Influence of the Compression Molding Temperature on VOCs and Odors Produced from Natural Fiber Composite Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
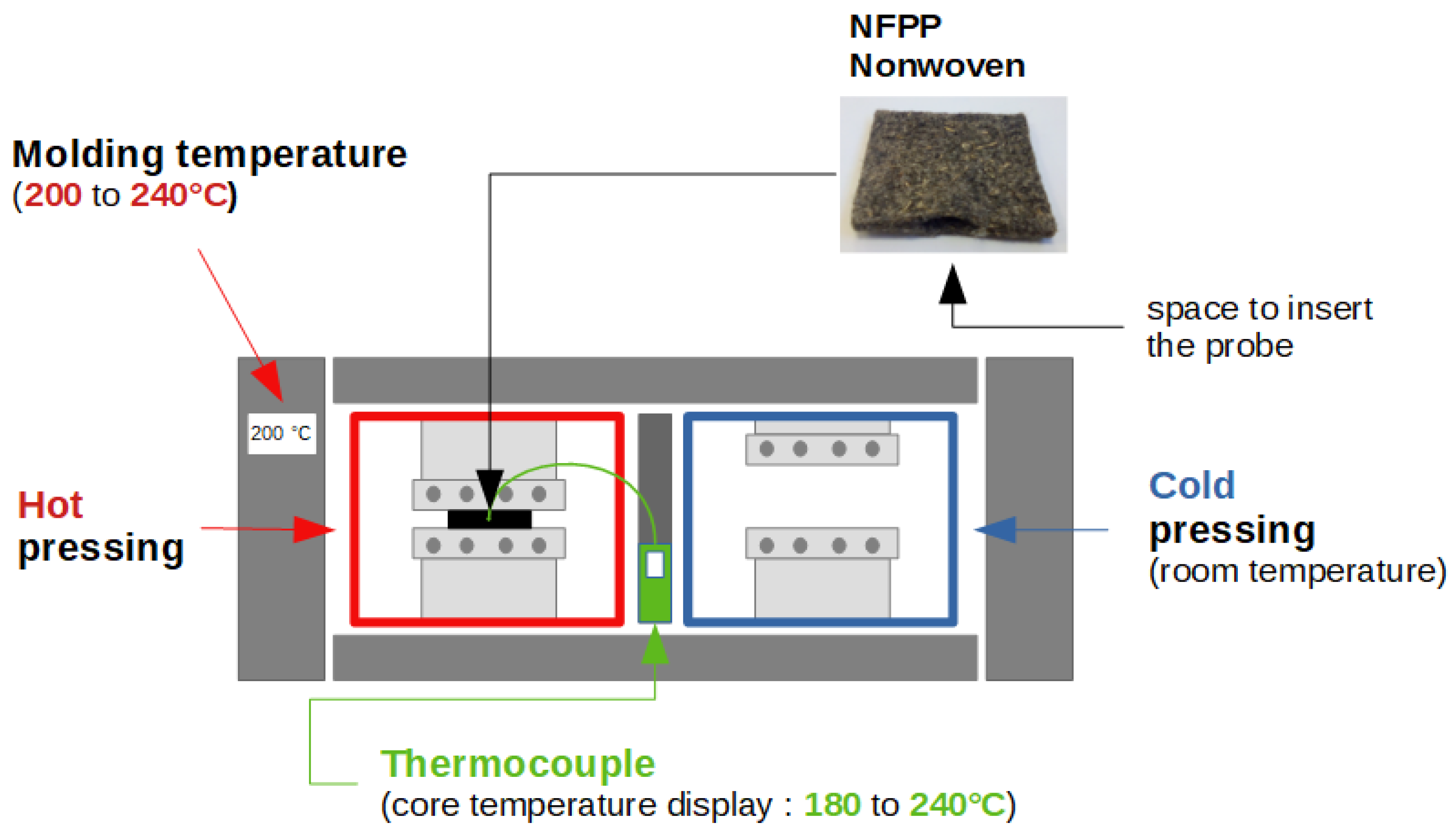
2.2.1. Composite Manufacturing
2.2.2. Temperature Measurement System
2.2.3. Samples Preparation and Conditioning
2.2.4. Extraction of Volatile Organic Compounds by the HS-SPME Method
2.2.5. GC-MS Analysis
2.2.6. Odor Analysis
2.2.7. Statistical Analysis
3. Results and Discussion
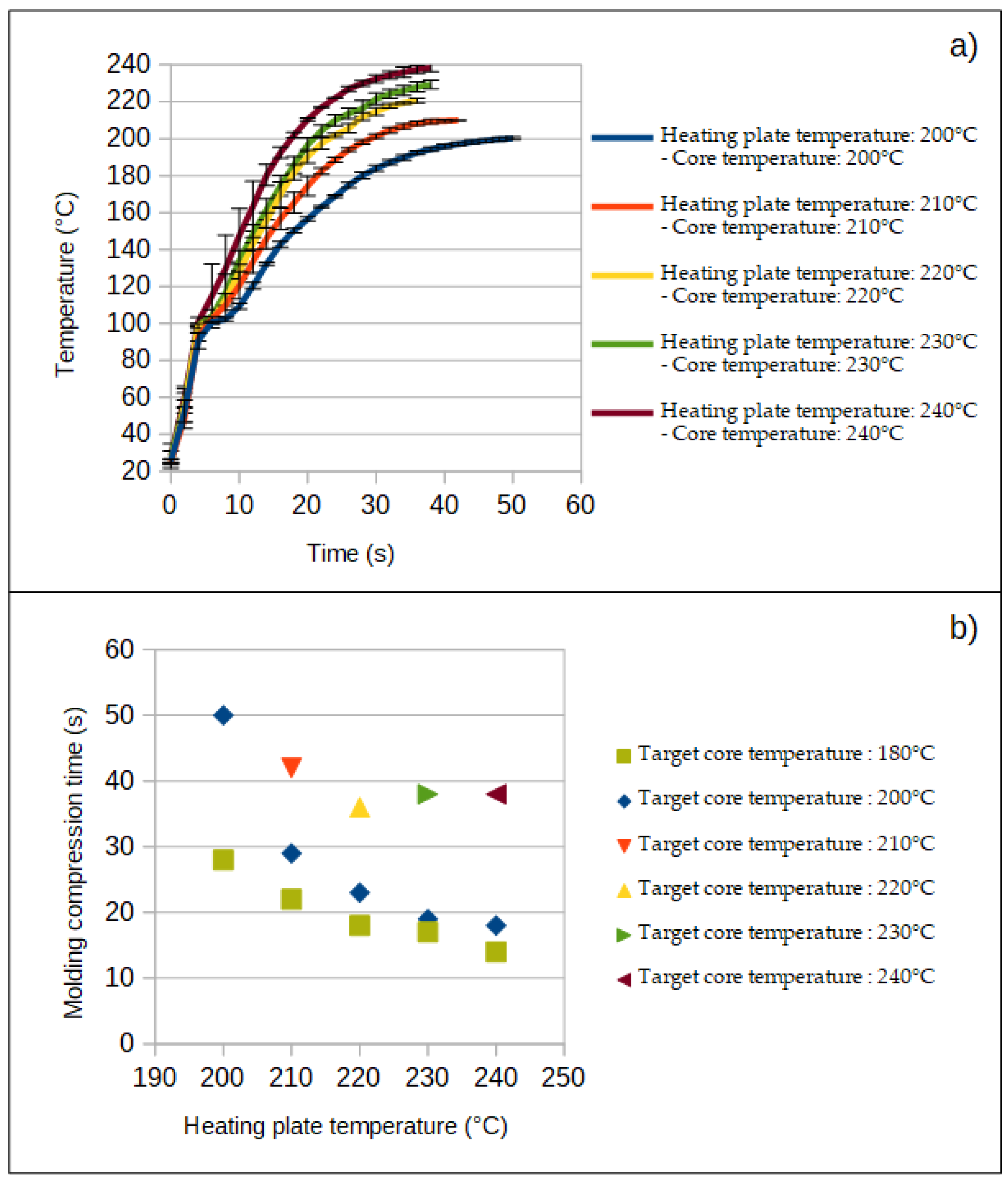
3.1. Evolution of the Temperature of the Nonwoven In Situ and in Real Time during the Compression Molding Process
3.2. Evolution of the Odor Intensity Coming from the NFC According to the Molding Temperature and Core Temperature of the Nonwoven In Situ during the Compression Molding Process
3.3. Evolution of VOCs Emission Coming from Fibers of NFC According to the Molding Temperature and Core Temperature
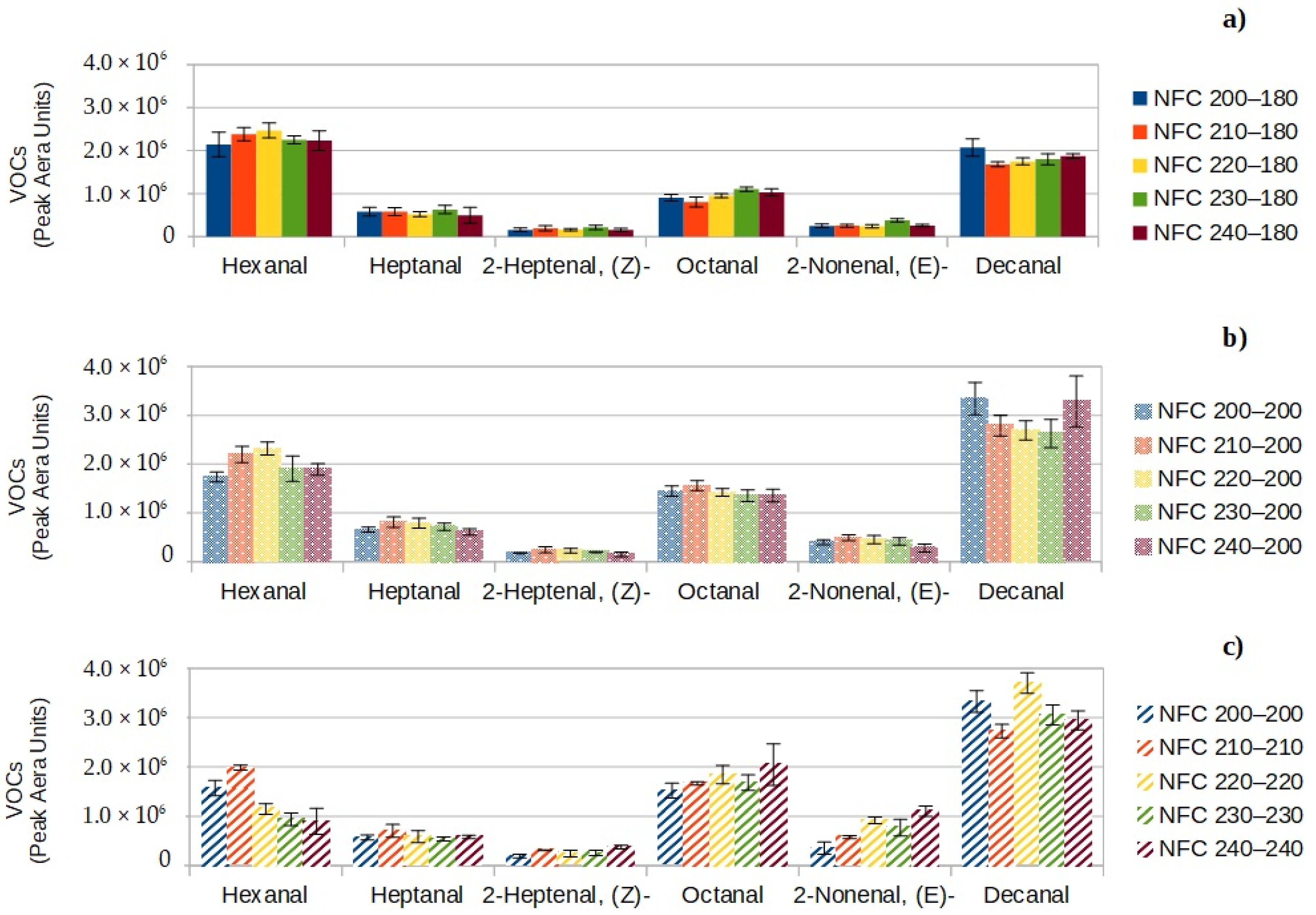
3.3.1. VOCs Coming from Lipophilic Extractives of Flax Fibers
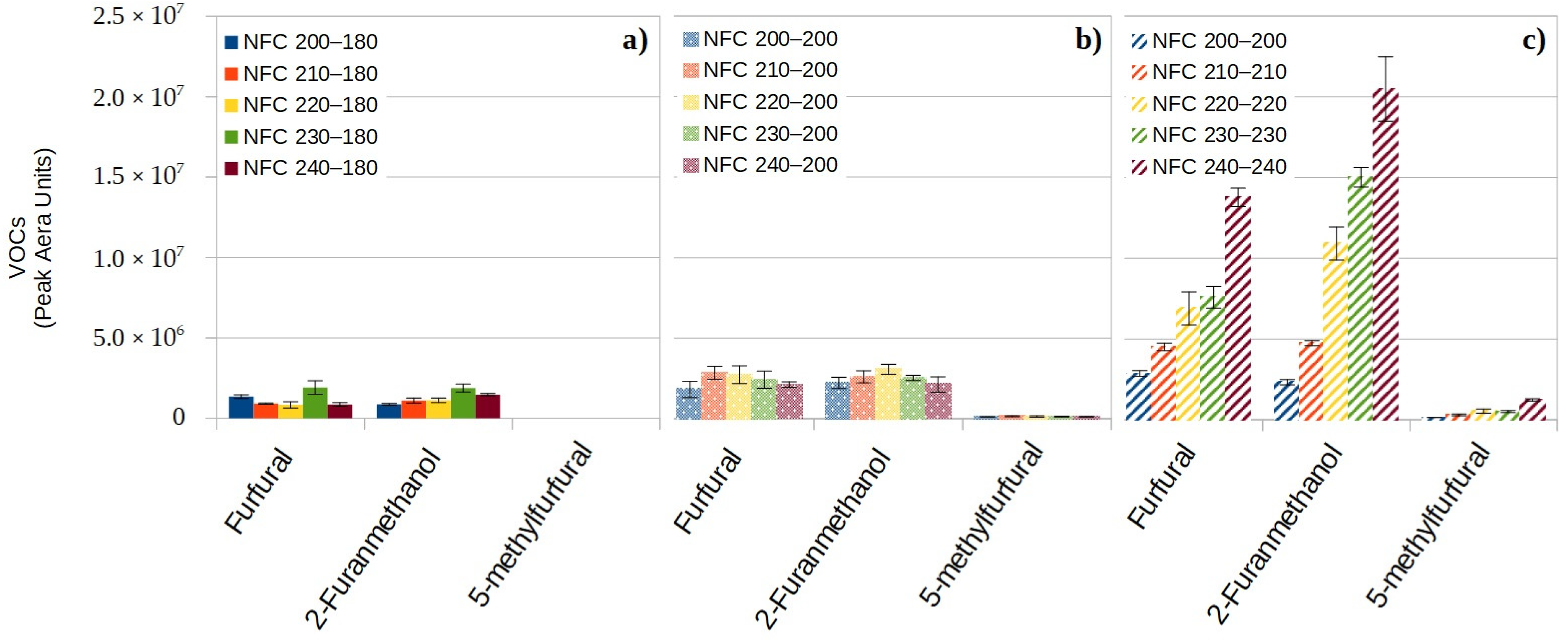
3.3.2. VOCs Coming from the Dehydration of the Primary Holocellulose Decomposition Products
3.3.3. VOCs Coming from the Primary Degradation of Lignin
4. Conclusions
- -
- For a target core temperature of 180 and 200 °C, heat nonwovens from 200 °C to 240 °C did not drastically change the odor intensity of NFCs. It is noteworthy that the lowest average odor intensity was obtained for material hot pressed at 200 °C.
- -
- For a target core temperature equal to the temperature of the heating plates of the hot press compression molding, heating nonwovens from 200 °C to 240 °C increased the odor intensity of NFCs. The lowest odor intensity is obtained for the heat press temperature of 200 °C and the targeted core temperature of 180 °C. Moreover, to avoid a high odor intensity (>4), the heat press temperature and targeted core temperature should not exceed 210 °C.
- -
- Some VOCs come from lipophilic extractives of flax fibers and are mainly aliphatic aldehydes (hexanal, heptanal, octanal and decanal) and unsaturated aldehydes (2-heptenal, 2-nonenal) associated with fatty and green odors.
- -
- Some VOCs come from the dehydration of the primary holocellulose decomposition products and are furan compounds (2-furanmethanol, furfural and 5-methylfurfural) associated with burnt sugar, baked bread and burnt odors.
- -
- Some VOCs come from the primary degradation of lignin, which are aromatic aldehydes (benzaldehyde and vanillin) associated with almond, burnt sugar and vanilla odors.
- -
- Switching from a target core temperature of 180 to 200 °C and heat nonwovens from 200 °C to 240 °C slightly increases all VOCs coming from flax fibers of NFC.
- -
- For a target core temperature equal to the temperature of the heating plates of hot press compression molding, heat nonwovens from 200 °C to 240 °C do not change the VOCs quantity coming from the lipophilic extractives of flax fibers but drastically increase the VOCs coming from the degradation of holocellulose and lignin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Odor Descriptors | Quantification (Mean Peak Area Units × 103 ± SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Core Temperature: 180 °C | |||||||||||
| VOCs Families | Compounds a,b | MSS(%) | RT exp. | LRI exp. | LRI lit. | NFC 200-180 | NFC 210-180 | NFC 220-180 | NFC 230-180 | NFC 240-180 | |
| Aldehyde | Hexenal | 98 | 6.37 | 806 | 804 | Green, fatty, aldehyde, herb c,d,e,f,g,h,i | 2142 ± 288 | 2383 ± 154 | 2466 ± 174 | 2253 ± 92 | 2233 ± 229 |
| Furfural | 98 | 7.00 | 831 | 836 | Almond, baked bread, woody, nut c,j,k,l,i | 1349 ± 116 | 1349 ± 116 | 834 ± 191 | 1912 ± 419 | 856 ± 115 | |
| Heptanal | 90 | 8.33 | 905 | 904 | Fatty, citrus, rancid, green c,g,h | 583 ± 98 | 581 ± 93 | 522 ± 56 | 631 ± 94 | 496 ± 185 | |
| 2-Heptenal, (Z)- | 92 | 9.38 | 913 | 960 | Fatty, fruit/mushroom, soapy h,i | 161 ± 38 | 195 ± 59 | 162 ± 28 | 215 ± 53 | 154 ± 39 | |
| 5-Methylfurfural | 94 | 9.46 | 920 | 964 | Caramel, almond, burnt sugar c | / | / | / | / | / | |
| Benzaldehyde | 96 | 9.52 | 982 | 967 | Almond, burnt sugar, sweet, caramel c,h,k,i | 209 ± 56 | 214 ± 79 | 241 ± 35 | 307 ± 72 | 223 ± 7 | |
| Octanal | 97 | 10.24 | 1005 | 1005 | Fatty, lemon, green, rancid, soap c,d,e,j,f,g,h | 908 ± 76 | 802 ± 115 | 950 ± 46 | 1105 ± 49 | 1031 ± 82 | |
| 2-Nonenal, (E)- | 95 | 13.74 | 1112 | 1162 | Cucumber, green, fatty c,d,j,f,gh | 251 ± 42 | 254 ± 34 | 244 ± 39 | 384 ± 38 | 259 ± 29 | |
| Decanal | 96 | 14.88 | 1204 | 1208 | Fatty, soapy, orange peel, sugar c,e,g,h | 2071 ± 200 | 1680 ± 57 | 1750 ± 80 | 1801 ± 128 | 1873 ± 53 | |
| Vanillin | 91 | 20.23 | 1392 | 1402 | Vanilla c,e,f,l | 90 ± 2 | 105 ± 9 | 104 ± 16 | 129 ± 7 | 109 ± 23 | |
| Alcohol | 2-Furanmethanol | 98 | 7.43 | 885 | 858 | Burnt, sweet, caramel, bread, coffee c,k,l | 860 ± 59 | 860 ± 59 | 1118 ± 138 | 1873 ± 249 | 1466 ± 72 |
| Carboxylicacid | Acetic acid | 98 | 3.44 | 656 | 576 | Sour, acid, sunflower seed, cheesy c,e,k | 7545 ± 677 | 7480 ± 1119 | 7584 ± 977 | 8800 ± 217 | 71761 ± 214 |
| Octanoic acid | 94 | 14.27 | 1183 | 1173 | Sweat, cheese, rancid c,k | 3318 ± 397 | 3106 ± 590 | 3585 ± 632 | 4555 ± 81 | 4099 ± 450 | |
| Furan | Furan, 2-pentyl- | 97 | 9.99 | 992 | 1040 | Fruity, green, earthy, orange c,g | 3006 ± 70 | 3136 ± 354 | 3137 ± 461 | 3605 ± 203 | 3215 ± 130 |
| Compounds a,b | Quantification (Mean Peak Area Units × 103 ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Core Temperature: 200 °C | Core Temperature: From 210 to 240 °C | ||||||||
| NFC 200-200 | NFC 210-200 | NFC 220-200 | NFC 230-200 | NFC 240-200 | NFC 210-210 | NFC 220-220 | NFC 230-230 | NFC 240-240 | |
| Hexenal | 1735 ± 102 | 2198 ± 165 | 2319 ± 132 | 1910 ± 261 | 1891 ± 117 | 1983 ± 52 | 1148 ± 104 | 942 ± 128 | 898 ± 261 |
| Furfural | 1814 ± 507 | 2847 ± 404 | 2731 ± 553 | 2419 ± 531 | 2118 ± 173 | 4518 ± 232 | 6898 ± 1025 | 7586 ± 672 | 13,777 ± 572 |
| Heptanal | 656 ± 52 | 809 ± 108 | 790 ± 102 | 712 ± 74 | 611 ± 65 | 707 ± 130 | 592 ± 122 | 543 ± 39 | 584 ± 34 |
| 2-Heptenal, (Z)- | 170 ± 11 | 244 ± 65 | 225 ± 50 | 196 ± 10 | 150 ± 48 | 314 ± 5 | 247 ± 68 | 260 ± 53 | 376 ± 36 |
| 5-Methylfurfural | 104 ± 9 | 138 ± 33 | 131 ± 47 | 119 ± 24 | 109 ± 15 | 296 ± 55 | 536 ± 132 | 507 ± 66 | 1230 ± 65 |
| Benzaldehyde | 279 ± 14 | 434 ± 50 | 434 ± 50 | 291 ± 25 | 267 ± 51 | 630 ± 29 | 765 ± 143 | 861 ± 111 | 1424 ± 41 |
| Octanal | 1445 ± 106 | 1557 ± 102 | 1420 ± 79 | 1353 ± 119 | 1355 ± 131 | 1663 ± 33 | 1843 ± 187 | 1678 ± 158 | 2045 ± 422 |
| 2-Nonenal, (E)- | 398 ± 51 | 490 ± 61 | 452 ± 83 | 415 ± 78 | 279 ± 83 | 580 ± 29 | 923 ± 67 | 772 ± 169 | 1104 ± 102 |
| Decanal | 3343 ± 332 | 2788 ± 213 | 2692 ± 195 | 2629 ± 289 | 3288 ± 519 | 2726 ± 136 | 3702 ± 208 | 3054 ± 201 | 944 ± 194 |
| Vanillin | 107 ± 6 | 205 ± 23 | 205 ± 23 | 151 ± 42 | 116 ± 16 | 261 ± 30 | 296 ± 80 | 326 ± 55 | 505 ± 90 |
| 2-Furanmethanol | 2214 ± 352 | 2596 ± 377 | 3063 ± 312 | 2531 ± 163 | 2123 ± 482 | 4744 ± 159 | 10,918 ± 1024 | 15,019 ± 598 | 20,480 ± 2001 |
| Acetic acid | 8523 ± 1719 | 9880 ± 219 | 8895 ± 1845 | 7893 ± 761 | 6458 ± 682 | 8579 ± 275 | 10,664 ± 1240 | 8,748 ± 1297 | 13,552 ± 1545 |
| Octanoic acid | 5986 ± 637 | 5821 ± 422 | 5078 ± 1188 | 5590 ± 2052 | 5678 ± 782 | 7059 ± 275 | 8378 ± 1031 | 7228 ± 995 | 14,051 ± 2381 |
| Furan, 2-pentyl- | 3375 ± 249 | 4148 ± 246 | 40,153 ± 81 | 3908 ± 121 | 3618 ± 348 | 4675 ± 149 | 4096 ± 530 | 4263 ± 619 | 4639 ± 324 |
References
- Paris Agreement, United Nations. 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 16 April 2020).
- CO2 Emission Performance Standards for Cars and Vans. Available online: https://climate.ec.europa.eu/eu-action/transport-emissions/road-transport-reducing-co2-emissions-vehicles/co2-emission-performance-standards-cars-and-vans_en (accessed on 7 November 2022).
- Proposal for a Regulation of the European Parliament and of the Council Amending Regulation (EU) 2019/631 as Regards Strengthening the CO2 Emission Performance Standards for New Passenger Cars and New Light Commercial Vehicles in Line with the Union’s Increased Climate Ambition; European Commission: Brussels, Belgium, 2021.
- La Transition Bas Carbone: Une Opportunité Pour l’industrie Automobile Française? The Shift Project: Paris, France, 2021; Rapport Final.
- Meilhan, N. Comment faire enfin baisser les émissions de CO2 des voitures. La Note D’analyse 2019, 78, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Ha, N.; Li, T. Research on Carbon Emissions of Electric Vehicles throughout the Life Cycle Assessment Taking into Vehicle Weight and Grid Mix Composition. Energies 2019, 12, 3612. [Google Scholar] [CrossRef]
- Ducreux, B.O.; Dore, N. AVIS de l’ADEME: Voitures Électriques et Bornes de Recharges. ADEME 2022, 1–10. [Google Scholar]
- Huda, M.K.; Widiastuti, I. Natural Fiber Reinforced Polymer in Automotive Application: A Systematic Literature Review. J. Phys.: Conf. Ser. 2021, 1808, 012015. [Google Scholar] [CrossRef]
- Issa, A.; Salihi, A.; Aliyu, A.B. Automotive Applications of Animal and Plant Fiber Based Thermoplastic Composite: A Review. Am. J. Eng. Res. 2022, 12. [Google Scholar]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Review: Raw Natural Fiber–Based Polymer Composites. Int. J. Polym. Anal. Charact. 2014, 19, 256–271. [Google Scholar] [CrossRef]
- Meirhaeghe, C.; Bewa, H. Evaluation de la disponibilité et de l’accessibilité de fibres végétales à usages matériaux en France. ADEME-FRD 2011, 84. [Google Scholar]
- Le Duigou, A.; Davies, P.; Baley, C. Environmental Impact Analysis of the Production of Flax Fibres to Be Used as Composite Material Reinforcement. J. Biobased Mater. Bioenergy 2011, 5, 153–165. [Google Scholar] [CrossRef]
- de Beus, N.; Barth, M.; Carus, M. Carbon Footprint and Sustainability of Different Natural Fibres for Biocomposites and Insulation Material. Nova Inst. 2019, 56. [Google Scholar]
- Gueudet, A. Analyse Du Cycle de Vie Comparative de Panneaux de Porte Automobiles Biosourcé (PP/Fibres de Lin et de Chanvre) et Petrosource (ABS). Quantis Frs Ecotechnilin 2016, 119. [Google Scholar]
- Merotte, J.; Le Duigou, A.; Bourmaud, A.; Behlouli, K.; Baley, C. Mechanical and Acoustic Behaviour of Porosity Controlled Randomly Dispersed Flax/PP Biocomposite. Polym. Test. 2016, 51, 174–180. [Google Scholar] [CrossRef]
- Zhang, J.; Khatibi, A.A.; Castanet, E.; Baum, T.; Komeily-Nia, Z.; Vroman, P.; Wang, X. Effect of Natural Fibre Reinforcement on the Sound and Vibration Damping Properties of Bio-Composites Compression Moulded by Nonwoven Mats. Compos. Commun. 2019, 13, 12–17. [Google Scholar] [CrossRef]
- Hadiji, H.; Assarar, M.; Zouari, W.; Pierre, F.; Behlouli, K.; Zouari, B.; Ayad, R. Damping Analysis of Nonwoven Natural Fibre-Reinforced Polypropylene Composites Used in Automotive Interior Parts. Polym. Test. 2020, 89, 106692. [Google Scholar] [CrossRef]
- Renouard, N.; Mérotte, J.; Kervoëlen, A.; Behlouli, K.; Baley, C.; Bourmaud, A. Exploring Two Innovative Recycling Ways for Poly-(Propylene)-Flax Non Wovens Wastes. Polym. Degrad. Stab. 2017, 142, 89–101. [Google Scholar] [CrossRef]
- Bourmaud, A.; Fazzini, M.; Renouard, N.; Behlouli, K.; Ouagne, P. Innovating Routes for the Reused of PP-Flax and PP-Glass Non Woven Composites: A Comparative Study. Polym. Degrad. Stab. 2018, 152, 259–271. [Google Scholar] [CrossRef]
- Shah, D.U. Developing Plant Fibre Composites for Structural Applications by Optimising Composite Parameters: A Critical Review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Engel, L. Biocomposites Performing Great—Not Only for Lightweight Construction. Nova-Institute. Renew. Carbon News 2019. [Google Scholar]
- Chen, X.; Zhang, G.; Chen, H. Controlling Strategies and Technologies of Volatile Organic Compounds Pollution in Interior Air of Cars. In Proceedings of the 2010 International Conference on Digital Manufacturing & Automation, Changcha, China, 18–20 December 2010; Volume 1, pp. 450–453. [Google Scholar]
- Faber, J. Air Quality inside Passenger Cars. AIMS Environ. Sci. 2017, 4, 112–133. [Google Scholar] [CrossRef]
- Badji, C.; Beigbeder, J.; Garay, H.; Bergeret, A.; Bénézet, J.-C.; Desauziers, V. Under Glass Weathering of Hemp Fibers Reinforced Polypropylene Biocomposites: Impact of Volatile Organic Compounds Emissions on Indoor Air Quality. Polym. Degrad. Stab. 2018, 149, 85–95. [Google Scholar] [CrossRef]
- Guidelines on Odour Pollution and Its Control. Central Pollution Control Board; Ministry of Environment & Forests, Govt. of India: New Delhi, India, 2008; 57p. [Google Scholar]
- Fischer, H.; Knittel, D.; Opwis, K. Verbesserung Der Geruchseigenschaften von Naturfasern Zur Öffnung Neuer Märkte in Den Bereichen Technische Textilien Und Verbundwerkstoffe.; University of Bremen: Bremen, Germany, 2008; ISBN 978-3-8370-7213-6. [Google Scholar]
- Savary, G.; Morel, A.; Picard, C.; Grisel, M. Effect of Temperature on the Release of Volatile and Odorous Compounds in Flax Fibers. J. Appl. Polym. Sci. 2016, 133, 43497. [Google Scholar] [CrossRef]
- Bledzki, A.; Al-Mamun, A.; Faruk, O. Abaca Fibre Reinforced PP Composites and Comparison with Jute and Flax Fibre PP Composites. Express Polym. Lett. —Express Polym Lett. 2007, 1, 755–762. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.; Liu, J.; Han, H.; Jia, H.; Song, J. Mechanical Properties and VOC Emission of Hemp Fibre Reinforced Polypropylene Composites: Natural Freezing-Mechanical Treatment and Interface Modification. Fibers Polym. 2021, 22, 1050–1062. [Google Scholar] [CrossRef]
- Morin, S.; Richel, A. Study of Chemical and Enzymatic Functionalization of Lignocellulosic Natural Fibers: Designing Natural Fibers for Biocomposites; Liège Université Gembloux Agro-Bio Tech: Liège, Belgium, 2021. [Google Scholar]
- Courgneau, C.; Rusu, D.; Henneuse, C.; Ducruet, V.; Lacrampe, M.F.; Krawczak, P. Characterisation of Low-Odour Emissive Polylactide/Cellulose Fibre Biocomposites for Car Interior. Express Polym. Lett. 2013, 7, 787. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, B.-H.; Kim, H.-J.; Yang, H.-S. Mechanical–Thermal Properties and VOC Emissions of Natural-Flour-Filled Biodegradable Polymer Hybrid Bio-Composites. J. Polym. Environ. 2011, 19, 628–636. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.; Al-Mamun, A. Influence of Compounding Processes and Fibre Length on the Mechanical Properties of Abaca Fibre-Polypropylene Composites. Polymery 2008, 53, 35–42. [Google Scholar] [CrossRef]
- Mahir, F.; Keya, K.N.; Sarker, B.; Nahiun, K.; Khan, R. A Brief Review on Natural Fiber Used as a Replacement of Synthetic Fiber in Polymer Composites. Mater. Eng. Res. 2019, 1, 88–99. [Google Scholar] [CrossRef]
- Radkar, S.; Amiri, A.; Ulven, C. Tensile Behavior and Diffusion of Moisture through Flax Fibers by Desorption Method. Sustainability 2019, 11, 3558. [Google Scholar] [CrossRef]
- Pintiaux, T.; Viet, D.; Vandenbossche, V.; Rigal, L.; Rouilly, A. Binderless Materials Obtained by Thermo-Compressive Processing of Lignocellulosic Fibers: A Comprehensive Review. BioResources 2015, 10, 1915–1963. [Google Scholar]
- Cristescu, C. Self-Bonding of Beech Veneers; Luleå Tekniska Universitet: Luleå, Sweden, 2015. [Google Scholar]
- Gager, V.; Le Duigou, A.; Bourmaud, A.; Pierre, F.F.; Behlouli, K.; Baley, C. Comportement Hygromécanique Des Biocomposites Non-Tissés Soumis à Des Variations d’humidité. In Proceedings of the JNC 21: 21ème Journées Nationales sur les Composites 2019; École Nationale Supérieure d’Arts et Métiers (ENSAM)-Bordeaux, Bordeaux, France, 1–3 July 2019. [Google Scholar]
- Evon, P.; Barthod-Malat, B.; Gregoire, M.; Vaca-Medina, G.; Labonne, L.; Ballas, S.; Véronèse, T.; Ouagne, P. Production of Fiberboards from Shives Collected after Continuous Fiber Mechanical Extraction from Oleaginous Flax. J. Nat. Fibers 2018, 16, 453–469. [Google Scholar] [CrossRef]
- Acera Fernández, J.; Le Moigne, N.; Caro-Bretelle, A.S.; El Hage, R.; Le Duc, A.; Lozachmeur, M.; Bono, P.; Bergeret, A. Role of Flax Cell Wall Components on the Microstructure and Transverse Mechanical Behaviour of Flax Fabrics Reinforced Epoxy Biocomposites. Ind. Crops Prod. 2016, 85, 93–108. [Google Scholar] [CrossRef]
- Gutiérrez, A.; del Río, J.C. Lipids from Flax Fibers and Their Fate in Alkaline Pulping. J. Agric. Food Chem. 2003, 51, 6911–6914. [Google Scholar] [CrossRef]
- Marques, G.; del Río, J.C.; Gutiérrez, A. Lipophilic Extractives from Several Nonwoody Lignocellulosic Crops (Flax, Hemp, Sisal, Abaca) and Their Fate during Alkaline Pulping and TCF/ECF Bleaching. Bioresour. Technol. 2010, 101, 260. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, E.; Fekete, E.; Tóth, A.; Bandi, E.K.; Koczka, B.; Sajó, I. Effect of Particle Size on the Surface Properties and Morphology of Ground Flax. Carbohydr. Polym. 2013, 94, 927–933. [Google Scholar] [CrossRef]
- Sachs, R.; Ihde, J.; Wilken, R.; Mayer, B. Treatment of Flax Fabric with AP-DBD in Parallel Plane Configuration. Plasma 2019, 2, 272–282. [Google Scholar] [CrossRef]
- Majcher, M.A.; Scheibe, M.; Jeleń, H.H. Identification of Odor Active Compounds in Physalis peruviana L. Molecules 2020, 25, 245. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the Smell of Wood-Identification of Odour-Active Compounds in Scots Pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 8294. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liu, X.; Tian, H.; Han, B.; Li, Y.; Tang, C.; Zhu, K.; Li, C.; Meng, Y. Odor-Active Volatile Compounds Profile of Triploid Rainbow Trout with Different Marketable Sizes. Aquac. Rep. 2020, 17, 100312. [Google Scholar] [CrossRef]
- Villavicencio, J.D.; Zoffoli, J.P.; Plotto, A.; Contreras, C. Aroma Compounds Are Responsible for an Herbaceous Off-Flavor in the Sweet Cherry (Prunus avium L.) Cv. Regina during Fruit Development. Agronomy 2021, 11, 2020. [Google Scholar] [CrossRef]
- Nie, C.; Gao, Y.; Du, X.; Bian, J.; Li, H.; Zhang, X.; Wang, C.; Li, S. Characterization of the Effect of Cis-3-Hexen-1-Ol on Green Tea Aroma. Sci. Rep. 2020, 10, 15506. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; He, Q.; Liu, P.; Che, Z.; Wang, X.; Huang, J. Characterization of Odor Components of Pixian Douban (Broad Bean Paste) by Aroma Extract Dilute Analysis and Odor Activity Values. Int. J. Food Prop. 2019, 22, 1223–1234. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, C.; Song, H.; Pan, W.; Gong, L. Characterization of Key Odor-Active Compounds in Thermal Reaction Beef Flavoring by SGC×GC-O-MS, AEDA, DHDA, OAV and Quantitative Measurements. J. Food Compos. Anal. 2022, 114, 104805. [Google Scholar] [CrossRef]
- Casassa, L.; Ceja, G.; Vega-Osorno, A.; Fresne, F.; Llodrá, D. Detailed Chemical Composition of Cabernet Sauvignon Wines Aged in French Oak Barrels Coopered with Three Different Stave Bending Techniques. Food Chem. 2020, 340, 127573. [Google Scholar] [CrossRef]
- Fereidoon, S.; Pegg, R.B. Hexanal as an Indicator of Meat Flavor Deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Clarke, H.J.; McCarthy, W.P.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods 2021, 10, 2315. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Kastrup Dalsgaard, T.; Sørensen, J.; Bakman, M.; Vognsen, L.; Nebel, C.; Albrechtsen, R.; Nielsen, J.H. Light-Induced Protein and Lipid Oxidation in Cheese: Dependence on Fat Content and Packaging Conditions. Dairy Sci. Technol. 2010, 90, 565–577. [Google Scholar] [CrossRef]
- Snyder, J.M.; Frankel, E.N.; Selke, E.; Warner, K. Comparison of Gas Chromatographic Methods for Volatile Lipid Oxidation Compounds in Soybean Oil. J. Amer. Oil Chem Soc. 1988, 65, 1617–1620. [Google Scholar] [CrossRef]
- Lipid Oxidation-2nd Edition. Available online: https://www.elsevier.com/books/lipid-oxidation/frankel/978-0-9531949-8-8 (accessed on 11 November 2022).
- Yang, S.; Lee, J.; Lee, J.; Lee, J. Effects of Riboflavin-Photosensitization on the Formation of Volatiles in Linoleic Acid Model Systems with Sodium Azide or D2O. Food Chem. 2007, 105, 1375–1381. [Google Scholar] [CrossRef]
- García-Martínez, M.C.; Márquez-Ruiz, G.; Fontecha, J.; Gordon, M.H. Volatile Oxidation Compounds in a Conjugated Linoleic Acid-Rich Oil. Food Chem. 2009, 113, 926–931. [Google Scholar] [CrossRef]
- Min, D.B.; Callison, A.L.; Lee, H.O. Singlet Oxygen Oxidation for 2-Pentylfuran and 2-Pentenyfuran Formation in Soybean Oil. J. Food Sci. 2003, 68, 1175–1178. [Google Scholar] [CrossRef]
- Krishnamurthy, R.G.; Smouse, T.H.; Mookherjee, B.D.; Reddy, B.R.; Chang, S.S. Identification of 2-Pentyl Furan in Fats and Oils and Its Relationship to the Reversion Flavor of Soybean Oil. J. Food Sci. 1967, 32, 372–374. [Google Scholar] [CrossRef]
- Cao, J.; Deng, L.; Zhu, X.-M.; Fan, Y.; Hu, J.-N.; Li, J.; Deng, Z.-Y. Novel Approach to Evaluate the Oxidation State of Vegetable Oils Using Characteristic Oxidation Indicators. J. Agric. Food Chem. 2014, 62, 12545–12552. [Google Scholar] [CrossRef] [PubMed]
- Domenek, S.; Berzin, F.; Ducruet, V.; Plessis, C.; Dhakal, H.; Richaud, E.; Beaugrand, J. Extrusion and Injection Moulding Induced Degradation of Date Palm Fibre-Polypropylene Composites. Polym. Degrad. Stab. 2021, 190, 109641. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shah, D.; Placet, V.; Baley, C. Towards the Design of High-Performance Plant Fibre Composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Paczkowski, S.; Paczkowska, M.; Dippel, S.; Schulze, N.; Schütz, S.; Sauerwald, T.; Weiß, A.; Bauer, M.; Gottschald, J.; Kohl, C.-D. The Olfaction of a Fire Beetle Leads to New Concepts for Early Fire Warning Systems. Sens. Actuators B: Chem. 2013, 183, 273–282. [Google Scholar] [CrossRef]
- González Martínez, M.; Anca Couce, A.; Dupont, C.; da Silva Perez, D.; Thiéry, S.; Meyer, X.; Gourdon, C. Torrefaction of Cellulose, Hemicelluloses and Lignin Extracted from Woody and Agricultural Biomass in TGA-GC/MS: Linking Production Profiles of Volatile Species to Biomass Type and Macromolecular Composition. Ind. Crops Prod. 2022, 176, 114350. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile Production from Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wang, C.-W.; Ong, H.C.; Show, P.L.; Hsieh, T.-H. Torrefaction, Pyrolysis and Two-Stage Thermodegradation of Hemicellulose, Cellulose and Lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Scheirs, J.; Camino, G.; Avidano, M.; Tumiatti, W. Origin of Furanic Compounds in Thermal Degradation of Cellulosic Insulating Paper. J. Appl. Polym. Sci. 1998, 69, 2541–2547. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the Pyrolytic Behaviour of Xylan-Based Hemicellulose Using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Wang, S.; Luo, Z. Interactions of Biomass Components during Pyrolysis: A TG-FTIR Study. J. Anal. Appl. Pyrolysis 2011, 90, 213–218. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.-M. Effect of Cellulose, Hemicellulose and Lignin Contents on Pyrolysis and Combustion of Natural Fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Chabi, M.; Goulas, E.; Leclercq, C.C.; de Waele, I.; Rihouey, C.; Cenci, U.; Day, A.; Blervacq, A.-S.; Neutelings, G.; Duponchel, L.; et al. A Cell Wall Proteome and Targeted Cell Wall Analyses Provide Novel Information on Hemicellulose Metabolism in Flax*. Mol. Cell. Proteom. 2017, 16, 1634–1651. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The Production of Furfural Directly from Hemicellulose in Lignocellulosic Biomass: A Review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Weil, J.; Dien, B.; Bothast, R.; Hendrickson, R.; Mosier, N.; Ladisch, M. Removal of Fermentation Inhibitors Formed during Pretreatment of Biomass by Polymeric Adsorbents. Ind. Eng. Chem. Res. 2002, 41, 132–6138. [Google Scholar] [CrossRef]
- Fahm, T.Y.A.; Mobarak, F. Advanced Binderless Board-like Green Nanocomposites from Undebarked Cotton Stalks and Mechanism of Self-Bonding. Cellulose 2013, 20, 1453. [Google Scholar] [CrossRef]
- Wakushie, N.; Woldeyes, B.; Demsash, H.; Jabasingh, S. An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation. Waste Biomass Valorization 2021, 12, 531–552. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and Composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Nitu, I.P.; Islam, M.N.; Ashaduzzaman, M.; Amin, M.K.; Shams, M.I. Optimization of Processing Parameters for the Manufacturing of Jute Stick Binderless Particleboard. J. Wood Sci. 2020, 66, 65. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Pintiaux, T.; Heuls, M.; Vandenbossche Maréchal, V.; Murphy, T.; Wuhrer, R.; Castignolles, P.; Gaborieau, M.; Rouilly, A. Cellulose Consolidation under High-Pressure and High-Temperature Uniaxial Compression. Cellulose 2019, 26, 2941–2954. [Google Scholar] [CrossRef]
- Badji, C.; Sotiropoulos, J.-M.; Beigbeder, J.; Garay, H.; Bergeret, A.; Bénézet, J.-C.; Desauziers, V. Under Glass Weathering of Hemp Fibers Reinforced Polypropylene Biocomposites: Degradation Mechanisms Based on Emitted Volatile Organic Compounds. Front. Mater. 2020, 7, 162. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Bridgwater, A.V.; Elliott, D.C.; Meier, D.; de Wild, P. Lignin Fast Pyrolysis: Results from an International Collaboration. J. Anal. Appl. Pyrolysis 2010, 88, 53–72. [Google Scholar] [CrossRef]
- Sallem-Idrissi, N.; Vanderghem, C.; Pacary, T.; Richel, A.; Debecker, D.P.; Devaux, J.; Sclavons, M. Lignin Degradation and Stability: Volatile Organic Compounds (VOCs) Analysis throughout Processing. Polym. Degrad. Stab. 2016, 130, 30–37. [Google Scholar] [CrossRef]


| Configuration | Molding Temperature (°C) | Core Temperature (°C) |
|---|---|---|
| NFC 200-180 | 200 | 180 |
| NFC 210-180 | 210 | 180 |
| NFC 220-180 | 220 | 180 |
| NFC 230-180 | 230 | 180 |
| NFC 240-180 | 240 | 180 |
| NFC 200-200 | 200 | 200 |
| NFC 210-200 | 210 | 200 |
| NFC 220-200 | 220 | 200 |
| NFC 230-200 | 230 | 200 |
| NFC 240-200 | 240 | 200 |
| NFC 210-210 | 210 | 210 |
| NFC 220-220 | 220 | 220 |
| NFC 230-230 | 230 | 230 |
| NFC 240-240 | 240 | 240 |
| Level | Odor Intensity | n-butanol Aqueous Solution (g/L) |
|---|---|---|
| 0 | No odor | 0 |
| 1 | Very weak | 1 × 10−2 |
| 2 | Weak | 5 × 10−2 |
| 3 | Strong | 5 × 10−1 |
| 4 | Very Strong | 2.5 |
| 5 | Very strong and insupportable | 10 |
| Factors of Variation and Interaction | dF | MS | F | p-Value (Pr > F) |
|---|---|---|---|---|
| Natural Fiber Composite (NFC) material | 13 | 1.63 | 6.26 | <0.0001 |
| Judge | 5 | 3.88 | 14.87 | <0.0001 |
| Session | 1 | 0.12 | 0.46 | 0.50 |
| NFC material × Judge | 65 | 0.42 | 1.62 | 0.03 |
| Session × NFC | 13 | 0.17 | 0.66 | 0.80 |
| Judge × Session | 5 | 0.31 | 1.19 | 0.33 |
| VOCs Families | Compounds a,b | LRI exp. | Odor Descriptors |
|---|---|---|---|
| Aldehyde | Hexenal | 806 | Green, fatty, aldehyde, herb c,d,e,f,g,h,i |
| Furfural | 831 | Almond, baked bread, woody, nut c,j,k,l,i | |
| Heptanal | 905 | Fatty, citrus, rancid, green c,g,h | |
| 2-Heptenal, (Z)- | 913 | Fatty, fruit/mushroom, soapy h,i | |
| 5-Methylfurfural | 920 | Caramel, almond, burnt sugar c | |
| Benzaldehyde | 982 | Almond, burnt sugar, sweet, caramel c,h,k,i | |
| Octanal | 1005 | Fatty, lemon, green, rancid, soap c,d,e,j,f,g,h | |
| 2-Nonenal, (E)- | 1112 | Cucumber, green, fatty c,d,j,f,g,h | |
| Decanal | 1204 | Fatty, soapy, orange peel, sugar c,e,g,h | |
| Vanillin | 1392 | Vanilla c,e,f,l | |
| Alcohol | 2-Furanmethanol | 885 | Burnt, sweet, caramel, bread, coffee c,k,l |
| Carboxylic acid | Acetic acid | 656 | Sour, acid, sunflower seed, cheesy c,e,k |
| Octanoic acid | 1183 | Sweat, cheese, rancid c,k | |
| Furan | Furan, 2-pentyl- | 992 | Fruity, green, earthy, orange c,g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthod-Malat, B.; Hauguel, M.; Behlouli, K.; Grisel, M.; Savary, G. Influence of the Compression Molding Temperature on VOCs and Odors Produced from Natural Fiber Composite Materials. Coatings 2023, 13, 371. https://doi.org/10.3390/coatings13020371
Barthod-Malat B, Hauguel M, Behlouli K, Grisel M, Savary G. Influence of the Compression Molding Temperature on VOCs and Odors Produced from Natural Fiber Composite Materials. Coatings. 2023; 13(2):371. https://doi.org/10.3390/coatings13020371
Chicago/Turabian StyleBarthod-Malat, Benjamin, Maxime Hauguel, Karim Behlouli, Michel Grisel, and Géraldine Savary. 2023. "Influence of the Compression Molding Temperature on VOCs and Odors Produced from Natural Fiber Composite Materials" Coatings 13, no. 2: 371. https://doi.org/10.3390/coatings13020371
APA StyleBarthod-Malat, B., Hauguel, M., Behlouli, K., Grisel, M., & Savary, G. (2023). Influence of the Compression Molding Temperature on VOCs and Odors Produced from Natural Fiber Composite Materials. Coatings, 13(2), 371. https://doi.org/10.3390/coatings13020371








