Abstract
The superatomic structure of film-forming sols obtained by the acid hydrolysis of tetraethoxysilane (TEOS) in an aqueous medium (free of organic solvents) was studied using the SAXS method. The formation of nanoparticles (NPs) was confirmed in alcohol-free silica sols with both a low (1 vol. %) content of TEOS and a high (10 vol. %) content of TEOS, hydrolyzed in an aqueous-alcoholic medium. A trimodal size distribution was revealed for the resulting NPs, with radii ranging from less than 1 nm to ~11 nm. The volume fraction of NPs tends to grow with increases in TEOS concentration, as well as with the introduction of magnetic NPs of iron oxides into silica sols. The synthesized silica sols and suspensions based on silica sols with FexOy NPs were used for the pre-sowing treatment of white and cauliflower cabbage seeds in order to provide a functional coating on their surfaces, thereby improving seed germination, stimulating their growth in the early stages of development, and suppressing the effect of phytopathogens. The effect of the pre-sowing seed treatment in sol-gel compositions on seed germination and the growth characteristics of plant seedlings is analyzed, including the influence of iron-oxide magnetic NPs’ compositions and concentrations in silica sols.
1. Introduction
Pre-sowing seed treatment is one of the ways to increase the productivity of agricultural crops, providing a positive effect on plants, starting from the early stages of their development [1,2,3]. This treatment is also important to prevent the loss of seeds and the deterioration of their properties during storage [1,4,5]. A promising research direction in this field is the search for new methods and approaches for seed treatment in order to stimulate plants’ growth and increase their resistance to phytopathogens or protect against pests during seed storage.
In particular, such techniques as pellets, encrusting or film coatings are successfully used for this purpose [1,4,5,6]. Thin film coatings are commonly obtained on the basis of film-forming polymers (poly(vinyl alcohol), poly(vinylpyrrolidone, polyethylene glycol, ethyl cellulose, cellulose diacetate, etc.), hydrogels based on hydrophilic polymers, and swelling in water, as well as biopolymers and the products of their processing (alginates, chitosan, gelatin, pectin, guar gum, etc.) [2,6,7]. The use of biodegradable biopolymers in agrotechnology is very promising. They can also be used to encapsulate plant-biocontrol bacteria. The presence of microorganisms beneficial to plants on the roots and leaves of plants prevents the development of phytopathogens and protects against pests [8,9].
Quite recently, nanotechnologies were introduced for seed treatment, including the use of biologically active nanoparticles (NPs), e.g., SiO2, TiO2, ZnO, FeOx, etc. [6,10,11,12,13,14], as well as electrospun polymer nanocomposite membrane [6,14,15,16]. The NPs provide a dual effect involving the protection of seeds from pests during storage and increases in seed germination, particularly under abiotic stress. Nanofiber polymers (e.g., polyvinyl alcohol, polyvinylpyrrolidone, cellulose diacetate, etc.) compare favorably with monolithic polymer coatings, enabling water and gas exchange, the controlled release of nutrients from the fiber scaffolds, and the enhancement of seeds’ ability to sprout [6,14,15].
Of particular interest for applications in agricultural technologies are silicon compounds and silicon-containing preparations, which have a positive effect on the growth, development, yield, and quality of various cultivated plants. Recently, much attention has been paid to the use of amorphous SiO2 NPs for pre-sowing seed treatment [13]. In particular, in [17], synthetic amorphous silica (based on 50 mg SiO2 per gram of seeds) was demonstrated to promote a stimulating effect on the seed germination, growth parameters, and physiological parameters of peas, corn, and cucumbers, and silica sol based on TEOS (the concentration is 2 g TEOS per liter of silica sol) increases the yield of carrots. The treatment of tomato seeds with aqueous suspensions of SiO2 NPs with a size of ~12 nm (8 g SiO2/L H2O) was reported [18] to improve the percentage of seed germination, average germination time, seed-germination index, seed-energy index, and weights of fresh and dry seedlings (increased seed-germination percentage, mean germination time, seed-germination index, seed-vigor index, and seedling fresh weight and dry weight). In [19], the pre-sowing treatment of tomato seeds with SiO2 NPs, coupled with useful additives, is shown as an effective strategy for improving seed germination. The authors associate these effects with improved water absorption by seeds and with the formation of an environment of beneficial microorganisms around the seeds. Furthermore, they found that the effectiveness of the influence on seed germination depends on the involved NPs’ sizes. The smaller SiO2 NPs (10–17 nm) were found to be more effective than the larger NPs (110–120 nm) in promoting seed germination and growth. However, the mechanisms of the effect of SiO2 NPs on seed germination and growth and the development of plants are still unclear [13,19].
In our earlier studies [20], film-forming polymer silica sols prepared by the hydrolysis of tetraethoxysilane (TEOS) were proposed, for the first time, instead of aqueous suspensions of amorphous SiO2 NPs and particulate aqueous sols.
The use of TEOS-based film-forming silica sol in combination with Azofoska fertilizer for seed treatment was recently reported in [21] to provide a direct application of nutrients directly to the seed environment, promoting faster germination and seedling development. Based on the obtained results, it was concluded that the use of sol-gel technology is a promising approach to increasing crop yields without imposing stress on the natural environment.
Our studies using scanning-electron microscopy and micro-X-ray spectral analysis confirmed the formation of quite continuous coatings on the surfaces of spring barley and Chinese cabbage seeds upon treatment with silica sols, depending on the concentration of TEOS and the pH of the environment (strongly acidic pH of 2–3 or weakly alkaline pH of 7–8). This effect was especially pronounced for Chinese cabbage seeds, in which silicon was initially not detected [20,22].
A positive effect of the pre-sowing treatment of spring barley and Chinese cabbage seeds with silica sols based on TEOS hydrolyzed in an acidic environment on the growth characteristics of both plants, as well as on the resistance of barley plants to the causative agent of root rot, was revealed [20]. It was also found that the introduction of small NP additives into such silica sols, e.g., a charge of detonation nanodiamond (0.1% DND) or titanium dioxide (0.003 g TiO2/mL silica sol), can further enhance the positive effects [22,23]. A pronounced synergistic effect on the formation of plants with higher biomass and the growth rates from using an additive of detonation nanodiamond batch together with silica sol was also reported in [23]. The pre-sowing seed treatment of Chinese cabbage with TEOS-based silica sols (1%) modified with TiO2 NPs provides the optimization of the composition of epiphytic microorganisms on the surfaces of seeds, increasing, in particular, the proportion of functionally useful bacteria able to decompose and transform organic and mineral forms of nitrogen [22].
Using sol-gel technology, thin film (nano-sized) coatings of a certain thickness can be obtained on the surfaces of diverse materials. The chemical composition and structure of the resulting coatings can be controlled, giving them useful target properties. For this purpose, alkoxy compounds with diverse functional groups and different structures, as well as alcohol-soluble inorganic additives (salts, acids, etc.), beneficial to plants are used [20,21,22,23,24,25]. The advantages of functional coatings obtained from silica sols on the surfaces of seeds include gas and water permeability, as well as the hydrophilicity of the surface. The positive influence of the hydrophilic surfaces of thin-film polymer coatings, both for seeds’ treatment and for their storage, is noted by the authors of [26].
In our research, we revealed a positive effect of silica sols with a low concentration of TEOS (1%) at pH 2–3. We also tried to exclude ethanol in the synthesis of sols in order to further increase the environmental safety of sol-gel compositions in relation to plant seeds. However, the questions arose as to how completely the hydrolysis and polycondensation reactions of TEOS proceed, the structure that is characteristic of such sol-gel compositions, and whether silica sol remains film-forming. Therefore, in this work, we studied the supramolecular structure of a silica sol with a low concentration of TEOS (1%) hydrolyzed in an alcohol-free acidic aqueous medium, which made it possible to identify and characterize fragments of the sol’s structural network. For comparison, the supramolecular structure of a silica sol with a higher content of TEOS (10%) hydrolyzed in an acidic aqueous-alcoholic medium, guaranteed to have film-forming ability, was studied.
In recent years, the use of magnetic NPs of iron oxides (magnetite, maghemite), along with other NPs of transition-metal oxides, has begun both for the root and for the foliar feeding of plants, which has increased the yields of important crops (rapeseed, barley, cucumber, tomato, muskmelon, etc.) [12,27,28,29,30]. As a rule, in agrotechnology, NPs are used in the form of aqueous suspensions. In this work, we introduced iron-oxide NPs into film-forming silica sols to obtain sol-gel compositions in the form of suspensions. For the pre-sowing treatment of plant seeds, both a silica sol and a suspension were used. It was assumed that the seed treatment with sol-gel systems would have a synergistic effect on the plant-growth characteristics and increase the resistance to stress and phytopathogens. In addition, the use of a film-forming sol instead of a water medium would provide better contact between the iron-oxide NPs and the surfaces of the seeds.
It was of interest to determine how the introduction of iron-oxide nanoparticles affects the supramolecular structure of the silica sol, which ultimately affects the structure of the functional coating formed on the surfaces of the seeds.
There is still no clear understanding as to whether it is better to use magnetite Fe3O4 or maghemite γ-Fe2O3 NPs for the pre-sowing treatment of vegetable seeds [29,30].
Thus, an alcohol-free silica sol (1% TEOS) and sol-gel compositions based on this sol, enriched with FexOy NPs with different ratios of Fe2+ and Fe3+, were tested for treating cabbage seeds of different subspecies (Brassica oleracea var. capitata L. cv. Penca de Povoa and Brassica oleracea var. botrytis L. cv. Hobart), in order to determine their effect on the seed-germination rates and plant growth in the initial period of their development, as well as on resistance to the dangerous phytopathogen causing “blackleg” disease.
2. Materials and Methods
Silica sols were synthesized by acid hydrolysis of tetraethoxysilane (TEOS) using the following precursors: tetraethoxysilane Si(OEt)4 (high purity grade), hydrochloric acid (HCl) (high purity grade) in the form of a 0.25 mol/L aqueous solution and solvents—distilled water and ethanol (high purity grade) only for the silica sol with the higher TEOS concentration. The TEOS content in the alcohol-free silica sols was 1 vol. % and in aqueous-alcohol-based silica sols, it was 10 wt. %. The precursors were used in the following molar ratios: TEOS:H2O:HCl = 1:1650:0.03 (for silica sol with TEOS concentration 1 vol. %) or TEOS:C2H5OH:H2O:HCl = 1:1.8:103:0.001 (for silica sol with TEOS concentration 10 wt. %).
To enhance the positive effect on the germination and development of cabbage plants of different varieties, a number of sol-gel compositions based on these silica sols were also prepared by adding magnetic FexOy NPs to them in a wide range of concentrations (0.0001, 0.001, 0.01, 0.1 1, 10, and 100 mg FexOy NPs/L). The resulting mixtures were dispersed in an ultrasonic bath for 40 min, resulting in stable suspensions (silica sol@FexOy NPs) subsequently used for pre-sowing treatment of seeds.
The magnetic FexOy NPs for introduction into silica sols were previously synthesized by precipitation from aqueous solutions of iron (II, III) chlorides with an aqueous ammonia solution, according to procedures described in detail in [31]. The phase composition of FexOy NPs corresponded to solid solutions of the magnetite–maghemite series with different ratios of Fe2+:Fe3+ cations. This was undertaken in order to find out which is more favorable for plant development—the predominance of iron in the oxidation state of 2+ or 3+. To obtain FexOy NPs with different Fe2+:Fe3+ ratios of cations, different techniques were used in the course of precipitation, including ultrasonic treatment (No. 1), sparging with and heating at 60 °C (No. 2), or long-term maturation in the mother liquor (No. 3). Experimental and calculated data [31] characterizing the compositions of the prepared FexOy NPs, as well as known research data, are summarized in Table 1.

Table 1.
Characteristics of the prepared FexOy NPs.
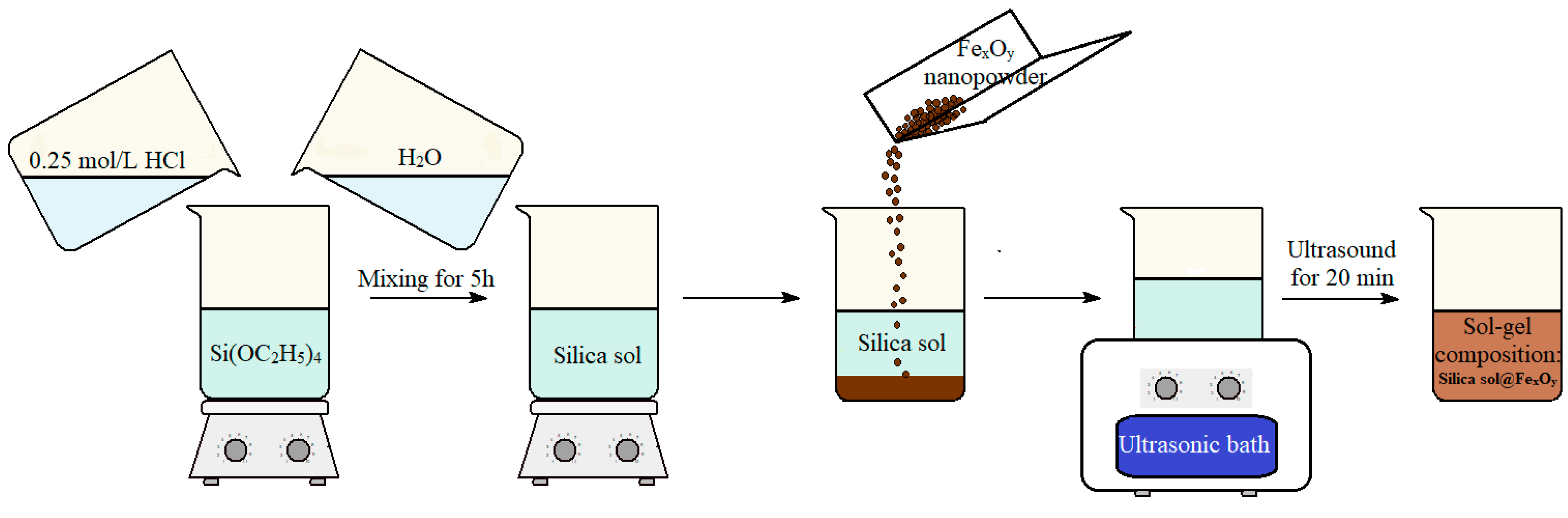
The synthesis scheme for silica sols and suspensions based on these sols is shown in Figure 1.
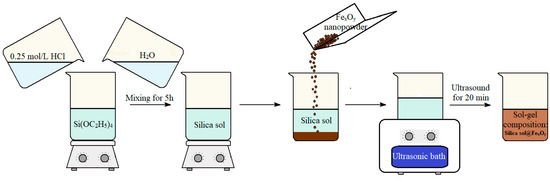
Figure 1.
Scheme for the synthesis of a silica sol and a suspension of silica sol@FexOyNPs based on this sol.
The TEM images of FexOy NPs were characterized from images obtained by high-resolution transmission-electron microscopy (TEM) performed using a ZEISS Libra 120 installation.
The supramolecular structures of the synthesized sols (containing both 1 and 10% TEOS) and the suspension based on silica sols with FexOy NPs (No. 2) were studied by small-angle X-ray scattering (SAXS) using an automatic small-angle X-ray diffractometer “AMUR-K” [35] with a single-coordinate position-sensitive detector ODZM at a fixed radiation wavelength of 0.1542 nm (CuKα line of a fine-focus tube with a copper anode, monochromator of pyrolytic graphite) and Kratky collimation system.
The cross-section of the X-ray beam s was 0.2 × 8 mm, and the range of scattering angles corresponded to the range of transmitted momentum values 0.2 < q < 0.3 nm−1, where q = 4πλ−1sin(θ/2), θ is the scattering angle.
The samples were placed into quartz capillaries with diameters of 1 mm. The sample –detector distance was 700 mm. The measurement time for one sample was 20 min. The experimental data were normalized to the intensity of the incident beam, after which a correction for collimation distortions was introduced. The scattering intensity measured for an empty capillary was subtracted from the sample scattering data. The measurement procedure was carried out according to a certified methodology approved for the AMUR-K installation [36].
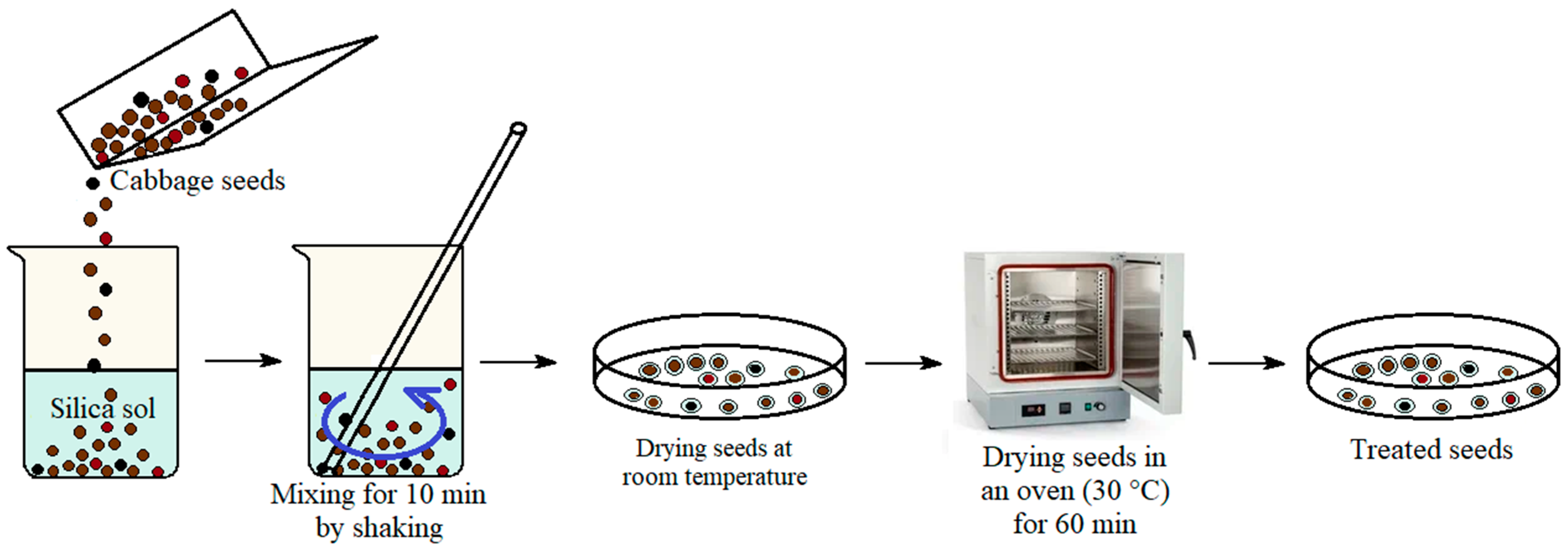
The biological-activity tests were performed using seeds of white cabbage Brassica oleracea var. capitata L. cv. Penca de Povoa and cauliflower cabbage Brassica oleracea var. botrytis L. cv. Hobart from the collection of the Federal Research Center–N.I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR). Pre-sowing seed treatment was carried out via mixing for 10 min by shaking the seeds in containers with water (control) and alcohol-free silica sol with 1 vol. % TEOS, as well as with the above suspensions based on silica sol and FexOy NPs with different Fe2+:Fe3+ ratios of cations (samples No. 1, No. 2, No. 3, see Figure 2). The seeds were dried at room temperature in air and then at 30 °C for 60 min in an oven. Seed-drying modes corresponded to the Russian National Standard [37]. The treated seeds were stored at room temperature before further use.
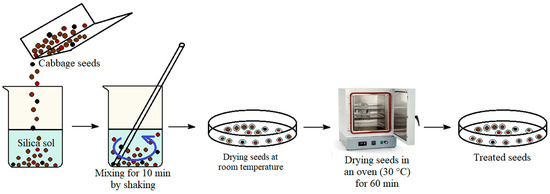
Figure 2.
Scheme for the pre-sowing treatment of cabbage seeds with silica sol and a suspension of silica sol@FexOyNPs.
The biological activity of the tested substances involved the determination of their effect on seed germination and biometric characteristics of seedlings. The seeds of white and cauliflower cabbage were germinated in Petri dishes with diameters of 10 cm on filter paper moistened with 10 mL of an aqueous solution of the test substance. In control variants, seeds were germinated in distilled water [38]. On the 3rd day, the energy of seed germination was assessed, and then, on the 7th day, their germination and the lengths of the sprouts and roots of the seedlings were measured. The studies were carried out in accordance with the rules of the International Seed Testing Association (ISTA). Repeatability of the experiment—400 seeds for each experiment variant. The experiment was repeated twice.
The effect of pre-sowing seed treatment of white cabbage Brassica oleracea var. capitata L. with the alcohol-free silica sol (1 vol. % TEOS) and suspensions (silica sol/FexOy NPs—Nos. 1, 2, 3) on the resistance of cabbage plants to damage by the main phytopathogenic microorganisms, including fungal pathogens (Pyhtium debaryanum Hesse, Olpidium brassicae Wor., Phizoctonia aderholdii Kolosh., Leptosphaeria maculans (Sowerby) P.Karst., fungi of the genus Fusarium sp.), which are causative agents of the “blackleg” disease, was assessed according to the following method.
Seeds of white cabbage were sown in vessels (20 seeds per vessel) filled with a substrate (300 cm3/vessel) based on low-decomposition high-peat Agrobalt (“Pindstrup” LLC, Moscow region, Russia) infected with blackleg-causing fungal pathogens (Pyhtium debaryanum Hesse, Olpidium brassicae Wor., Phizoctonia aderholdii Kolosh., Leptosphaeria maculans (Sowerby) P.Karst., fungi of the genus Fusarium sp.). The considered substrate was selected from areas in the greenhouse where plants were severely affected (more than 85% of plants affected) by the blackleg disease. A test performed before the experiment indicated that 88.7% of the cabbage plants grown on the infected substrate were affected by the “blackleg” disease. The experiment involved the following control series: negative or infected (seeds treated with water, sown on an infected substrate) and positive or uninfected (seeds treated with water, sown on an autoclaved, uninfected substrate). The substrate humidity in the vessels was maintained at 70 ± 5%.
Vessels with seeds sown into the substrate were placed in a Fisons Fitotron 600H phytotron in day/night (16 h/8 h) light conditions and at a temperature 24 °C. The assessment was carried out on the 21st day after germination according to the following two indicators:
- -
- number of living plants.
- -
- degree of damage on the VIR scale:
- 1—no lesion;
- 3—mild symptoms;
- 5—typical symptoms;
- 7—severe symptoms, necrosis;
- 9—absence of shoots.
The test results were summarized in tables while taking into account the effects of treatment components on the manifestation of disease symptoms. The weighted average lesion score (M) for variants was calculated according to the following formula:
where a is the number of affected variants, b is the corresponding lesion scores, N is the total number of variants [39].
M = Σ (a × b)/N,
3. Experimental Results
3.1. FexOy NP Morphology
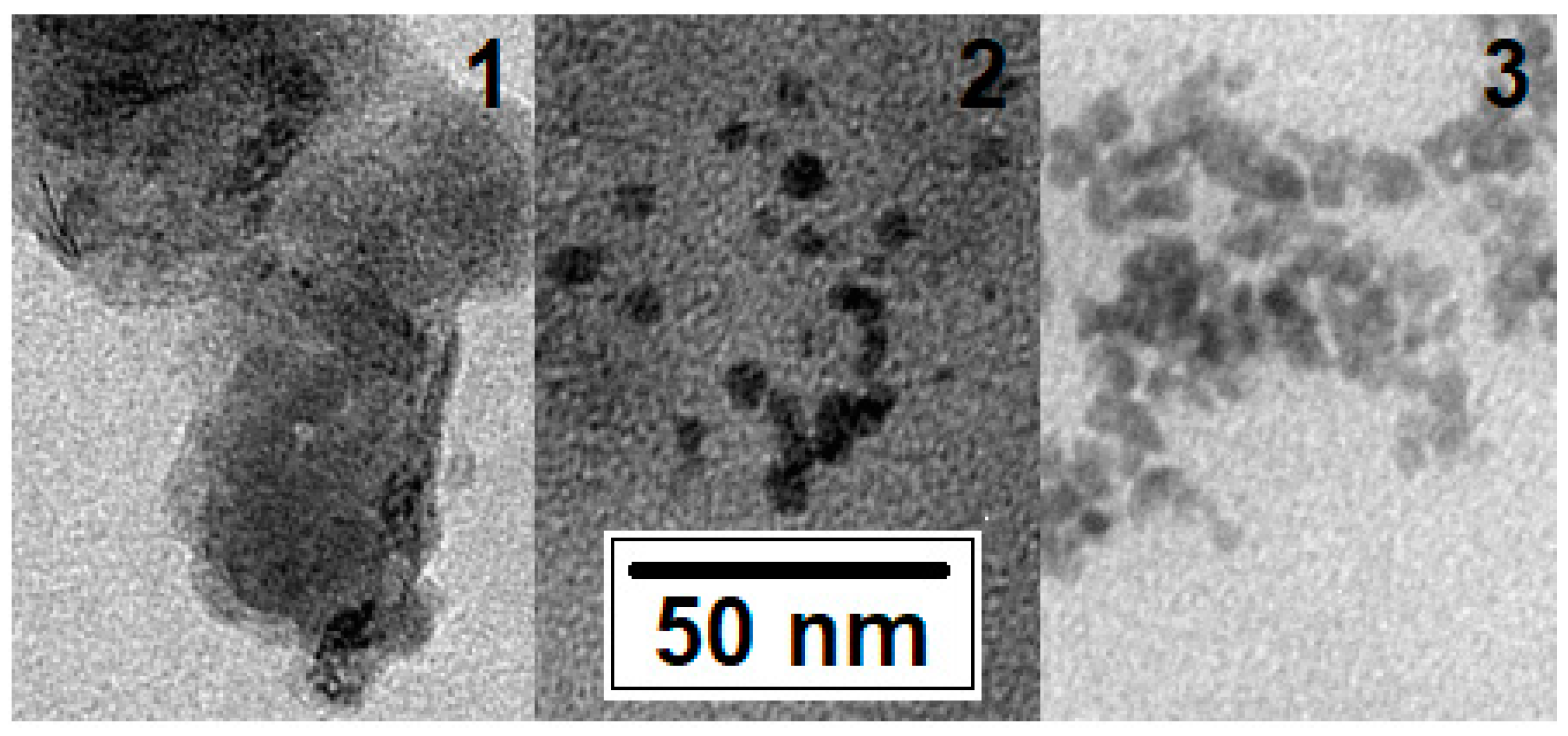
The shape, size, and aggregation tendency of the FexOy NPs were characterized from the TEM images (Figure 3).
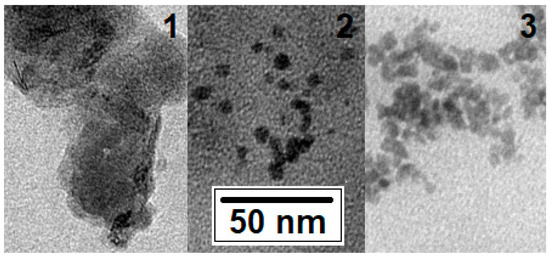
Figure 3.
TEM image of FexOy NPs No. 1, No. 2, and No. 3 (see Table 1).
The sizes of the NPs for all three variants were approximately the same and correlated with the X-ray phase-analysis data (from ~5 to 20 nm). The NP Nos. 1 and 3 were found to be the most aggregated. The least aggregated and those with clear boundaries were those from No. 2, the composition of which corresponded to a solid solution in the middle of the magnetite–maghemite series.
3.2. Structural Characterization of Sol-Gel Compositions
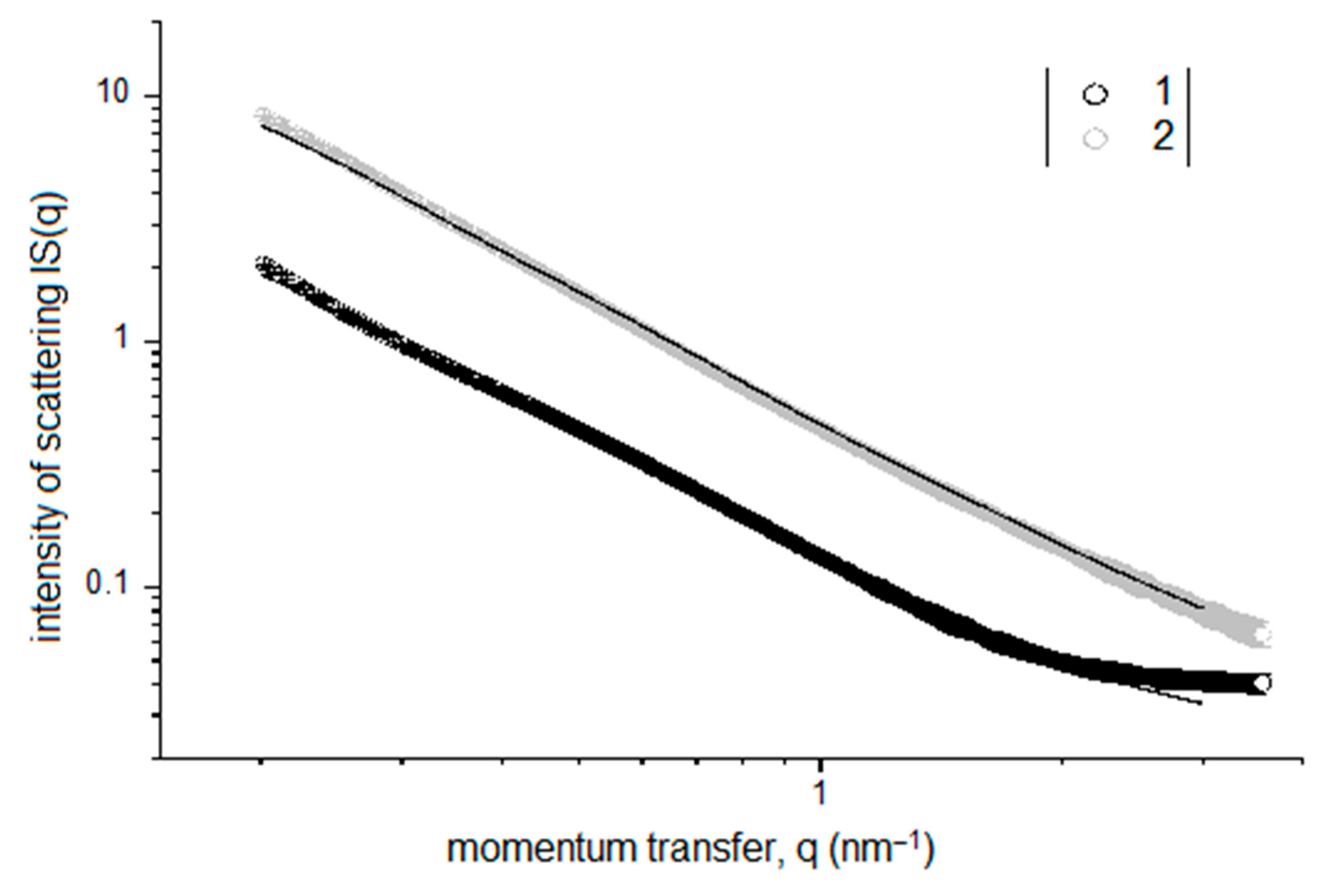
Double logarithmic-scale plots of the SAXS intensity IS(q) for the silica sols with TEOS concentrations of 1 and 10 vol. % are presented in Figure 4. These data show that an increase in the TEOS concentration in the sol leads to a significant increase in the observed small-angle scattering, which is apparently due to an increase in the number of SiO2 NPs in the sol and can be interpreted as an increase in the number of structural fragments responsible for film formation.
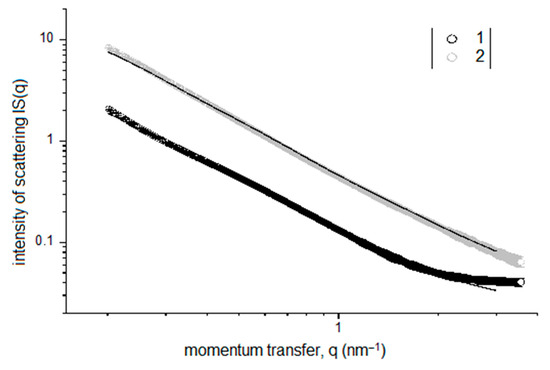
Figure 4.
SAXS intensity IS(q) for silica sols with TEOS concentrations of 1 vol. % (1) and 10 vol. % (2).
Based on the analysis of the SAXS plots, the size distribution of scattering inhomogeneities (NPs) in the silica sols was derived:
where DN(R) is the distribution-density function along the particle radius and V is the particle volume.
DV(R) = DN(R)V
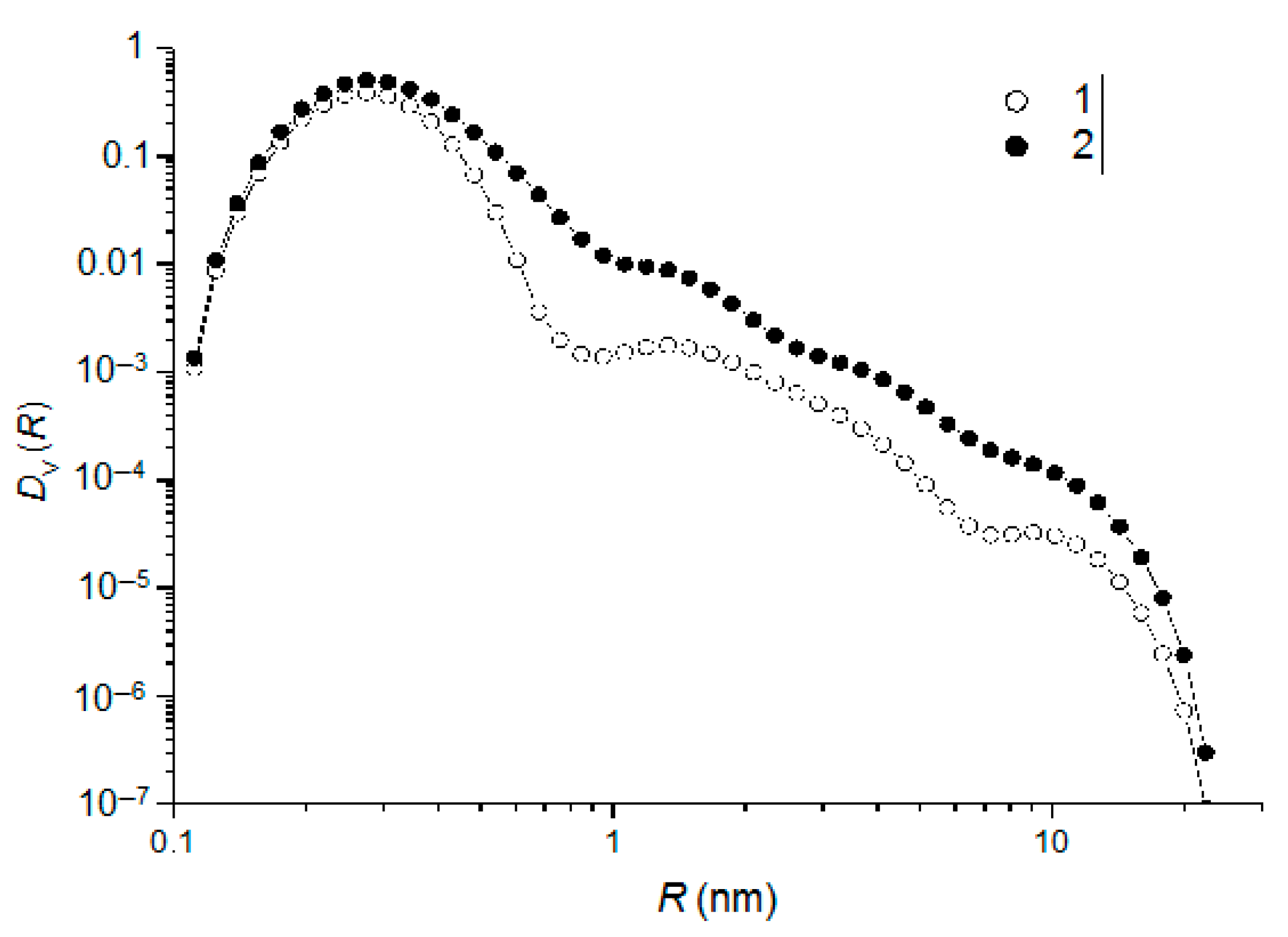
A direct modeling of the scattering curves using the form factor of individual non-interacting spherical particles while taking into account the commonly used lognormal particle-distribution function does not give reliable results, which indicates the significant polydispersity of the particles in the system. Therefore, to estimate the function DV(R), we used the indirect Fourier transformation [24,25], which is less sensitive to termination at small radii, which occurs in real systems. The obtained particle-size-distribution functions DV(R) for the samples of the studied silica sols are presented in Figure 5.

Figure 5.
Particle-size-distribution functions DV(R) for silica sols with TEOS concentrations of 1 vol. % (1) and 10 vol. % (2).
The above data suggest that silica sols with a TEOS concentration 1 vol. % feature a trimodal particle-size distribution DV(R) with peaks at R1 ≈ 0.3 nm, R2 ≈ 1.5 nm, and R3 ≈ 10 nm, with the largest volume fraction corresponding to small NPs. An increase in the TEOS concentration in the sol to 10 vol. % leads to a shift in the distribution to larger sizes and an increase in the volume fractions of large NPs, which is apparently due to their aggregation or an increase in the size of the fragments of the silica-sol structural network occurring during the hydrolytic polymerization of the TEOS.
Thus, the SAXS characterization revealed that even at low TEOS concentrations (1 vol. %), its hydrolysis in acidic aqueous solutions followed by polycondensation provides a dispersed nanoscale solid phase, i.e., silica sols. The resulting silica sols are quite stable over time and do not turn into gels, even after long-term storage at room temperature (more than a year). However, the presence of SiO2 NPs in these sols allowed them to be deposited on the surfaces of the seeds during pre-sowing treatment. As a result, a quite continuous functional coating formed on the surfaces of the seeds. As mentioned above, the beneficial properties of such a coating can be enhanced by introducing other useful NPs into sols, such as magnetic FexOy NPs.
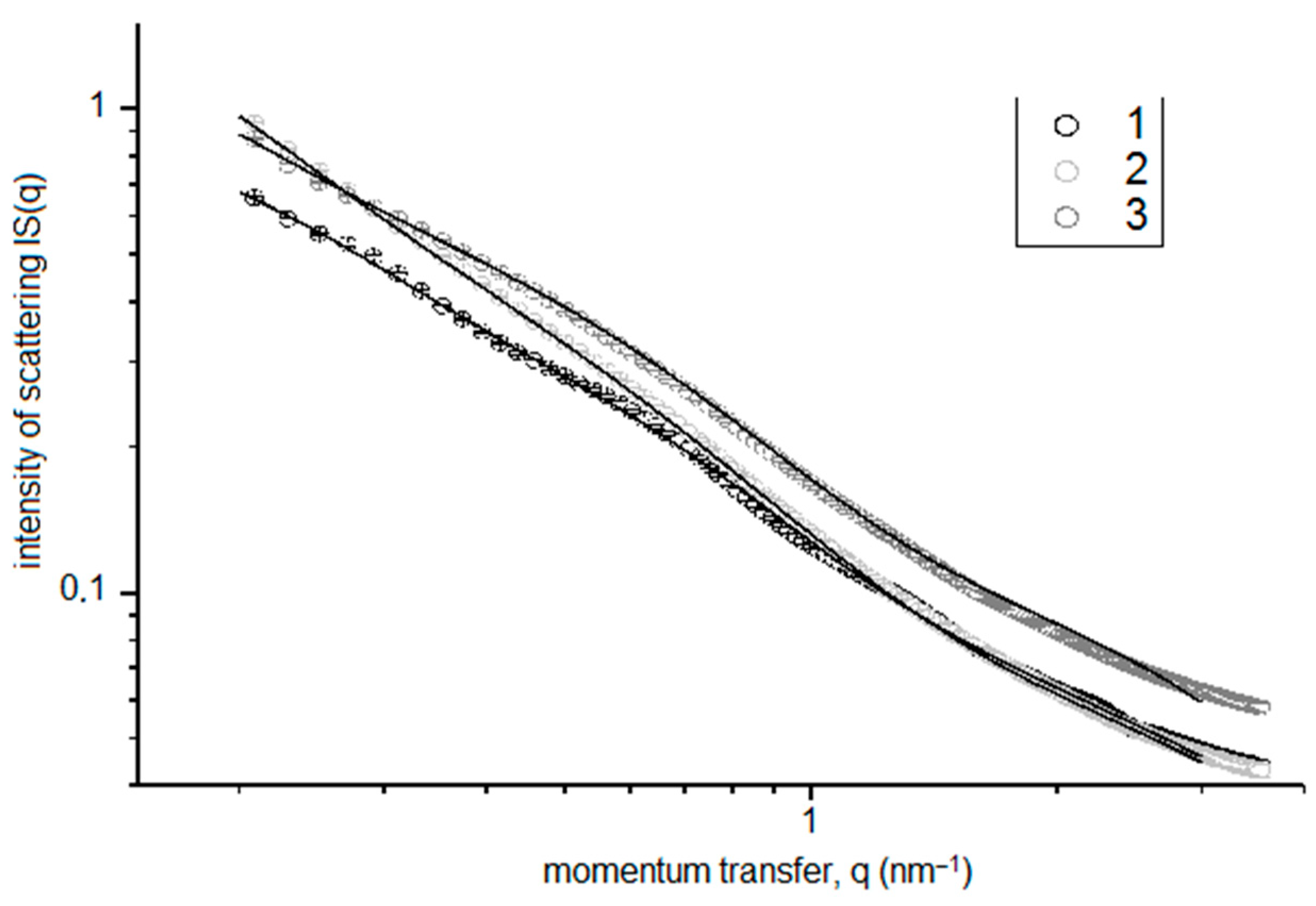
The SANS intensity IS(q) plots for the silica sol/FexOy NPs with FexOy NP (No. 2) concentrations of 0.001, 0.1, and 10 mg FexOy NP No. 2/L silica sol are shown in Figure 6. The presented data indicate that the increase in the FexOy NPs concentration in the sol-gel compositions resulted, as expected, in the growth of the observed SANS intensity due to an increase in the total amount of NPs caused by the addition of FexOy NP No. 2 to the sol-gel compositions.
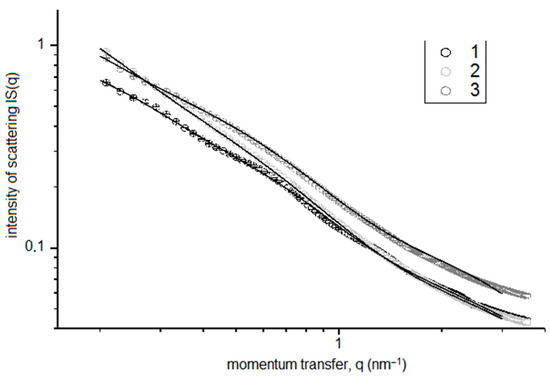
Figure 6.
SAXS intensity IS(q) for silica sol/FexOy NP No. 2 compositions with FexOy NP concentrations of 0.001 mg/L(1), 0.1 mg/L (2), and 10 mg/L (3).
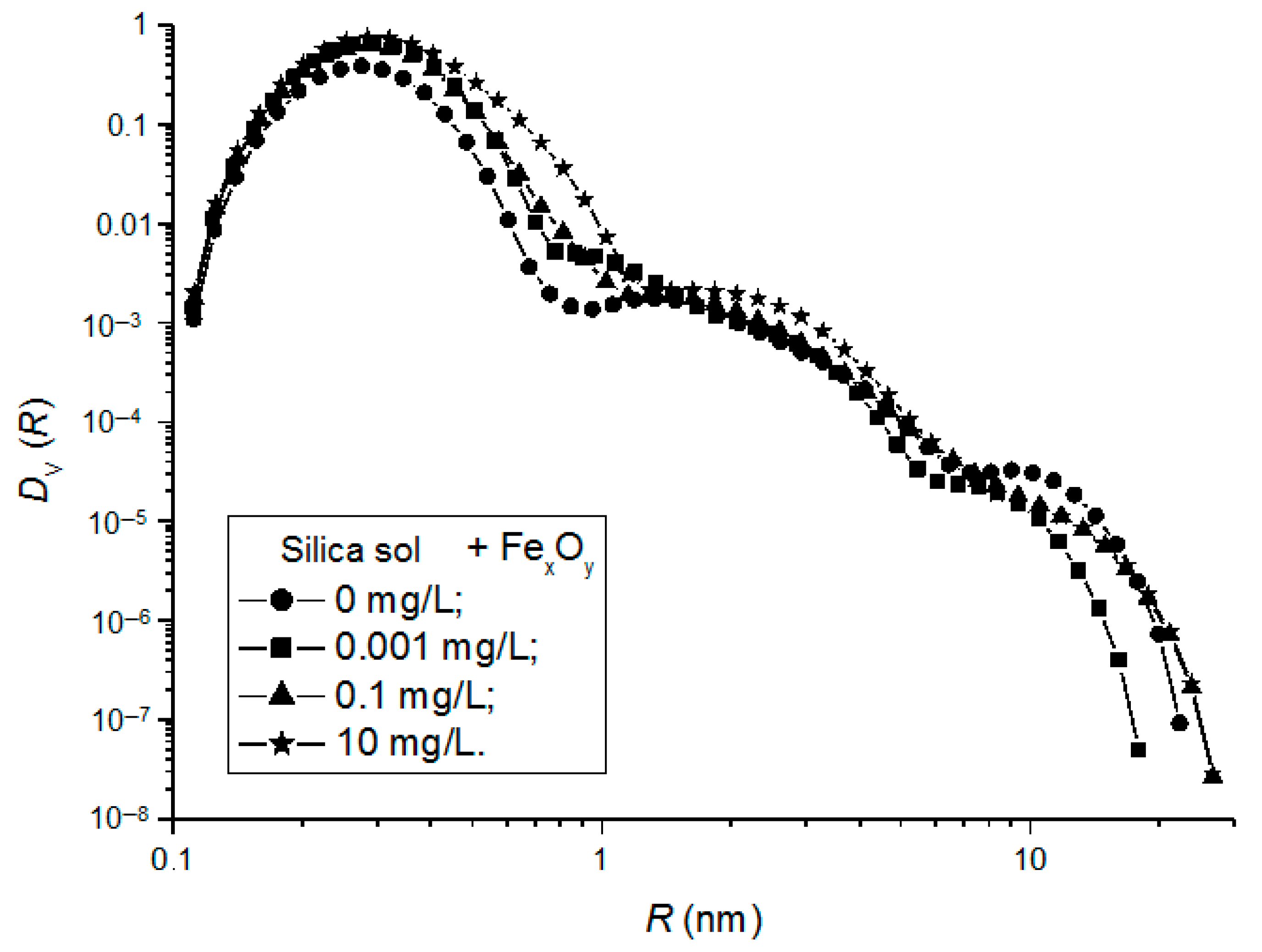
Similar to the silica sols, the distribution functions DV(R) of the scattering particles in the silica sol/FexOy NP compositions were derived and shown in Figure 7.
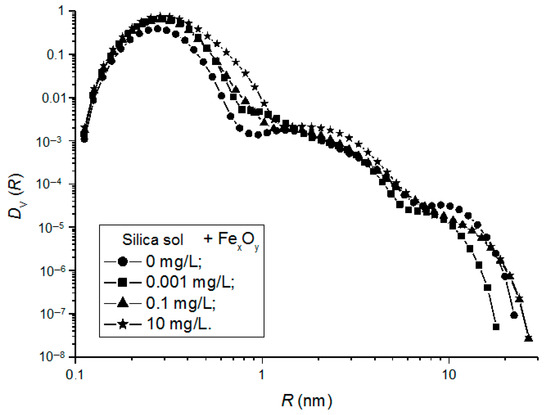
Figure 7.
Particle-size-distribution functions DV(R) for silica sol/FexOy NP No. 2 compositions with FexOy NP concentrations of 0.001 mg/L (1), 0.1 mg/L (2), and 10 mg/L (3).
All the studied samples featured a trimodal particle-size distribution. The increase in the concentrations of magnetic FexOy NPs (No. 2) with the phase composition corresponding to the middle of the magnetite–maghemite solid-solution series led to a shift in the DV(R) distribution to larger particle sizes and to an increase in the volume fraction of larger particles, which was most likely due to their aggregation in the dispersion medium of the silica sol.
3.3. Study of Biological Activity of the Synthesized Sol-Gel Compositions
The data on the effect of the pre-sowing seed treatment of the white cabbage Brassica oleracea var. capitata L. cv. Penca de Povoa with the synthesized sol-gel compositions on germination energy, germination, and biometric parameters of the seedlings are summarized in Table 2.

Table 2.
Effect of white cabbage Brassica oleracea var. capitata L. cv. Penca de Povoa seed treatment with silica sol and sol-gel compositions with magnetic FexOy NPs with different Fe2+/Fe3+ cation ratios *.
The data in Table 2 suggest that the treatment of the seeds of the white cabbage variety Penca de Povoa with silica provides a trend of or a significant decrease in their germination energy and germination compared to similar indicators for seeds treated with water. However, the introduction of magnetic FexOy NPs into silica provides a prominent mitigation of this adverse effect and, as a result, no significant differences from the control in the values of these indicators were observed.
The pre-sowing treatment of the Penca de Povoa white cabbage seeds with silica sol@FexOy NP No. 1 composition resulted in a significant reduction in the root-length-growth index compared with the corresponding control (the seeds treated with water). In the case of the treatment with the silica-sol@FexOy NP No. 2 and silica sol@FexOy NP No. 3 compositions, certain trends were observed towards a decrease in this indicator compared to the seeds treated with water. Along with a decrease in germination, this effect indicates that the applied treatment causes stress for these cabbage seeds. At the same time, the seed treatment in the sol-gel compositions with concentrations of FexOy NPs (No. 1) of 0.0001, 0.01, 0.1, 10, and 100 mg/L and concentrations of FexOy NPs (No. 3) of 0.0001, 0.001, 0.01, and 100 mg/L in relation to the plant roots had, in most cases, a similar stress effect as in the variants with the silica sol. However, a predominantly significant decrease in root length was observed for FexOy NP No. 2 (silica sol + 0.0001, 0.001, 0.01, 10.0 mg/L) and No. 1 (Silica sol + 1.0, 100.0 mg/L). None of the other experimental options had a significant effect on this indicator.
With respect to the biometric indicator, sprout length, the tested seed-treatment options did not have a significant effect relative to the corresponding control, with the exception of the seed-treatment option of silica sol + 0.01 mg/l FexOy NPs (No. 3), with the largest Fe3+ content providing a significant increase in this indicator. The absence of differences from the control in the other assessed indicators in this treatment option indicates its positive effect on the growth of the cv. Penca de Povoa cabbage plants at an early stage of their development.
Table 3 presents the effect of the pre-sowing treatment of the cv. Hobart cauliflower cabbage seeds with the synthesized sol-gel compositions on the germination energy, the germination, and the biometric indicators of the plant seedlings.

Table 3.
Effect of seed treatment of cauliflower cabbage Brassica oleracea var. botrytis L. cv. Hobart with silica sols and sol-gel compositions with various concentrations of magnetic FeOx NPs on their germination energy, germination, and biometric indicators of seedlings.
The data in Table 3 show that the treatment of the cv. Hobart cauliflower cabbage seeds with silica sol, as well as the silica sols containing FexOy NPs, provides a trend of or a significant increase in their germination energy and germination compared to similar indicators for the seeds treated with water. The considered effect is especially pronounced in relation to the germination energy for the series of silica sol + 0.0001–100.0 mg FexOy NP No. 2/L and silica sol + 0.1, 10.0 mg FexOy NP No. 3/L and in relation to the germination for silica-sol variants + 0.0001–0.01 mg FexOy NP No. 2/L and silica sol + 0.001 mg FexOy NP No. 3/L.
The assessment of the effect of the pre-sowing treatment of the cauliflower cabbage seeds on the biometric characteristics of the seedling growth revealed a difference between series No. 3 and other two series. This difference may have been due to the fact that the experiments were carried out in the same laboratory conditions but during different seasons of the year. In general, no significant effects of the tested drugs on the lengths of the sprouts were detected, while the root lengths changed significantly under the influence of the suspensions of the tested substances. Thus, reduced values of root length compared to the control series were noted for the treatments with silica sol + 0.0001, 0.01 mg FexOy NP No. 1/L, silica sol + 0.1 mg FexOy NP No. 2/L, and silica sol + 0.1 mg FexOy Ns No. 3/L FexOy NPs. A significantly increased value of root length relative to that in the control was noted only in the variant of the seed treatment with a suspension of silica sol + 0.1 mg FexOy NP No. 1/L.
Regarding the whole set of obtained data and taking into account the stimulation of the germination energy and seed germination of the test crops in combination with almost no change in the biometric growth indicators from the control series, it is possible to predict higher plant yields per area unit upon seed treatment with the following suspensions of series No. 2: silica sol + 0.0001–0.001 mg FexOy NP No. 2/L and silica sol + 0.001 mg FexOy NP No. 3/L.
Furthermore, in view of the species-specific response of cabbage to seed treatment, a certain composition and concentration of NPS in silica sol should be selected for each plant species and variety.
The effect of the pre-sowing seed treatment with suspensions of synthesized silica sols and sol-gel compositions on the resistance of the white cabbage plants to damage by phytopathogens was investigated. Table 4 presents the obtained data on the survival of the white cabbage Brassica oleracea var. capitata L. cv. Penca de Povoa and the incidence of the “black leg” disease when the cabbage was grown from seeds treated with sol-gel compositions on a substrate infected with fungal phytopathogens that cause this disease when the plants were grown in the conditions of the VIR-greenhouse complex.

Table 4.
Survival of white cabbage plants (Brassica oleracea var. capitata L. cv. Penca de Povoa) and their susceptibility to the “blackleg” disease when grown on the substrate infected with fungal pathogen substrate from seeds treated with silica-sol compositions.
The data obtained showed that in the infected control, the seed germination in the white cabbage decreased by 61.1% relative to the uninfected control. At the same time, the weighted average scores of the damage (according to the scale by E. Goiman) to the plants were 8 and 1 in the indicated controls, respectively. The treatment of the seeds with tested suspensions of silica sols separately and in combination with FexOy NPs in various concentration ratios showed that none of them provided a phytoprotective effect in combination with the compensation of the negative effect of infection on the germination and survival of the phytotest objects relative to the uninfected control. However, certain substances and options for their use provide a protective effect and an increase in plant survival above those of the infected control, but they are significantly inferior in ensuring plant survival compared to the uninfected control. In particular, the treatment of cabbage seeds with a suspension of silica sol + 10 mg/L FexOy NP No. 2 provided the plants with fairly high resistance to fungal attack, but the percentage of surviving plants was only 45.7%. The remaining options were significantly inferior to the latter in terms of resistance or survival.
4. Discussion
4.1. Structure Formation in Silicas and Sol-Gel Compositions
In this work, the supramolecular structure of silica sol obtained from a low-concentration aqueous solution of TEOS (1 vol. %) was studied for the first time. The SAXS characterization revealed that even at low TEOS concentrations, its hydrolysis in acidic aqueous solutions followed by polycondensation provides a dispersed nanoscale solid phase, i.e., silica sols. According to our long-term phenomenological observations, the resulting silica sols do not lose fluidity and maintain their sedimentation stability over the long term and do not turn into gels, even after long-term storage at room temperature (more than a year). This stability of the silica sol over time makes it promising for subsequent commercialization and use in agricultural technology.
The presence of SiO2 NPs in these sols allows them to be deposited on the surfaces of seeds during pre-sowing treatment. As a result, a quite continuous functional coating can be formed on the surfaces of the seeds.
As mentioned above, the beneficial properties of such a coating can be enhanced by introducing other useful NPs into the sols, such as magnetic FexOy NPs. The incorporation of FexOy NPs into the silica sol leads to the enlargement of the nanoparticles in direct proportion to the concentration of incorporated NPs. In this case, the process can be considered as the heterocoagulation of SiO2 NPs and FexOy NPs, i.e., the deposition of smaller SiO2 NPs on the surfaces of larger FexOy NPs. Similar processes were observed for various aqueous suspensions of NPs in [40,41], as well as in our earlier studies involving the introduction of micron-sized alumina powders into silica sols [42]. Heterocoagulation can afford finely dispersed or nanodispersed powders with core-shell structures. In [43], similar structures were obtained in hybrid dispersions as a result of the interaction of titanium-dioxide nanoparticles and cellulose nanocrystals. The presence of a sufficient amount of TiO2 NPs in the hybrid dispersions makes it possible to completely cover cellulose nanocrystals’ surfaces and form core-shell structures. In a similar way, the prepared silica sols (SiO2 NPs) and FexOy NPs@SiO2 NP sol-gel compositions can interact with the surfaces of seeds to form functional shells, whose continuity depends on the NP concentration.
4.2. The Prospects for Using Silica Sols and Sol-Gel Compositions for Pre-Sowing Treatment of Cabbage Seeds of Different Subspecies
Species specificity was revealed in the reactions of the white and cauliflower cabbage plants of the indicated cultivars to the seed treatment with both the silica sol (1 vol. % TEOS), and the silica sol containing various concentrations of FexOy NPs corresponding to the compositions of solid solutions of the magnetite–maghemite series with different ratios of cations Fe2+:Fe3+. The cauliflower was found to be more responsive to the treatment of the seeds with sol-gel compositions with magnetic FexOy NPs in certain concentrations and ratios of iron oxides from the magnetite–maghemite series, in which a pronounced stimulation of germination energy and germination was noted. Significant changes relative to controls in the values of sprout growth were predominantly not observed in the experimental variants for both subspecies of cabbage, with the exception of the treatment of white cabbage seeds with the sol-gel composition of silica sol + 0.01 mg FexOy NP No. 3/L, with a significant increase in this indicator observed relative to the control. The observed changes in the biometric indicators of seedling-root growth suggest the necessity of determining certain ranges of concentrations of dopants in the compositions of silica sols that cause no inhibitory effects of seed treatment on the growth of plant roots in the early stages of their development.
In general, the magnetic FexOy NPs corresponded to the compositions of solid solutions of the magnetite–maghemite series with different ratios of F2+/Fe3+ cations, as part of the silica sols enhanced their biocompatibility without any negative effects on the bioactive properties of the seeds of both subspecies and cultivars of cabbage. Furthermore, a shift in the composition of the NPs towards magnetite in a wide range of their concentrations was found to provide a beneficial effect on germination rates and is useful for improving the biometric parameters of seedlings.
A general tendency was revealed towards the increasing resistance of white cabbage plants to phytopathogens that cause the “blackleg” disease when seeds are treated with sol-gel compositions containing 10 mg FexOy NPs/L. However, in the future, additional in-depth studies are required to enhance the positive effect of the tested substances on the survival of plants under infection conditions. In this regard, from our point of view [22,23], a promising approach can be based on the complex use of such additives to the composition of silica sol as titanium dioxide and detonation nanodiamond charge, including those doped with B or other dopants.
5. Conclusions
For the first time, silica sol intended for the pre-sowing treatment of seeds was obtained through acid hydrolysis in an aqueous medium without organic co-solvents and with a low content of TEOS (1 vol. %). The formation of nanoparticles with a wide trimodal size distribution in this silica sol was confirmed. It was shown that an increase in the TEOS concentration from 1% to 10% and the use of an ethanol co-solvent during hydrolysis leads to an increase in the sizes of nanoparticles, which is apparently associated with a more complete occurrence of TEOS-hydrolysis processes. To enhance the positive effect on the plants, iron-oxide nanoparticles were added to the silica sol. For the first time, the synthesized silica sols based on TEOS (1 vol. %) hydrolyzed in a strongly acidic aqueous medium without organic co-solvents and sol-gel composition silica sol@FexOy NPS with different concentrations of magnetic NPs were used for the pre-sowing treatment of seeds of white and cauliflower cabbage (Brassica oleracea var. capitata L. cv. Penca de Povoa and Brassica oleracea var. botrytis L. cv. Hobart). The magnetic FexOy NPs, as parts of the silica sols, enhanced their biocompatibility without any negative effects on the bioactive properties of the seeds of both subspecies and cultivars of cabbage. A general tendency was revealed towards an increase in the resistance of the white cabbage plants to phytopathogens that cause the “blackleg” disease when the seeds were treated with the sol-gel compositions.
Author Contributions
Conceptualization, O.A.S. and G.G.P.; synthesis, A.M.N., T.V.K. and A.S.K.; investigation, A.S.K., T.V.K., G.P.K., A.M.N., A.A.S., O.R.U. and D.L.K.; writing—original draft preparation, O.A.S., T.V.K., G.P.K., G.G.P. and A.M.A.; review and editing, A.M.N., O.A.S. and A.M.A.; supervision, G.G.P. and O.A.S.; project administration, G.G.P. and O.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Institute of Silicate Chemistry of the Russian Academy of Sciences (No. 0081-2022-0006) in the theoretical part and in ensuring the performance of the synthesis and the study of the physicochemical properties of the compounds, and by the Agrophysical Research Institute, scientific and technical state task no. FGEG-2022-0005 in the theoretical part and in ensuring the performance of the biological experiments. The research was carried out using equipment from the center for collective use at Voronezh State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors appreciate Nadezhda Shabanova for her assistance in interpreting the results on the coagulation and heterocoagulation of nanoparticles in sol-gel systems and V.V. Volkov for support in the SAXS measurements performed using the equipment from the Shared Research Center FSRC “Crystallography and Photonics” RAS, supported by the Russian Ministry of Education and Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taylor, A.G.; Amirkhani, M.; Hill, H. Modern seed technology. Agriculture 2021, 11, 630. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Mayton, H.; Amirkhani, M.; Yang, D.; Donovan, S.; Taylor, A.G. Tomato seed coat permeability: Optimal seed treatment chemical properties for targeting the embryo with implications for internal seed-borne pathogen control. Agriculture 2021, 11, 199. [Google Scholar] [CrossRef]
- Mallick, S.; Mohanty, S.; Das, S.; Beura, J.K.; Jyoth, G.B.N. Effect of polymer coating on seed quality during storage of groundnut seeds. Pharma Innov. J. 2022, 11, 1396–1401. [Google Scholar]
- Reddy, B.P.; Bara, B.M.; Krishina, R.Y. Effect of polymer seed coating and seed treatment on seed quality parameters and yield attributing characters of hybrid maize (Zea mays L.). Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1175–1182. [Google Scholar] [CrossRef]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.A. Recent advances in seed coating technologies: Transitioning toward sustainable agriculture. Green Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Fu, Y.; Bhunia, A.K.; Yao, Y. Alginate-based antimicrobial coating reduces pathogens on alfalfa seeds and sprouts. Food Microbiol. 2022, 103, 103954. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Moradi Pour, M.; Ait Barka, E. A Novel Route for Double-Layered Encapsulation of Streptomyces fulvissimus Uts22 by Alginate–Arabic Gum for Controlling of Pythium aphanidermatum in Cucumber. Agronomy 2022, 12, 655. [Google Scholar] [CrossRef]
- Aisvarya, S.; Kalyanasundaram, M.; Kannan, M.; Lakshmanam, A.; Srinivasan, T. Dual activity of biogenic zinc oxide nanoparticles as a protectant against seed weevil and growth promoter in maize seeds. In Book of Extended Abstracts, Proceedings of the GPCP 2021, Coimbatory, India, 8–10 December 2021; Centre for Plant Protection Studies, Tamil Nadu Agriculture University: Coimbatory, India, 2021; pp. 137–138. [Google Scholar]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Agrawal, S.; Ghazaryan, K.; Minkina, T.; Al-Tawaha, A.R.M.; Al-Zoubi, O.M.; Habeeb, T. Impact of zinc oxide nanoparticles on seed germination characteristics in rice (Oryza sativa L.) under salinity stress. J. Ecol. Eng. 2023, 24, 142–156. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanosc. Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef]
- Goswami, P.; Mathur, J.; Srivastava, N. Silica nanoparticles as novel sustainable approach for plant growth and crop protection. Heliyon 2022, 8, e09908. [Google Scholar] [CrossRef]
- Zaim, N.S.H.B.H.; Tan, H.L.; Rahman, S.M.A.; Rahman, S.M.A.; Fitrah, A.B.N.; Osman, M.S.; Thakur, V.K.; Radacsi, N. Recent Advances in Seed Coating Treatment Using Nanoparticles and Nanofibers for Enhanced Seed Germination and Protection. J. Plant Growth Regul. 2023, 42, 7374–7402. [Google Scholar] [CrossRef]
- Chakkalakkal, N.D.; Thomas, M.; Chittillapilly, P.S.; Sujith, A.; Anjali, P. Electrospun polymer nanocomposite membrane as a promising seed coat for controlled release of agrichemicals and improved germination: Towards a better agricultural prospect. J. Clean. Prod. 2022, 377, 134479. [Google Scholar] [CrossRef]
- Ismail, I.; Bakar, F.A.; Ling, T.H.; Zain ZH, M.; Radacsi, N. Morphology Conductivity Evaluation of Electrospun Polyacrylic Acid (PAA) Microfiber. Mater. Today Proc. 2019, 17, 574–583. [Google Scholar] [CrossRef]
- Lozhnikova, V.N.; Slastya, I.V. Growth of spring barley and activity endogenous phytohormones under the influence of silicon compounds. Agric. Biol. (Sel’skokhozyaistvennaya Biol.) 2010, 3, 102–107. Available online: http://www.agrobiology.ru/3-2010lozhnikova.html (accessed on 20 November 2023).
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef]
- Sembada, A.A.; Maki, S.; Faizal, A.; Fukuhara, T.; Suzuki, T.; Lenggoro, I.W. The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds. Nanomaterials 2023, 13, 2110. [Google Scholar] [CrossRef]
- Shilova, O.A.; Khamova, T.V.; Panova, G.G.; Anikina, L.M.; Artem’eva, A.M.; Kornyukhin, D.L. Using the sol–gel technology for the treatment of barley seeds. Glass Phys. Chem. 2018, 44, 26–32. [Google Scholar] [CrossRef]
- Borak, B. Sol-gel coatings with Azofoska fertilizer deposited onto pea seed. Polymers 2022, 14, 4119. [Google Scholar] [CrossRef]
- Shilova, O.A.; Khamova, T.V.; Panova, G.G.; Kornyukhin, D.L.; Anikina, L.M.; Artemyeva, A.M.; Udalova, O.R.; Galushko, A.S.; Baranchikov, A.E. Synthesis and research of functional layers based on titanium dioxide nanoparticles and silica sols formed on the surface of seeds of Chinese Cabbage. Russ. J. Appl. Chem. 2020, 93, 25–34. [Google Scholar] [CrossRef]
- Shilova, O.; Dolmatov, V.; Panova, G.; Khamova, T.; Baranchikov, A.; Gorshkova, Y.; Udalova, O.; Zhuravleva, A.; Kopitsa, G. Nanodiamond batch enriched with boron: Properties and prospects for use in agriculture. Biointerface Res. Appl. Chem. 2022, 12, 6134–6147. [Google Scholar] [CrossRef]
- Jacob, S.R.; Kumar, M.B.A.; Varghese, E.; Sinha, S.N. Hydrophilic polymer film coat as a micro-container of individual seedfacilitates safe storage of tomato seeds. Sci. Hortic. 2016, 204, 116–122. [Google Scholar] [CrossRef]
- Purcar, V.; Răditoiu, V.; Răditoiu, A.; Manea, R.; Raduly, F.M.; Ispas, G.C.; Frone, A.N.; Nicolae, C.A.; Gabor, R.A.; Anastasescu, M.; et al. Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates. Coatings 2021, 11, 11. [Google Scholar] [CrossRef]
- Ribicki, A.C.; Chemin, B.G.; Van Haandel, V.J.; Winiarski, J.P.; de Castro Rozada, T.; Pessoa, C.A.; Estrada, R.A.; Fiorin, B.C.; Fujiwara, S.T. Sol gel synthesis of 3-n-propyl(4-aminomethyl)pyridinium silsesquioxane chloride and the enhanced electrocatalytic activity of LbL films. J. Sol-Gel Sci. Technol. 2018, 87, 216–229. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Tian, D.-D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.-R. Recent Trends in NanoFertilizers for Sustainable Agriculture under Climate Change for Global Food Security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Amanzadeh, T.; Dashti, S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric. Slov. 2016, 107, 265–276. [Google Scholar] [CrossRef]
- Kovalenko, A.S.; Nikolaev, A.M.; Khamova, T.V.; Udalova, O.R.; Zhuravleva, A.S.; Kopitsa, G.P.; Sinel’nikov, A.A.; Tsvigun, N.V.; Khomyakov, Y.V.; Panova, G.G.; et al. Synthesis of iron oxide magnetic nanoparticles and their effect on growth, productivity, and quality of tomato. Glass Phys. Chem. 2021, 47 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Wang Yu Wang, S.; Xu, M.; Xiao, L.; Dai, Z.; Li, J. The impacts of γ-Fe2O3 and Fe3O4 nanoparticles on the physiology and fruit quality of muskmelon (Cucumis melo) plants. Environ. Pollut. 2019, 249, 1011–1018. [Google Scholar] [CrossRef]
- Kovalenko, A.S.; Shilova, O.A.; Nikolaev, A.M.; Myakin, S.V. Comparative characterization of aqueous suspensions of magnetic iron oxide nanoparticles with different phase compositions. Colloid. J. 2023, 85, 389–397. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Raman, A. The application of infrared spectroscopy to the study of rust systems—II. Study of cation deficiency in magnetite (Fe3O4) produced during its transformation to maghemite (γ-Fe2O3) and hematite (α-Fe2O3). Corros. Sci. 1993, 34, 1355–1365. [Google Scholar] [CrossRef]
- Pecharroman, C.; Gonzalez-Carreno, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, γ-Fe2O3, from reflectance measurement on pressed powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W. Magnetite. In Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2018. [Google Scholar]
- Mogilevsky, L.Y.; Dembo, A.T.; Svergun, D.I.; Feigin, L.A. Automatic small-angle X-ray diffractometer with a linear position-sensitive detector. Crystallography 1984, 29, 587–591. [Google Scholar]
- GOST R 8.698-2010; State System for Ensuring the Uniformity of Measurements. Dimensional Parameters of Nanoparticles and Thin Films. Method for Measurement by Means of a Small Angle X-ray Scattering Diffractometer. Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2010.
- GOST 11230-95; Seeds of Vetch. Varietal and Sowing Characteristics. Specifications. Committee of the Russian Federation for Standardization, Metrology and Certification: Moscow, Russia, 1995.
- International Seed Testing Association. International Rules for Seed Testing; ISTA: Basserdorf, Switzerland, 2008. [Google Scholar]
- VIR Guidelines. In Study and Maintenance of a Collection of Vegetable Plants (Carrots, Celery, Parsley, Parsnips, Radishes, Radishes); Sazonova, L.V.; Levandovskaya, L.I.; Krivchenko, V.I.; Vlasova, E.A.; Ermakov, A.I.; Voskresenskaya, V.V. (Eds.) VIR: Leningrad, Russia, 1981; 190p. (In Russian) [Google Scholar]
- Golikova, E.V.; Burdina, N.M.; Vysokovskaya, N.A. Aggregation stability of SiO2, FeOOH, ZrO2, CeO2, and natural diamond sols and their binary mixtures: 2. The photometric study of heterocoagulation of SiO2–FeOOH, SiO2–ZrO2, SiO2–CeO2, and CeO2–natural diamond binary systems in KCl solutions. Colloid J. 2002, 64, 142–148. [Google Scholar] [CrossRef]
- Yates, P.D.; Franks, G.V.; Biggs, S.; Jameson, G.J. Heteroaggregation with nanoparticles: Effect of particle size ratio on optimum particle dose. Colloids Surf. A Physicochem. Eng. Aspects 2005, 255, 85–90. [Google Scholar] [CrossRef]
- Khamova, T.V.; Shilova, O.A.; Golikova, E.V. Investigation of the structuring in the Sol-Gel systems based on tetraethoxysilane. Glass Phys. Chem. 2006, 32, 448–459. [Google Scholar] [CrossRef]
- Martakov, I.S.; Torlopov, M.A.; Mikhaylov, V.I.; Krivoshapkina, E.F.; Silant’ev, V.E.; Krivoshapkin, P.V. Interaction of cellulose nanocrystals with titanium dioxide and peculiarities of hybrid structures formation. J. Sol-Gel Sci. Technol. 2018, 88, 13–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).