Atmospheric Pressure Plasma Treatment of Magnesium Alloy for Enhanced Coating Adhesion and Corrosion Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
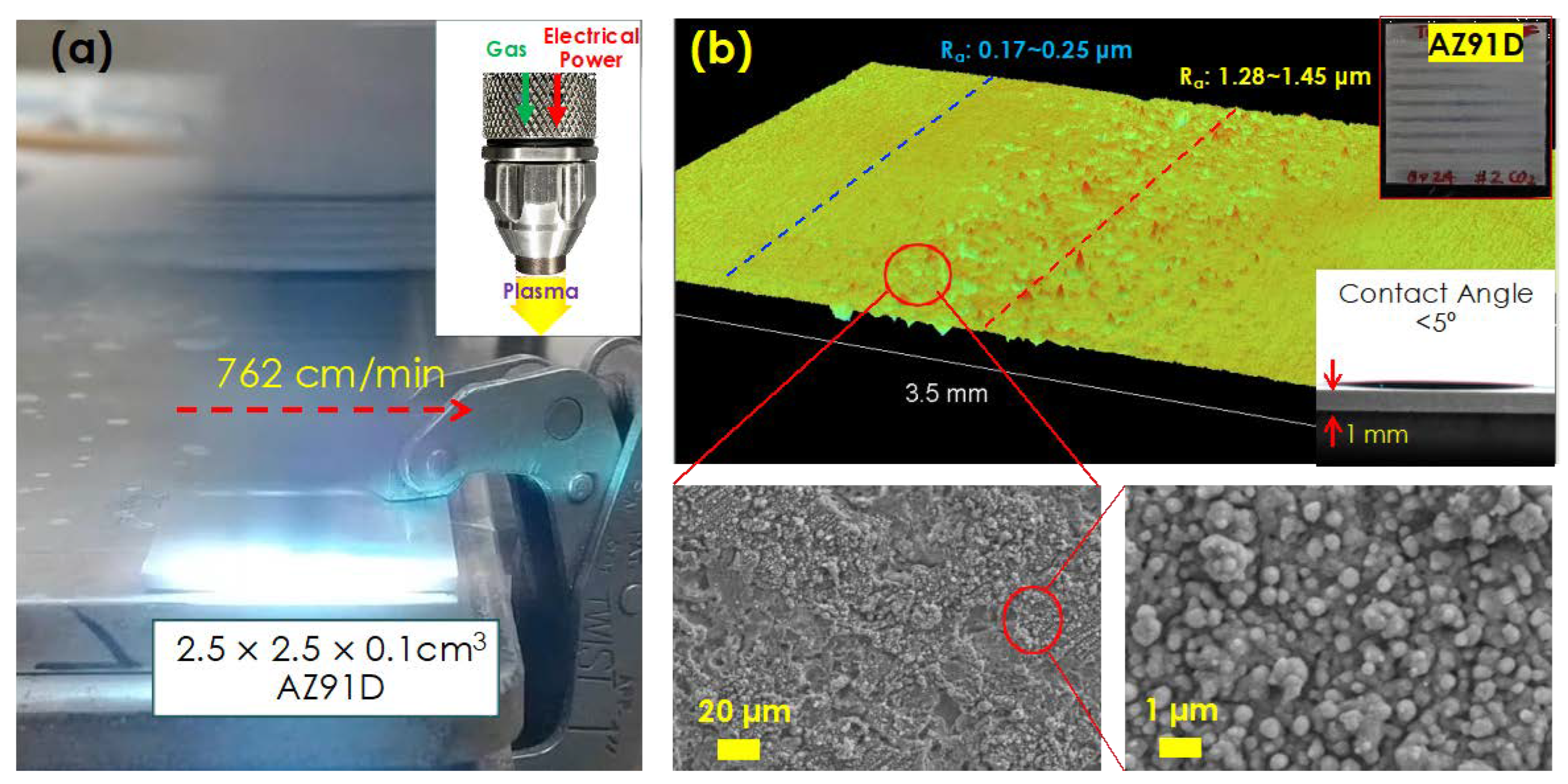
2.2. CO2/Air Atmospheric Pressure Plasma Treatment of AZ91D Substrate
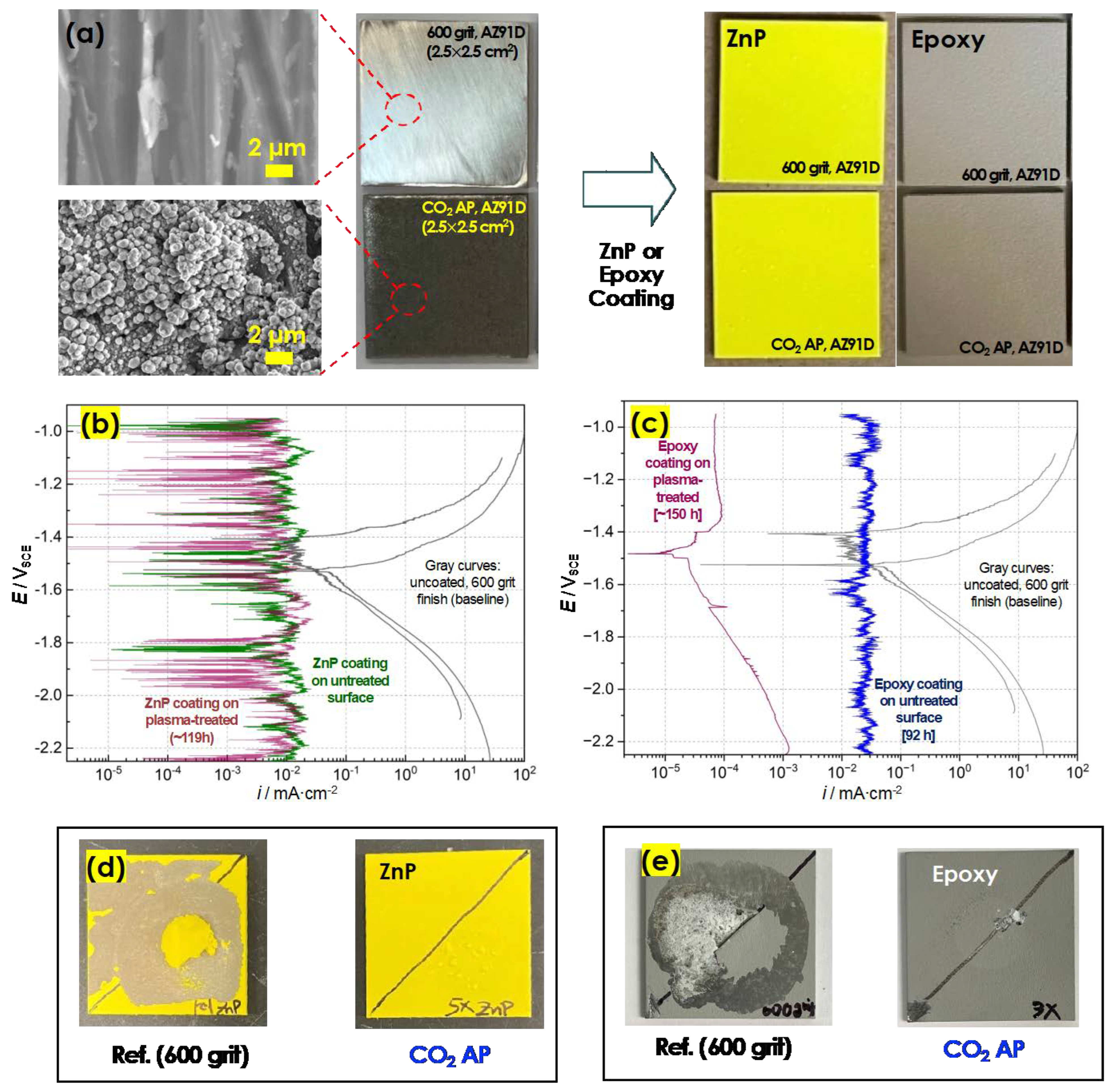
2.3. Primer Coatings
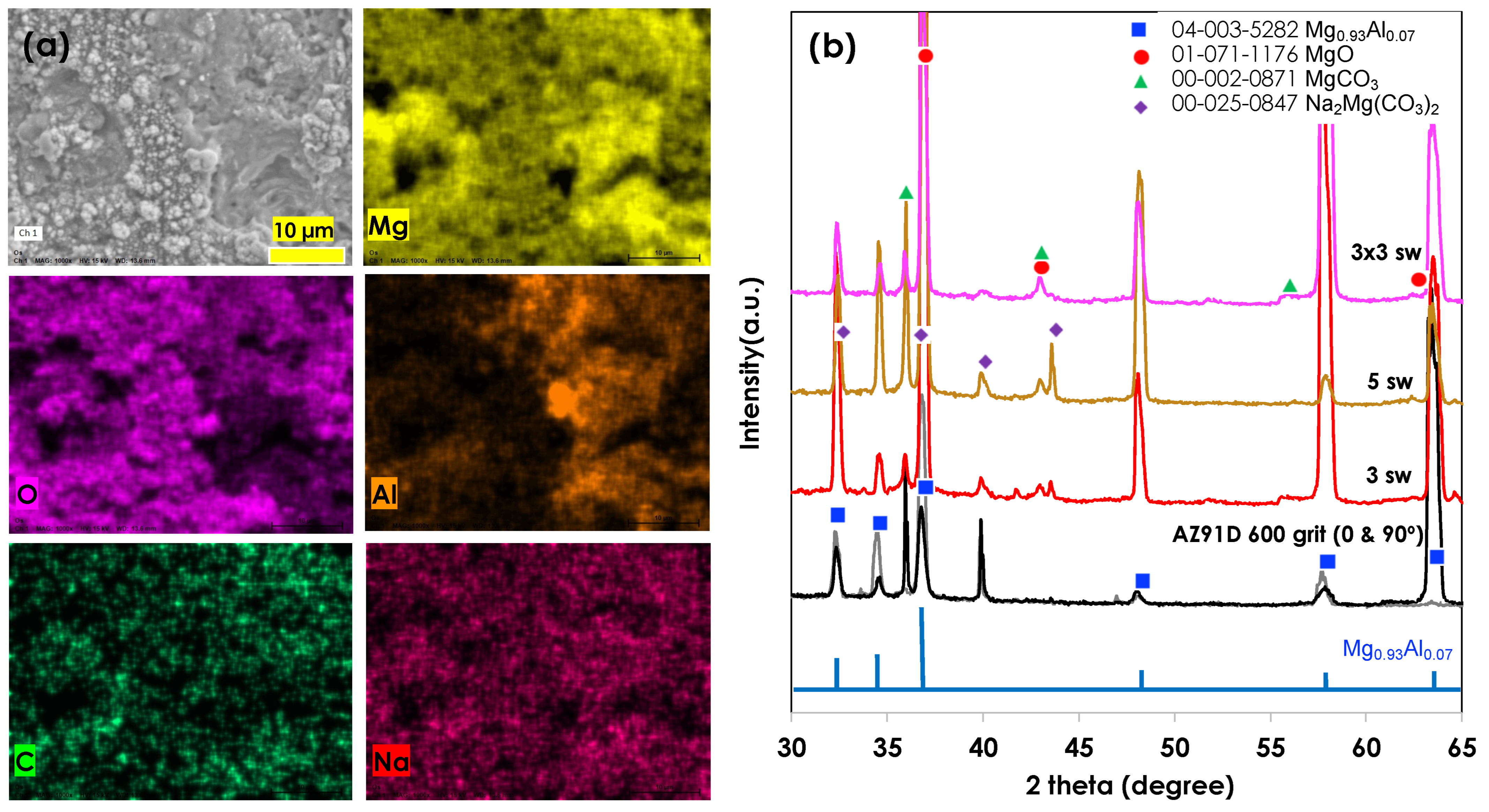
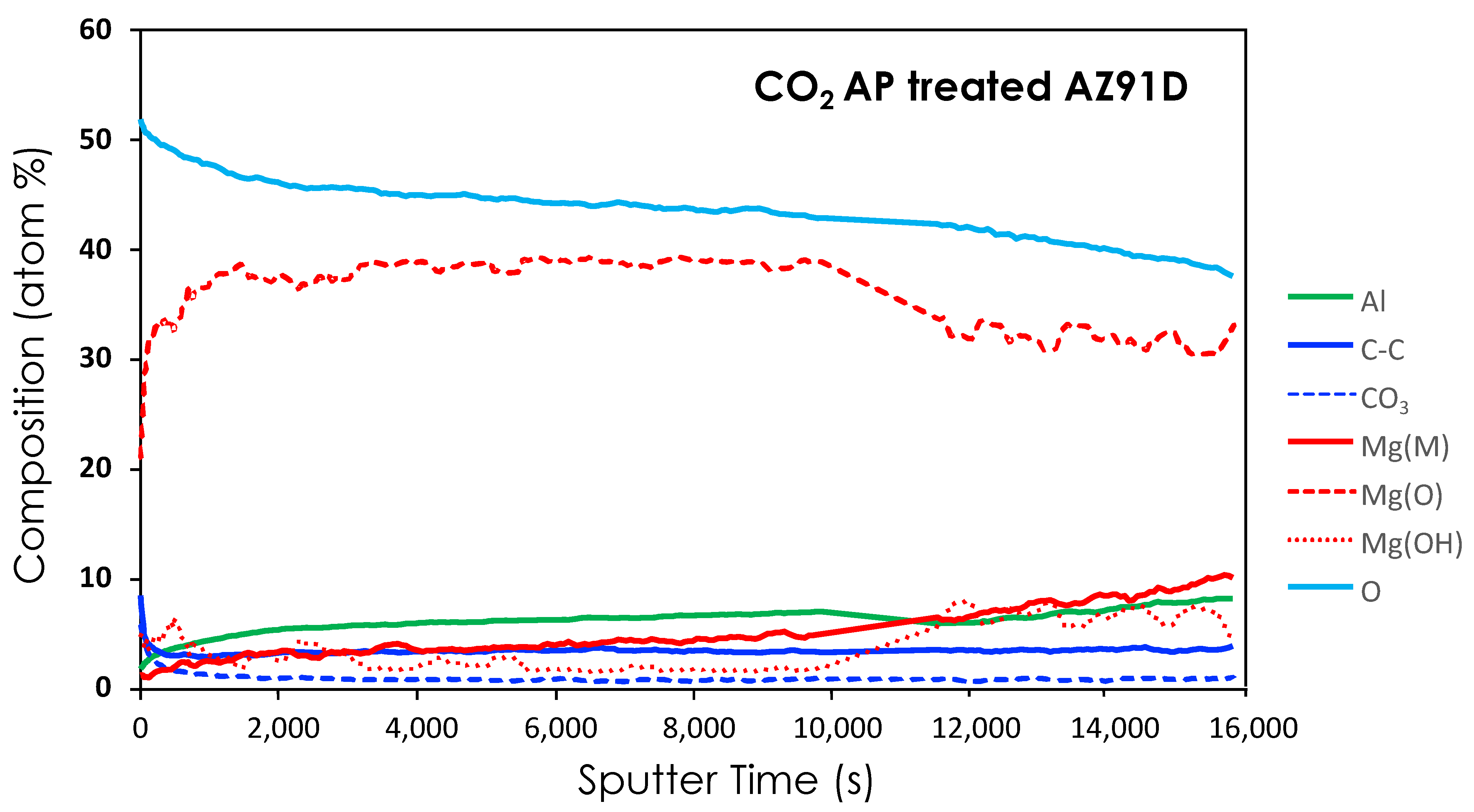
2.4. Characterization of Materials
2.5. Corrosion Evaluation
2.6. Preliminary Cost Estimation
3. Results and Discussion
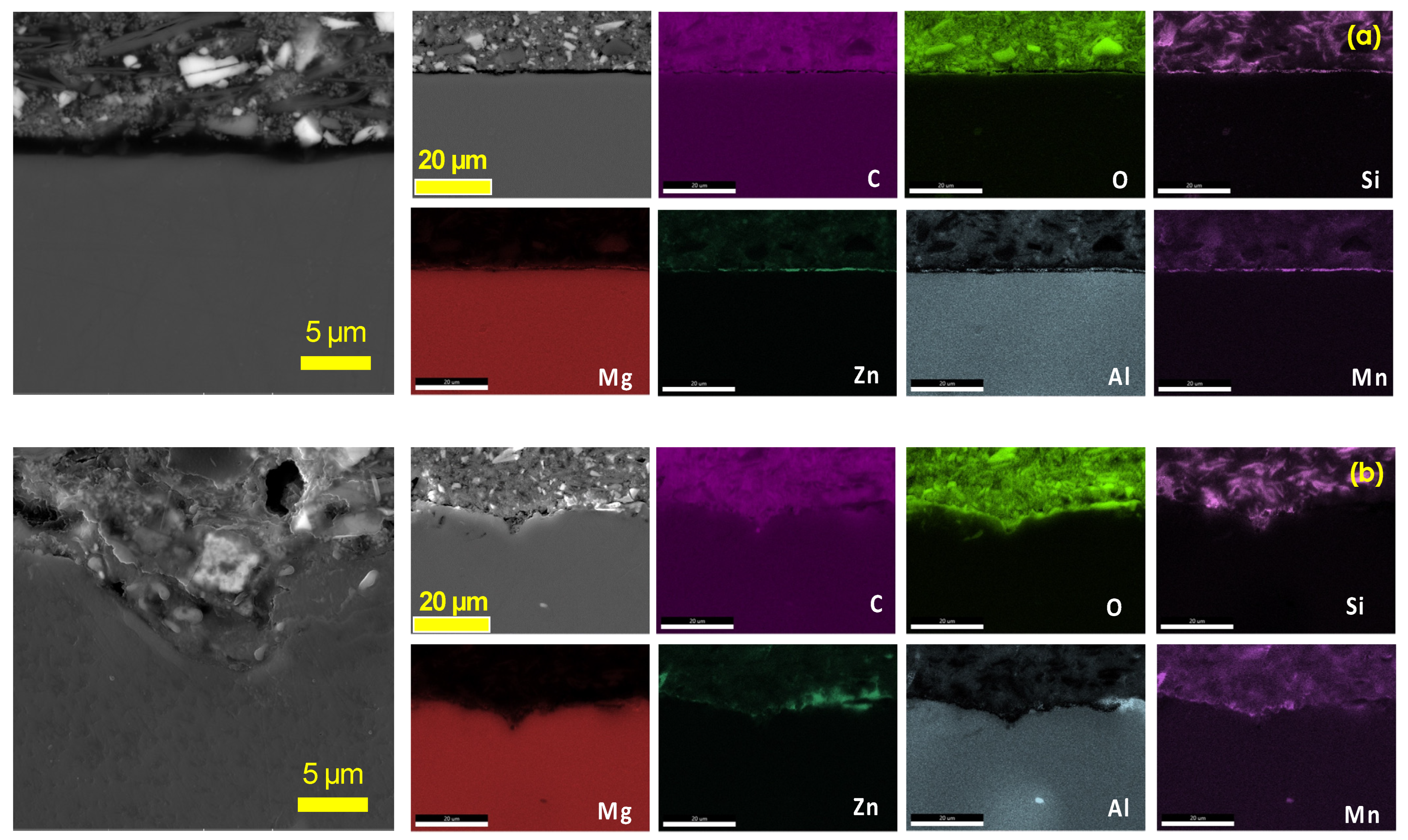
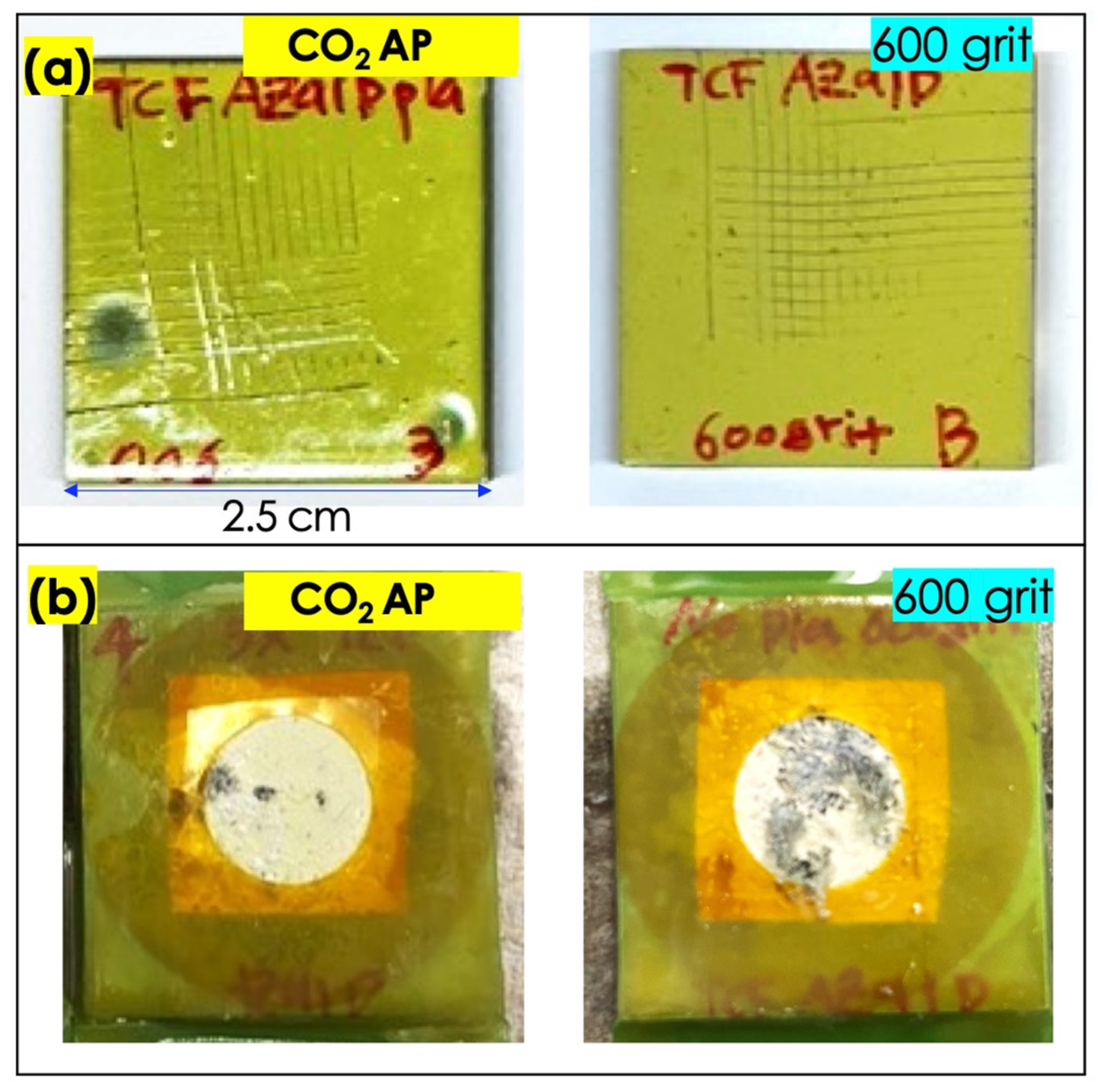
3.1. CO2 Atmospheric Plasma Treatment
3.2. Enhancement of Adhesion Bonding
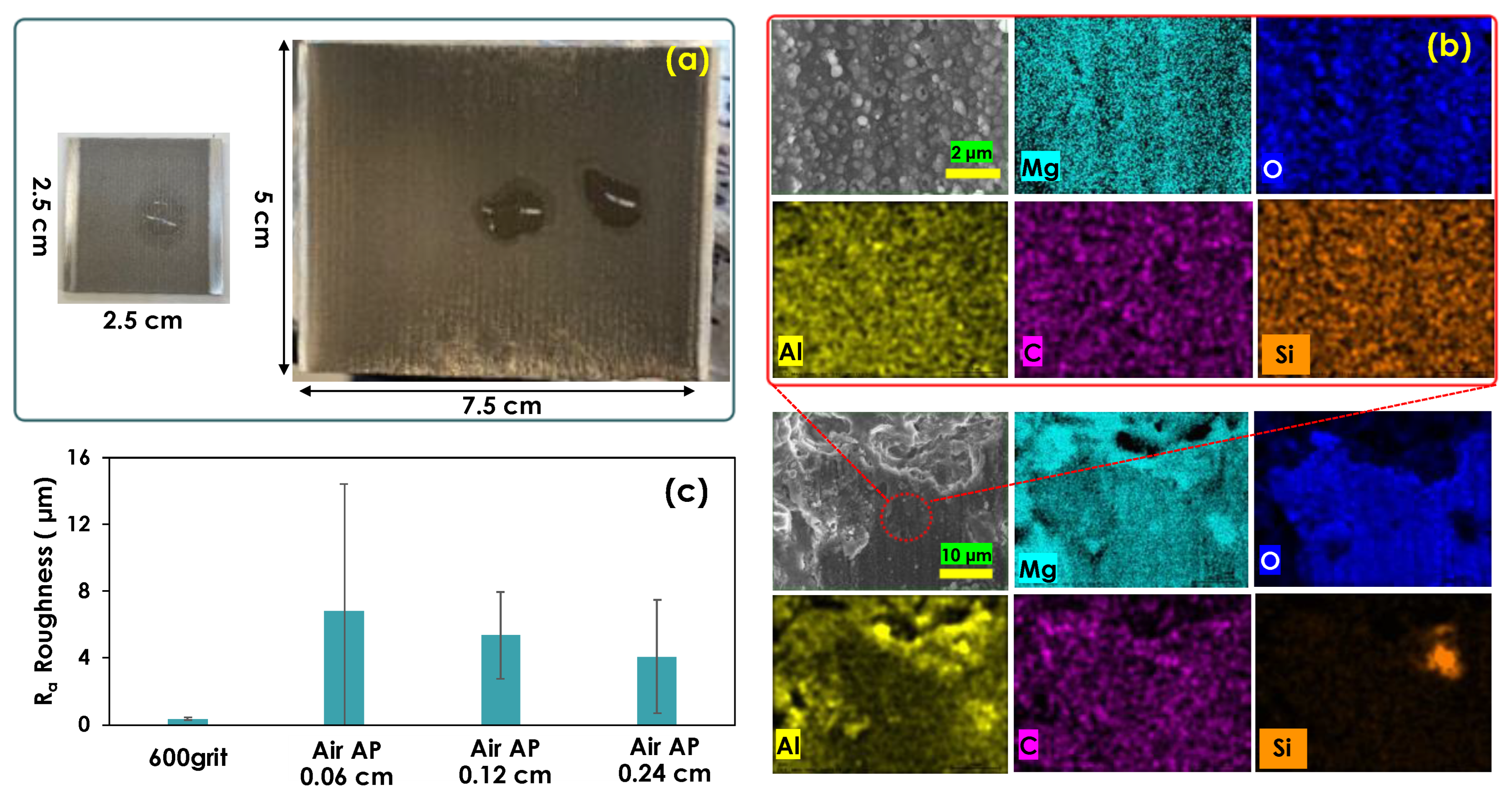
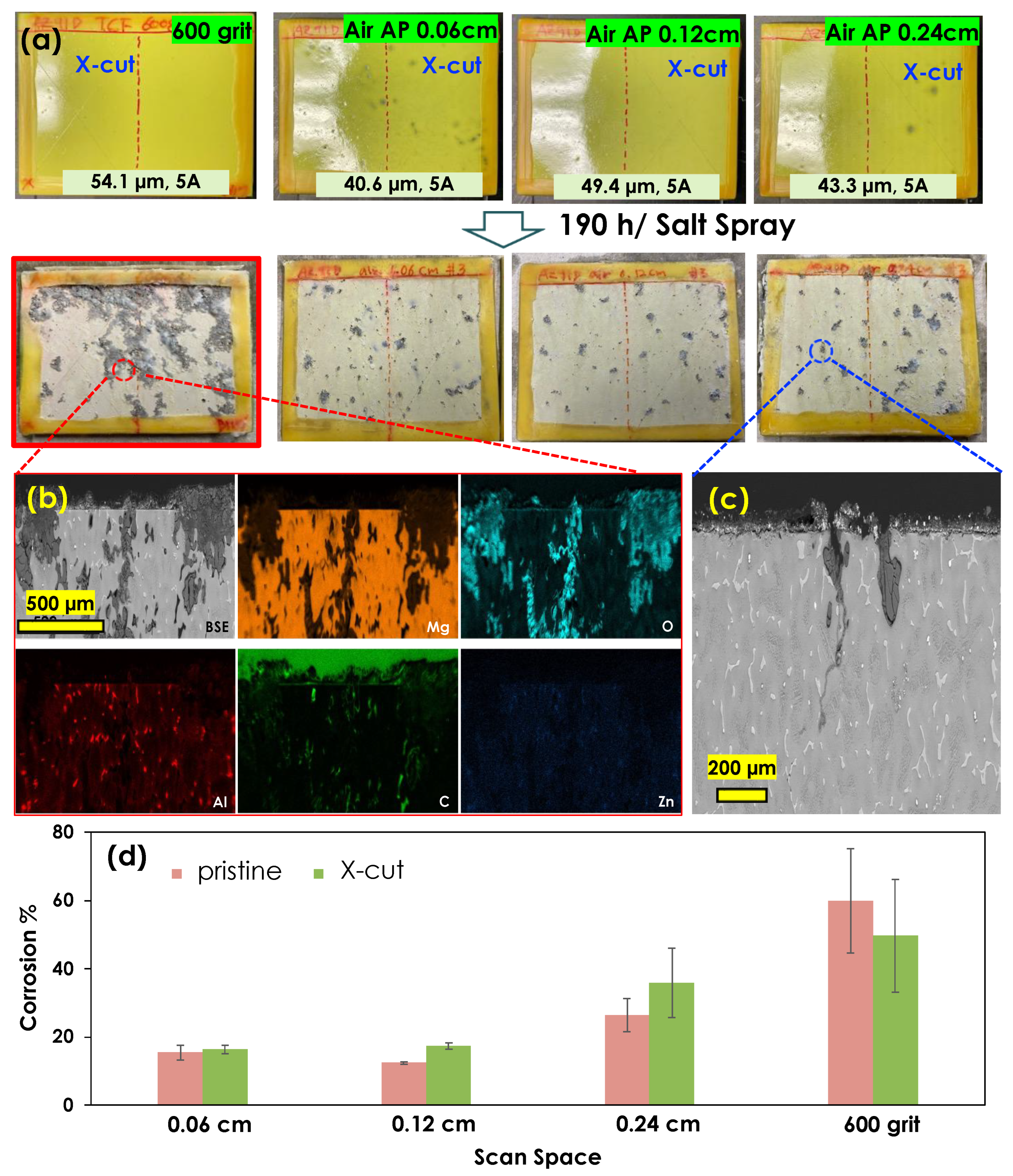
3.3. Air Atmospheric Plasma Treatment
3.4. Nitrogen Atmospheric Plasma Treatment
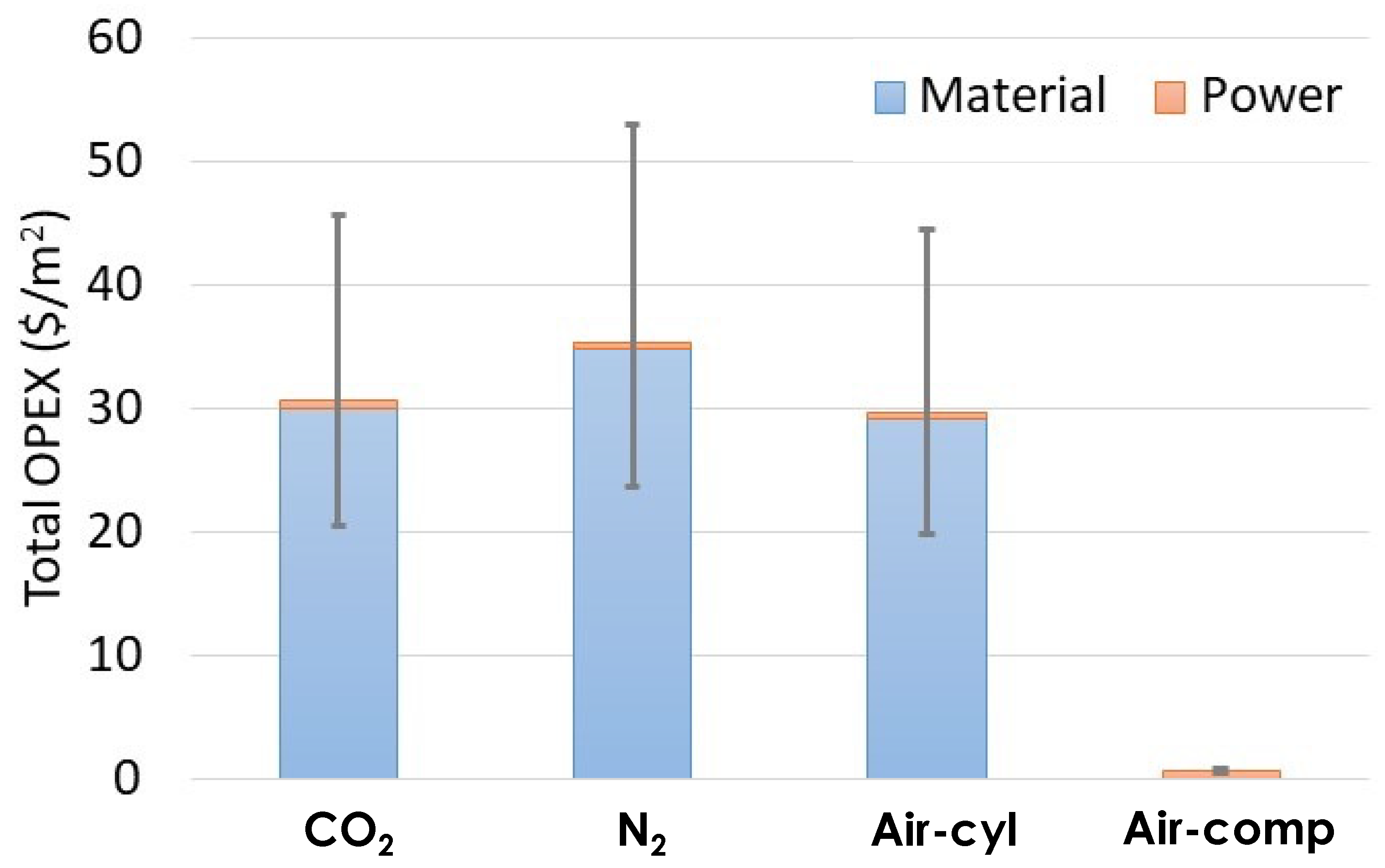
3.5. Cost Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czerwinski, F. Current Trends in Automotive Lightweighting Strategies and Materials. Materials 2021, 14, 6631. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Phanden, R.K.; Thakur, L. A review on environment friendly and lightweight Magnesium-Based metal matrix composites and alloys. Mater Today-Proc. 2021, 38, 359–364. [Google Scholar] [CrossRef]
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. Scr. Mater. 2017, 128, 107–112. [Google Scholar] [CrossRef]
- Brady, M.P.; Joost, W.J.; Warren, C.D. Insights from a Recent Meeting: Current Status and Future Directions in Magnesium Corrosion Research. Corrosion 2017, 73, 452–462. [Google Scholar] [CrossRef]
- Bender, S.; Goellner, J.; Heyn, A.; Boese, E. Corrosion and corrosion testing of magnesium alloys. Mater. Corros. 2007, 58, 977–982. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Y.; Wang, J.; Yergin, M. Corrosion avoidance in lightweight materials for automotive applications. NPJ Mater. Degrad. 2018, 2, 24. [Google Scholar] [CrossRef]
- Xu, W.Q.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.; Lamaka, S. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: PART I-Pre-Treatment and Conversion Coating. Materials 2022, 15, 8676. [Google Scholar] [CrossRef] [PubMed]
- Dzikova, J.; Fintova, S.; Kajanek, D.; Florkova, Z.; Wasserbauer, J.; Dolezal, P. Characterization and Corrosion Properties of Fluoride Conversion Coating Prepared on AZ31 Magnesium Alloy. Coatings 2021, 11, 675. [Google Scholar] [CrossRef]
- Cipriano, A.F.; Lin, J.J.; Miller, C.; Lin, A.; Alcaraz, M.C.C.; Soria, P.; Liu, H.N. Anodization of magnesium for biomedical applications—Processing, characterization, degradation and cytocompatibility. Acta Biomater. 2017, 62, 397–417. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Mehner, T.; Lampke, T. Characterisation Method of the Passivation Mechanisms during the pre-discharge Stage of Plasma Electrolytic Oxidation Indicating the Mode of Action of Fluorides in PEO of Magnesium. Coatings 2020, 10, 965. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Yu, G.; Ouyang, Y.J.; He, X.M.; Hu, B.N.; Zhang, J.; Wu, Z.J. Studies on influence of zinc immersion and fluoride on nickel electroplating on magnesium alloy AZ91D. Appl. Surf. Sci. 2009, 255, 7773–7779. [Google Scholar] [CrossRef]
- Lu, F.F.; Ma, K.; Li, C.X.; Yasir, M.; Luo, X.T.; Li, C.J. Enhanced corrosion resistance of cold-sprayed and shot-peened aluminum coatings on LA43M magnesium alloy. Surf. Coat. Tech. 2020, 394, 125865. [Google Scholar] [CrossRef]
- Yao, H.L.; Hu, X.Z.; Wang, H.T.; Chen, Q.Y.; Bai, X.B.; Zhang, M.X.; Ji, G.C. Microstructure and Corrosion Behavior of Thermal-Sprayed Hydroxyapatite/Magnesium Composite Coating on the Surface of AZ91D Magnesium Alloy. J. Therm. Spray Technol. 2019, 28, 495–503. [Google Scholar] [CrossRef]
- Polizos, G.; Jang, G.G.; Smith, D.B.; List, F.A.; Lassiter, M.G.; Park, J.; Datskos, P.G. Transparent superhydrophobic surfaces using a spray coating process. Sol. Energy Mater. Sol. Cells 2018, 176, 405–410. [Google Scholar] [CrossRef]
- Jang, G.G.; Smith, D.B.; Polizos, G.; Collins, L.; Keum, J.K.; Lee, D.F. Transparent superhydrophilic and superhydrophobic nanoparticle textured coatings: Comparative study of anti-soiling performance. Nanoscale Adv. 2019, 1, 1249–1260. [Google Scholar] [CrossRef]
- Jang, G.G.; Smith, D.B.; List, F.A.; Lee, D.F.; Ievlev, A.V.; Collins, L.; Park, J.; Polizos, G. The anti-soiling performance of highly reflective superhydrophobic nanoparticle-textured mirrors. Nanoscale 2018, 10, 14600–14612. [Google Scholar] [CrossRef]
- Saji, V.S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloy. 2021, 9, 748–778. [Google Scholar] [CrossRef]
- Lu, X.Y.; Zuo, Y.; Zhao, X.H.; Tang, Y.M.; Feng, X.G. The study of a Mg-rich epoxy primer for protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 153–160. [Google Scholar] [CrossRef]
- Lu, X.Y.; Sun, S.C.; Fan, Q.Q.; Pei, X.J.; Dun, Y.C.; Feng, X.G.; Zou, C.; Lu, W. Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain. Coatings 2019, 9, 649. [Google Scholar] [CrossRef]
- Akafuah, N.K.; Poozesh, S.; Salaimeh, A.; Patrick, G.; Lawler, K.; Saito, K. Evolution of the Automotive Body Coating Process-A Review. Coatings 2016, 6, 24. [Google Scholar] [CrossRef]
- Boerio, F.J.; Roby, B.; Dillingham, R.G.; Bossi, R.H.; Crane, R.L. Effect of grit-blasting on the surface energy of graphite/epoxy composites. J. Adhes. 2006, 82, 19–37. [Google Scholar] [CrossRef]
- Maroofi, A.; Safa, N.N.; Ghomi, H. Atmospheric air plasma jet for improvement of paint adhesion to aluminium surface in industrial applications. Int. J. Adhes. Adhes. 2020, 98, 102554. [Google Scholar] [CrossRef]
- Yim, J.H.; Rodriguez-Santiago, V.; Williams, A.A.; Gougousi, T.; Pappas, D.D.; Hirvonen, J.K. Atmospheric pressure plasma enhanced chemical vapor deposition of hydrophobic coatings using fluorine-based liquid precursors. Surf. Coat. Tech. 2013, 234, 21–32. [Google Scholar] [CrossRef]
- Jha, S.; Bhowmik, S.; Bhatnagar, N.; Bhattacharya, N.K.; Deka, U.; Iqbal, H.M.S.; Benedictus, R. Experimental Investigation into the Effect of Adhesion Properties of PEEK Modified by Atmospheric Pressure Plasma and Low Pressure Plasma. J. Appl. Polym. Sci 2010, 118, 173–179. [Google Scholar] [CrossRef]
- Dupuis, A.; Ho, T.H.; Fahs, A.; Lafabrier, A.; Louarn, G.; Bacharouche, J.; Airoudj, A.; Aragon, E.; Chailan, J.F. Improving adhesion of powder coating on PEEK composite: Influence of atmospheric plasma parameters. Appl. Surf. Sci. 2015, 357, 1196–1204. [Google Scholar] [CrossRef]
- Wu, C.C.; Demaree, J.D.; Weerasooriya, A.; Bujanda, A.; Robinette, E.J. Enhanced Interfacial Adhesion of Nylon 66 to Epoxy Resin EPON 825 by Non-thermal Atmospheric Pressure Dielectric Barrier Discharge Plasmas. Coatings 2022, 12, 919. [Google Scholar] [CrossRef]
- Munoz, J.; Bravo, J.A.; Calzada, M.D. Aluminum metal surface cleaning and activation by atmospheric-pressure remote plasma. Appl. Surf. Sci. 2017, 407, 72–81. [Google Scholar] [CrossRef]
- Rodriguez-Villanueva, C.; Encinas, N.; Abenojar, J.; Martinez, M.A. Assessment of atmospheric plasma treatment cleaning effect on steel surfaces. Surf. Coat. Tech. 2013, 236, 450–456. [Google Scholar] [CrossRef]
- Favaro, M.; Patelli, A.; Ceccato, R.; Dire, S.; Callone, E.; Fredi, G.; Quaranta, A. Thin Films of Plasma-Polymerized n-Hexane and ZnO Nanoparticles Co-Deposited via Atmospheric Pressure Plasma Jet. Coatings 2021, 11, 167. [Google Scholar] [CrossRef]
- Jang, G.G.; Yeom, S.; Keum, J.K.; Yoon, M.; Meyer, H.I.; Su, Y.F.; Jun, J. Formation of carbon and oxygen rich surface layer on high purity magnesium by atmospheric carbon dioxide plasma. J. Magnes. Alloy. 2023, 11, 88–99. [Google Scholar] [CrossRef]
- Pappas, D.; Guist, S.; Ben Salem, D. Plasma Surface Engineering: An Enabling Technology Designed to Clean and Protect Printed Circuit Boards. In International Symposium on Microelectronics; International Microelectronics Assembly and Packaging Society: Pittsburgh, PA, USA, 2020; Volume 2020, pp. 000197–000200. [Google Scholar]
- ASTM B117-16; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- KUB. Business Electric Rates as of May 2022. Available online: https://www.kub.org/bills-payments/understand-your-bill/business-rates/business-electric-rate-schedules/ (accessed on 14 June 2022).
- Industrial Grade Carbon Dioxide, 50 Pound High Pressure Steel Cylinder, CGA-320. Available online: https://www.airgas.com/product/Gases/Carbon-Dioxide/p/CD%2050S (accessed on 5 April 2022).
- Industrial Grade Nitrogen, Size 300 High Pressure Steel Cylinder, CGA-580. Available online: https://www.airgas.com/product/Gases/Nitrogen/p/NI%20300 (accessed on 26 September 2022).
- Breathing Grade Air, Size 300 High Pressure Steel Cylinder, CGA-346. Available online: https://www.airgas.com/p/AI%20B300 (accessed on 2 February 2023).
- MSG® Centac® C400 Centrifugal Air Compressor. Available online: https://www.ingersollrand.com/en-us/air-compressor/centrifugal-air-compressors/45-70-m3min-1600-2350-cfm (accessed on 14 February 2023).
- Hu, Y.C.; Guo, Y.F.; Sun, J.; Li, H.L.; Liu, W.Q. Progress in MgO sorbents for cyclic CO2 capture: A comprehensive review. J. Mater. Chem. A 2019, 7, 20103–20120. [Google Scholar] [CrossRef]
- Association, E. Applied Costs of Electrocoating. 2016. Available online: https://electrocoat.wildapricot.org/resources/Pictures/ECA_CostsBrochure_EmailPages.pdf (accessed on 4 January 2023).
- Kurczewski, N. How Much Does It Cost to Paint a Car? Car and Driver. 14 May 2019. Available online: https://www.caranddriver.com/features/a27438340/cost-to-paint-car/ (accessed on 4 January 2023).
- Jackson, L. Why there won’t be a solar powered car. The Washington Times, 26 July 2008. Available online: https://www.washingtontimes.com/blog/spinning-wheels-community-car-lovers/2008/jul/26/why-there-wont-be-a-solar-powered-car/ (accessed on 4 January 2023).


| Variable | Gas (CO2/N2) | Air | Unit |
|---|---|---|---|
| Density | 1.870/1.251 | 1.225 | kg/m3 |
| Area scan rate | 0.00456 | 0.00456 | m2/min |
| Gas flow | 70 | 0 | SLPM |
| Airflow | 0 | 33 | SLPM |
| Power | 1.3 | 1.3 | kW |
| Mg | O | C | Al | Cu | F | Na | |
|---|---|---|---|---|---|---|---|
| Beginning of beam sputter | 22.7 | 46.4 | 22.4 | 0 | 1.2 | 7.2 | 0.2 |
| 30 s | 33.1 | 50.5 | 10.3 | 0 | 1.4 | 4.7 | 0.1 |
| 16,000 s | 48.0 | 39.6 | 8.9 | 0.3 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, G.G.; Jun, J.; Yeom, S.; Yoon, M.; Su, Y.F.; Wade, J.; Stephens, M.S.; Keum, J.K. Atmospheric Pressure Plasma Treatment of Magnesium Alloy for Enhanced Coating Adhesion and Corrosion Resistance. Coatings 2023, 13, 897. https://doi.org/10.3390/coatings13050897
Jang GG, Jun J, Yeom S, Yoon M, Su YF, Wade J, Stephens MS, Keum JK. Atmospheric Pressure Plasma Treatment of Magnesium Alloy for Enhanced Coating Adhesion and Corrosion Resistance. Coatings. 2023; 13(5):897. https://doi.org/10.3390/coatings13050897
Chicago/Turabian StyleJang, Gyoung Gug, Jiheon Jun, Sinchul Yeom, Mina Yoon, Yi Feng Su, John Wade, Michael S. Stephens, and Jong K. Keum. 2023. "Atmospheric Pressure Plasma Treatment of Magnesium Alloy for Enhanced Coating Adhesion and Corrosion Resistance" Coatings 13, no. 5: 897. https://doi.org/10.3390/coatings13050897
APA StyleJang, G. G., Jun, J., Yeom, S., Yoon, M., Su, Y. F., Wade, J., Stephens, M. S., & Keum, J. K. (2023). Atmospheric Pressure Plasma Treatment of Magnesium Alloy for Enhanced Coating Adhesion and Corrosion Resistance. Coatings, 13(5), 897. https://doi.org/10.3390/coatings13050897






