Effect of Fluorocarbon Polymers on Hydrophobicity, Wear Resistance and Corrosion Resistance of Epoxy Resins
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
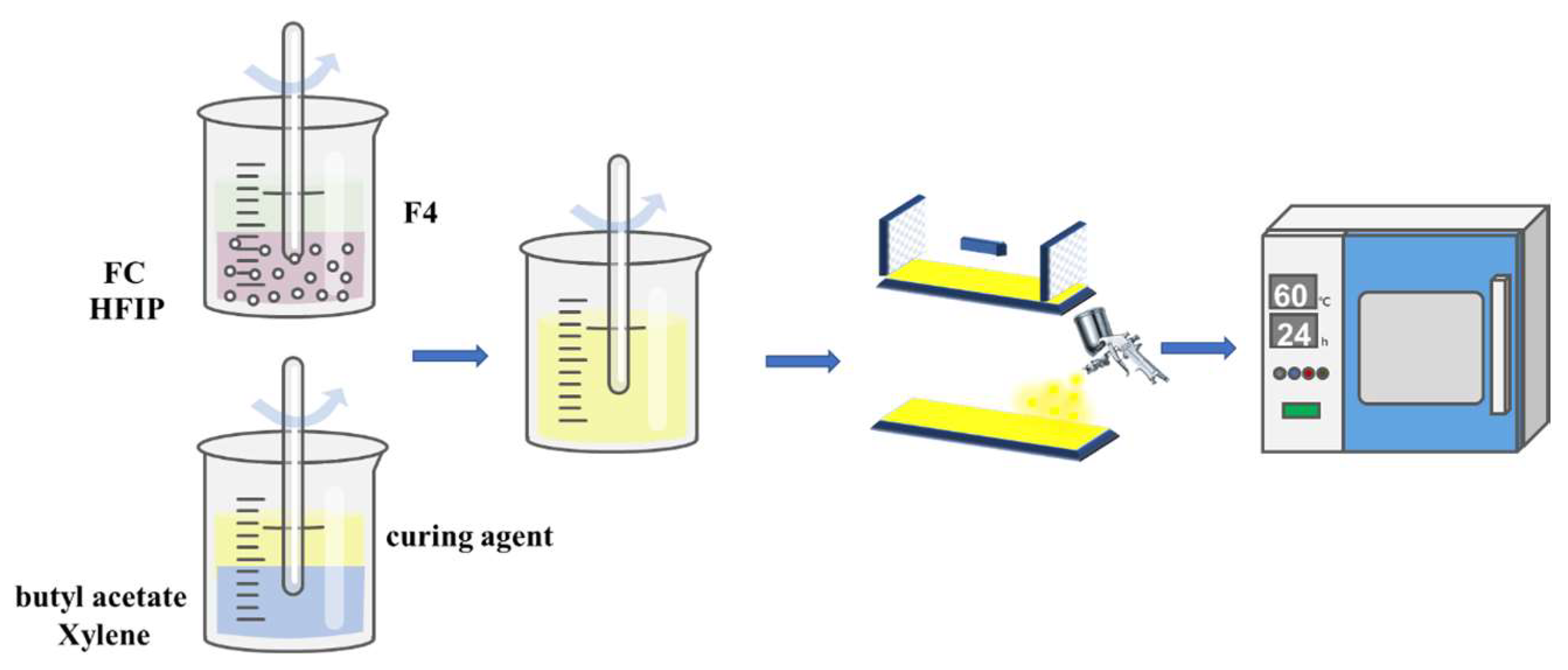
2.2. Preparation of FC Copolymer
2.3. Preparation of Composite Coatings
2.4. Characterization Testing
2.5. Surface Wettability Testing
2.6. Friction Reduction Performance Test
2.7. Corrosion Resistance Test
3. Results and Discussion
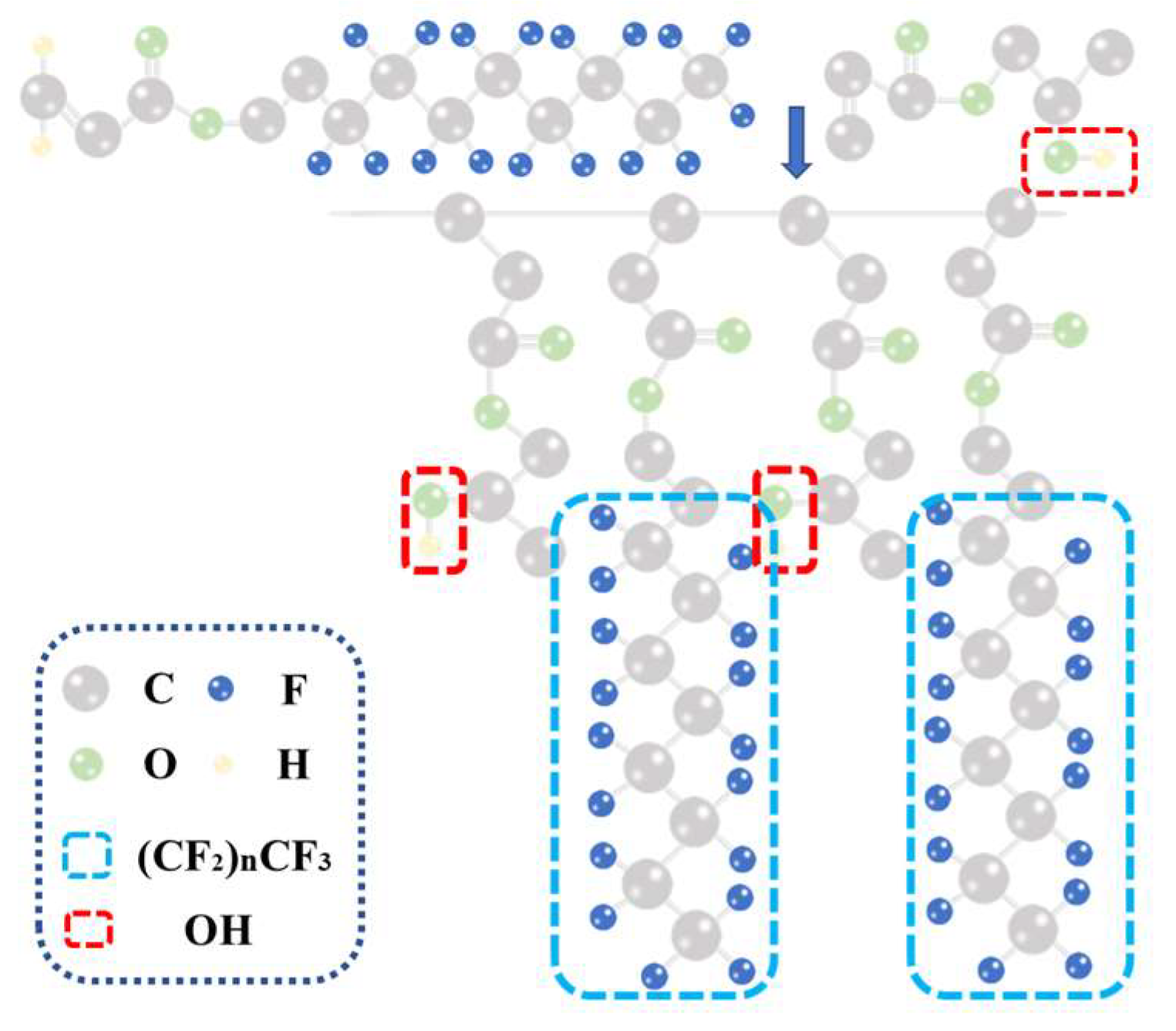
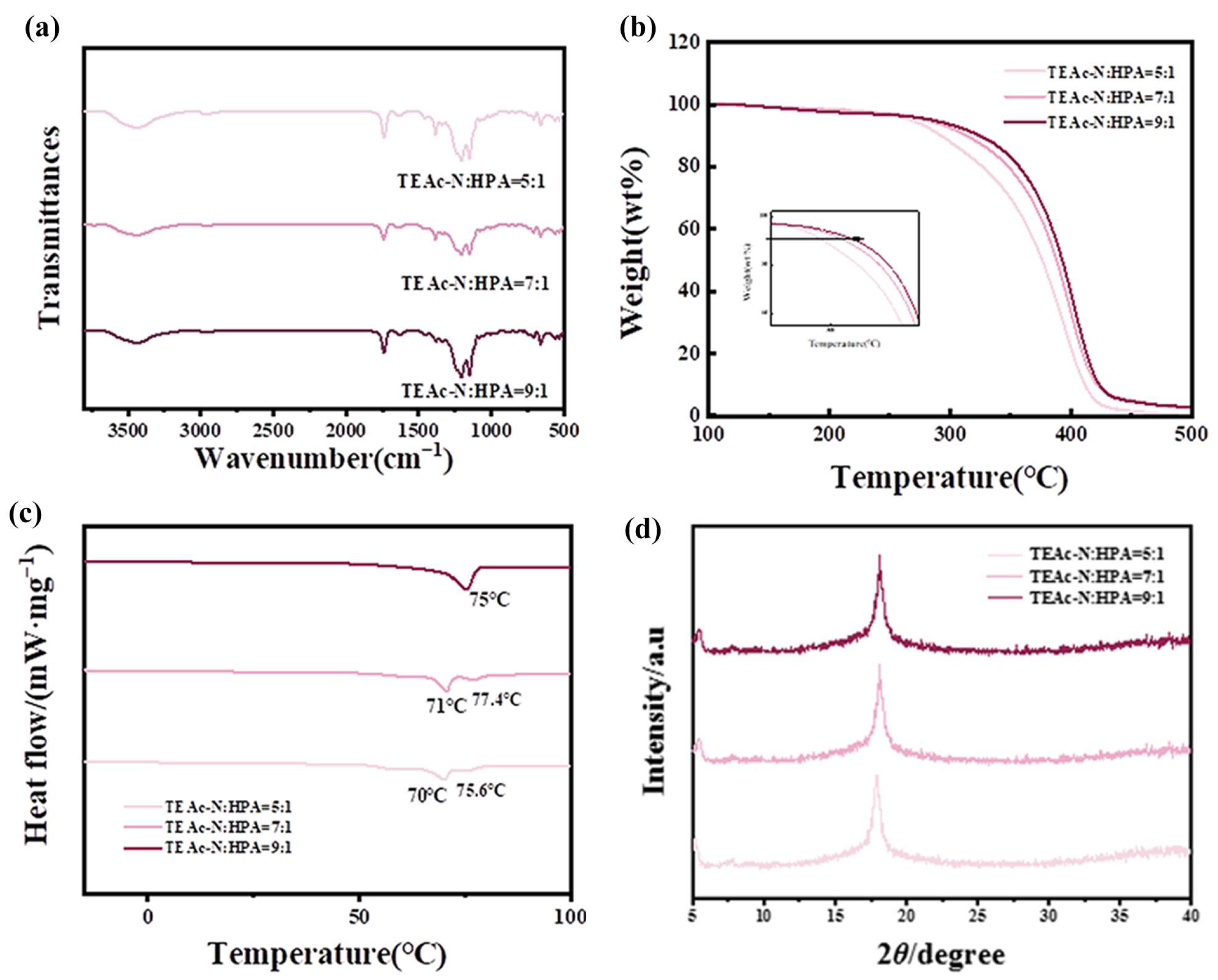
3.1. Characterization of FC
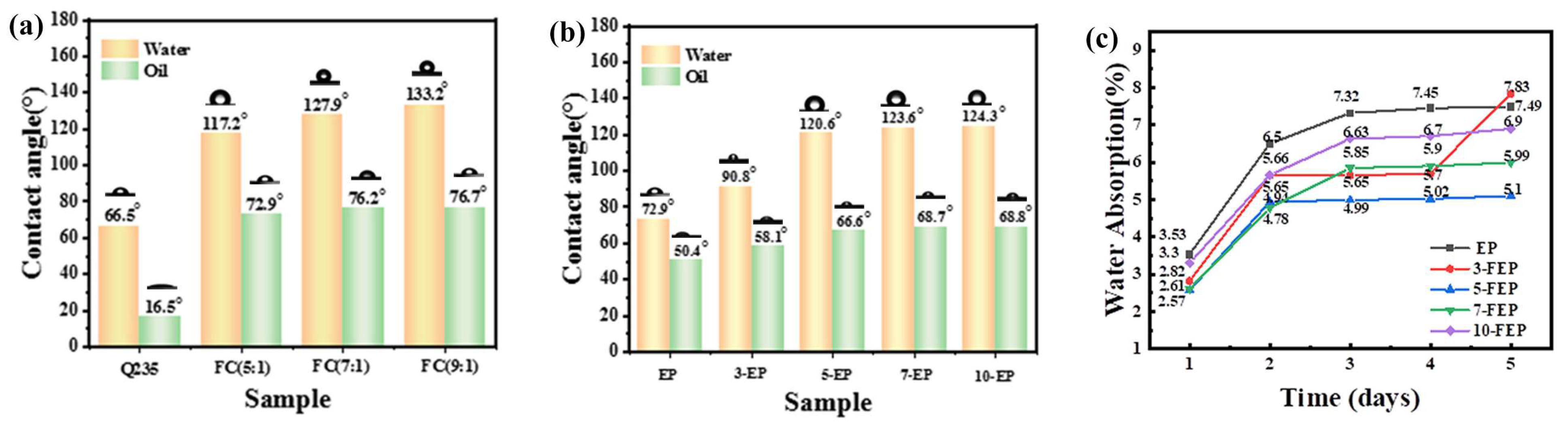
3.2. Wettability of Composite Coating
3.3. Dispersibility of Composite Coatings
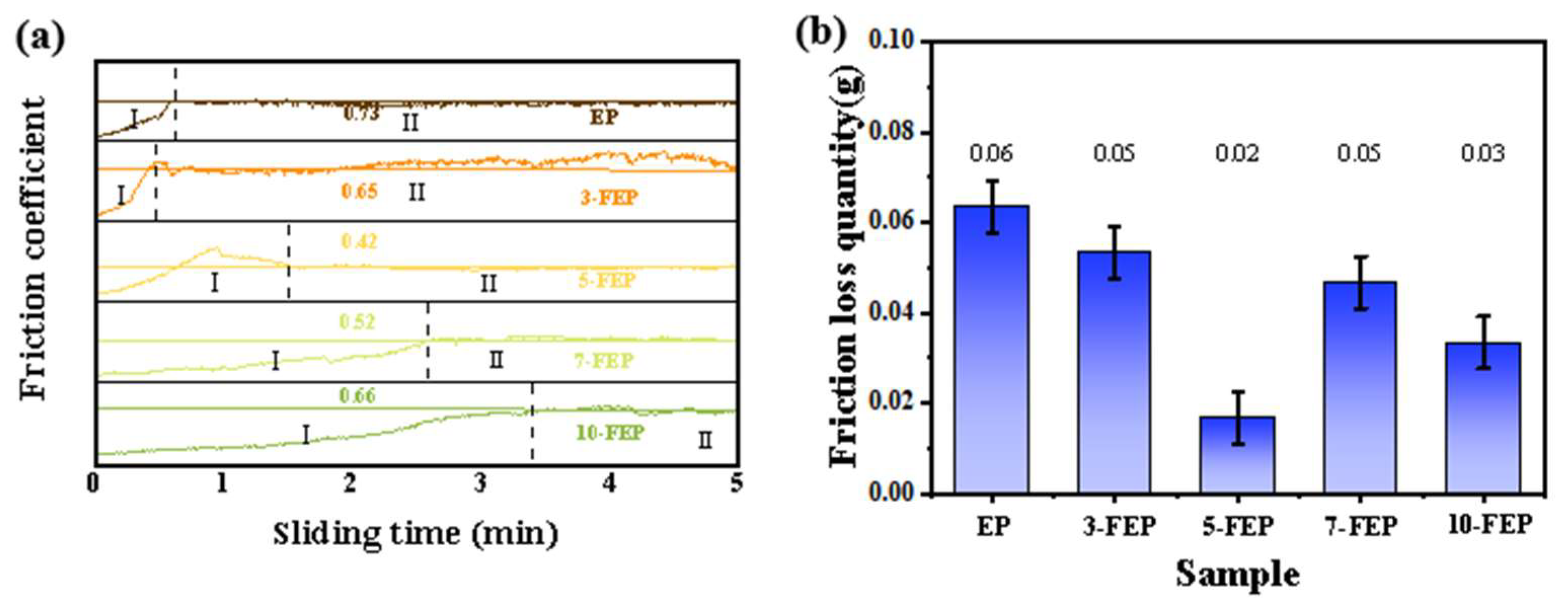
3.4. Tribological Properties of Composite Coatings
3.5. Anticorrosion Performance of Composite Coatings
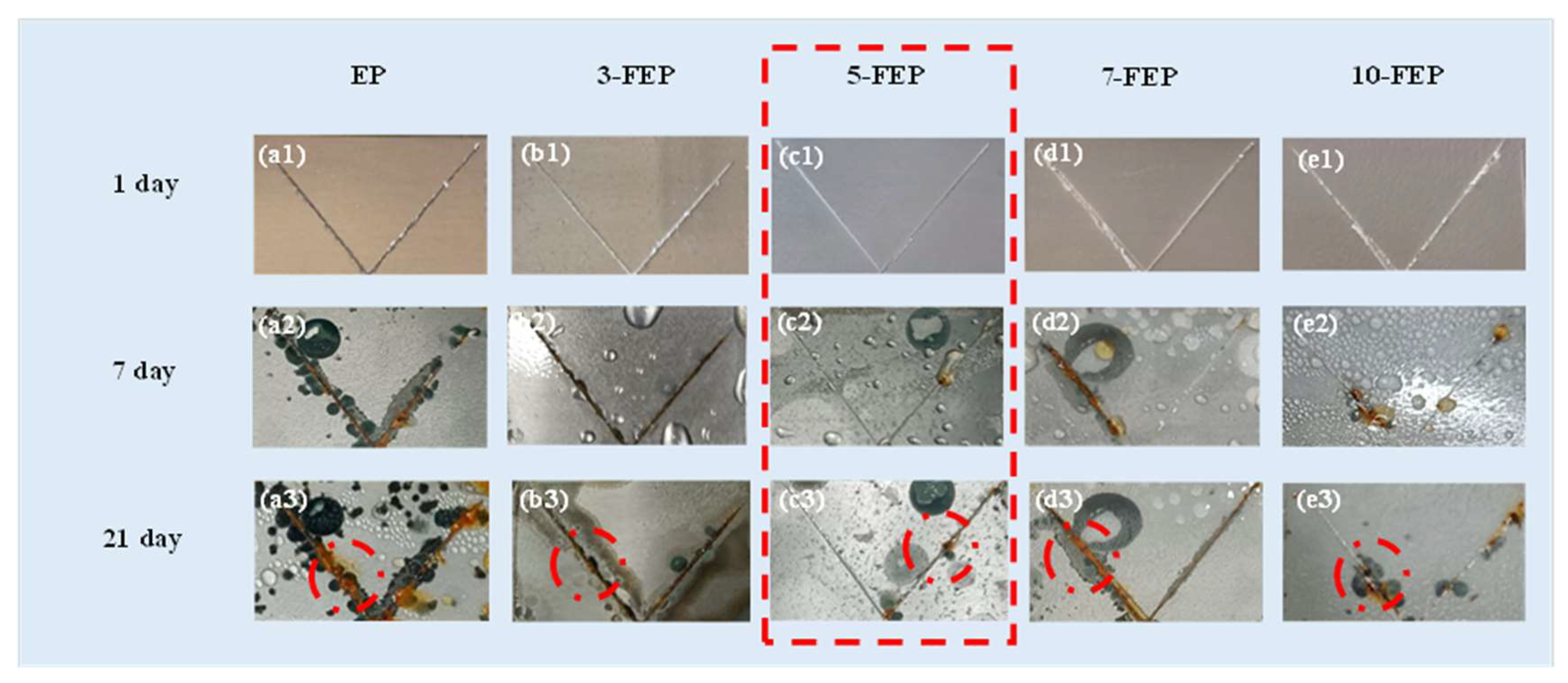
3.6. Analysis of Neutral Salt Spray Test
4. Conclusions
- Fluorinated acrylic resins were synthesized by free radical copolymerization, and their structures were confirmed by FTIR, XPS and GPC measurements. The oil contact angle and water contact angle of FC synthesized with different TEAc-N monomers were tested, and 9:1 was selected as the best FC.
- FC was added to epoxy resin and cured with isocyanate compound to obtain the composite coating. The performance of the composite coating was comprehensively evaluated by contact angle, scanning electron microscope, super depth of field microscope, tribometer, electrochemical impedance spectroscopy and salt spray test. It was found that the addition of FC changed the epoxy resin from hydrophilic to hydrophobic, reduced the friction coefficient and loss, and enhanced the anticorrosion performance.
- 5-FEP had the best effect. The friction coefficient was 0.42, which was lower than that of pure EP (0.73). The friction loss was only 0.02 g. 5-FEP had the largest corrosion potential (−0.03 V) and the smallest corrosion current density (3.42−14 A/cm2). Compared with pure EP, the corrosion potential was one order of magnitude different, and the corrosion current was two orders of magnitude different. After 21 days of immersion, the impedance of the EP coating was nearly six orders of magnitude higher than that of the EP coating.
- FEP can be of great value as a long-term anticorrosion and hydrophobic wear-resistant method for the inner coating of transport pipelines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ijaola, A.O.; Farayibi, P.K.; Asmatulu, E. Superhydrophobic coatings for steel pipeline protection in oil and gas industries: A comprehensive review. J. Nat. Gas Sci. Eng. 2020, 83, 103544. [Google Scholar] [CrossRef]
- Cheng, S.; Yuan, W. Corrosion mechanism and anticorrosion technology of oil and gas gathering and transportation pipeline. IOP Conf. Ser. Earth Environ. Sci. 2021, 859, 012115. [Google Scholar] [CrossRef]
- Yang, G.R.; Song, W.M.; Sun, X.M.; Ma, Y.; Lu, J.J.; Hao, Y. The Wear Behavior of Electroless Ni-P/SiC Composite Coating. Adv. Mater. Res. 2011, 239–242, 954–957. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, J.; Tang, Y.; Yu, Z. EIS study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Lusk, D.; Gore, M.; Boardman, B.; Upadhyaya, D.; Casserly, T.; Oppus, M. A High Corrosion and Wear Resistant Interior Surface Coating for Use in Oilfield Applications. Adv. Mater. Res. 2010, 83–86, 592–600. [Google Scholar] [CrossRef]
- Ling, Z.; Qing, Y.; Huang, X. Research and application progress of natural gas pipeline resistance reduction and wear-resistant coatings. Corros. Protect. 2003, 24, 4. [Google Scholar]
- Shibuichi, S.; Yamamoto, T.; Onda, T.; Tsujii, K. Super Water- and Oil-Repellent Surfaces Resulting from Fractal Structure. J. Colloid Interface Sci. 1998, 208, 287–294. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Zhang, H.; Liu, X.; Chen, H. Preparation and Properties of Fluorinated Poly(ethyl methacrylate-co-butyl acrylate). Polym. Sci. Ser. B 2019, 61, 163–169. [Google Scholar]
- Yao, M.; Gao, P.; Zhao, H.; Nie, J.; He, Y. Photo-patternable F-containing acrylic copolymers as passivation materials. Mater. Chem. Phys. 2020, 253, 123404. [Google Scholar] [CrossRef]
- Ding, Z.; Ping, X.; Pan, R.; Lin, X.; Jiang, B. Preparation and Surface Properties Study of Novel Fluorine-Containing Methacrylate Polymers for Coating. Materials 2018, 11, 2258. [Google Scholar]
- Maeztu, J.; Berlanga, C.; Bastidas, D.; Palacio, J.; Rodriguezet, R. Effect of graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Interf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Zhang, J.; Shen, L.; Bao, N. Tribological and Corrosion Behaviors of Fluorocarbon Coating Reinforced with Sodium Iron Titanate Platelets and Whiskers. J. Appl. Polym. Sci. 2020, 13, 48936. [Google Scholar] [CrossRef]
- Cichomski, M.; Burnat, B.; Prowizor, M.; Jedrzejczak, A.; Batory, D.; Piwoński, I.; Kozłowski, W.; Szymanski, W.; Dudek, M. Tribological and corrosive investigations of perfluoro and alkylphosphonic self-assembled monolayers on Ti incorporated carbon coatings, Tribology International. Tribol. Int. 2019, 130, 359–365. [Google Scholar] [CrossRef]
- Zhong, S.; Qin, K.; Hou, Y.; Xu, T.; Cai, Y.; Yi, L. Waterborne corrosion-resistant hydrophobic alkyd resin composite coatings modified with fluorinated acrylate–siloxane and submicron-sheet zinc phosphate pigment. J. Coat. Technol. Res. 2021, 18, 1309–1320. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, C.; Hu, L. Dual functional low surface energy coating of anti-corrosion / fouling via crosslinking polysilazane preceramic precursor incorporated with fluorine. Prog. Org. Coat. 2023, 177, 107409. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, W.; Liang, L.; Liu, C.; He, S.; Zhang, F.; Shi, H.; Yang, M. Enhancement of anticorrosion property and hydrophobicity of modified epoxy coatings with fluorinated polyacrylate, Coll, Surf, A: Physicochemi, Engineer. Aspects 2019, 579, 123659. [Google Scholar] [CrossRef]
- Su, Y.C.; Chang, F.C. Synthesis and characterization of fluorinated polybenzoxazine material with low dielectric constant. Polymer 2003, 44, 7989–7996. [Google Scholar] [CrossRef]
- Suda, Y.; Morimoto, T. Molecularly adsorbed water on the bare surface of titania (rutile). Langmuir 1987, 3, 786–788. [Google Scholar] [CrossRef]
- Nakayama, T. Structure of TiO2/ SiO2 Multilayer Films. J. Electrochem. Soc. 1993, 141, 237–241. [Google Scholar] [CrossRef]
- Tang, W.; Matyjaszewski, K.; Dong, H. Well-Defined High-Molecular-Weight Polyacrylonitrile via Activators Regenerated by Electron Transfer ATRP. Macromolecules 2007, 40, 2974–2977. [Google Scholar]
- Jiao, C.; Shao, Q.; Wu, M.; Zheng, B.; Guo, Z. 2-(3,4-Epoxy) ethyltriethoxysilane-modified waterborne acrylic resin: Preparation and property analysis. Polymer 2020, 190, 122196. [Google Scholar] [CrossRef]
- Chen, H.; Tian, X.; Liu, J. Unsaturated Polyester Resin Nanocomposites Containing ZnO Modified with Oleic Acid Activated by N,N′-Carbonyldiimidazole. Polymers 2018, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kan, Y.; Pan, Y.; Yuan, Y.; Liew, K.M.; Hu, Y. Urchin-Like Shells of TiO2 Hollow Spheres for Improving the Fire Safety of Epoxy Resin. Ind. Eng. Chem. Res. 2016, 56, 1341–1348. [Google Scholar] [CrossRef]
- Williams, B.L.; Ding, H.; Hou, Z.; Paul, P.O.; Lewis, F.A.; Smith, A.T.; Sun, L. Highly efficient polyvinyl alcohol/montmorillonite flame retardant nanocoating for corrugated cardboard. Adv. Compos. Hybrid Mater. 2021, 4, 662–669. [Google Scholar] [CrossRef]
- Fang, C.; Jing, Y.; Zong, Y.; Lin, Z. Preparation and characterization of fluorine-containing acrylic latex PSAs using a reactive surfactant. J. Fluor. Chem. 2016, 192, 113–119. [Google Scholar] [CrossRef]
- Fang, C.; Zhu, K.; Zhu, X.; Lin, Z. Preparation and characterization of self-crosslinking fluorinated polyacrylate latexes and their pressure sensitive adhesive applications. Int. J. Adhes. Adhes. 2019, 95, 102417. [Google Scholar] [CrossRef]
- Azémard, C.; Vieillescazes, C.; Ménager, M. Effect of photodegradation on the identification of natural varnishes by FT-IR spectroscopy. Microchem. J. 2014, 112, 137–149. [Google Scholar] [CrossRef]
- Tian, H.; Wang, C.; Guo, M.; Cui, Y.; Tang, Z. Microstructures and high-temperature self-lubricating wear-resistance mechanisms of graphene-modified WC-12Co coatings. Friction 2021, 9, 331–335. [Google Scholar] [CrossRef]
- Xia, W.; Xue, H.; Wang, J.; Wang, T.; Song, L.; Guo, H.; Fan, X.; Gong, H.; He, J. Functionlized graphene serving as free radical scavenger and corrosion protection in gamma-irradiated epoxy composites. Carbon 2016, 101, 315–323. [Google Scholar] [CrossRef]
- Marcus, P. Corrosion Mechanisms in Theory and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; p. 742. [Google Scholar]
- Meng, F.; Liu, L.; Li, Y.; Wang, F. The failure behaviour of a commercial highly pigmented epoxy coating under marine alternating hydrostatic pressure. Prog. Org. Coat. 2015, 82, 101–112. [Google Scholar]







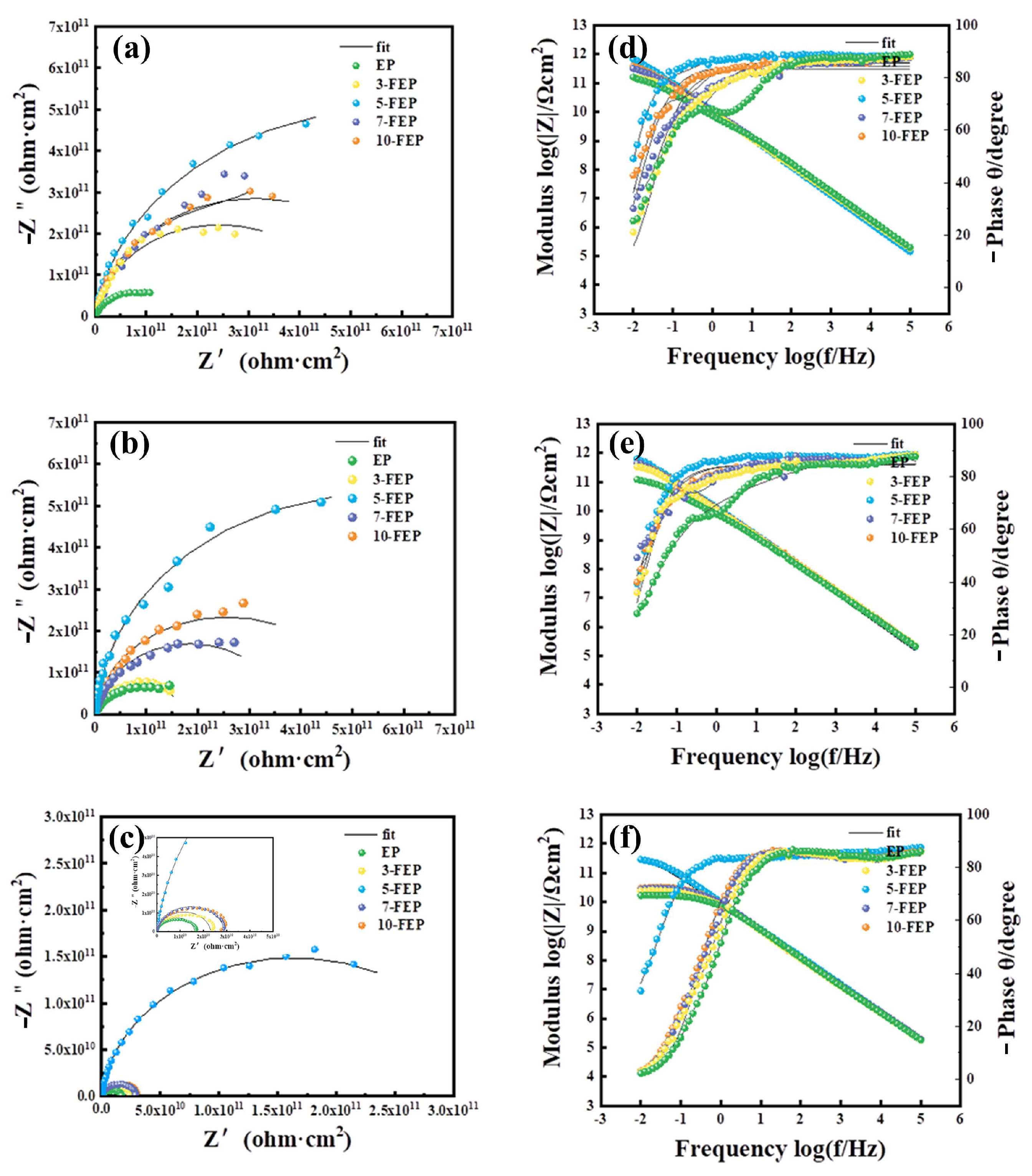
| Sample | Ecorr (V vs. SCE) | Icorr (A/cm2) | βa (V/dec) | −βc (V/dec) |
|---|---|---|---|---|
| EP | −0.39 | 1.14 × 10−12 | 0.07 | 0.05 |
| 3-FEP | −0.27 | 1.56 × 10−13 | 0.16 | 0.14 |
| 5-FEP | −0.03 | 3.42 × 10−14 | 0.03 | 0.04 |
| 7-FEP | −0.20 | 1.52 × 10−13 | 0.07 | 0.05 |
| 10-FEP | −0.32 | 1.61 × 10−13 | 0.09 | 0.06 |
| Coatings | Time | Rc (Ω∙cm2) | Qcd | Rct (Ω∙cm2) |
|---|---|---|---|---|
| Y0 (Ω−1cm−2sn) | ||||
| EP | 1d | 1.28 × 1010 | 1.56 × 10−10 | 4.44 × 109 |
| 7d | 2.00 × 107 | 7.78 × 10−12 | 2.45 × 1011 | |
| 21d | 8.54 × 105 | 7.70 × 10−12 | 1.88 × 1011 | |
| 3-FEP | 1d | 7.04 × 1011 | 1.31 × 10−10 | 1.99 × 109 |
| 7d | 1.44 × 1011 | 8.73 × 10−12 | 4.69 × 1011 | |
| 21d | 7.04 × 1011 | 1.30 × 10−10 | 1.38 × 1011 | |
| 5-FEP | 1d | 8.63 × 1011 | 6.52 × 10−12 | 1.16 × 1012 |
| 7d | 5.93 × 1011 | 1.68 × 10−12 | 1.17 × 1012 | |
| 21d | 3.56 × 1011 | 7.75 × 10−12 | 2.48 × 1010 | |
| 7-FEP | 1d | 6.58 × 1011 | 2.53 × 10−7 | 1.81 × 107 |
| 7d | 7.78 × 1010 | 8.56 × 10−12 | 2.86 × 1011 | |
| 21d | 5.68 × 107 | 1.04 × 10−12 | 2.89 × 1010 | |
| 10-FEP | 1d | 5.81 × 1011 | 7.99 × 10−11 | 9.99 × 107 |
| 7d | 5.13 × 1011 | 2.80 × 10−12 | 3.97 × 107 | |
| 21d | 2.44 × 1010 | 1.62 × 10−10 | 6.09 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Tang, J.; Zhao, N.; Qi, F.; Ouyang, X. Effect of Fluorocarbon Polymers on Hydrophobicity, Wear Resistance and Corrosion Resistance of Epoxy Resins. Coatings 2023, 13, 685. https://doi.org/10.3390/coatings13040685
Tan Y, Tang J, Zhao N, Qi F, Ouyang X. Effect of Fluorocarbon Polymers on Hydrophobicity, Wear Resistance and Corrosion Resistance of Epoxy Resins. Coatings. 2023; 13(4):685. https://doi.org/10.3390/coatings13040685
Chicago/Turabian StyleTan, Yali, Jun Tang, Nie Zhao, Fugang Qi, and Xiaoping Ouyang. 2023. "Effect of Fluorocarbon Polymers on Hydrophobicity, Wear Resistance and Corrosion Resistance of Epoxy Resins" Coatings 13, no. 4: 685. https://doi.org/10.3390/coatings13040685
APA StyleTan, Y., Tang, J., Zhao, N., Qi, F., & Ouyang, X. (2023). Effect of Fluorocarbon Polymers on Hydrophobicity, Wear Resistance and Corrosion Resistance of Epoxy Resins. Coatings, 13(4), 685. https://doi.org/10.3390/coatings13040685






