Oxidation Behaviors of the NiCrAlY Bond Coats in the Thermal Barrier Coatings under External Loads
Abstract
1. Introduction
2. Procedures
2.1. Experiment
2.2. Model
3. Results
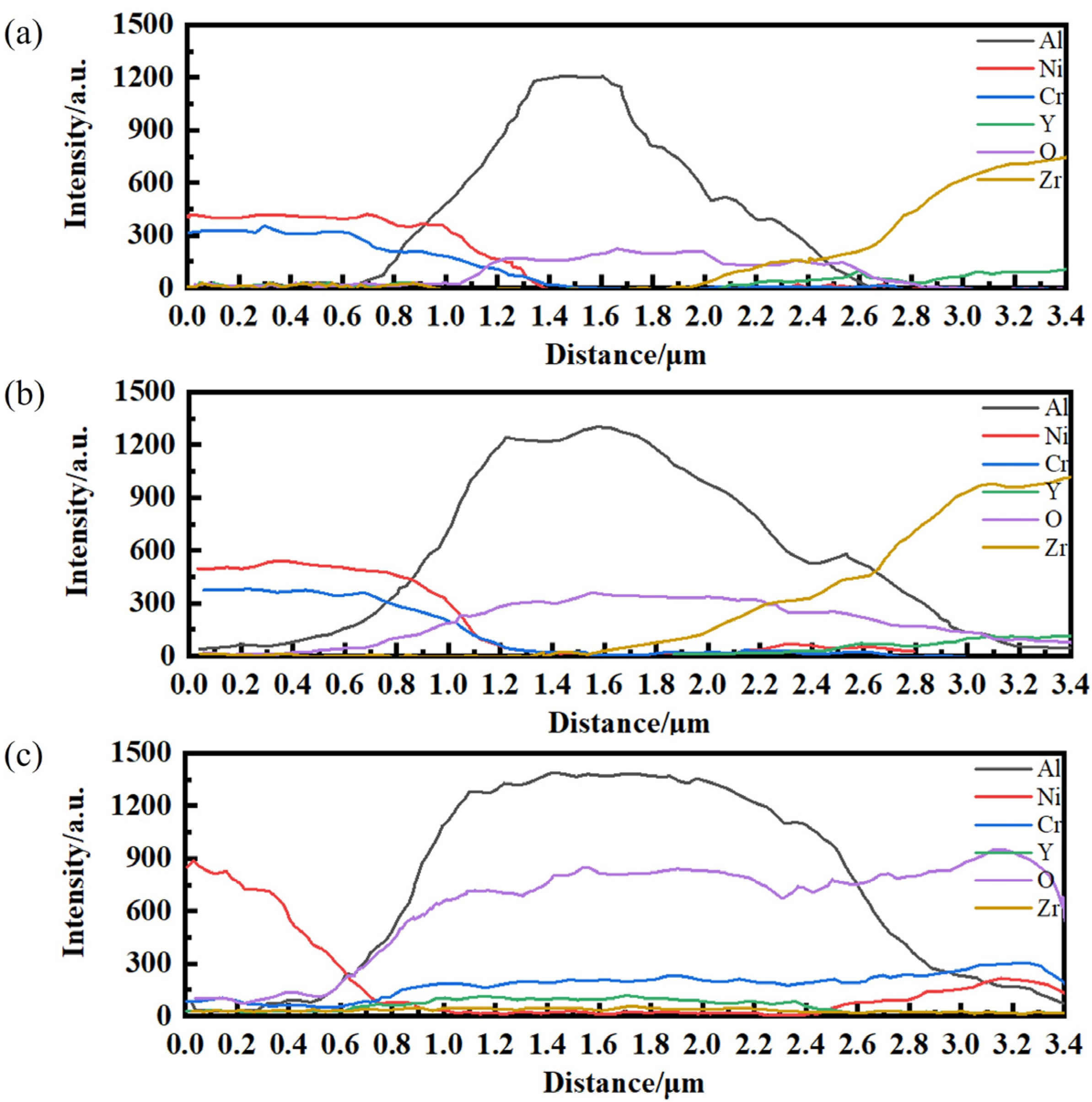
3.1. Experimental Results
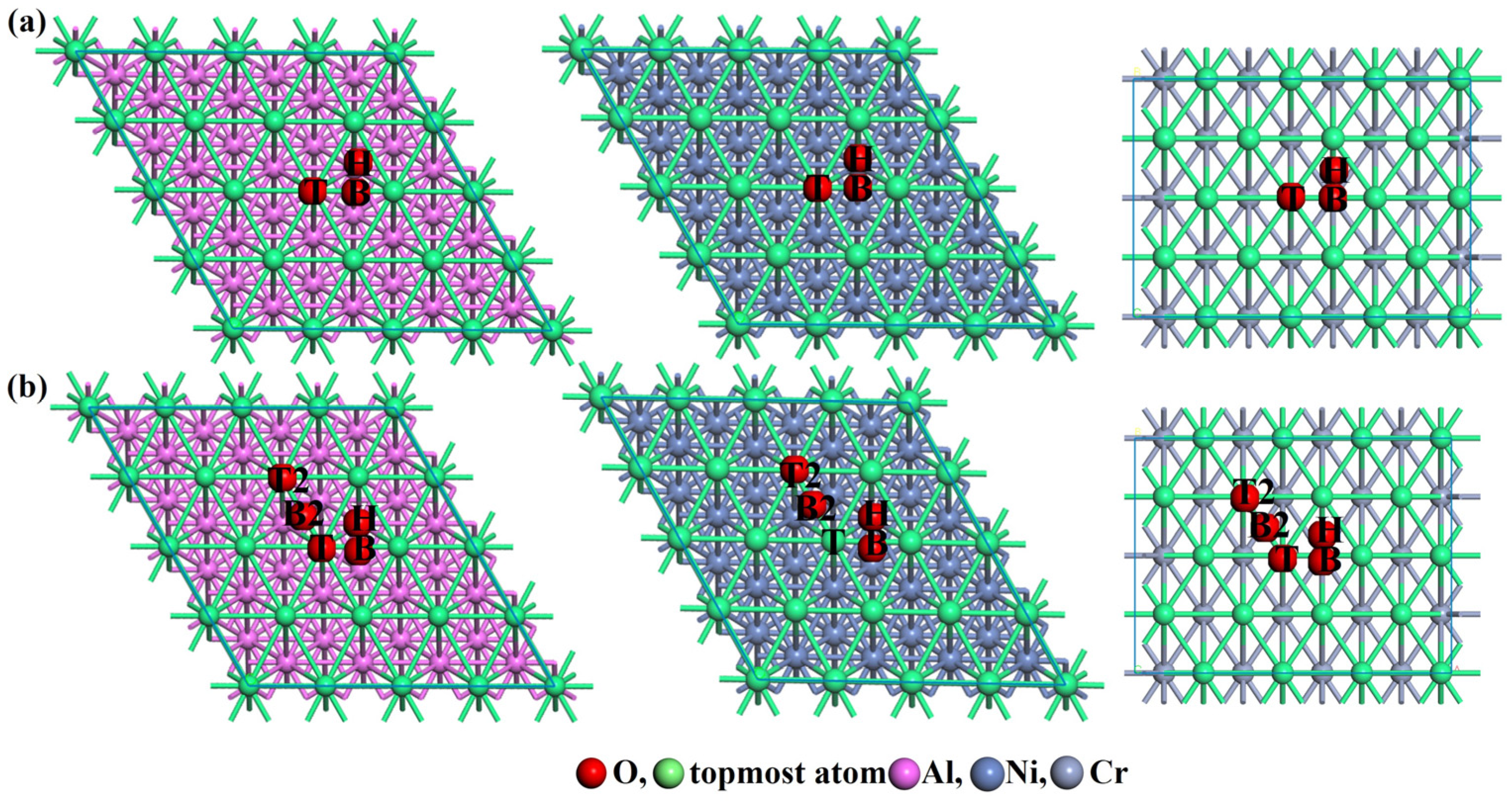
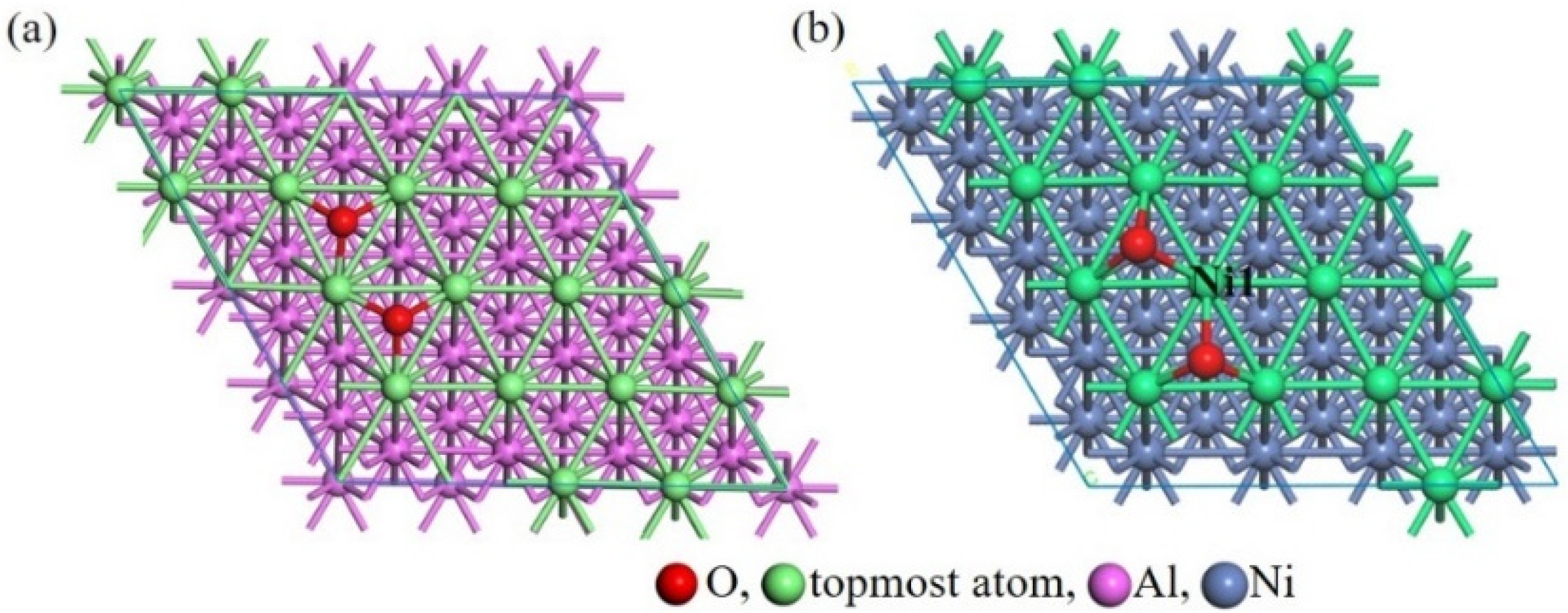
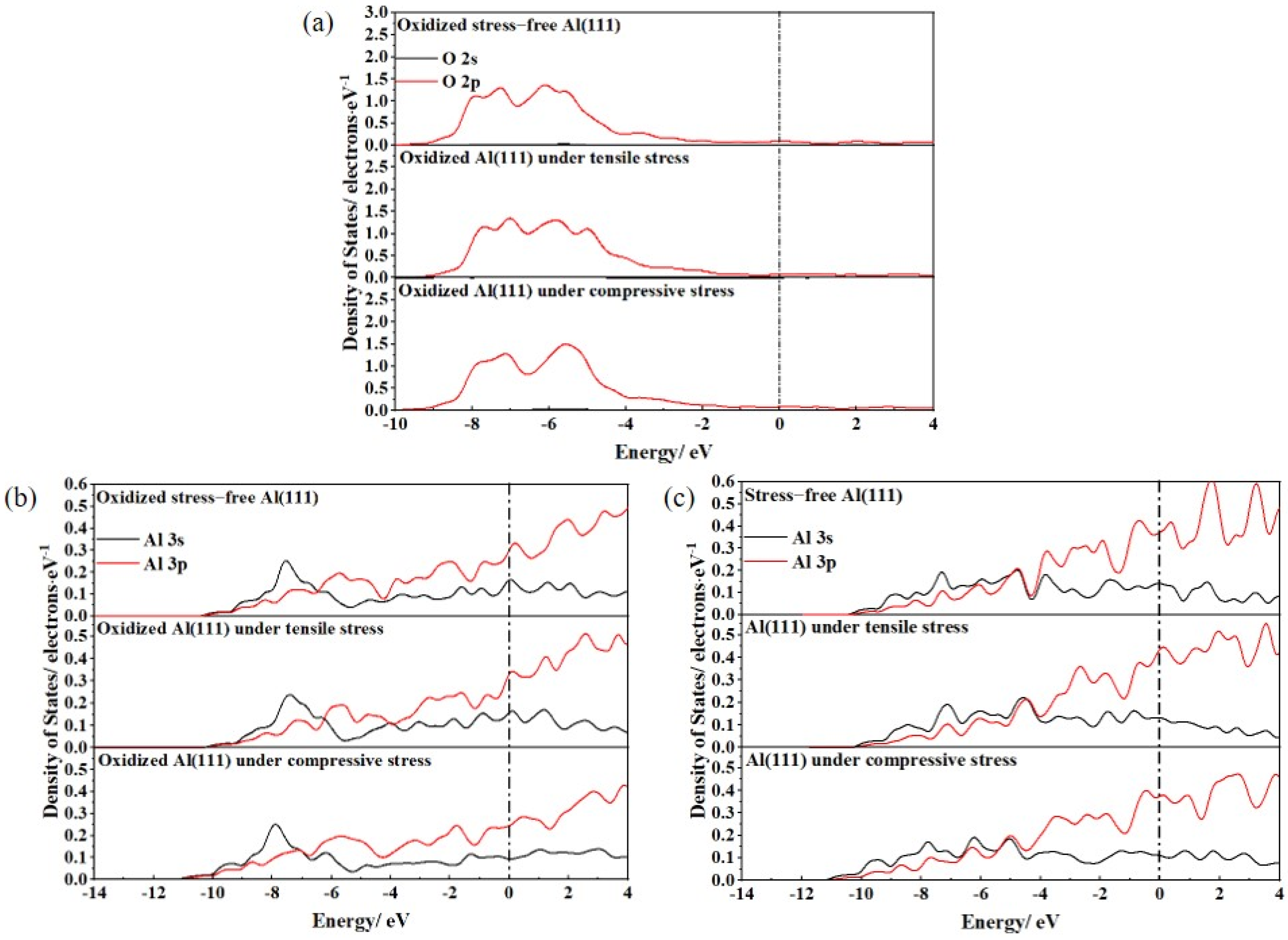
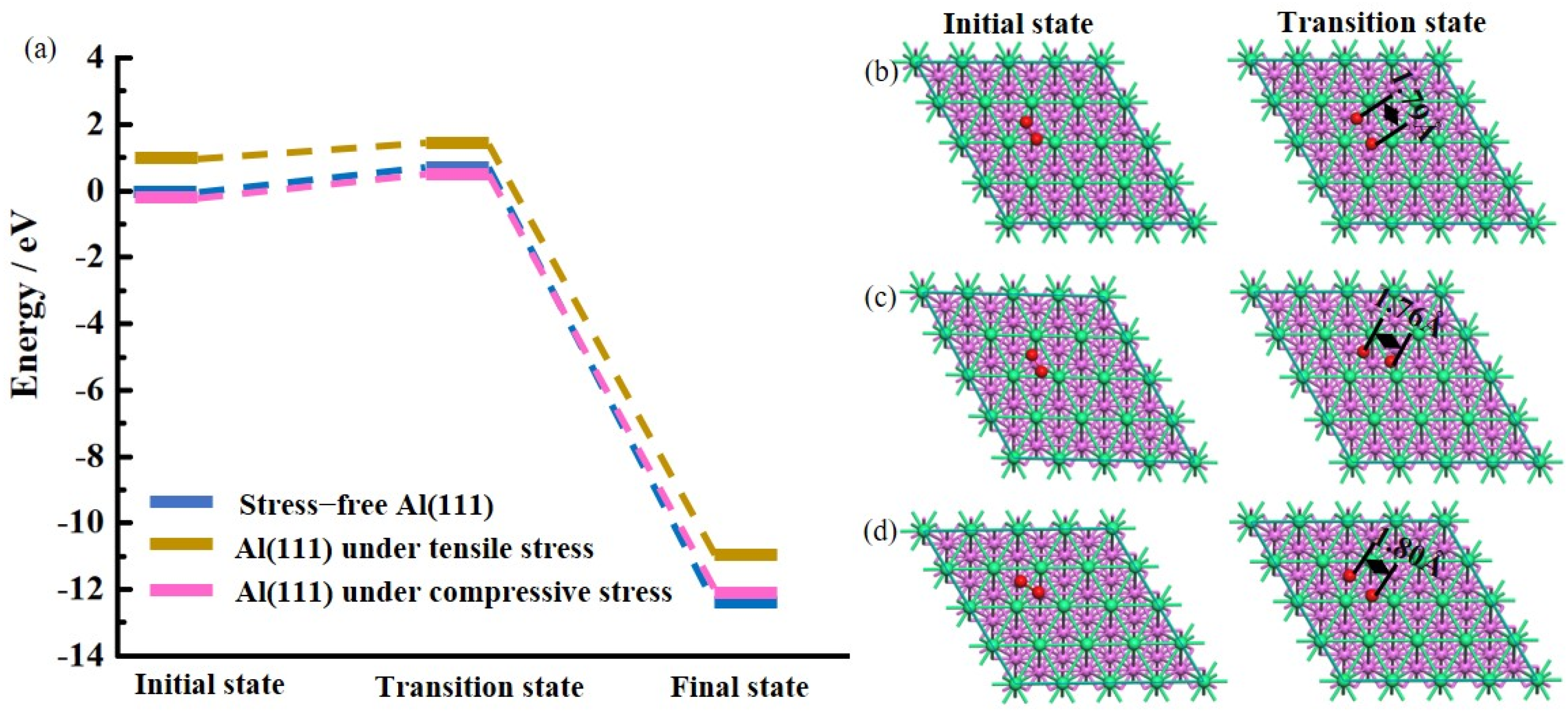
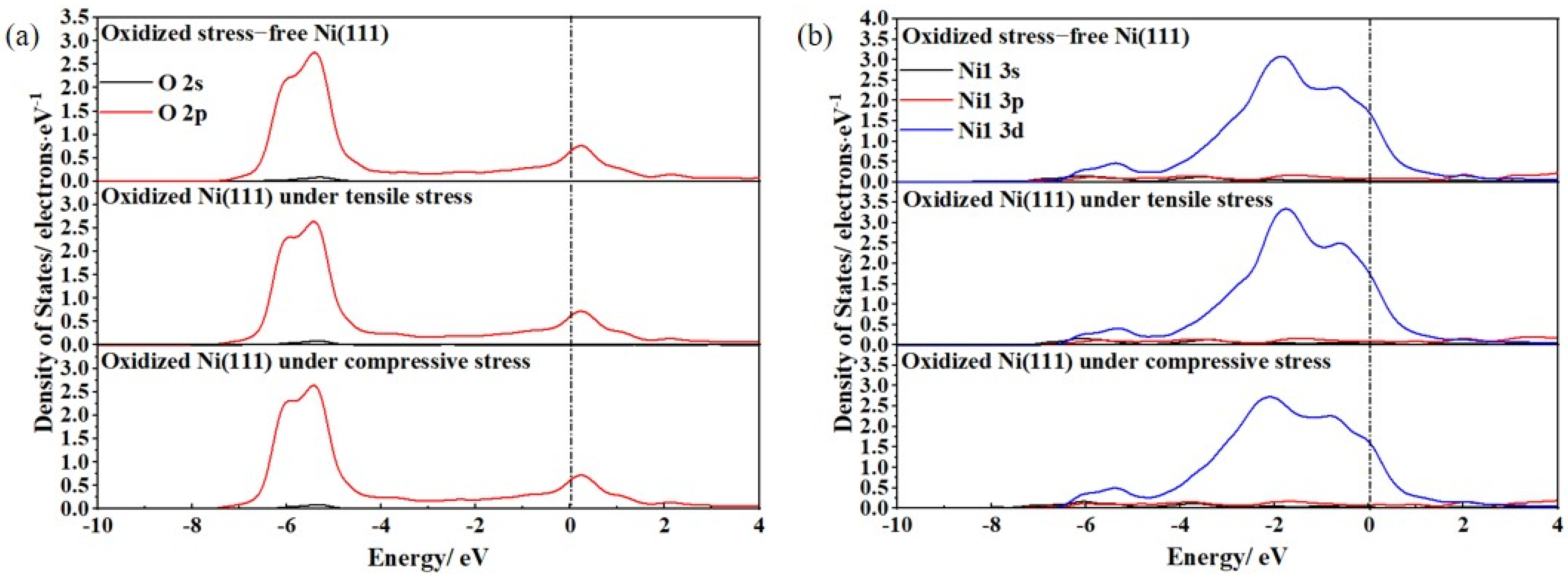
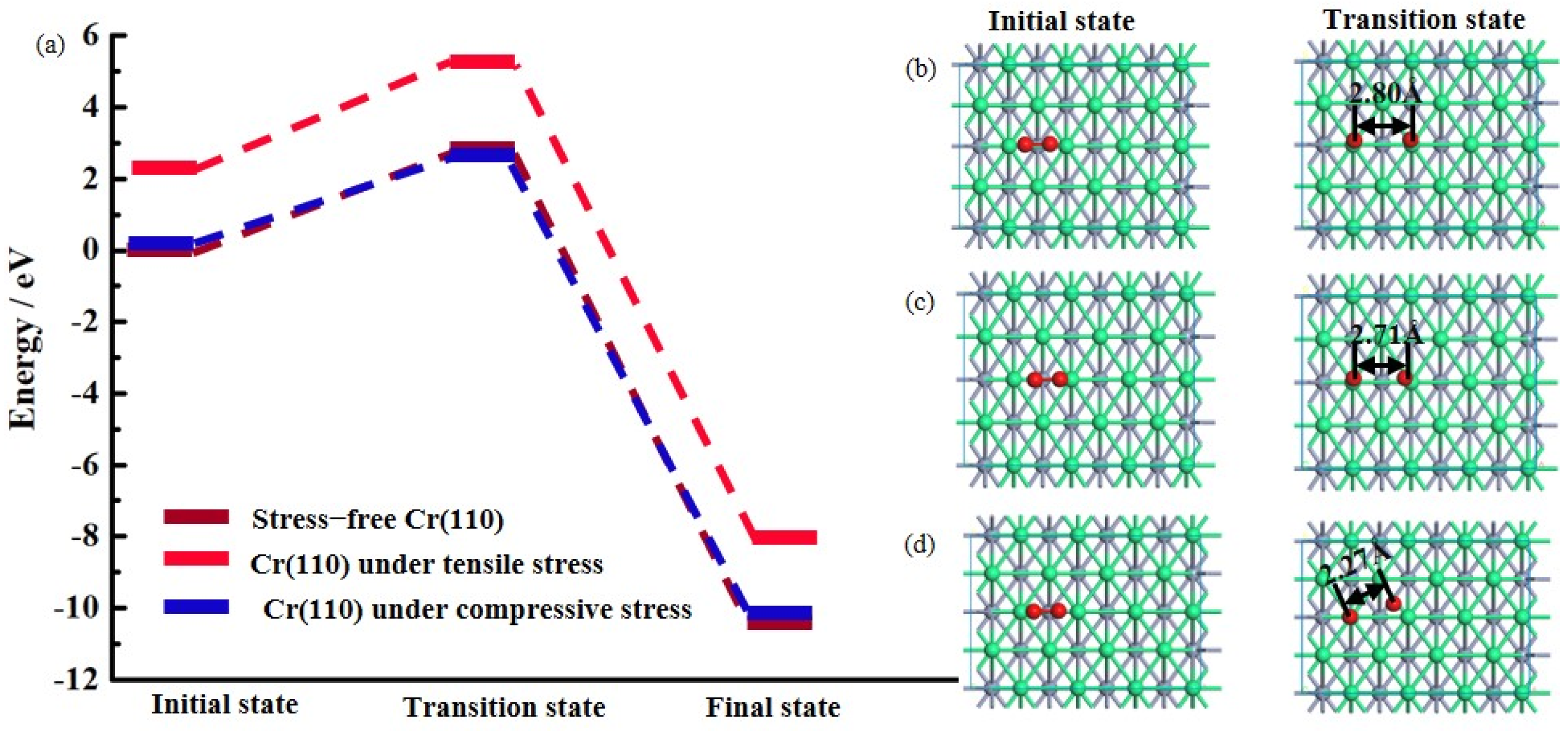
3.2. Ideal Oxidation of Al(111), Ni(111), and Cr(110) under Different Stresses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Gao, Y.; Cheng, Y.; Sun, J.; Ye, F.; Wang, L. Microstructure design of the laser glazed layer on thermal barrier coatings and its effect on the CMAS corrosion. Corros. Sci. 2021, 192, 109847. [Google Scholar] [CrossRef]
- Lyu, G.; Song, D.; Kim, J.; Lu, Z.; Yang, B.; Yang, S.; Byeun, Y.; Jung, Y. Multidisciplinary approach for investigating the hot corrosion behavior of thermal barrier coatings. J. Eur. Ceram. Soc. 2021, 41, 324–333. [Google Scholar] [CrossRef]
- Cong, L.; Li, W.; Wang, J.; Gu, S.; Zhang, S. High-entropy (Y0.2Gd0.2 Dy0.2Er0.2Yb0.2)2Hf2O7 ceramic: A promising thermal barrier coating material. J. Mater. Sci. Technol. 2022, 101, 199–204. [Google Scholar] [CrossRef]
- Xu, G.N.; Yang, L.; Zhou, Y.C. Investigating the Interface Cracking Mechanism of CMAS-Corroded Thermal Barrier Coatings Based on the Cohesive Zone Model. Corros. Sci. 2022, 204, 110337. [Google Scholar] [CrossRef]
- Padture, N.P. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, M.M.; Zhang, C.Y.; Bao, Z.B.; Yang, Y.F.; Zhu, S.L.; Wang, F.H. Effect of pre-oxidation on the failure mechanisms of EB-PVD thermal barrier coatings with (Ni, Pt)Al bond coats. Corros. Sci. 2021, 193, 109873. [Google Scholar] [CrossRef]
- Deng, S.; He, G.; Yang, Z.; Wang, J.; Li, J.; Jiang, L. Calcium-magnesium-alumina-silicate (CMAS) resistant high entropy ceramic (Y0.2Gd0.2Er0.2Yb0.2Lu0.2)2Zr2O7 for thermal barrier coatings. J. Mater. Sci. Technol. 2022, 107, 259–265. [Google Scholar] [CrossRef]
- Torkashvand, K.; Poursaeidi, E.; Mohammadi, M. Effect of TGO thickness on the thermal barrier coatings life under thermal shock and thermal cycle loading. Ceram. Int. 2018, 44, 9283–9293. [Google Scholar] [CrossRef]
- Shen, Z.; He, L.; Mu, R.; Xu, Z.; Huang, G. Effects of gradient transitional layer on thermal cycling life and failure of LaZrCeO/YSZ thermal barrier coatings. Corros. Sci. 2020, 163, 108221–108224. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Liu, Y.; Cheng, B.; Tahir, A. Influence of non-uniform feature of thermally grown oxide thickness on the local stress state and cracking behavior in TBC. Surf. Coat. Technol. 2022, 443, 128607. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Xing, C.; Hu, L.; Zhao, X. Preparation of long-lifetime thermal barrier coatings and toughening mechanism by using hierarchy structured zirconia-based microspheres. J. Eur. Ceram. Soc. 2021, 41, 4625–4636. [Google Scholar] [CrossRef]
- Xu, M.M.; Li, Y.Y.; Zhang, C.Y.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Synergistic effect of Re-base diffusion barrier and Pt modification on the oxidation behaviour of NiCrAlY coatings. Corros. Sci. 2022, 194, 109919. [Google Scholar] [CrossRef]
- Zhang, X.; Watanabe, M.; Kuroda, S. Effects of residual stress on the mechanical properties of plasma-sprayed thermal barrier coatings. Eng. Fract. Mech. 2013, 110, 314–327. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Long, Y.; Zhao, Y.; Xiong, X.; Zhao, X.; Xiao, P. Evolution of residual stress in air plasma sprayed yttria stabilised zirconia thermal barrier coatings after isothermal treatment. Surf. Coat. Technol. 2014, 251, 98–105. [Google Scholar] [CrossRef]
- Yu, Q.M.; He, Q. Effect of material properties on residual stress distribution in thermal barrier coatings. Ceram. Int. 2018, 44, 3371–3380. [Google Scholar] [CrossRef]
- Lin, A.Q.; Liu, G.W.; Yu, X.X.; Chang, R.; Feng, Q. Comprehensive investigations on fluid flow and heat transfer characteristics of a high-speed rotating turbine disk cavity system of aero-engine. Int. Commun. Heat Mass Transf. 2022, 136, 106170. [Google Scholar] [CrossRef]
- Lin, A.Q.; Ma, J.L.; Fan, G.Y.; Lei, Z.; Fawzy, H.; Liu, G.W. Aerothermal performances of a rotating turbine disk cavity with non-uniform wall temperature profiles in an aero-engine. Therm. Sci. Eng. Prog. 2023, 38, 101582. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Yang, Z.H.; Zhu, W.; Yang, L.; Zhou, Y.C.; Hu, X.P. Mechanical properties and calcium-magnesium-alumino-silicate (CMAS) corrosion behavior of a promising Hf6Ta2O17 ceramic for thermal barrier coatings. Ceram. Int. 2020, 46, 25242–25248. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Z.Y.; Yang, L.; Zhou, Y.C.; Wei, J.F. Real-time detection of CMAS corrosion failure in APS thermal barrier coatings under thermal shock. Exp. Mech. 2020, 60, 775–785. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Clark, S.; Segall, M.; Pickard, C.; Hasnip, P.; Probert, M.; Refson, K.; Payne, M. First principles methods using CASTEP. Z. Fur Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Cheng, C.; Li, H.; Fu, Q. Effect of stretching on the initial oxidation of 3C-SiC nanowire by first-principle simulation. Appl. Surf. Sci. 2019, 483, 170–177. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jo Nsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Yu, C.; Liu, H.; Jiang, C.; Bao, Z.; Zhu, S.; Wang, F. Modification of NiCoCrAlY with Pt: Part II. Application in TBC with pure metastable tetragonal(t’) phase YSZ and thermal cycling behavior. J. Mater. Sci. Technol. 2019, 35, 350–359. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, W.; Zhao, K.; Fang, L. Comparison of stress evolution under TGO growth simulated by two different methods in thermal barrier coatings. Ceram. Int. 2020, 46, 2915–2922. [Google Scholar] [CrossRef]
- RöSler, J.; BäKer, M.; Aufzug, K. A parametric study of the stress state of thermal barrier coatings: Part I: Creep relaxation. Acta Mater. 2004, 52, 4809–4817. [Google Scholar]
- Shen, Q.; Yang, L.; Zhou, Y.C.; Wei, Y.G.; Zhu, W. Effects of growth stress in finite-deformation thermally grown oxide on failure mechanism of thermal barrier coatings. Mech. Mater. 2017, 114, 228–242. [Google Scholar] [CrossRef]

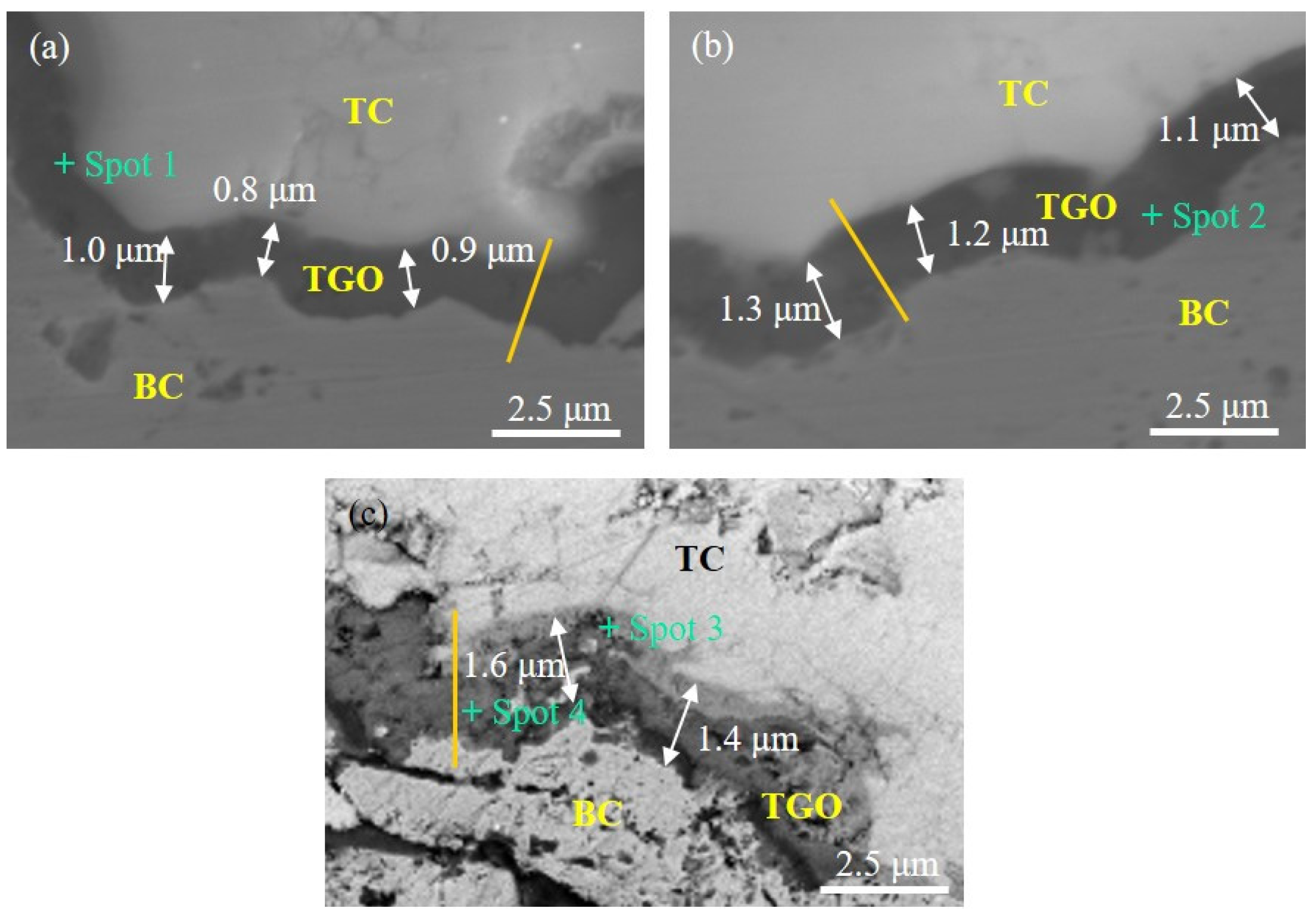



| Spot | Ni | Cr | Al | Y | O |
|---|---|---|---|---|---|
| Composition/at. % | |||||
| 1 | 2.7 | 3.5 | 35.2 | 0.0 | 58.6 |
| 2 | 3.1 | 4.2 | 33.7 | 0.0 | 59.0 |
| 3 | 9.6 | 16.4 | 22.1 | 0.3 | 51.6 |
| 4 | 2.3 | 3.9 | 36.9 | 0.0 | 56.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Nie, M.; Tang, Z.; Chai, Y.; Li, C.; Yang, L.; Zhou, Y. Oxidation Behaviors of the NiCrAlY Bond Coats in the Thermal Barrier Coatings under External Loads. Coatings 2023, 13, 862. https://doi.org/10.3390/coatings13050862
Cheng C, Nie M, Tang Z, Chai Y, Li C, Yang L, Zhou Y. Oxidation Behaviors of the NiCrAlY Bond Coats in the Thermal Barrier Coatings under External Loads. Coatings. 2023; 13(5):862. https://doi.org/10.3390/coatings13050862
Chicago/Turabian StyleCheng, Chunyu, Min Nie, Zhili Tang, Yadong Chai, Cong Li, Li Yang, and Yichun Zhou. 2023. "Oxidation Behaviors of the NiCrAlY Bond Coats in the Thermal Barrier Coatings under External Loads" Coatings 13, no. 5: 862. https://doi.org/10.3390/coatings13050862
APA StyleCheng, C., Nie, M., Tang, Z., Chai, Y., Li, C., Yang, L., & Zhou, Y. (2023). Oxidation Behaviors of the NiCrAlY Bond Coats in the Thermal Barrier Coatings under External Loads. Coatings, 13(5), 862. https://doi.org/10.3390/coatings13050862







