Characteristics of Corrosion Products of Friction-Type High-Strength Bolted Joints of Steel Bridge: A Case Study
Abstract
1. Introduction
2. Node Diseases
3. Analysis of Node Corrosion Products
3.1. Samples of Test Pieces
3.2. Micromorphology
3.3. Chemical Composition
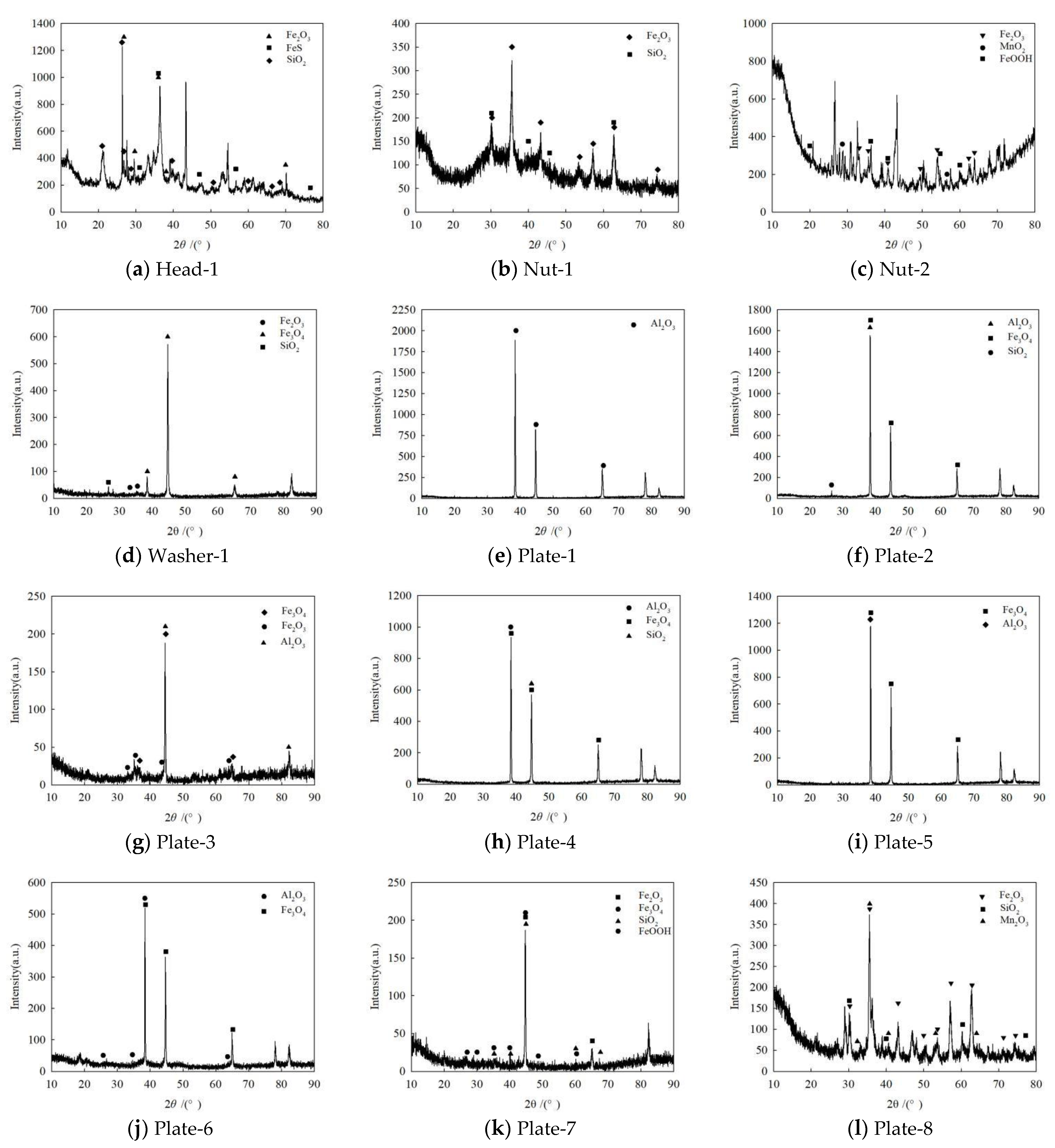
3.4. Phase Analysis
4. Corrosion Causes and Prevention
4.1. Corrosion Causes
4.2. Corrosion Prevention
5. Conclusions
- (1)
- After the aluminum coating corroded, the contact surface changed from uneven and dense to smooth and powdery. As the steel substrate corroded further, the contact surface transformed from fluffy and stratified to surface-bonded. These changes in the corroded contact surface significantly impacted the slip-bearing capacity of the FHSB joint by altering the friction coefficient;
- (2)
- Almost all samples detected SiO2 from Yellow River soil as well as elements such as Mg, Ca, K, and Na, largely matching Yellow River soil composition. Cracking of asphalt pavement and anti-slip layers allowed corrosive media and soil to invade the joints. Joint tightness was impaired by construction issues, accelerating corrosion;
- (3)
- Corrosion products contained large amounts of S and Cl as well as oxides of Mn, Si, and other elements and FeS, indicating atmospheric corrosion (e.g., acid rain) and industrial dust contributed to joint corrosion;
- (4)
- Sandblasting and coating the corroded contact surface and deck steel plate with inorganic zinc-rich paint prevented media penetration and delayed substrate corrosion. Adopting UHPC as the deck structure reduced top plate tensile stress, deck cracking, and media invasion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, J.K.; Braga, H.B.F.; Mukherjee, A.; Uy, B. Ultrasonic monitoring of corroding bolted joints. Eng. Fail. Anal. 2019, 102, 7–19. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Su, M.Z.; Zhu, C.H.; Wang, M.M. Bearing performance of high-strength bolted corrugated steel longitudinal seams. J. Constr. Steel Res. 2022, 198, 107538. [Google Scholar] [CrossRef]
- Xu, G.N.; Wang, Y.Z.; Du, Y.F.; Zhao, W.S.; Wang, L.Y. Static Strength of Friction-Type High-Strength Bolted T-Stub Connections under Shear and Compression. Appl. Sci. 2020, 10, 3600. [Google Scholar] [CrossRef]
- Li, M.; Yao, L.B.; Zhang, S.S.; Wang, D.P.; He, Z.Q.; Sun, G.H. Study on bolt head corrosion influence on the clamping force loss of high strength bolt. Eng. Fail. Anal. 2021, 129, 105660. [Google Scholar] [CrossRef]
- Kim, T.S.; Lee, H.S.; Yoo, J.H.; Tae, S.H.; Oh, S.H.; Lim, Y.C.; Lee, S.B. Slip Coefficient in High-Strength Bolt Joints Coated with Corrosion-Resistant Zn/Al Metal Spray Method. Mater. Manuf. Process. 2011, 26, 14–21. [Google Scholar] [CrossRef]
- Jiang, C.; Xiong, W.; Cai, C.S.; Zhu, Y.J.; Liu, Z.X. Experimental study on the shear behavior of friction connections with corrosion damage. J. Constr. Steel Res. 2022, 197, 107449. [Google Scholar] [CrossRef]
- Wang, H.L.; Tang, F.J.; Qin, S.F.; Tu, K.Y.; Guo, J.Q. Corrosion-Induced Mechanical Degradation of High-Strength Bolted Steel Connection. J. Mater. Civil. Eng. 2020, 32, 04020203. [Google Scholar] [CrossRef]
- Kim, I.-T.; Lee, J.M.; Huh, J.W.; Ahn, J.-H. Tensile behaviors of friction bolt connection with bolt head corrosion damage: Experimental research B. Eng. Fail. Anal. 2016, 59, 526–543. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Guo, T.M.; Song, Z.T.; Nan, X.L.; Xu, X.J.; Dong, Z.L. Corrosion Behavior of Q345q Steel with Oxide Scale in Simulated Typical Atmospheric Environment in Northwest China. J. Mater. Res. 2019, 33, 749–762. [Google Scholar]
- Li, N.; Zhang, W.F.; Xu, H.; Cai, Y.K.; Yan, X.J. Corrosion Behavior and Mechanical Properties of 30CrMnSiA High-Strength Steel under an Indoor Accelerated Harsh Marine Atmospheric Environment. Materials 2022, 15, 629. [Google Scholar] [CrossRef]
- Křivý, V.; Kubzová, M.; Konečný, P.; Kreislová, K. Corrosion Processes on Weathering Steel Bridges Influenced by Deposition of De-Icing Salts. Materials 2019, 12, 1089. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Allahkaram, S.R.; Kermani, M.B. The effects of temperature and pH on the characteristics of corrosion product in CO2 corrosion of grade X70 steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Felix, D.E.; Li, C.; Wang, C.G.; Dong, J.H.; Ime, U.I.; Chukwuemeka, O.P.; Zhang, D.J.; Zhong, W.A.; Zhong, S. Insights into the characteristics of corrosion products formed on the contact and exposed regions of C1045 steel bolt and nut fasteners exposed to aqueous chloride environments. J. Mater. Sci. Technol. 2023, 135, 250–264. [Google Scholar]
- Wen, J.; Chen, L.; Duan, X.; Yang, J.; Liu, Q.C.; Liu, L. Fracture Failure Performance of 35VB Steel High-Strength Bolts Used in Subtropical Humid Climate. J. Mater. Civil. Eng. 2021, 33, 04021359. [Google Scholar] [CrossRef]
- Yang, X.K.; Zhang, L.W.; Zhang, S.Y.; Zhou, K.; Li, M.; He, Q.Y.; Wang, J.C.; Wu, S.; Yang, H.M. Atmospheric Corrosion Behaviour and Degradation of High-Strength Bolt in Marine and Industrial Atmosphere Environments. Int. J. Electrochem. Sci. 2021, 16, 151015. [Google Scholar]
- Lachowicz Maciej, B.; Lachowicz Marzena, M. Influence of Corrosion on Fatigue of the Fastening Bolts. Materials 2021, 14, 1485. [Google Scholar]
- Jiang, C.; Xiong, W.; Cai, C.S.; Zhou, X.Y.; Zhu, Y.J. Corrosion effects on the fatigue performance of high-strength bolted friction connections. Int. J. Fatigue 2023, 168, 107392. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Yang, F.; Jin, Y.; Hong, S.Z.; Wang, X.Q.; Vu, Q.-V.; Truong, V.-H.; Yu, B.; Kim, S.-E. Experimental study on bearing capacity of corroded high-strength bolt connections under shear force. Constr. Build. Mater. 2021, 309, 125117. [Google Scholar] [CrossRef]
- Goran, V.; Goran, V.; Spiro, I. Tensile strength behaviour of steel plates with corrosion-induced geometrical deteriorations. Ships Offshore Struc. 2022, 17, 2611–2619. [Google Scholar]
- Resen, A.M.; Hanoon, M.M.; Alani, W.K.; Kadhim, A.; Mohammed, A.A.; Gaaz, T.S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Takriff, M.S. Exploration of 8-piperazine-1-ylmethylumbelliferone for application as a corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Corros. Scale Inhib. 2021, 10, 368–387. [Google Scholar]
- Kadhim, A.; Betti, N.; Al-Bahrani, H.A.; Al-Ghezi, M.K.S.; Gaaz, T.; Kadhum, A.H.; Alamiery, A. A mini review on corrosion, inhibitors and mechanism types of mild steel inhibition in an acidic environment. Int. J. Corros. Scale Inhib. 2021, 10, 861–884. [Google Scholar]
- Alamiery, A.; Shaker, L.M.; Gaaz, T.S.; Kadhum, A.H.; Takriff, M.S. A study of acidic corrosion behavior of Furan-Derived schiff base for mild steel in hydrochloric acid environment: Experimental, and surface investigation. Mater. Today. 2020, 44, 2337–2341. [Google Scholar] [CrossRef]
- Eltmimi, A.J.M.; Alamiery, A.; Allami, A.J.; Yusop, R.M.; Kadhum, A.H.; Allami, T. Inhibitive effects of a novel efficient Schiff base on mild steel in hydrochloric acid environment. Int. J. Corros. Scale Inhib. 2021, 10, 441–850. [Google Scholar]
- AlBaghdadi Shaimaa, B.; AlAmiery Ahmed, A.; Gaaz Tayser, S.; Kadhum Abdul Amir, H. Terephthalohydrazide and isophthalo-hydrazide as new corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical approaches. KOM–Corros. Mater. Prot. J. 2021, 65, 12–22. [Google Scholar]
- GB 699-1965; Grade and General Technical Conditions of High-Quality Carbon Structural Steel. National Standards of the People’s Republic of China. Ministry of Metallurgical Industry: Beijing, China, 1965.
- YB (T) 10-1981; Structural Steel for Bridge. Ministry of Metallurgical Industry: Beijing, China, 1981.
- YB6-1971; Technical Conditions for Alloy Structural Steel. Ministry of Metallurgical Industry: Beijing, China, 1971.
- Wen, J.; Wu, Q.R.; Zhang, L.Y.; Zhang, S.; Xiao, Y.J. Research on the Corrosion Products of Fracture Failure High-Strength Bolt Used in Steel Structure Bridge. Surf. Tech. 2021, 50, 321–328. [Google Scholar]

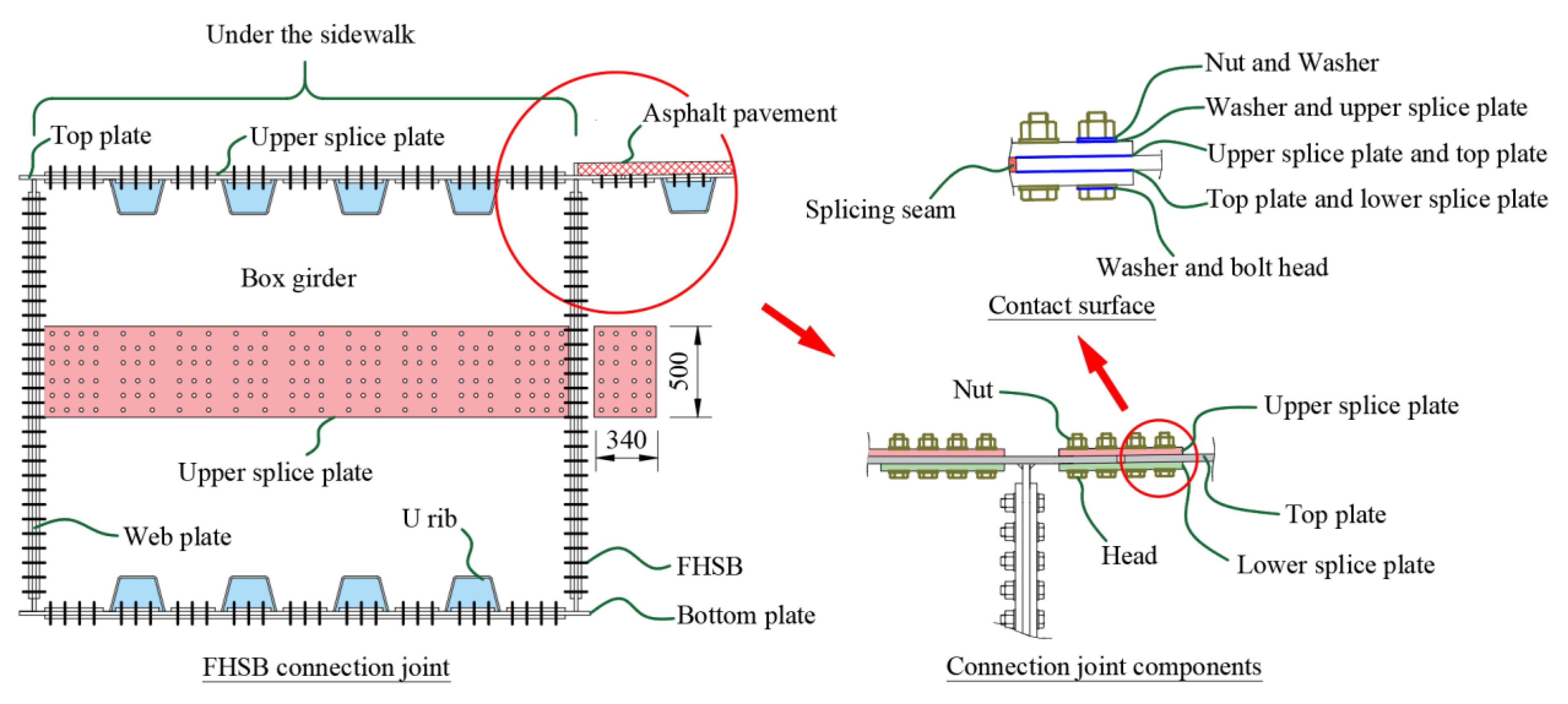
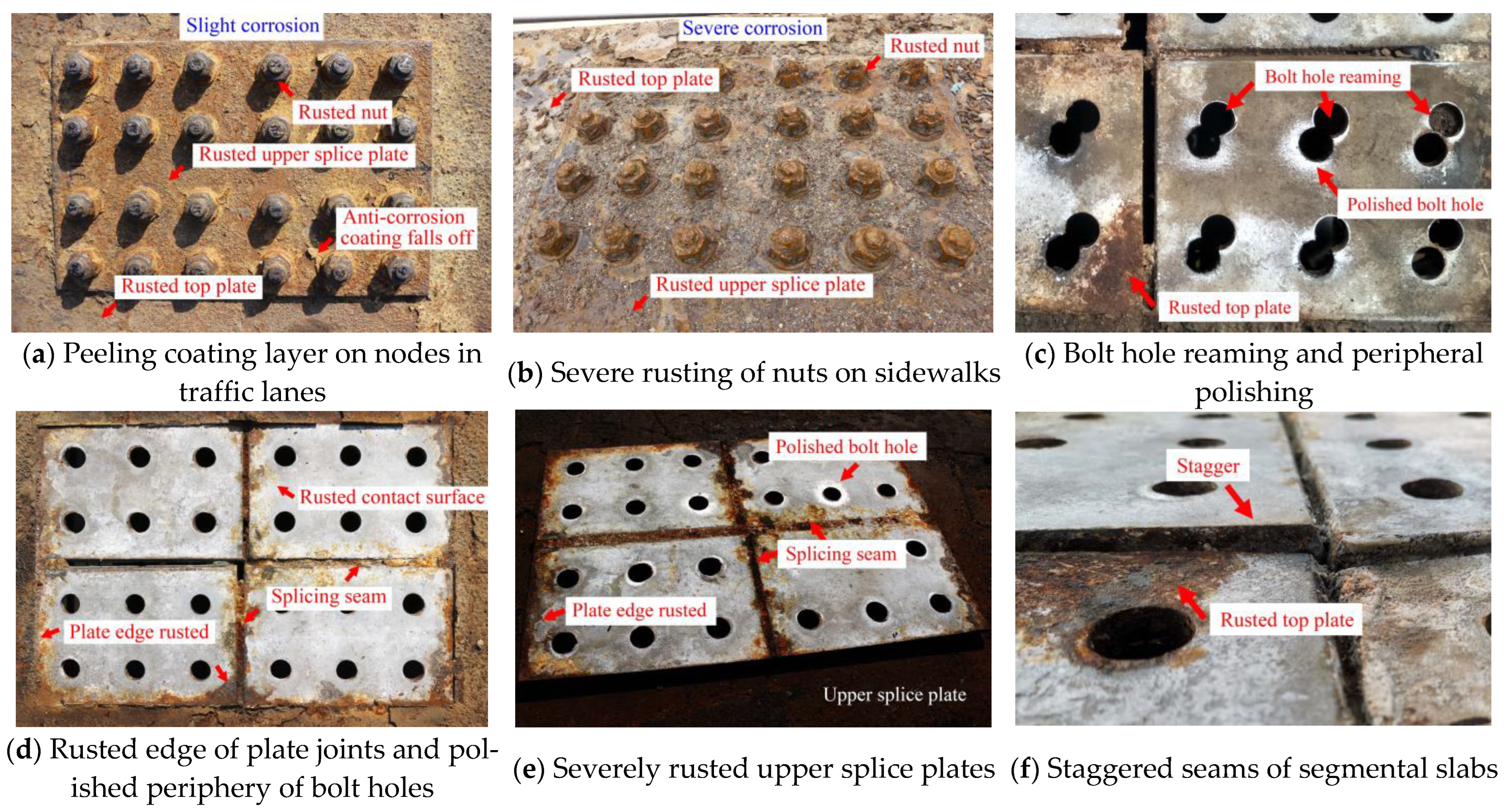
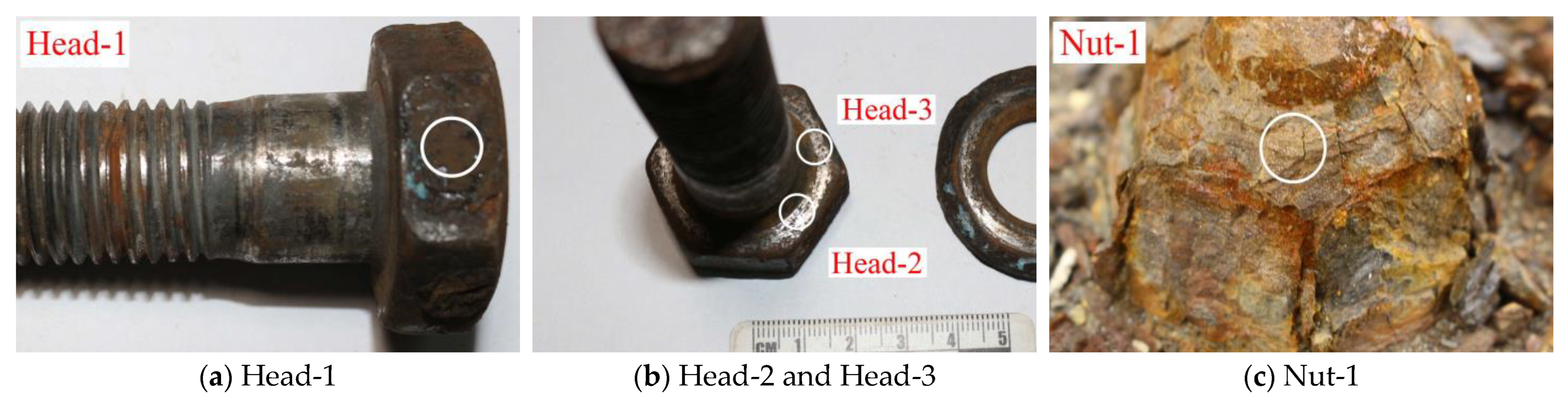
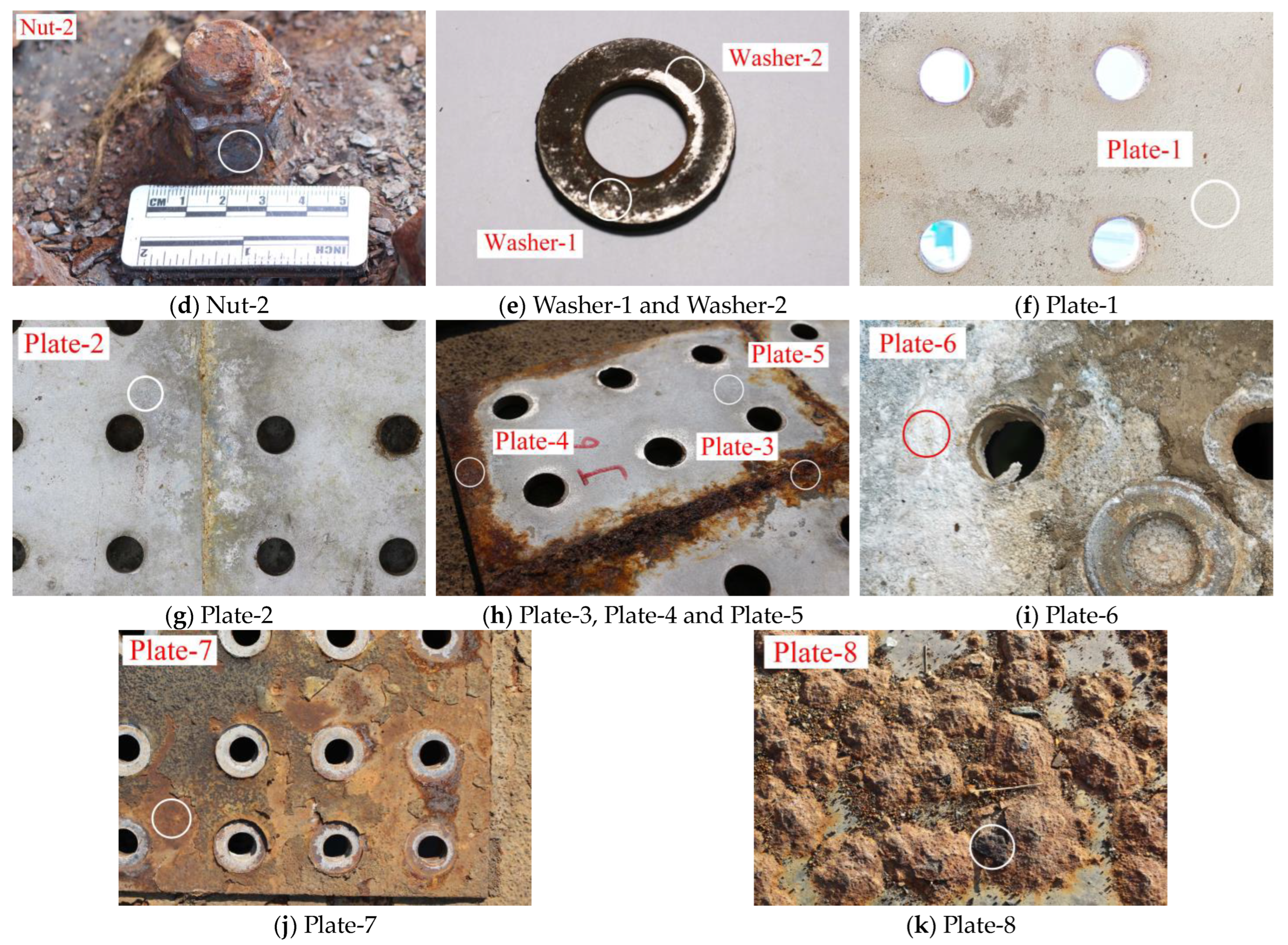

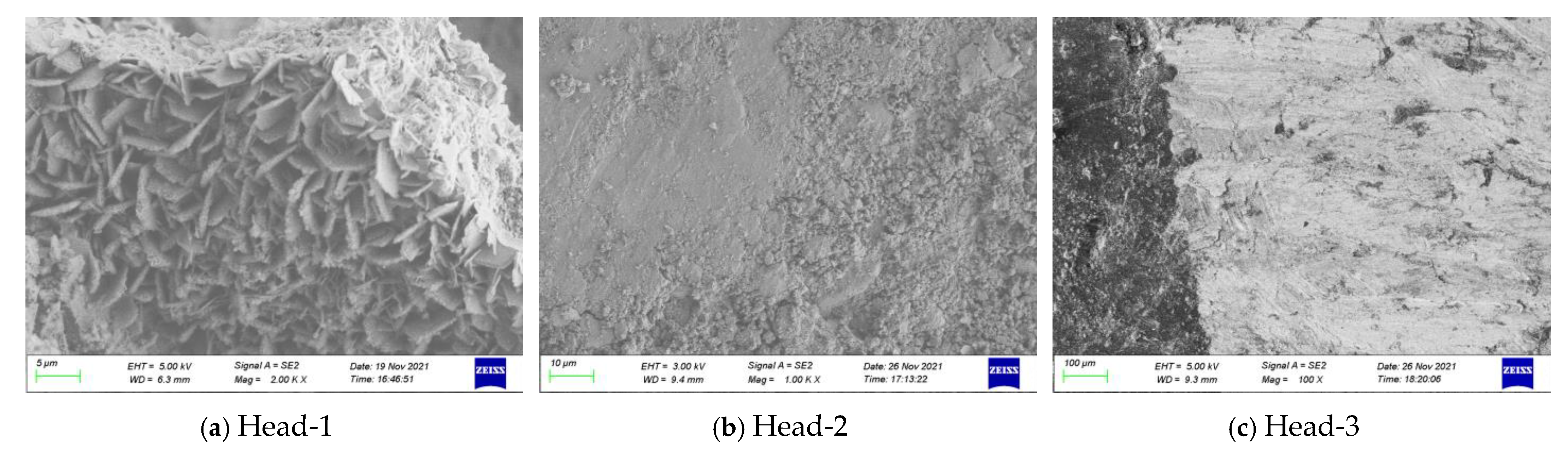
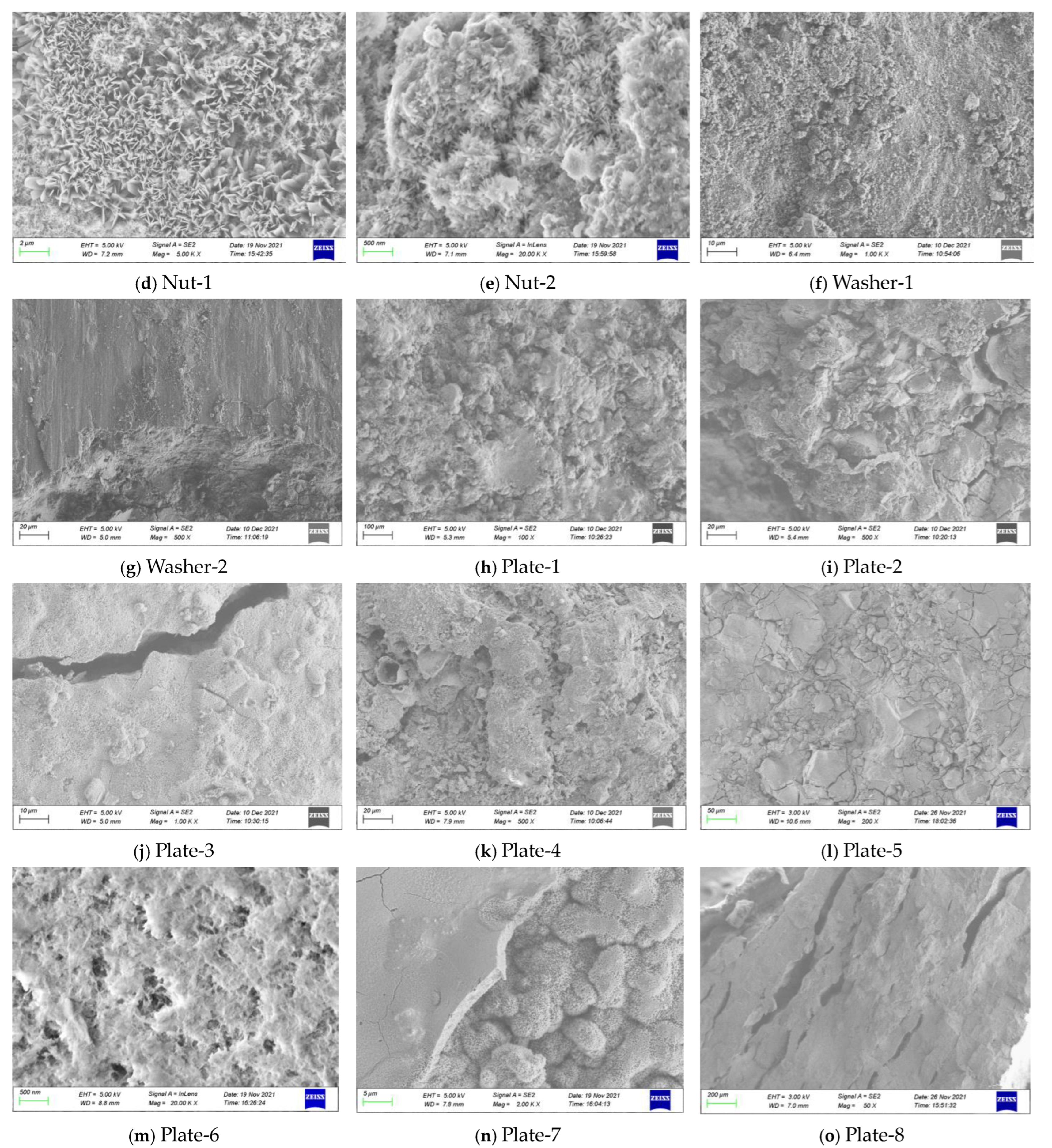
| Joint Components | Specimen ID | Material | Selection Position | Contact Medium | Description of Surface Features |
|---|---|---|---|---|---|
| FHSB connection pair | Head-1 | 40B | Bolt head | Atmosphere | With the anti-corrosion coating falling off, the surface is slightly rusted and red. |
| Head-2 | Washer | No rust is found by naked eye, and local parts are polished and shiny. | |||
| Head-3 | Washer | No rust is found by naked eye, and local polishing is shiny, with obvious boundary. | |||
| Nut-1 | 45 | Nut | Atmosphere | The outer layer of the nut is severely rusted and reddish brown. | |
| Nut-2 | Atmosphere | The inner layer of the nut is severely rusted and brown-black. | |||
| Washer-1 | Washer | Upper splice plate | Slightly rusted, partially polished, and shiny. | ||
| Washer-2 | Upper splice plate | No rust is found with naked eye, and there is obvious boundary. | |||
| Steel plate | Plate-1 | 16Mnq | Lower splice plate | Roof | No rust is found by naked eye, and the aluminum coating is shiny. |
| Plate-2 | Roof | The aluminum coating is slightly rusted and gray. | |||
| Plate-3 | Upper splice plate | Atmosphere | The aluminum spraying layer is severely rusted and reddish brown. | ||
| Plate-4 | Roof | The aluminum spraying layer is severely rusted and reddish brown. | |||
| Plate-5 | Roof | The aluminum coating is slightly rusted and gray. | |||
| Plate-6 | Roof | The aluminum spraying layer is severely rusted and has gray-white powder. | |||
| Plate-7 | Atmosphere | With the anti-corrosion coating falling off, the surface is slightly rusted, and red. | |||
| Plate-8 | Top plate | Atmosphere | Severely rusted, and brown-black. |
| Specimen | Fe | O | C | Mn | S | Mg | Ti | Ca | Al | Si | P | Cl | Zn | K | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | 0.37~0.44 | 0.60~0.90 | 0.20~0.40 | ||||||||||||
| Head-1 | 52.79~93.69 | 3.33~38.84 | 1.70~9.43 | 0.41~0.75 | 0.13~0.55 | 0.36~0.72 | 0.16~0.34 | 0.08~1.10 | 0.20~1.05 | 0.11~0.28 | 0.24~0.36 | 1.11~2.37 | |||
| Head-2 | 41.29~86.89 | 32.62~34.31 | 11.21~18.07 | 0.67~0.83 | 0.19~0.38 | 0.27~0.32 | 0.25~0.94 | 0.23~1.70 | 0.37~2.90 | 0.26~2.62 | 2.34~2.53 | ||||
| Head-3 | 48.62~64.35 | 10.40~32.18 | 8.14~22.56 | 0.45~0.58 | 0.28~1.30 | 0.25~0.34 | 0.94~1.95 | ||||||||
| Standard | 0.42~0.50 | 0.50~0.80 | ≤0.04 | 0.17~0.37 | ≤0.04 | ||||||||||
| Nut-1 | 54.96~60.08 | 36.40~37.27 | 2.97~5.71 | 0.55~1.80 | 0.15~0.27 | 0.17~0.24 | 0.14~0.37 | 0.38~0.51 | 0.85~2.54 | ||||||
| Nut-2 | 57.11~63.11 | 32.79~37.14 | 3.69~5.01 | 0.41~0.74 | 0.14~0.32 | 0.13~0.21 | |||||||||
| Standard | 0.42~0.50 | 0.50~0.80 | ≤0.04 | 0.17~0.37 | ≤0.04 | ||||||||||
| Washer-1 | 6.01~26.69 | 14.23~28.51 | 53.50~54.51 | 0.23~1.38 | 0.51~0.86 | 0.87~2.61 | 0.53~2.37 | 0.57~1.87 | 0.42~1.75 | 0.39~1.99 | 0.11~1.46 | 0.30~0.40 | 0.46~1.28 | ||
| Washer-2 | 14.89~25.26 | 17.53~24.53 | 44.43~56.46 | 0.39~0.79 | 0.26~0.54 | 0.28~2.32 | 3.01~4.56 | 0.76~2.29 | 0.16~0.53 | ||||||
| Standard | ≤0.20 | 1.20~1.35 | ≤0.004 | 1.20~1.35 | ≤0.015 | ||||||||||
| Plate-1 | 3.51~3.99 | 3.93~5.86 | 0.18~0.64 | 88.09~90.05 | 0.35~0.84 | ||||||||||
| Plate-2 | 6.52~15.62 | 7.92~21.21 | 1.64~4.30 | 0.44~0.65 | 0.08~0.13 | 1.25~2.00 | 11.31~42.51 | 2.76~3.61 | 0.73~1.12 | ||||||
| Plate-3 | 42.42~88.59 | 7.19~46.19 | 2.87~6.27 | 0.24~0.31 | 0.32~1.43 | 0.37~1.75 | 0.98~5.13 | 0.83~1.29 | |||||||
| Plate-4 | 25.16~56.62 | 23.88~35.11 | 4.64~5.63 | 0.61~2.34 | 0.42~0.94 | 0.41~0.84 | 1.32~2.41 | 1.20~3.24 | 0.58~1.17 | 0.78~1.77 | 0.78~1.37 | 0.53~1.95 | 0.99~4.99 | 0.56~2.03 | |
| Plate-5 | 0.85~4.95 | 34.38~54.53 | 4.71~10.83 | 0.16~0.61 | 0.29~0.79 | 0.15~0.26 | 0.42~3.45 | 5.10~39.09 | 0.34~2.02 | 0.12~0.16 | 1.24~3.65 | 0.28~1.16 | 0.13~4.10 | ||
| Plate-6 | 54.33~62.83 | 4.61~7.49 | 0.57~0.81 | 1.88~2.35 | 26.74~36.72 | 0.26~1.42 | |||||||||
| Plate-7 | 67.81~87.48 | 7.94~26.31 | 1.36~3.48 | 0.43~1.40 | 0.18~0.22 | 0.17~1.27 | 0.35~1.64 | 0.35~2.36 | 0.67~1.62 | ||||||
| Plate-8 | 61.56~80.96 | 16.52~32.86 | 1.82~4.34 | 0.60~1.73 | 0.10~0.82 | 0.15~0.21 | 1.42~3.05 | ||||||||
| Soil | 0.96~2.08 | 45.31~51.02 | 8.84~10.95 | 0.36~0.53 | 0.44~3.29 | 0.84~1.50 | 23.62~32.84 | 0.41~4.33 | 2.24~4.62 |
| Specimen | Contact Medium | Products | ||
|---|---|---|---|---|
| This Study | Ref. [9] | Ref. [28] | ||
| Head | Atmosphere | Fe2O3, FeS, SiO2 | / | / |
| Nut | Atmosphere | Fe2O3, MnO2, FeOOH, SiO2 | / | / |
| Washer | Interface | Fe2O3, Fe3O4, SiO2 | / | / |
| Plate | Interface | Al2O3, Fe3O4, SiO2 | / | / |
| Atmosphere | Al2O3, Fe2O3, Fe3O4, Mn2O3, FeOOH, SiO2 | Fe2O3, Fe3O4, FeOOH | Fe3O4, FeOOH | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Xu, W.; Dong, X.; Fan, S.; Wang, X. Characteristics of Corrosion Products of Friction-Type High-Strength Bolted Joints of Steel Bridge: A Case Study. Coatings 2023, 13, 1023. https://doi.org/10.3390/coatings13061023
Xu G, Xu W, Dong X, Fan S, Wang X. Characteristics of Corrosion Products of Friction-Type High-Strength Bolted Joints of Steel Bridge: A Case Study. Coatings. 2023; 13(6):1023. https://doi.org/10.3390/coatings13061023
Chicago/Turabian StyleXu, Gangnian, Wenpeng Xu, Xu Dong, Shengwei Fan, and Xianggang Wang. 2023. "Characteristics of Corrosion Products of Friction-Type High-Strength Bolted Joints of Steel Bridge: A Case Study" Coatings 13, no. 6: 1023. https://doi.org/10.3390/coatings13061023
APA StyleXu, G., Xu, W., Dong, X., Fan, S., & Wang, X. (2023). Characteristics of Corrosion Products of Friction-Type High-Strength Bolted Joints of Steel Bridge: A Case Study. Coatings, 13(6), 1023. https://doi.org/10.3390/coatings13061023




