Bilayer Coating Composed of Starch and Methyl Cellulose-Nanoscale TiO2 for the Protection of Historic Paper from UV
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Process
2.1.1. The Bottom Layer
2.1.2. The Top Layer
2.2. Characterization Methods
2.2.1. Visual Assessment
2.2.2. Colour Measurement and Fluorescence Intensity
2.2.3. Surface Properties
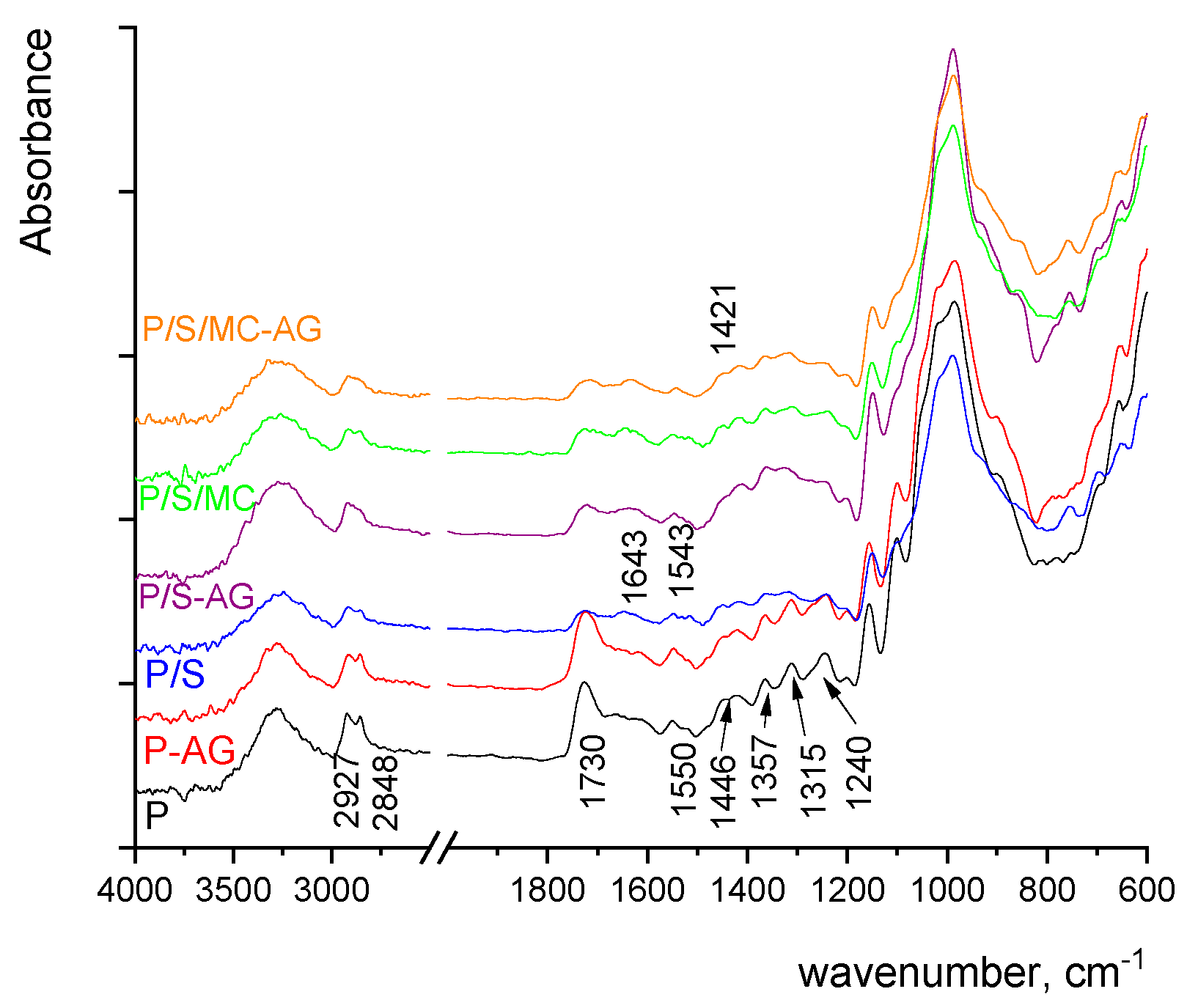
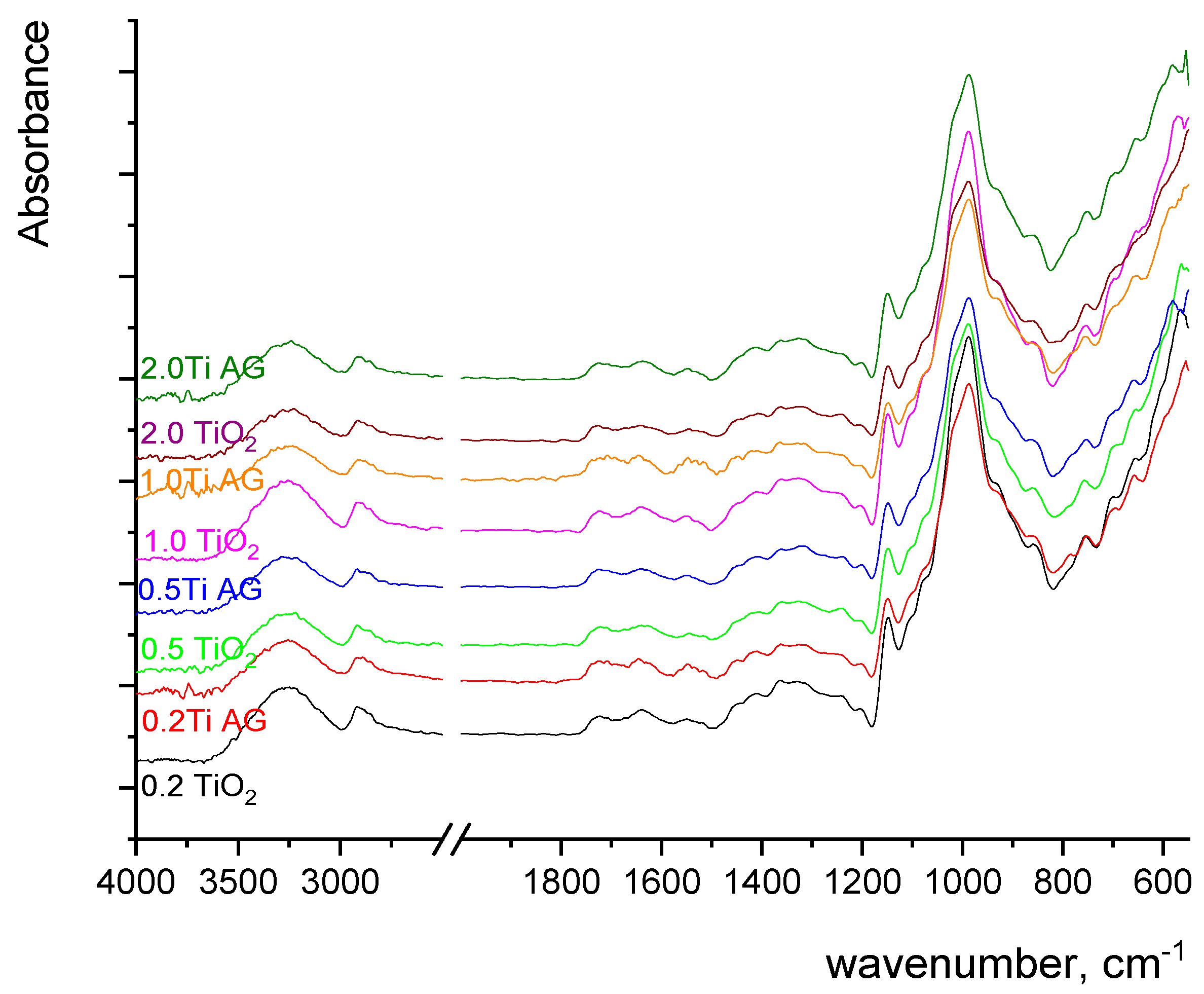
2.2.4. Fourier Transform Infrared (FTIR) Spectroscopy
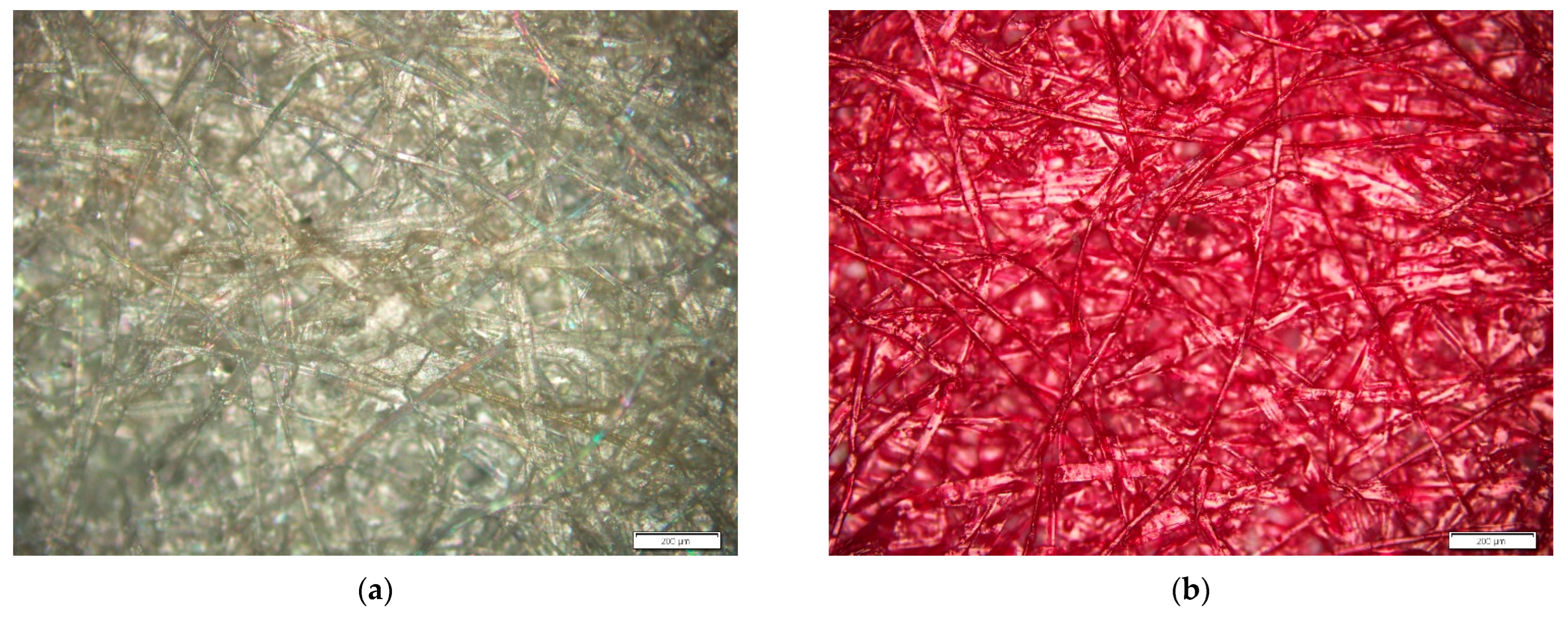
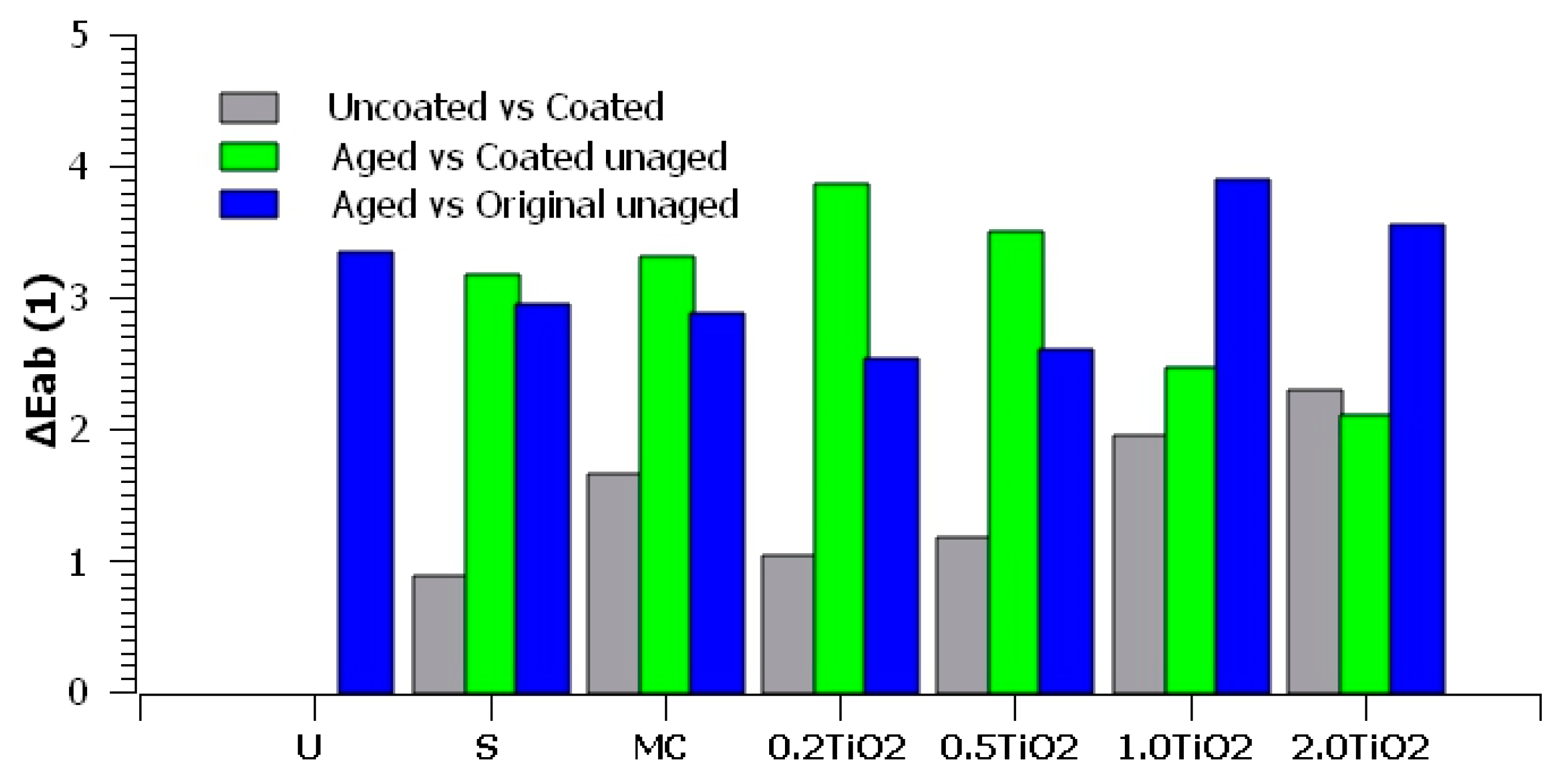
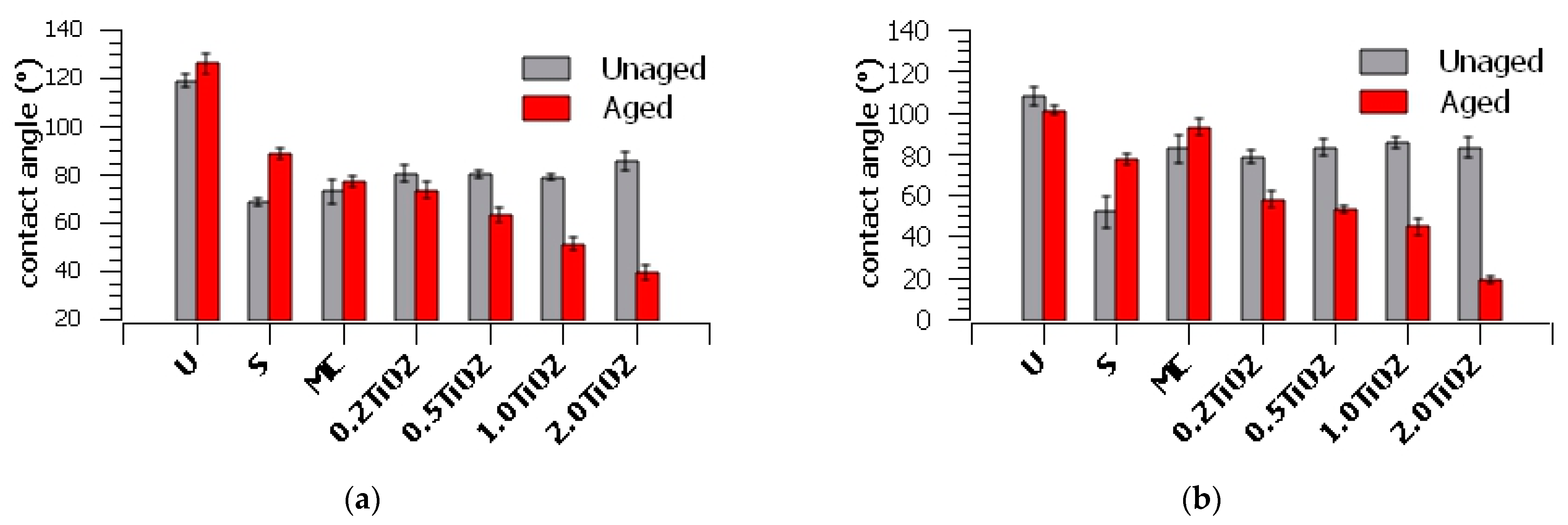
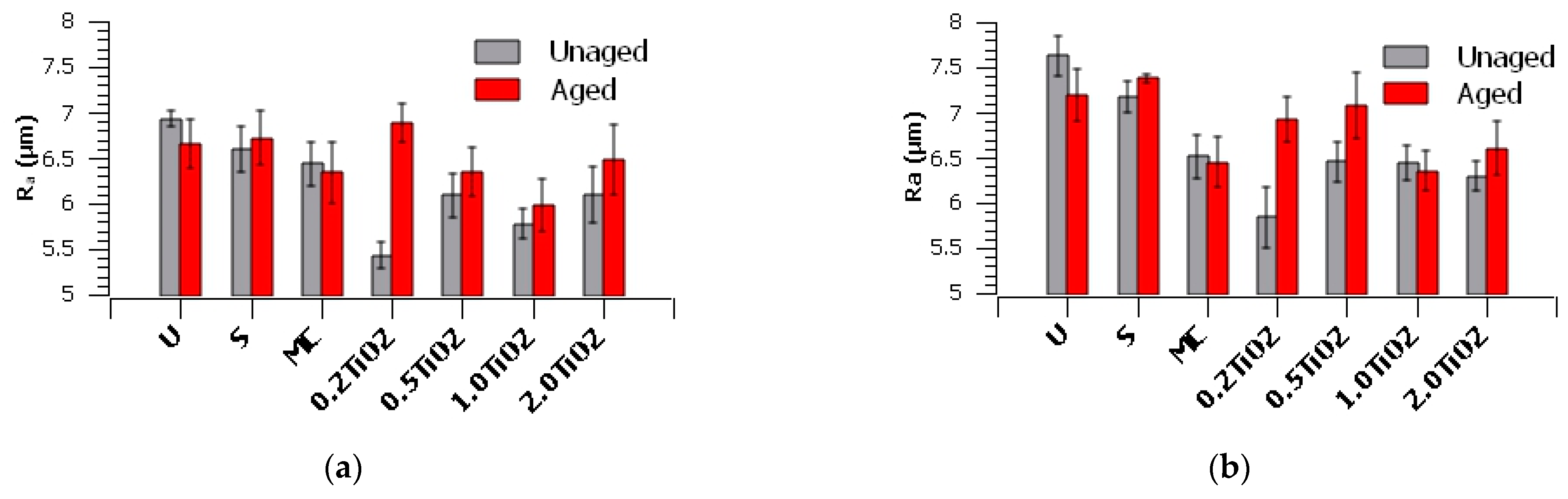
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Canadian Conservation Institute Agents of Deterioration. Available online: https://www.canada.ca/en/conservation-institute/services/agents-deterioration.html (accessed on 25 March 2023).
- Kolar, J.; Strlič, M.; Malešič, J.; Lemaire, J.; Fromageot, D. Photooxidative Degradation. In Ageing and Stabilisation of Paper; Strlič, M., Kolar, J., Eds.; National and University Library: Ljubljana, Slovenia, 2005; pp. 149–162. [Google Scholar]
- Izdebska, J. Aging and Degradation of Printed Materials. In Printing on Polymers: Fundamentals and Applications; Izdebska, J., Thomas, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 353–370. ISBN 9780323374682. [Google Scholar]
- Michalski, S. Agent of Deterioration: Light, Ultraviolet and Infrared. Available online: https://www.canada.ca/en/conservation-institute/services/agents-deterioration/light.html (accessed on 20 March 2023).
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Deacidification of Paper, Canvas and Wood. In Nanotechnologies in the Conservation of Cultural Heritage: A Compendium of Materials and Techniques; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; pp. 117–144. [Google Scholar]
- IFLA Core Programme on Preservation and Conservation (PAC); Council on Library and Information Resources (CLIR); Adcock, E.P.; Varlamoff, M.-T.; Kremp, V. IFLA Principles for the Care and Handling of Library Material; International Preservation Issues: No. 1; IFLA: Edinburgh, UK, 1998. [Google Scholar]
- Kroftová, K.; Šmidtová, M.; Kuřitka, I.; Škoda, D. Nanotechnology in the Cultural Heritage—Influence of Nanosuspensions Adopted by Nanoparticles of TiO2 for Cleaning the Surface of Historical Plasters. Stavební Obz. Civ. Eng. J. 2017, 26, 216–221. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Innovative Nanomaterials: Principles, Availability and Scopes. In Nanotechnologies in the Conservation of Cultural Heritage: A Compendium of Materials and Techniques; Springer Science + Business Media: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Bonini, M.; Baglioni, P.; Chelazzi, D. Inorganic Nanomaterials: Synthesis and Properties. In Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 315–344. [Google Scholar]
- Wójciak, A. Deacidification of Paper with Mg(OH)2 Nanoparticles: The Impact of Dosage on Process Effectiveness. Wood Res. 2016, 61, 937–950. [Google Scholar]
- Malešič, J.; Kadivec, M.; Kunaver, M.; Skalar, T.; Cigić, I. Nano Calcium Carbonate versus Nano Calcium Hydroxide in Alcohols as a Deacidification Medium for Lignocellulosic Paper. Herit. Sci. 2019, 7, 50. [Google Scholar] [CrossRef]
- Afsharpour, M.; Imani, S. Preventive Protection of Paper Works by Using Nanocomposite Coating of Zinc Oxide. J. Cult. Herit. 2017, 25, 142–148. [Google Scholar] [CrossRef]
- Afsharpour, M.; Imani, S.; Abdolmohammadi, S. Zno Nanocomposites: Control of Enviromental Effects for Preservation of Old Manuscripts. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2011, 5, 298–300. [Google Scholar]
- Poggi, G.; Sistach, M.C.; Marin, E.; Garcia, J.F.; Giorgi, R.; Baglioni, P. Calcium Hydroxide Nanoparticles in Hydroalcoholic Gelatin Solutions (GeolNan) for the Deacidification and Strengthening of Papers Containing Iron Gall Ink. J. Cult. Herit. 2016, 18, 250–257. [Google Scholar] [CrossRef]
- Poggi, G.; Toccafondi, N.; Melita, L.N.; Knowles, J.C.; Bozec, L.; Giorgi, R.; Baglioni, P. Calcium Hydroxide Nanoparticles for the Conservation of Cultural Heritage: New Formulations for the Deacidification of Cellulose-Based Artifacts. Appl. Phys. A Mater. Sci. Process. 2014, 114, 685–693. [Google Scholar] [CrossRef]
- Baglioni, P.; Baglioni, M.; Bonelli, N.; Chelazzi, D.; Giorgi, G. Smart Soft Nanomaterials for Cleaning. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Lazzara, G., Fakhrullin, R., Eds.; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2019; pp. 171–204. [Google Scholar]
- Cavallaro, G.; Lazzara, G.; Parisi, F.; Riela, S.; Milioto, S. Nanoclays for Conservation. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Lazzara, G., Fakhrullin, R., Eds.; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2019; pp. 149–170. [Google Scholar]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. Chempluschem 2022, 87, 1–26. [Google Scholar] [CrossRef]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium Dioxide in Our Everyday Life; Is It Safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-Cleaning Applications of TiO2 by Photo-Induced Hydrophilicity and Photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Balliana, E.; Ricci, G.; Pesce, C.; Zendri, E. Assessing the Value of Green Conservation for Cultural Heritage: Positive and Critical Aspects of Already Available Methodologies. Int. J. Conserv. Sci. 2016, 7, 185–202. [Google Scholar]
- Seiß, V.; Thiel, S.; Eichelbaum, M. Preparation and Real World Applications of Titania Composite Materials for Photocatalytic Surface, Air, and Water Purification: State of the Art. Inorganics 2022, 10, 139. [Google Scholar] [CrossRef]
- Afsharpour, M.; Hadadi, M. Titanium Dioxide Thin Film: Environmental Control for Preservation of Paper-Art-Works. J. Cult. Herit. 2014, 15, 569–574. [Google Scholar] [CrossRef]
- Wang, H.; Lu, G.; Zhang, J.; Zheng, D. Multifunctional Nanocomposites for Paper Conservation. Stud. Conserv. 2013, 58, 23–29. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Dal Santo, V.; Naldoni, A. Titanium Dioxide Photocatalysis. Catalysts 2018, 8, 591. [Google Scholar] [CrossRef]
- Horie, C.V. Materials for Conservation: Organic Consolidants, Adhesives and Coatings, 2nd ed.; Rees-Jones, S.G., Linstrum, D., Eds.; Butterworth-Heinemann Series in Conservation and Museology: London, UK, 1987. [Google Scholar]
- Gemmellaro, P. Titanium Dioxide Nanoparticles in the Field of Conservation of Cultural Heritage. Ph.D. Thesis, Università degli Studi di Catania Facoltà di Scienze, Catania, Italy, 2011. [Google Scholar]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for Antimicrobial Nanomaterials in Cultural Heritage Conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- The European Commission Goodbye E171: The EU Bans Titanium Dioxide as a Food Additive. Available online: https://ec.europa.eu/newsroom/sante/items/732079/en (accessed on 29 March 2023).
- Verleysen, E.; Ledecq, M.; Siciliani, L.; Cheyns, K.; Vleminckx, C.; Blaude, M.N.; De Vos, S.; Brassinne, F.; Van Steen, F.; Nkenda, R.; et al. Titanium Dioxide Particles Frequently Present in Face Masks Intended for General Use Require Regulatory Control. Sci. Rep. 2022, 12, 2529. [Google Scholar] [CrossRef]
- Irfan, F.; Tanveer, M.U.; Moiz, M.A.; Husain, S.W.; Ramzan, M. TiO2 as an Effective Photocatalyst Mechanisms, Applications, and Dopants: A Review. Eur. Phys. J. B 2022, 95, 184. [Google Scholar] [CrossRef]
- Shu, H.; Yang, M.; Liu, Q.; Luo, M. Study of TiO2-Modified Sol Coating Material in the Protection of Stone-Built Cultural Heritage. Coatings 2020, 10, 179. [Google Scholar] [CrossRef]
- Ahmed, O.B.; Alamro, T. Evaluation of the Antibacterial Activities of Face Masks Coated with Titanium Dioxide Nanoparticles. Sci. Rep. 2022, 12, 18739. [Google Scholar] [CrossRef] [PubMed]
- Dei, L.; Chelazzi, D. Biomineralization, Geopolymers and Hybrid Nanocomposites. In Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 372–395. [Google Scholar]
- Afsharpour, M.; Rad, F.T.; Malekian, H. New Cellulosic Titanium Dioxide Nanocomposite as a Protective Coating for Preserving Paper-Art-Works. J. Cult. Herit. 2011, 12, 380–383. [Google Scholar] [CrossRef]
- Ariafar, A.A.; Afsharpour, M.; Samanian, K. Use of TiO2/Chitosan Nanoparticles for Enhancing the Preservative Effects of Carboxymethyl Cellulose in Paper-Art-Works against Biodeterioration. Int. Biodeterior. Biodegrad. 2018, 131, 67–77. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with MgO for Paper Reinforcement and Deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite Nanotubes as Sustainable Nanofiller for Paper Consolidation and Protection. J. Therm. Anal. Calorim. 2014, 117, 1293–1298. [Google Scholar] [CrossRef]
- Iwamiya, Y.; Nishio-Hamane, D.; Akutsu-Suyama, K.; Arima-Osonoi, H.; Shibayama, M.; Hiroi, Z. Photocatalytic Silica-Resin Coating for Environmental Protection of Paper as a Plastic Substitute. Ind. Eng. Chem. Res. 2022, 61, 6967–6972. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Consolidation of Wall Paintings and Stone. In Nanotechnologies in the Conservation of Cultural Heritage; Springer: Berlin/Heidelberg, Germany, 2015; pp. 15–59. [Google Scholar]
- da Silva Borges, I.; Casimiro, M.H.; Macedo, M.F.; Sequeira, S.O. Adhesives Used in Paper Conservation: Chemical Stability and Fungal Bioreceptivity. J. Cult. Herit. 2018, 34, 53–60. [Google Scholar] [CrossRef]
- IPCI-Canada Colophon. Available online: https://www.ipci-canada.org/files/IPCI-Canada%20Colophon.pdf (accessed on 19 April 2023).
- Werner, S. Studying Early Printed Books 1450–1800: A Practical Guide, 1st ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mizumura, M.; Kubo, T.; Moriki, T. Japanese Paper: History, Development and Use in Western Paper Conservation. In Proceedings of the International Conference of the Icon Book & Paper Group, London, UK, 8–10 April 2015; The Institute of Conservation: London, UK, 2017; pp. 43–59. [Google Scholar]
- Szczepanowska, H.M. Conservation of Cultural Heritage: Key Principles and Approaches, 1st ed.; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Aleksić, G.; Cigula, T.; Itrić Ivanda, K. Influence of Multilayered Films Containing Cellulose Nanocrystals on the Properties of Japanese Paper. In Proceedings of the Eleventh International Symposium GRID 2022; Vladić, G., Ed.; University of Novi Sad, Faculty of Technical Sciences, Department of Graphic Engineering and Design: Novi Sad, Serbia, 2022; pp. 459–466. [Google Scholar]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile? Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Talas Methyl Cellulose. Available online: https://www.talasonline.com/Methyl-Cellulose (accessed on 5 March 2023).
- The Conservation Unit Museums and Galleries Commission. The Science For Conservators Series Volume 1: An Introduction to Materials, 2nd ed.; Ashley-Smith, J., Wilks, H., Eds.; Routledge: London, UK, 1992; Volume 1. [Google Scholar]
- Fairbrass, S. Sticky Problems for Conservators of Works of Art on Paper. Int. J. Adhes. Adhes. 1995, 15, 115–120. [Google Scholar] [CrossRef]
- Vodopivec Tomašič, J.; Avguštin Florjančič, B.; Grkman, S.; Černič, M.; Ljuba, M.; Haraurer, D.; Kotar, M.; Planinc, L.; Petelin, N.; Rahovsky Šuligoj, T. The Dalmatin Bible—Structure and Conservation. Vjesn. Bibl. Hrvat. 2015, 58, 67–100. [Google Scholar]
- Mokrzycki, W.; Tatol, M. Color Difference Delta E—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar] [CrossRef]
- Xiong, B.; Li, J.; He, C.; Tang, X.; Lv, Z.; Li, X.; Yan, X. Effect of Pore Morphology and Surface Roughness on Wettability of Porous Titania Films. Mater. Res. Express 2020, 7, 115013. [Google Scholar] [CrossRef]
- Caminati, G. Cultural Heritage Artefacts and Conservation: Surfaces and Interfaces. In Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 1–48. [Google Scholar]
- Cigula, T.; Hudika, T.; Donevski, D. Color Reproduction on Varnished Cardboard Packaging by Using Lower Ink Coverages Due to the Gray Component Replacement Image Processing. Color Res. Appl. 2022, 47, 172–181. [Google Scholar] [CrossRef]
- Hynninen, V.; Patrakka, J. Nonappa Methylcellulose–Cellulose Nanocrystal Composites for Optomechanically Tunable Hydrogels and Fibers. Materials 2021, 14, 5137. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Dmitrenko, E.; Penkova, A.V.; Zou, L.; Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Elfaghi, A.M.; et al. Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers 2022, 15, 63. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Moreno, S.M.P.; Bolívar, J.P. TiO2 as White Pigment and Valorization of the Waste Coming from Its Production. In Titanium Dioxide (Tio2) and Its Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 311–335. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Del Campo, A.; Fernández, J.F. Enhancement of UV Absorption Behavior in ZnO–TiO2 Composites. Boletín Soc. Española Cerámica Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Vukoje, M.; Kulčar, R.; Ivanda, K.I.; Bota, J.; Cigula, T. Improvement in Thermochromic Offset Print UV Stability by Applying PCL Nanocomposite Coatings. Polymers 2022, 14, 1484. [Google Scholar] [CrossRef]
- Irie, H.; Tsuji, K.I.; Hashimoto, K. Hydrophobic Anatase TiO2-Based Thin Films Modified with Al, Cr Derivatives to Reach Reversible Wettability Control. Phys. Chem. Chem. Phys. 2008, 10, 3072–3076. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Topolski, A.; Śmigiel, J.; Piszczek, P. The Photocatalytic Activity of Titania Coatings Produced by Electrochemical and Chemical Oxidation of Ti6Al4V Substrate, Estimated According to ISO 10678:2010. Materials 2020, 13, 2649. [Google Scholar] [CrossRef] [PubMed]
- Tkalčec, M.M.; Bistričić, L.; Leskovac, M. Influence of Adhesive Layer on the Stability of Kozo Paper. Cellulose 2016, 23, 853–872. [Google Scholar] [CrossRef]
- Ferreira, P.J.; Gamelas, J.A.; Moutinho, I.M.; Ferreira, A.G.; Gómez, N.; Molleda, C.; Figueiredo, M.M. Application of FT-IR-ATR Spectroscopy to Evaluate the Penetration of Surface Sizing Agents into the Paper Structure. Ind. Eng. Chem. Res. 2009, 48, 3867–3872. [Google Scholar] [CrossRef]
- Łojewski, T.; Miśkowiec, P.; Molenda, M.; Lubańska, A.; Łojewska, J. Artificial versus Natural Ageing of Paper. Water Role in Degradation Mechanisms. Appl. Phys. A Mater. Sci. Process 2010, 100, 625–633. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, T.; He, F.; Ou, Z.; Xu, J.; Ren, L. Preparation and Characterization of Functional Films Based on Chitosan and Corn Starch Incorporated Tea Polyphenols. Coatings 2021, 11, 817. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Applications and Trends. In Biopolymers: Applications and Trends; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–604. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksić, G.; Cigula, T.; Vukoje, M.; Itrić Ivanda, K. Bilayer Coating Composed of Starch and Methyl Cellulose-Nanoscale TiO2 for the Protection of Historic Paper from UV. Coatings 2023, 13, 899. https://doi.org/10.3390/coatings13050899
Aleksić G, Cigula T, Vukoje M, Itrić Ivanda K. Bilayer Coating Composed of Starch and Methyl Cellulose-Nanoscale TiO2 for the Protection of Historic Paper from UV. Coatings. 2023; 13(5):899. https://doi.org/10.3390/coatings13050899
Chicago/Turabian StyleAleksić, Gabriela, Tomislav Cigula, Marina Vukoje, and Katarina Itrić Ivanda. 2023. "Bilayer Coating Composed of Starch and Methyl Cellulose-Nanoscale TiO2 for the Protection of Historic Paper from UV" Coatings 13, no. 5: 899. https://doi.org/10.3390/coatings13050899
APA StyleAleksić, G., Cigula, T., Vukoje, M., & Itrić Ivanda, K. (2023). Bilayer Coating Composed of Starch and Methyl Cellulose-Nanoscale TiO2 for the Protection of Historic Paper from UV. Coatings, 13(5), 899. https://doi.org/10.3390/coatings13050899









