Photocatalytic Oxidation of Amoxicillin in CPC Reactor over 3D Printed TiO2-CNT@PETG Static Mixers
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
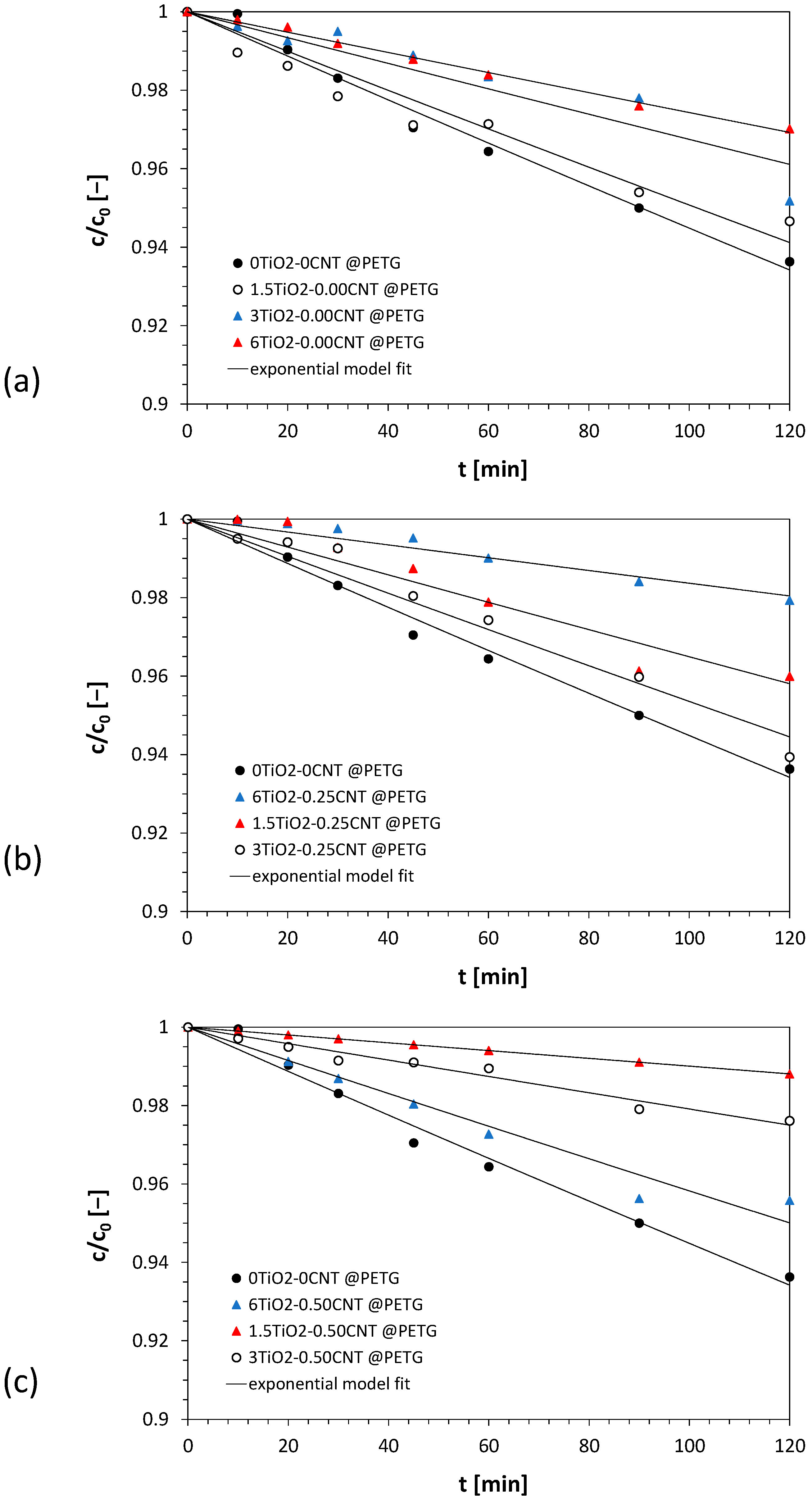
3.1. Results of Photocatalytic Oxidation of Amoxicillin
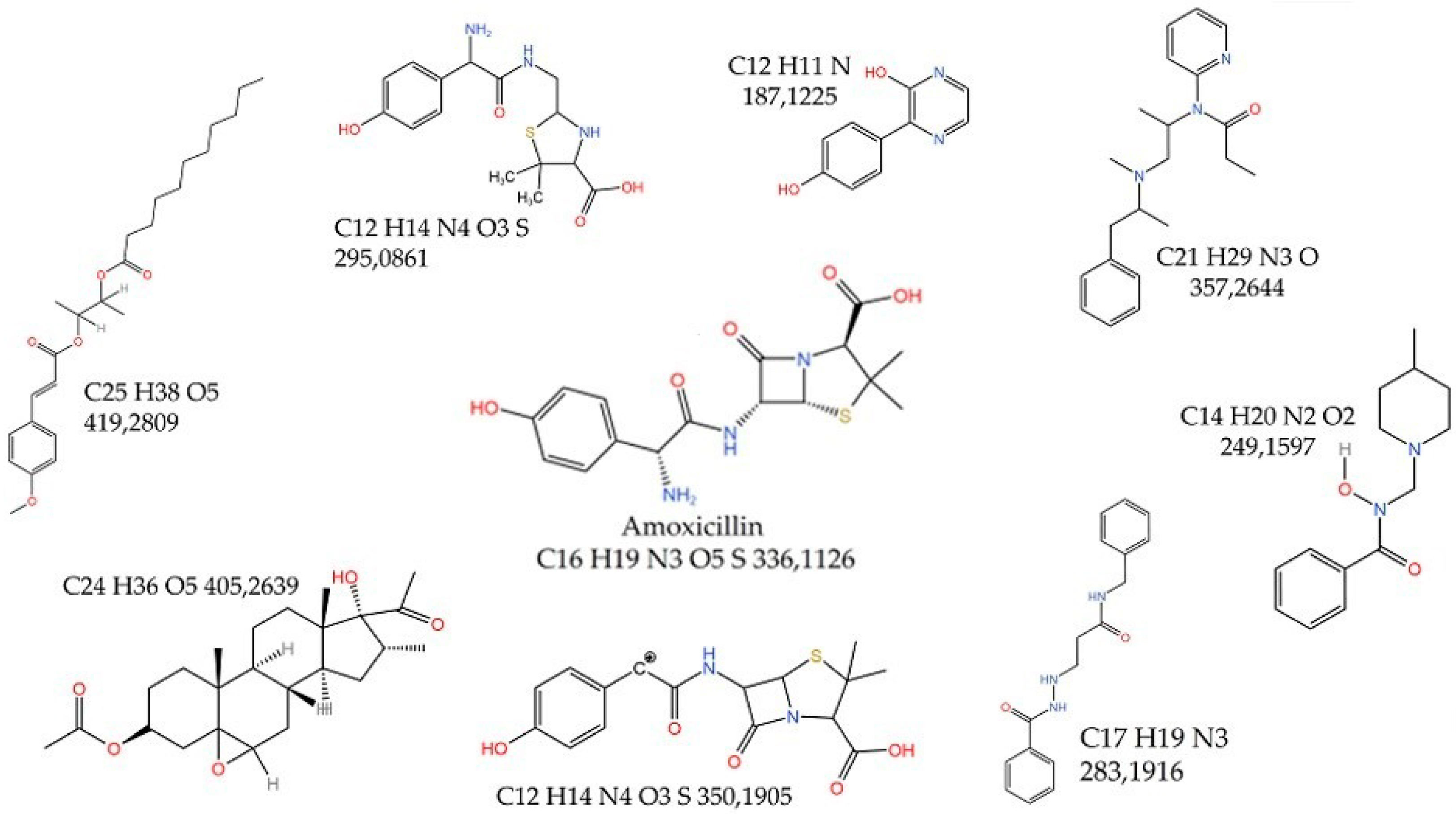
3.2. Identification of Amoxicillin Degradation Products
- Amoxicillin (as a parent drug) at a retention time of 6175 min and m/z of 366.1126;
- Photocatalysis product #1 at a retention time of 17,421 min and m/z of 419.2809;
- 2-Amino-2-(4-hydroxyphenyl)-N-{(Z)-[3-(4-hydroxyphenyl)-2-oxo-2,3,6,7-tetrahydro1H-1,4-diaze-pin-5-yl]methylidene}-acetamide at a retention time of 16,969 min and m/z of 381.2037;
- Dehydrocarboxylated amoxicillin penilloic acid at a retention time of 11,898 min and m/z if 315.0790;
- Amoxicillin penicilloic acid at a retention time of 16,617 min and m/z of 301.1427;
- Photocatalysis product #2 at a retention time of 14,910 min and m/z of 283.1916;
- Photocatalysis product #3 at a retention time of 15,161 min and m/z of 172,1709;
- Photocatalysis product #4 at a retention time of 9237 min and m/z of 249.1603;
- Photocatalysis product #5 at a retention time of 12,701 min and m/z of 369.1725.
- Photolysis product #1 at a retention time of 17,523 min and m/z of 419.2809;
- Photolysis product #2 at a retention time of 17,172 min and m/z of 405.2639;
- Photolysis product #3 at a retention time of 17,122 min and m/z of 357.2644;
- Photolysis product #4 at a retention time of 9138 min and m/z of 350.1905;
- Photolysis product #5 at a retention time of 13,456 min and m/z of295.0861;
- Photolysis product #6 at a retention time of 14,912 min and m/z of 283.1906;
- Photolysis product #7 at a retention time of 9239 min and m/z of 249.1597;
- Photolysis product #8 at a retention time of 11,800 min and m/z of 187.1225.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, A.I.; Becker, J.A. A Review of Emerging Contaminants in Water: Classification, Sources, and Potential Risks. In Impact of Water Pollution on Human Health and Environmental Sustainability; IGI Global: Hershey, PA, USA, 2016; pp. 55–80. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Rodayan, A.; Berk, D.; Yargeau, V. Removal of the antibiotic levofloxacin (LEVO) in water by ozonation and TiO2 photocatalysis. Chem. Eng. J. 2012, 189–190, 41–48. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. The feasibility of using combined TiO2 photocatalysis-SBR process for antibiotic wastewater treatment. Desalination 2011, 272, 218–224. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Demirezen, D.A.; Yıldız, Y.S.; Yılmaz, D.D. Amoxicillin degradation using green synthesized iron oxide nanoparticles: Kinetics and mechanism analysis. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100219. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Hirte, K.; Seiwert, B.; Schüürmann, G.; Reemtsma, T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Baranauskaite-Fedorova, I.; Dvarioniene, J. Management of Macrolide Antibiotics (Erythromycin, Clarithromycin and Azithromycin) in the Environment: A Case Study of Environmental Pollution in Lithuania. Water 2023, 15, 10. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. 2019, 378, 7. [Google Scholar] [CrossRef]
- Hrvatske vode. Report on Monitoring of Substances from the Second Watch List in Surface Waters of the Republic of Croatia for 2019; Hrvatske vode (Croatian Waters): Zagreb, Croatia, 2020. [Google Scholar]
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-degradation products formed under controlled environmental conditions: Identification and determination in the aquatic environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef]
- Ellepola, N.; Rubasinghege, G. Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts. Environments 2022, 9, 77. [Google Scholar] [CrossRef]
- Brinkmann, T.; Giner Santonja, G.; Yükseler, H.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Common Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector; European Commission: Brussels, Belgium, 2016.
- Roets, E.; De Pourcq, P.; Toppet, S.; Hoogmartens, J.; Vanderhaeghe, H.; Williams, D.H.; Smith, R.J. Isolation and structure elucidation of ampicillin and amoxicillin oligomers. J. Chromatogr. A 1984, 303, 117–129. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Kockler, J.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B Environ. 2015, 166–167, 45–55. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies, 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, 2nd ed.; Springer: New York, NY, USA, 2015; pp. 1–374. [Google Scholar] [CrossRef]
- Ahmadifar, M.; Benfriha, K.; Shirinbayan, M.; Tcharkhtchi, A. Additive Manufacturing of Polymer-Based Composites Using Fused Filament Fabrication (FFF): A Review. Appl. Compos. Mater. 2021, 28, 1335–1380. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Cuan-Urquizo, E.; Barocio, E.; Tejada-Ortigoza, V.; Pipes, R.B.; Rodriguez, C.A.; Roman-Flores, A. Characterization of the Mechanical Properties of FFF Structures and Materials: A Review on the Experimental, Computational and Theoretical Approaches. Materials 2019, 12, 895. [Google Scholar] [CrossRef]
- Marković, M.-P.; Cingesar, I.K.; Keran, L.; Prlić, D.; Grčić, I.; Vrsaljko, D. Thermal and Mechanical Characterization of the New Functional Composites Used for 3D Printing of Static Mixers. Materials 2022, 15, 6713. [Google Scholar] [CrossRef]
- Karthika, V.; Arumugam, A. Synthesis and characterization of MWCNT/TiO2/Au nanocomposite for photocatalytic and antimicrobial activity. IET Nanobiotechnol. 2017, 11, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.L.; Yu, W.J. CVD-Grown Carbon Nanotube Branches on Black Silicon Stems for Ultrahigh Absorbance in Wide Wavelength Range. Sci. Rep. 2020, 10, 3441. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Lee, J.; Liu, Y.; Qi, H. Preparation and Photocatalytic Performance of MWCNTs/TiO2 Nanocomposites for Degradation of Aqueous Substrate. J. Chem. 2016, 2016, 1262017. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Wu, F.; Shi, M.; Liu, X. Oxidative Degradation of Amoxicillin in Aqueous Solution by Thermally Activated Persulfate. J. Chem. 2019, 2019, 2505823. [Google Scholar] [CrossRef]
- Latko-Durałek, P.; Dydek, K.; Boczkowska, A. Thermal, Rheological and Mechanical Properties of PETG/rPETG Blends. J. Polim. Environ. 2019, 27, 2600–2606. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Irska, I.; Taraghi, I.; Pilawka, R.; Gu, J.; Li, X.; Tu, Y.; Piesowicz, E. Synthesis and characterization of poly(ethylene terephthalate-co-1,4-cyclohexanedimethylene terephtlatate)-block-poly(tetramethylene oxide) copolymers. RSC Adv. 2017, 7, 41745–41754. [Google Scholar] [CrossRef]
- Da Silva, A.K.; Amador, J.; Cherchi, C.; Miller, S.M.; Morse, A.N.; Pellegrin, M.-L.; Wells, M.J.M. Emerging pollutants - part I: Occurrence, fate and transport. Water Environ. Res. 2013, 85, 1978–2021. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Bazzan Arsand, J.; Barcellos Hoff, R.; Jank, L.; Meirelles, L.N.; Diaz-Cruz, M.S.; Mara Pizzolato, T.; Barcelo, D. Transformation products of amoxicillin and ampicillin after photolysis in aqueous matrices: Identification and kinetics. Sci. Total Environ. 2018, 642, 954–967. [Google Scholar] [CrossRef]
- Nägele, E.; Moritz, R. Structure elucidation of degradation products of the antibiotic amoxicillin with ion trap MSn and accurate mass determination by ESI TOF. J. Am. Soc. Mass Spectrom. 2005, 16, 1670–1676. [Google Scholar] [CrossRef]
- Frański, R.; Czerniel, J.; Kowalska, M.; Frańska, M. Electrospray ionization collision induced dissociation tandem mass spectrometry of amoxicillin and ampicillin and their degradation products. Rapid Commun. Mass Spectrom. 2014, 28, 713–722. [Google Scholar] [CrossRef]
- Weng, X.; Cai, W.; Lin, S.; Chen, Z. Degradation mechanism of amoxicillin using clay supported nanoscale zerovalent iron. Appl. Clay Sci. 2017, 147, 137–142. [Google Scholar] [CrossRef]
- Trovó, A.G.; Pupo, N.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef]
- Dou, M.; Wang, J.; Gao, B.; Xu, C.; Yang, F. Photocatalytic difference of amoxicillin and cefotaxime under visible light by mesoporous g-C3N4: Mechanism, degradation pathway and DFT calculation. Chem. Eng. J. 2020, 383, 123–134. [Google Scholar] [CrossRef]
- Timm, A.; Borowska, E.; Majewsky, M.; Merel, S.; Zwiener, C.; Bräse, S.; Horn, H. Photolysis of four β-lactam antibiotics under simulated environmental conditions: Degradation, transformation products and antibacterial activity. Sci. Total Environ. 2019, 651, 1605–1612. [Google Scholar] [CrossRef]




| Substances | Maximum Acceptable Detection Limit | Monitoring Results of Substances |
|---|---|---|
| (µg/L) | (µg/L) | |
| Erythromycin | 0.19 | <0.00323 |
| Clarithromycin | 0.19 | <0.00259–<0.00931 |
| Azithromycin | 0.19 | <0.00309–<0.00623 |
| Amoxicillin | 0.078 | <0.00442–0.004729 |
| Ciprofloxacin | 0.089 | 0.0345925–0.1416513 |
| No. of Experiment | Static Mixer | Content of TiO2 (%) | Content of CNT (%) |
|---|---|---|---|
| 0 | No Static Mixer (blank) | 0 | 0 |
| 1 | Reference Static Mixer | 0 | 0 |
| 2 | Static Mixer with fillers (PETG) | 1.50 | 0.00 |
| 3 | 1.50 | 0.25 | |
| 4 | 1.50 | 0.50 | |
| 5 | 3.00 | 0.00 | |
| 6 | 3.00 | 0.25 | |
| 7 | 3.00 | 0.50 | |
| 8 | 6.00 | 0.00 | |
| 9 | 6.00 | 0.25 | |
| 10 | 6.00 | 0.50 |
| Parameter | Value |
|---|---|
| Flow | 0.5 mL/min |
| Injection Volume | 20 µL |
| Sheath Gas | 400 °C |
| Flow Rate | 12 L/min |
| Capillary Voltage | 4 kV |
| Nebulizer Pressure | 60 psi |
| Drying Gas | 5 L/min |
| Gas Temperature | 250 °C |
| Skimmer Voltage | 65 V |
| Octopole RF peak | 750 V |
| Fragmentor Voltage | 90 V |
| m/z Range | 40–950 |
| Resolution | 4 GHz |
| Compound Name | Formula | m/z (exp) | m/z (theo) |
|---|---|---|---|
| Photocatalysis with 3TiO2–0.25CNT@PETG static mixer | |||
| Amoxicillin | C16H19N3O5 S | 366.1126 | 366.1116 [35] |
| Photocatalysis product #1 | C25H38O5 | 419.2809 | 419.2809 |
| 2-Amino-2-(4-hydroxyphenyl)-N-{(Z)- [3-(4-hydroxyphenyl)-2-oxo-2,3,6,7-tetrahydro1H-1,4-diazepin-5-yl]methylidene}-acetamide | C20H21N4O4 | 381.2037 | 381.1563 [8] |
| Dehydrocarboxylated amoxicillin penilloic acid | C14H19N3O2NaS | 315.0790 | 316.1096 [8] |
| Amoxicillin penicilloic acid | C14H24N2O3S | 301.1427 | 300 [36] |
| Photocatalysis product #2 | C17H19N3 | 283.1916 | 283.1916 |
| Photocatalysis product #3 | C10H18O | 172.1709 | 172.1709 |
| Photocatalysis product #4 | C13H17NO4 | 249.1603 | 252.1230 [37] |
| Photocatalysis product #5 | C16H20N3O5S | 369.1725 | 368 [38] |
| Photolysis with reference static mixer | |||
| Photolysis product #1 | C25H38O5 | 419.28 | 419.28 |
| Photolysis product #2 | C24H36O5 | 405.2639 | 405.2639 |
| Photolysis product #3 | C21H29N3O | 357.2644 | 357.244 |
| Photolysis product #4 | C14H24N2O7 | 350.1905 | 349 [36] |
| Photolysis product #5 | C12H14N4O3S | 295.0861 | 295.0861 |
| Photolysis product #6 | C17H19N3 | 283.1906 | 283.1906 |
| Photolysis product #7 | C14H20N2O2 | 249.1597 | 249.1597 |
| Photolysis product #8 | C12H11N | 187.1225 | 187.1225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklec, K.; Grčić, I.; Radetić, L.; Cingesar, I.K.; Vrsaljko, D. Photocatalytic Oxidation of Amoxicillin in CPC Reactor over 3D Printed TiO2-CNT@PETG Static Mixers. Coatings 2023, 13, 386. https://doi.org/10.3390/coatings13020386
Miklec K, Grčić I, Radetić L, Cingesar IK, Vrsaljko D. Photocatalytic Oxidation of Amoxicillin in CPC Reactor over 3D Printed TiO2-CNT@PETG Static Mixers. Coatings. 2023; 13(2):386. https://doi.org/10.3390/coatings13020386
Chicago/Turabian StyleMiklec, Kristina, Ivana Grčić, Lucija Radetić, Ivan Karlo Cingesar, and Domagoj Vrsaljko. 2023. "Photocatalytic Oxidation of Amoxicillin in CPC Reactor over 3D Printed TiO2-CNT@PETG Static Mixers" Coatings 13, no. 2: 386. https://doi.org/10.3390/coatings13020386
APA StyleMiklec, K., Grčić, I., Radetić, L., Cingesar, I. K., & Vrsaljko, D. (2023). Photocatalytic Oxidation of Amoxicillin in CPC Reactor over 3D Printed TiO2-CNT@PETG Static Mixers. Coatings, 13(2), 386. https://doi.org/10.3390/coatings13020386








