Effect of Glymo on the Morphological and Optical Properties of Eu3+-Doped Lu2SiO5 Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Films
2.2. Preparation of Powders
2.3. Characterization
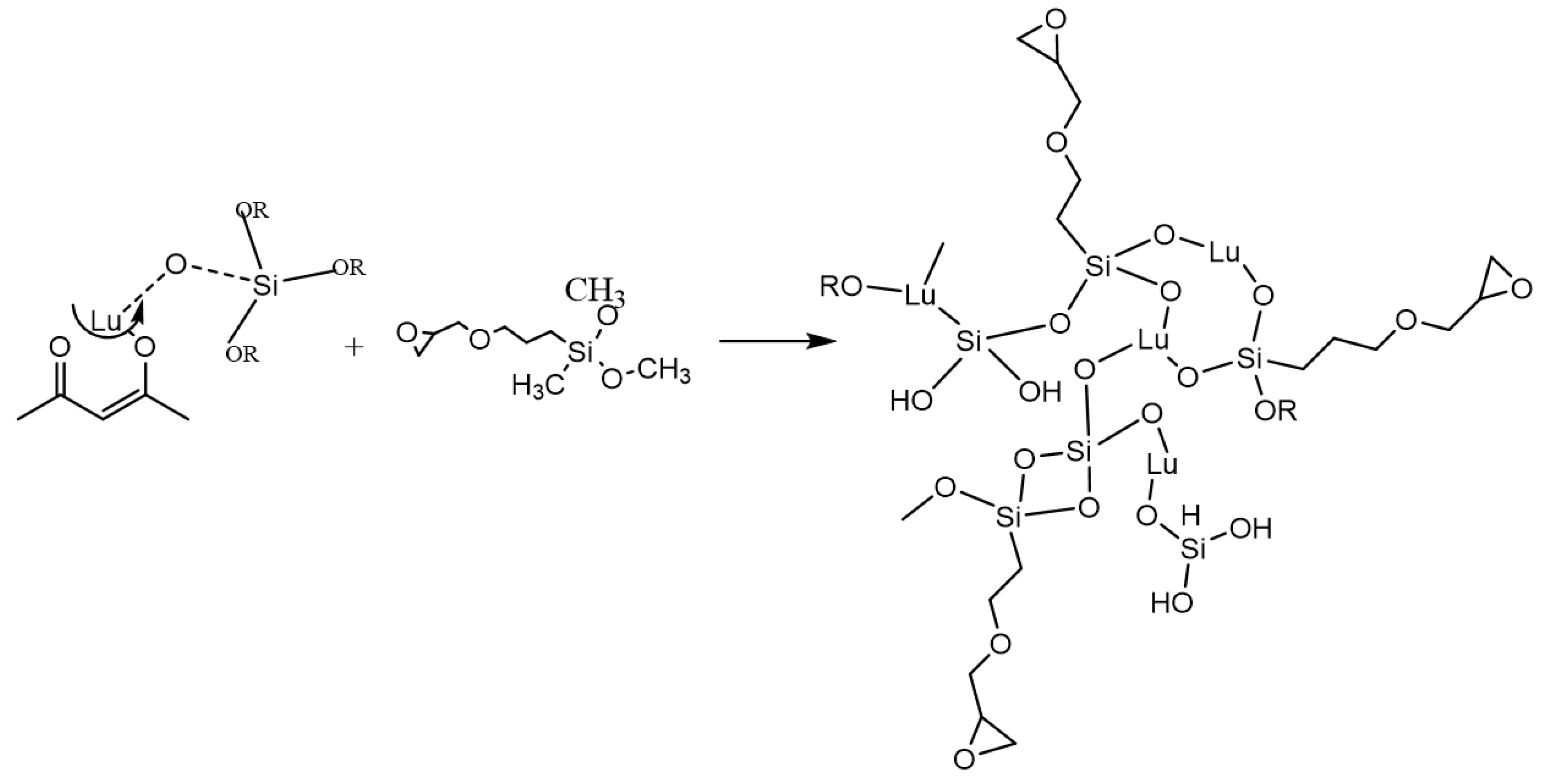
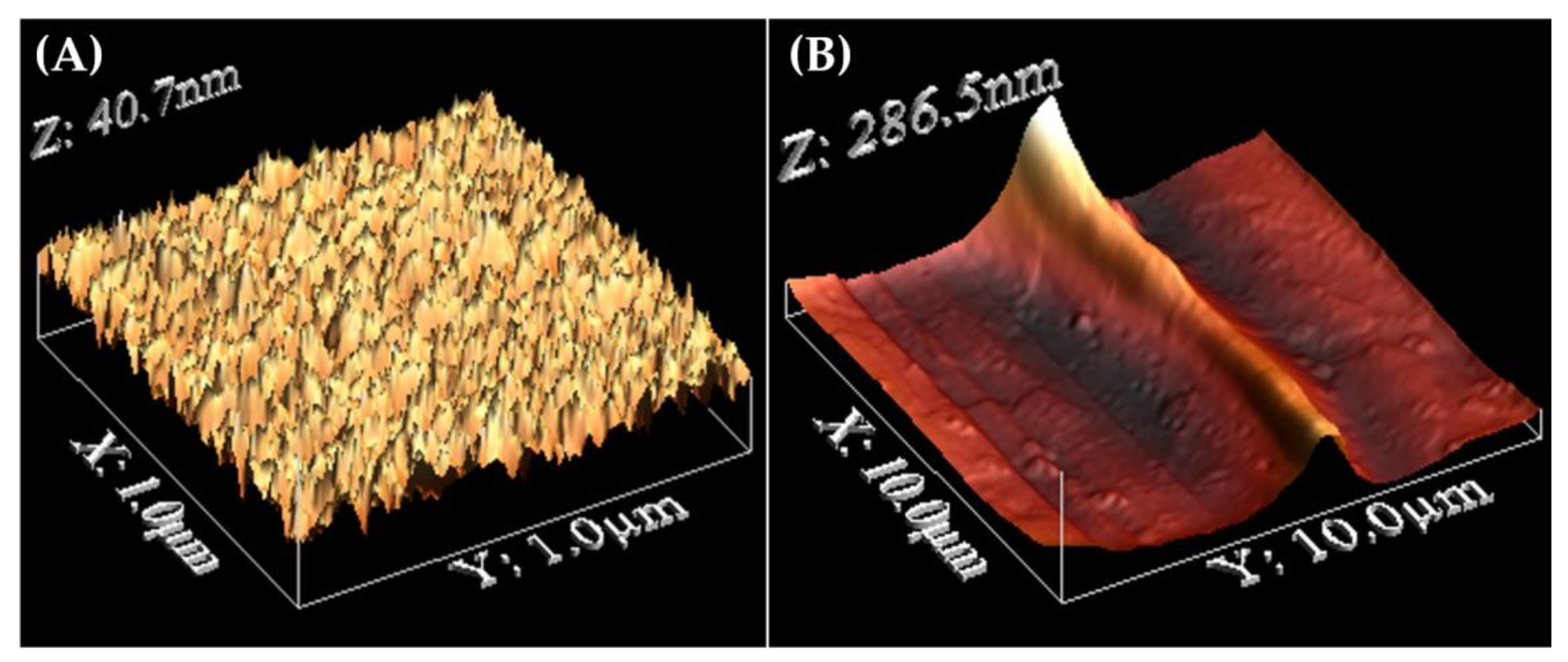
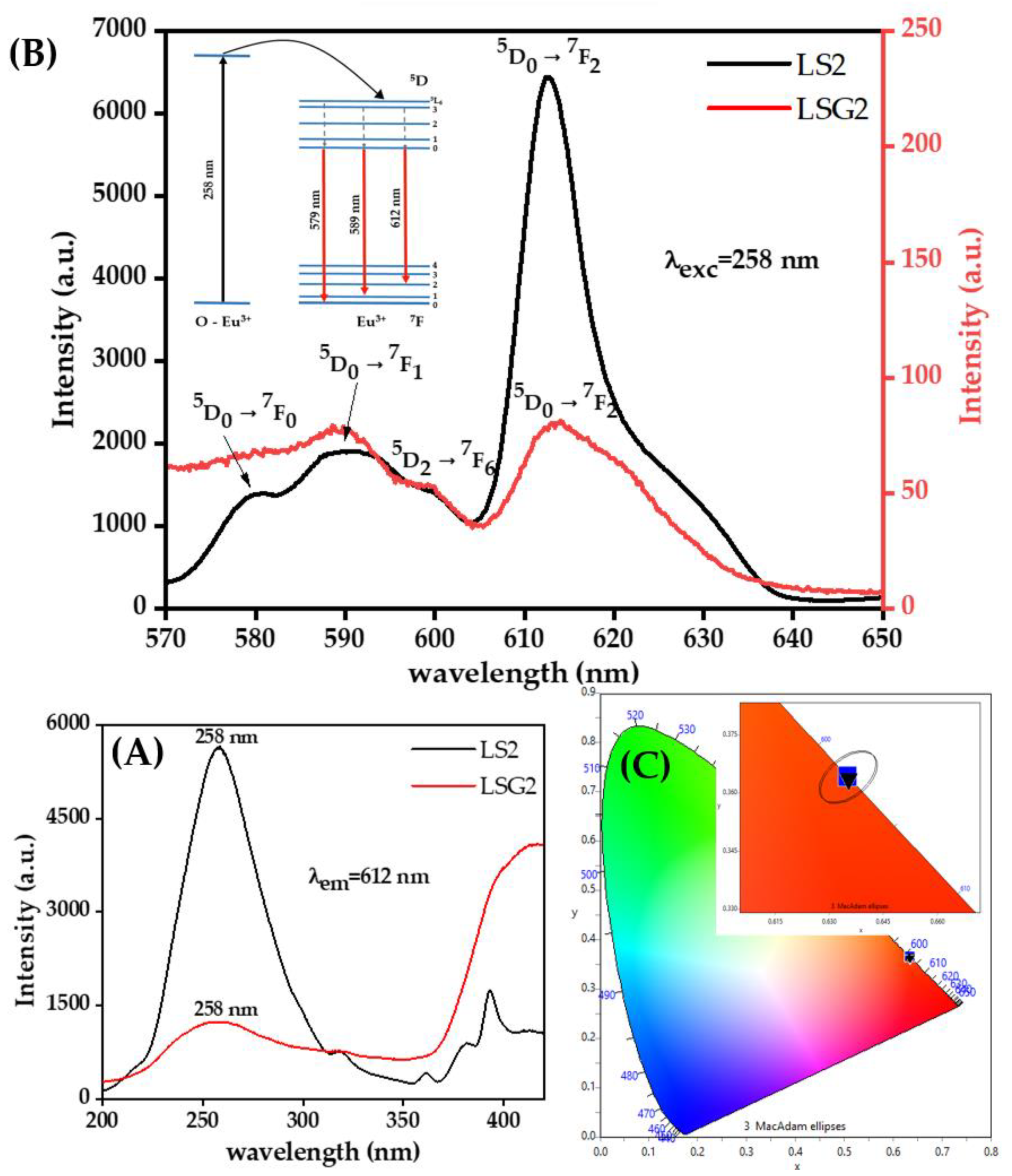
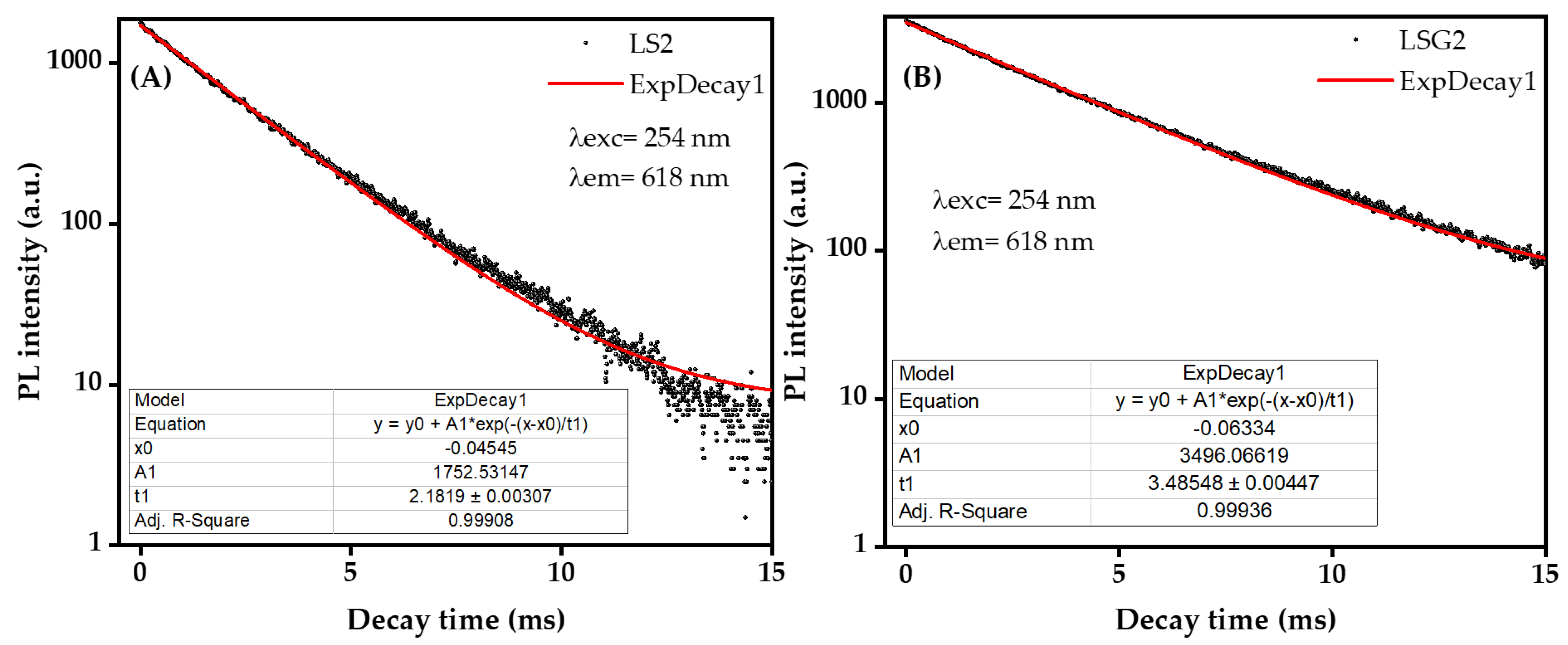
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Sahu, K.; Singh, R.; Som, T.; Kotnala, R.K.; Mohapatra, S. Thermal annealing induced strong photoluminescence enhancement in Ag-TiO2 plasmonic nanocomposite thin films. J. Alloys Compd. 2019, 786, 750–757. [Google Scholar] [CrossRef]
- Meng, Q.; Lin, J.; Fu, L.; Zhang, H.; Wang, S.; Zhou, Y. Sol-gel deposition of calcium silicate red-emitting luminescent films doped with Eu3+. J. Mater Chem. 2001, 11, 3382–3386. [Google Scholar] [CrossRef]
- Shen, S.Q.; Xu, Z.B.; Ma, Q.; Xie, J.J.; Shi, Y.; Xu, J.Y.; Ai, F. Pechini sol-gel fabrication and luminescent properties of Lu2SiO5:Ln3+ (Ln = Tb,Ce) thin films. Appl. Mech. Mater. 2011, 84–85, 631–634. [Google Scholar] [CrossRef]
- Melcher, C.; Schweitzer, J. Cerium-doped Lutetium Oxyorthosilicate: A Fast, Efficient New Scintillator. IEEE Trans. Nucl. Sci. 1992, 39, 502–505. [Google Scholar] [CrossRef]
- Kitaura, M.; Tanaka, S.; Itoh, M. Optical properties and electronic structure of Lu2SiO5 crystals doped with cerium ions: Thermally-activated energy transfer from host to activator. J. Lumin. 2015, 158, 226–230. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Moszynski, M. Scintillation Properties of Lu3Al5O12, Lu2SiO5 and LaBr3 Crystals Activated with Cerium. Phys. Procedia 2011, 22, 218–226. [Google Scholar] [CrossRef]
- Dominiak-Dzik, G.; Ryba-Romanowski, W.; Lisiecki, R.; Solarz, P.; Berkowski, M. Dy-doped Lu2SiO5 single crystal: Spectroscopic characteristics and luminescence dynamics. Appl. Phys. B 2010, 99, 285–297. [Google Scholar] [CrossRef]
- Mansuy, C.; Tomasella, E.; Mahiou, R.; Grimblot, J.; Nedelec, J.-M. Surface characterization of sol-gel derived scintillating rare-earth doped Lu2SiO5 thin films. J. Physics Conf. Ser. 2008, 100, 012037. [Google Scholar] [CrossRef]
- Cooke, D.; Muenchausen, R.; McClellan, K.; Bennett, B. Spectral emission of rare-earth doped Lu2SiO5 single crystals. Opt. Mater. 2005, 27, 1781–1786. [Google Scholar] [CrossRef]
- Mansuy, C.; Leroux, F.; Mahiou, R.; Nedelec, J.M. Preferential site substitution in sol-gel derived Eu3+ doped Lu2SiO5: A combined study by X-ray absorption and luminescence spectroscopies. J. Mater. Chem. 2005, 15, 4129–4135. [Google Scholar] [CrossRef]
- Yun, P.; Shi, Y.; Zhou, D.; Xie, J. Hydrothermal synthesis of Ce:Lu2SiO5 scintillator powders. J. Rare Earths 2009, 27, 801–805. [Google Scholar] [CrossRef]
- Lee, J.-K.; Muenchausen, R.E.; Jia, Q.X.; Nastasi, M.; Valdez, J.A.; Bennett, B.L.; Cooke, D.W.; Lee, S.Y. Structure and optical properties of Lu2SiO5:Ce phosphor thin films. Appl. Phys. Lett. 2006, 89, 101905. [Google Scholar] [CrossRef]
- Felsche, J. The Crystal Chemistry of the Rare-Earth Silicates; Springer: Berlin/Heidelberg, Germany, 1973; pp. 99–197. [Google Scholar]
- Lu, Q.; Liu, Q.; Wei, Q.; Liu, G.; Zhuang, J. Preparation and characterization of Lu2SiO5:Ce3+ luminescent ceramic fibers via electrospinning. Ceram. Int. 2013, 39, 8159–8164. [Google Scholar] [CrossRef]
- Fan, L.; Lin, D.; Zhang, X.; Shi, Y.; Zhang, J.; Xie, J.; Lei, F.; Zhang, L. Local structures of Lu atoms in a core-shell approach for synthesis of Lu2SiO5 phase. Chem. Phys. Lett. 2016, 644, 41–44. [Google Scholar] [CrossRef]
- Jota, M.L.C.; Hernández, M.G.; Murillo, A.G.; Romo, F.D.J.C.; Becerril, E.R.; Ramírez, A.D.J.M.; Enrriquez, H.D.; De La Rosa Cruz, E. Synthesis of Lu2O3:Eu3+ Luminescent Ceramic Powder Embedded in SiO2 Matrix. Mater. Trans. 2014, 55, 1867–1871. [Google Scholar] [CrossRef]
- Jota, M.C.; Murillo, A.G.; Romo, F.C.; Hernández, M.G.; Ramírez, A.D.J.M.; Velumani, S.; Cruz, E.D.L.R.; Kassiba, A. Lu2O3:Eu3+ glass ceramic films: Synthesis, structural and spectroscopic studies. Mater. Res. Bull. 2014, 51, 418–425. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Cao, G.; Kim, K.-N. Prepration and photoluminescence properties of Ce doped lutetium silicate nanopowders by sol-gel method. Curr. Appl. Phys. 2011, 11, S309–S312. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non-Crystalline Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Bahtat, M.; Mugnier, J.; Lou, L.; Bovier, C.; Serughetti, J.; Genet, M. La Spectroscopie Raman Tres Basse Frequence Utilisee Pour La Caracterisation Structurale De Guides D ’Ondes Plans. J. Opt. 1992, 23, 215–222. [Google Scholar] [CrossRef]
- Bishnoi, A.; Kumar, S.; Joshi, N. Wide-Angle X-ray Diffraction (WXRD). Microscopy Methods in Nanomaterials Characterization; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Khamis, F.; Arafah, D.E. Thermoluminescence Characteristics of Natural Quartz and Synthesized Silica Glass Prepared by Sol-Gel Technique. Asian J. Phys. Chem. Sci. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Arunkumar, P.; Ramaseshan, R.; Dash, S.; Babu, K.S. Tunable transport property of oxygen ion in metal oxide thin film: Impact of electrolyte orientation on conductivity. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Liu, H.; Wan, D.; Ishaq, A.; Chen, L.; Guo, B.; Shi, S.; Luo, H.; Gao, Y. Sputtering Deposition of Sandwich-Structured V2O5/Metal (V, W)/V2O5 Multilayers for the Preparation of High-Performance Thermally Sensitive VO2 Thin Films with Selectivity of VO2 (B) and VO2 (M) Polymorph. ACS Appl. Mater. Interfaces 2016, 8, 7884–7890. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J. Preparation and molten salt corrosion research of composite environmental barrier coatings of Lu2Si2O7 and Lu2SiO2. Mater. Res. Innov. 2014, 18, S4958–S4962. [Google Scholar] [CrossRef]
- Xie, J.; Shi, Y.; Fan, L.; Xu, Z. Microstructure and luminescent properties of Ce:Lu2SiO5 ceramic scintillator by spark plasma sintering. Opt. Mater. 2013, 35, 744–747. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Chu, B. Synthesis and characterization of Ce-doped Lu2SiO5 powders by the solid-state reaction with Li2SO4 flux. J. Alloys Compd. 2009, 479, 704–706. [Google Scholar] [CrossRef]
- Fan, L.; Shi, Y.; Xu, J.; Xie, J.; Lei, F. Consolidation of translucent Ce3+-doped Lu2SiO5 scintillation ceramics by pressureless sintering. J. Mater. Res. 2014, 29, 2252–2259. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Chen, S.; Gu, M.; Ni, C.; Liu, B.; Huang, S. Luminescence properties of Li-codoped Lu2SiO5:Ce thin-film phosphors prepared by sol-gel processing. Mater. Res. Bull. 2013, 48, 2370–2374. [Google Scholar] [CrossRef]
- Mansuy, C.; Dujardin, C.; Mahiou, R.; Nedelec, J.M. Characterization and scintillation properties of sol-gel derived Lu2SiO5:Ln3+ (Ln = Ce, Eu and Tb) powders. Opt. Mater. 2009, 31, 1334–1336. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Chen, X.; Fan, L.; Lin, D.; Wang, Y.; Shi, Y. Fabrication and luminescence properties of polycrystalline Pr3+-doped Lu2SiO5 thin films by sol-gel method. J. Alloys Compd. 2016, 656, 735–739. [Google Scholar] [CrossRef]
- Hoefdraad, H.E. The charge-transfer absorption band of Eu3+ in oxides. J. Solid. State Chem. 1975, 14, 217. [Google Scholar]
- Rajagukguk, J.; Kaewkhao, J.; Djamal, M.; Hidayat, R.; Suprijadi; Ruangtaweep, Y. Structural and optical characteristics of Eu3+ ions in sodium-lead-zinc-lithium-borate glass system. J. Mol. Struct. 2016, 1121, 180–187. [Google Scholar] [CrossRef]
- Shangda, X.; Yimin, C. Effect of J-mixing on the intensities of f-f transitions of the rare earth ions. J. Lumin. 1984, 32, 204–206. [Google Scholar] [CrossRef]
- Souza, A.; Santos, M. The J-mixing effect in Ln3+ ions crystal field levels. Chem. Phys. Lett. 2012, 521, 138–141. [Google Scholar] [CrossRef]
- Sakthivel, T.; Sun, L.; Devakumar, B.; Li, B.; Huang, X. Novel high-efficiency Eu3+-activated Na2Gd2B2O7 red-emitting phosphors with high color purity. RSC Adv. 2018, 8, 32948–32955. [Google Scholar] [CrossRef]
- Nixon, M.S.; Aguado, A.S. Appendix 4: Color Images. Feature Extraction & Image Processing for Computer Vision; Elsevier Ltd.: Amsterdam, The Netherlands, 2012. [Google Scholar]
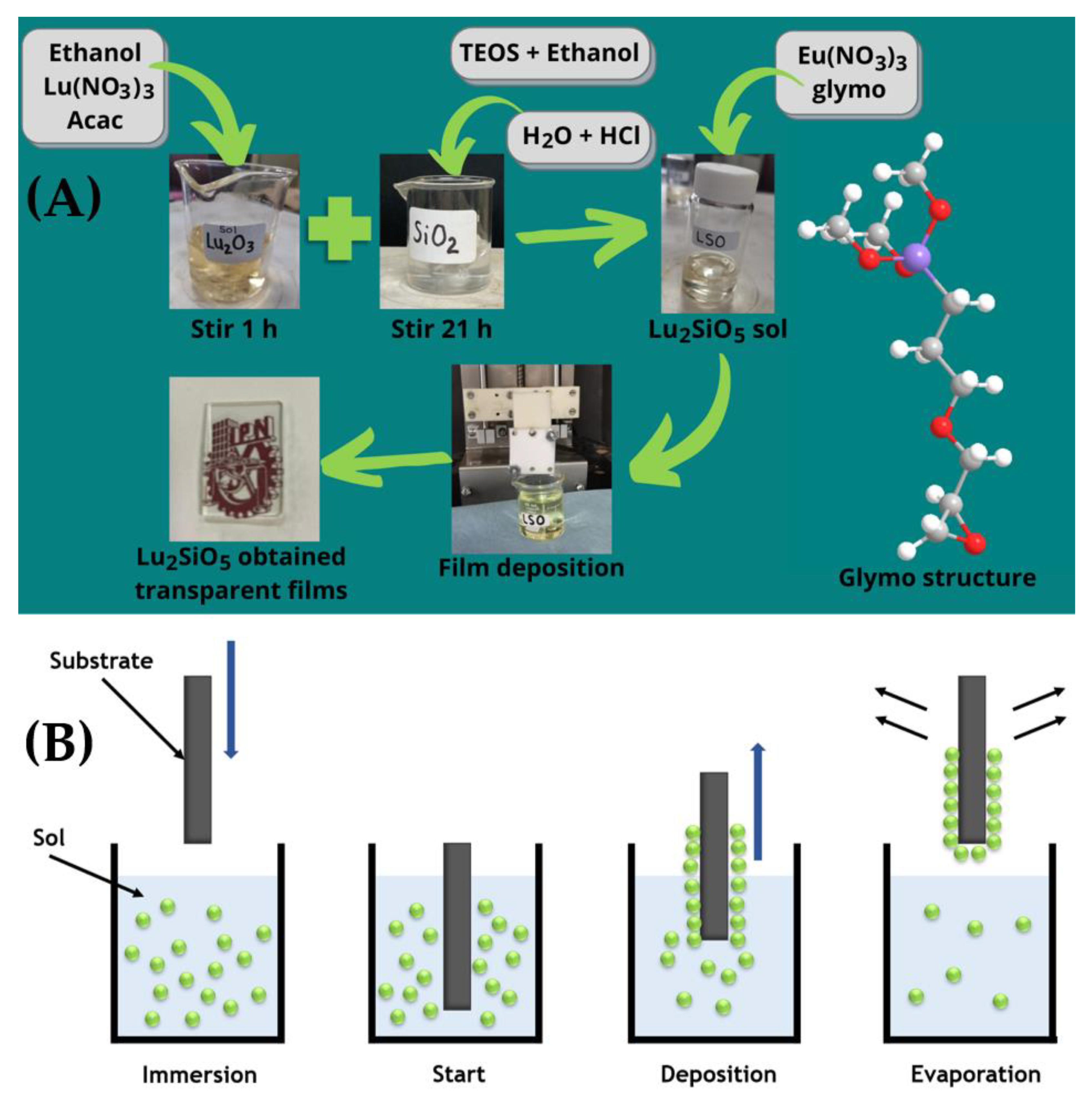
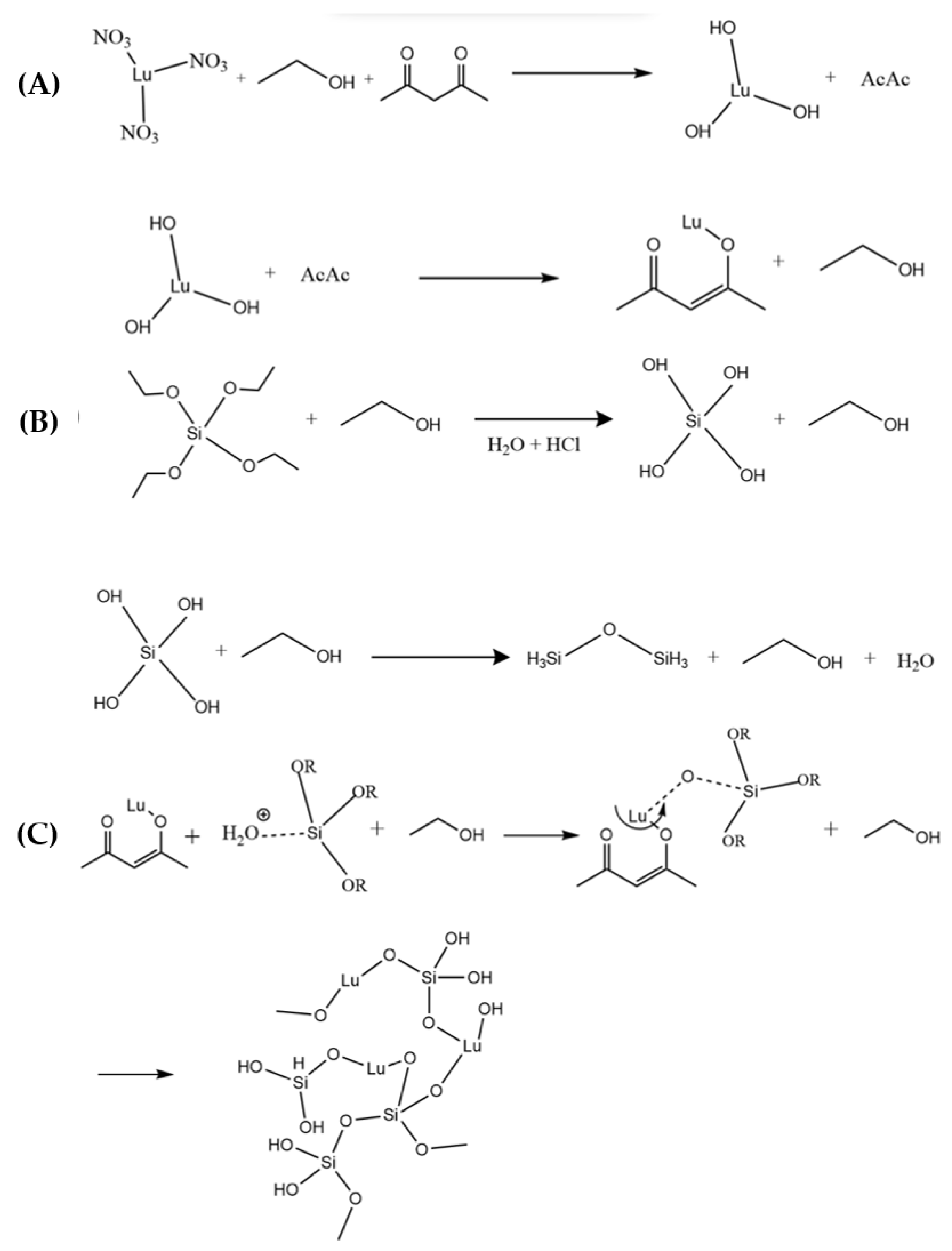
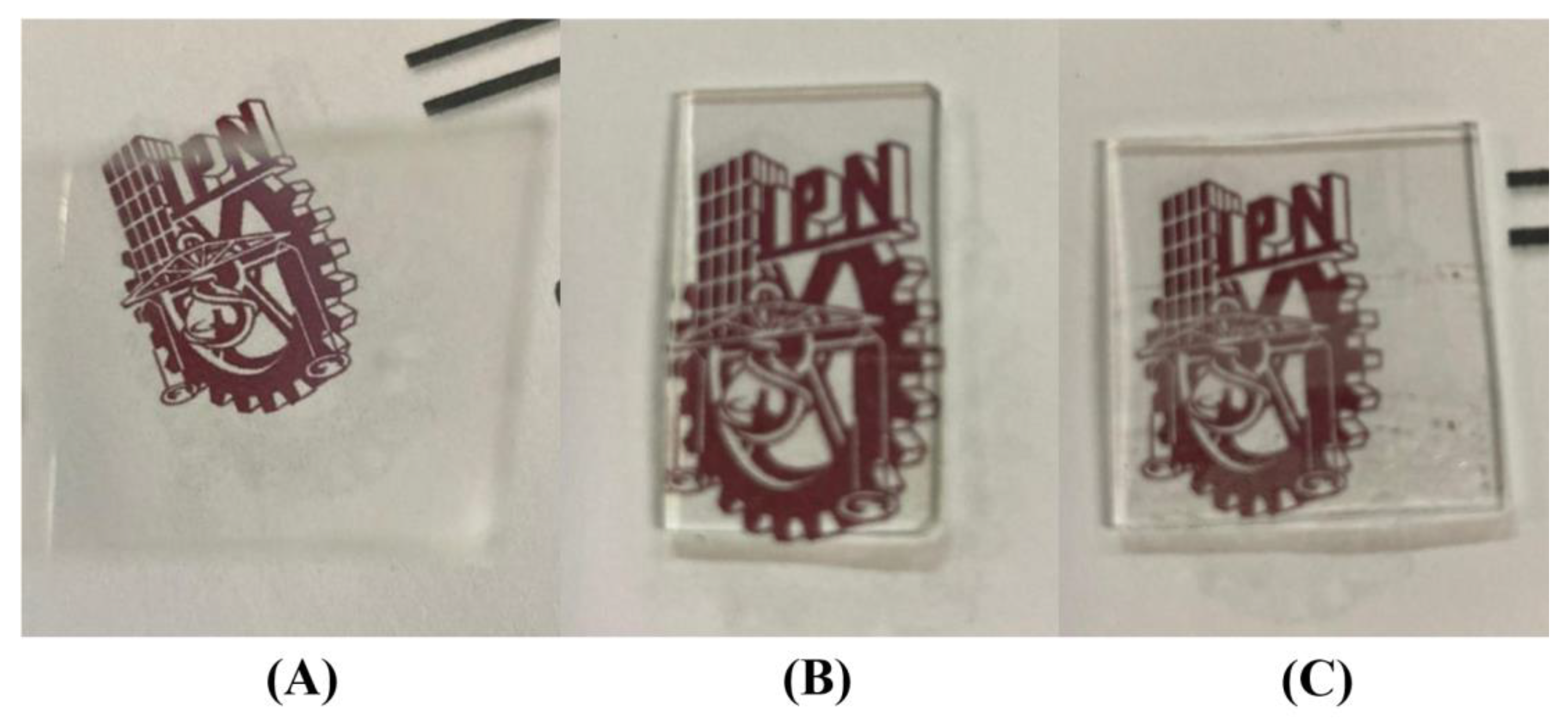
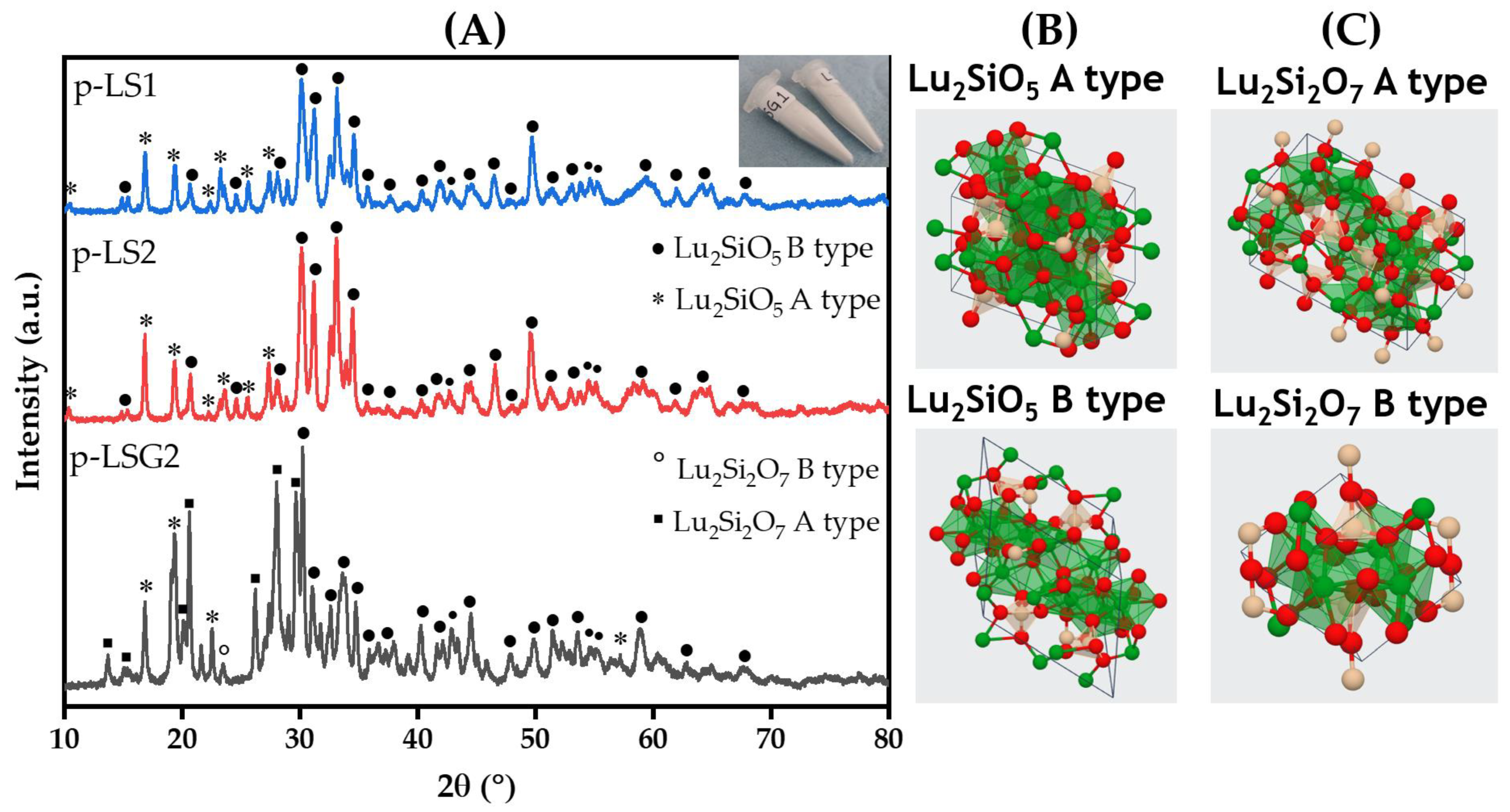


| Type | Matrix | Sample | Eu3+ (%Mol) | Glymo:Lu2SiO5 (Molar Ratio) | # Dips |
|---|---|---|---|---|---|
| Film | Lu2SiO5 | LS1 | - | - | 5 |
| Film | Lu2SiO5 | LS2 | 5 | - | 5 |
| Film | Lu2SiO5 | LSG2 | 5 | 1.3 | 5 |
| Powder | Lu2SiO5 | p-LS1 | - | - | - |
| Powder | Lu2SiO5 | p-LS2 | 5 | - | - |
| Powder | Lu2SiO5 | p-LSG2 | 5 | 1.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancino-Moreno, A.D.; López-Marure, A.; Luna-Domínguez, J.H.; Morales-Ramírez, Á.d.J.; Ortega-Avilés, M.; Álvarez-Chávez, J.A.; García-Hernández, M. Effect of Glymo on the Morphological and Optical Properties of Eu3+-Doped Lu2SiO5 Films. Coatings 2023, 13, 915. https://doi.org/10.3390/coatings13050915
Cancino-Moreno AD, López-Marure A, Luna-Domínguez JH, Morales-Ramírez ÁdJ, Ortega-Avilés M, Álvarez-Chávez JA, García-Hernández M. Effect of Glymo on the Morphological and Optical Properties of Eu3+-Doped Lu2SiO5 Films. Coatings. 2023; 13(5):915. https://doi.org/10.3390/coatings13050915
Chicago/Turabian StyleCancino-Moreno, Andrea Danielle, Arturo López-Marure, Jorge Humberto Luna-Domínguez, Ángel de Jesús Morales-Ramírez, Mayahuel Ortega-Avilés, José Alfredo Álvarez-Chávez, and Margarita García-Hernández. 2023. "Effect of Glymo on the Morphological and Optical Properties of Eu3+-Doped Lu2SiO5 Films" Coatings 13, no. 5: 915. https://doi.org/10.3390/coatings13050915
APA StyleCancino-Moreno, A. D., López-Marure, A., Luna-Domínguez, J. H., Morales-Ramírez, Á. d. J., Ortega-Avilés, M., Álvarez-Chávez, J. A., & García-Hernández, M. (2023). Effect of Glymo on the Morphological and Optical Properties of Eu3+-Doped Lu2SiO5 Films. Coatings, 13(5), 915. https://doi.org/10.3390/coatings13050915









