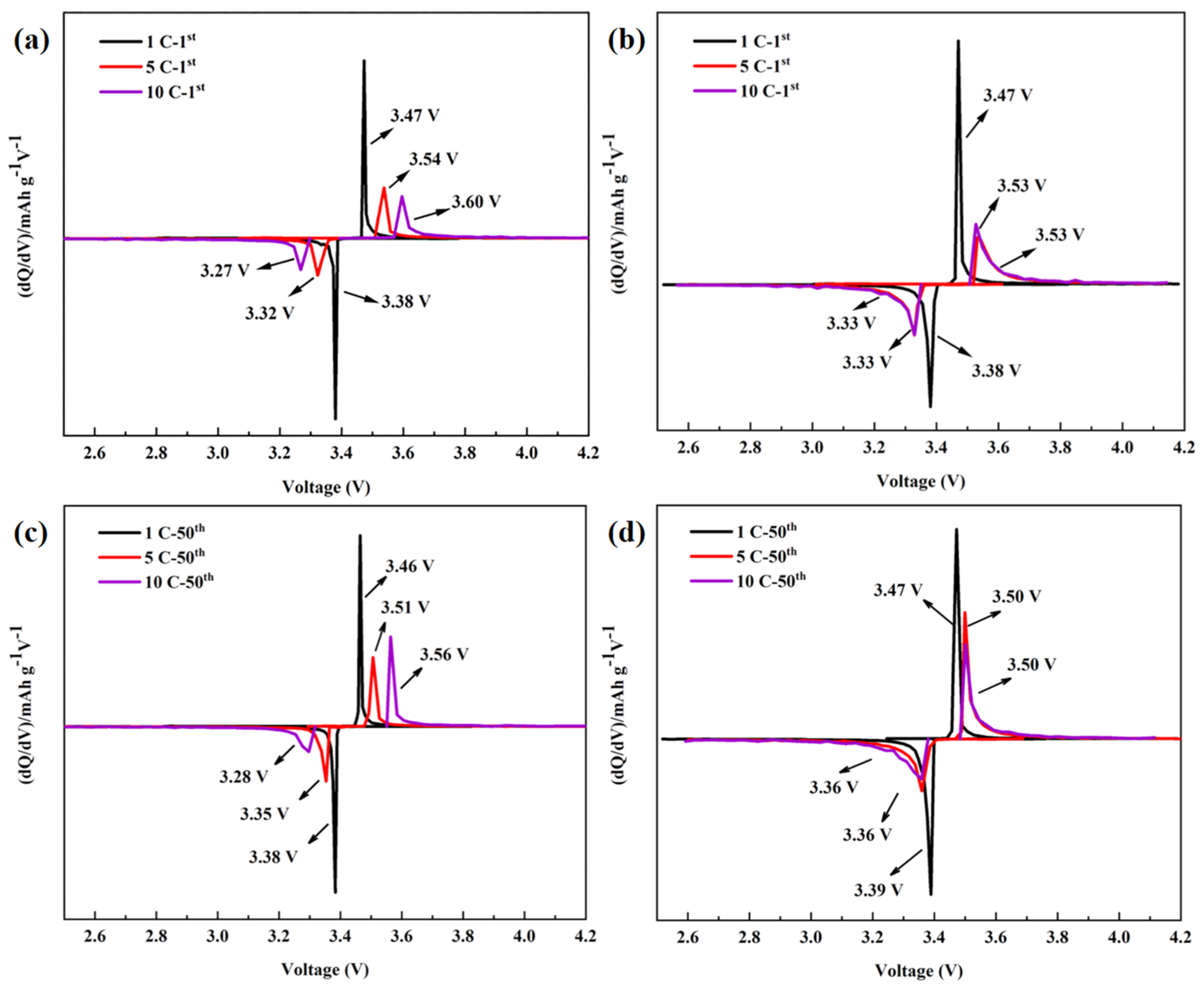
3.2. Characterization of LiFePO4/C
4. Conclusions
- The precursor FePO4 prepared via the air oxidation precipitation method has high purity, the particle size is about 1 um, and the ratio of iron to phosphorus is close to 1.
- The reduction of the primary particle size to 92.7 nm significantly improves the rate performance of LiFePO4. The discharge specific capacity of small grain LFP−b is about 155 mAh g−1 at low current density. When the current density is increased to 10 C, the discharge specific capacity remains approximately 130 mAh g−1 and the capacity retention is 83.5%. When the current density is restored to 0.2 C, the discharge specific capacity attenuation is 5.13%.
- Further studies show that grain refinement shortens the migration path of electrons and Li+, which reduces the charge transfer resistance and promotes the diffusion of Li+.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Li, Y.; Guo, Z.-P.; Dang, T. Experimental Study on Charging and Discharging Characteristics of Lithium Ion Power Battery for Electrochemical Energy Storage. Mech. Eng. Autom. 2022, 235, 10–11+5. [Google Scholar]
- Mei, J.; Zhang, J.; Liu, S.-Y.; Qiu, L.-C. Development Status of Battery Energy Storage Technology. Zhejiang Electr. Power 2020, 39, 75–81. [Google Scholar]
- Li, Z.-M.; Li, B.; Feng, D.; Zeng, T.-B. Research progress of cathode materials for lithium-ion battery. Res. Prog. Cathode Mater. Lithium-Ion Battery 2022, 39, 513–527. [Google Scholar]
- Yang, K.-X. Progress in the preparation of lithium iron phosphate cathode materials. Xinxi Jilu Cailiao 2022, 23, 37–40. [Google Scholar]
- Ma, Z.-M.; Xiao, R.-G.; Liao, X.; Ke, X. Preparation of the Lamellar Nanostructure Iron Phosphate and Its Effect on the Electrochemical Performance of Lithium Iron Phosphate. Cailiao Daobao 2018, 32, 3325–3331. [Google Scholar]
- Chen, S.-P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Wang, L. Ningde Era overseas business layout speed up. Xinnengyuan Keji 2022, 30, 17–18. [Google Scholar]
- Novikova, S.A.; Yaroslavtsev, A.B. Lithium deintercalation/intercalation processes in cathode materials based on lithium iron phosphate with the olivine structure. Russ. Chem. Bull. 2017, 66, 1336–1344. [Google Scholar] [CrossRef]
- Hu, J.-T.; Zheng, J.-X.; Pan, F. Research Progress into the Structure and Performance of LiFePO4 Cathode Materials. Acta Phys. -Chim. Sin. 2019, 35, 361–370. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Wu, F.; Song, S.; Zhang, X.; Fu, C.; Yuan, D.; Xiang, Y. Review—Recent Research Progress in Surface Modification of LiFePO4 Cathode Materials. J. Electrochem. Soc. 2017, 164, A2138. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Li, Y.; Sun, P.; Yang, C.; Wang, D.; Liu, L. Review: Phase transition mechanism and supercritical hydrothermal synthesis of nano lithium iron phosphate. Ceram. Int. 2020, 46, 27922–27939. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Wang, S.; Yu, J. How to make lithium iron phosphate better: A review exploring classical modification approaches in-depth and proposing future optimization methods. J. Mater. Chem. A 2016, 4, 18210–18222. [Google Scholar] [CrossRef]
- Dathar, G.K.; Sheppard, D.; Stevenson, K.J.; Henkelman, G. Calculations of Li-Ion Diffusion in Olivine Phosphates. Chem. Mater. 2011, 23, 4032–4037. [Google Scholar] [CrossRef]
- Liu, R.; Guo, H.; Gu, H.; Li, W.; Cao, J.; Ren, X.; Fu, Q.; Liang, G. Influence of PAN/PANI polymer on low-temperature rate performance of LiFePO4. Ionics 2023, 29, 2175–2189. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Meng, Y.; Xiao, M.; Kang, T.; Gao, H.; Huang, L.; Zhu, F. Preparation and electrochemical properties of Co doped core-shell cathode material on a lithium iron phosphate surface. J. Alloys Compd. 2022, 923, 166326. [Google Scholar] [CrossRef]
- Gao, C.; Liu, S.; Yan, P.; Zhu, M.; Qiu, T. Enhanced electrochemical kinetics and three dimensional architecture lithium iron phosphate/carbon nanotubes nanocomposites for high rate lithium-ion batteries. Colloids Surf. A 2022, 643, 128718. [Google Scholar] [CrossRef]
- Li, F.; Tao, R.; Tan, X.; Xu, J.; Kong, D.; Shen, L.; Mo, R.; Li, J.; Lu, Y. Graphite-Embedded Lithium Iron Phosphate for High-Power–Energy Cathodes. Nano Lett. 2021, 21, 2572–2579. [Google Scholar] [CrossRef]
- Longoni, G.; Panda, J.K.; Gagliani, L.; Brescia, R.; Manna, L.; Bonaccorso, F.; Pellegrini, V. In situ LiFePO4 nano-particles grown on few-layer graphene flakes as high-power cathode nanohybrids for lithium-ion batteries. Nano Energy 2018, 51, 656–667. [Google Scholar] [CrossRef]
- Han, J.-J.; Guo, A.-R.; Wang, Y.-F. Synthesis of PANI and its application in LiFePO4 cathode material. Ionics 2022, 28, 1073–1080. [Google Scholar] [CrossRef]
- Adepoju, A.A.; Doumbia, M.; Williams, Q.L. Graphene Nanoplatelet Additives for High C-rate LiFePO4 Battery Cathodes. JOM 2020, 72, 3170–3175. [Google Scholar] [CrossRef]
- Li, W.; Hwang, J.; Chang, W.; Setiadi, H.; Chung, K.Y.; Kim, J. Ultrathin and uniform carbon-layer-coated hierarchically porous LiFePO4 microspheres and their electrochemical performance. J. Supercrit. Fluids 2016, 116, 164–171. [Google Scholar] [CrossRef]
- Hwang, J.; Kong, K.C.; Chang, W.; Jo, E.; Nam, K.; Kim, J. New liquid carbon dioxide based strategy for high energy/power density LiFePO4. Nano Energy 2017, 36, 398–410. [Google Scholar] [CrossRef]
- Liu, X.; Sun, L.; Vu, N.H.; Linh, D.T.; Dien, P.T.; Nang, H.X.; Dao, V.D. Synthesis of LiFePO4/carbon/graphene for high-performance Li-ion battery. J. Electroanal. Chem. 2023, 932, 117205. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Feng, S.; Shen, W.; Guo, S. Improving the electrochemical properties of lithium iron(II) phosphate through surface modification with manganese ion(II) and reduced graphene oxide. J. Solid State Electrochem. 2018, 22, 285–292. [Google Scholar] [CrossRef]
- Wang, Q.; Deng, S.-X.; Liu, J.-B.; Wang, H. Research progress in rate performance of LiFePO4 cathode materials. Huagong Jinzhan 2011, 30, 2652–2657. [Google Scholar]
- Oh, J.; Lee, J.; Hwang, T.; Kim, J.M.; Seoung, K.D.; Piao, Y. Dual Layer Coating Strategy Utilizing N-doped Carbon and Reduced Graphene Oxide for High-Performance LiFePO4 Cathode Material. Electrochim. Acta 2017, 231, 85–93. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhao, X.; Wang, L.; Liang, G. Effect of Spherical Particle Size on the Electrochemical Properties of Lithium Iron Phosphate. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 549–557. [Google Scholar] [CrossRef]
- Ding, R.; Liu, H.; Wang, L.; Liang, G. Effect of spray drying technological conditions on the performance of LiFePO4/C cathode materials with high energy density. Ionics 2019, 25, 5633–5642. [Google Scholar] [CrossRef]
- Ke, X.; Xiao, R.G.; Liao, X.; Ma, Z.M.; Wang, S.D.; Xu, D. LiFePO4/C cathode material prepared with sphere mesoporous-FePO4 as precursors for lithium-ion batteries. J. Electroanal. Chem. 2018, 820, 18–23. [Google Scholar] [CrossRef]
- Guo, J.; Liang, C.; Cao, J.; Jia, S. Synthesis and electrochemical performance of lithium iron phosphate/carbon composites based on controlling the secondary morphology of precursors. Int. J. Hydrogen Energy 2020, 45, 33016–33027. [Google Scholar] [CrossRef]
- Cheng, W.H.; Wang, L.; Zhang, Q.B.; Wang, Z.J.; Xu, J.B.; Ren, W.; Bian, L.; Chang, A.M. Preparation and characterization of nanoscale LiFePO4 cathode materials by a two-step solid-state reaction method. J. Mater. Sci. 2017, 52, 2366–2372. [Google Scholar] [CrossRef]
- Satyavani, T.V.S.L.; Srinivas Kumar, A.; Subba Rao, P.S.V. Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: A review. Eng. Sci. Technol. Int. J. 2016, 19, 178–188. [Google Scholar] [CrossRef]
- Wang, X.-F.; Feng, Z.-J.; Zhang, S.-H.; Cong, X.-Q.; Zeng, Y.-P. Research process in rate performance of LiFePO4 cathode materials. Dianyuan Jishu 2017, 41, 1202–1205. [Google Scholar]
- Niu, B.; Man, L.-Y.; Qi, E.-L. Study on hydrothermal synthesis of lithium iron phosphate powder. Liulin Sheji Yu Fenti Gongcheng 2010, 14–16. [Google Scholar] [CrossRef]
- Li, W.; Xi, X.-M.; Zhan, Z.-K.; Hu, C.-B. Study on Hydrothermal Synthesis and Performance of LiFePO4 Cathode for Lithium Ion Batteries. Kuangye Gongcheng (Chang. China) 2011, 31, 88–91. [Google Scholar]
- Gaberscek, M.; Dominko, R.; Jamnik, J. Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem. Commun. 2007, 9, 2778–2783. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Sun, W.; Wang, J.; Li, Y.; Fan, S. Crystal Orientation Tuning of LiFePO4 Nanoplates for High Rate Lithium Battery Cathode Materials. Nano Lett. 2012, 12, 5632–5636. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, W.-J.; Yuan, M.-M.; Wang, Y.-K.; Yao, Y.-C. Preparation of battery grade nano iron phosphate by using ferro-phosphorus as raw material. Huagong Jinzhan 2019, 38, 5015–5023. [Google Scholar]


| Sample | Fe (ppm) | P (ppm) | Fe/P (Molar Ratio) |
|---|---|---|---|
| FP-a | 72.682 | 40.375 | 0.9984 |
| FP-b | 75.535 | 41.079 | 1.0198 |
| Sample | Rct (Ω) | Rs | D(Li+) (cm2 s−1) |
|---|---|---|---|
| LFP−a | 70.8 | 2.8 | 2.98 × 10−14 |
| LFP−b | 56.7 | 1.3 | 3.40 × 10−14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Qin, Z.; He, P.; Ren, X.; Li, Y.; Wu, F.; Cheng, Y.; He, Z. Refined Grain Enhancing Lithium-Ion Diffusion of LiFePO4 via Air Oxidation. Coatings 2023, 13, 1038. https://doi.org/10.3390/coatings13061038
Shen X, Qin Z, He P, Ren X, Li Y, Wu F, Cheng Y, He Z. Refined Grain Enhancing Lithium-Ion Diffusion of LiFePO4 via Air Oxidation. Coatings. 2023; 13(6):1038. https://doi.org/10.3390/coatings13061038
Chicago/Turabian StyleShen, Xinjie, Zijun Qin, Peipei He, Xugang Ren, Yunjiao Li, Feixiang Wu, Yi Cheng, and Zhenjiang He. 2023. "Refined Grain Enhancing Lithium-Ion Diffusion of LiFePO4 via Air Oxidation" Coatings 13, no. 6: 1038. https://doi.org/10.3390/coatings13061038
APA StyleShen, X., Qin, Z., He, P., Ren, X., Li, Y., Wu, F., Cheng, Y., & He, Z. (2023). Refined Grain Enhancing Lithium-Ion Diffusion of LiFePO4 via Air Oxidation. Coatings, 13(6), 1038. https://doi.org/10.3390/coatings13061038






