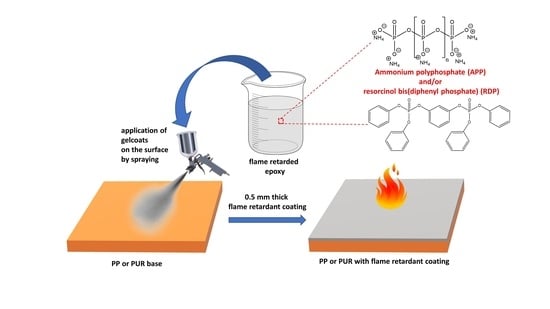
Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
3. Results and Discussion
3.1. Characterisation of the Gel Coatings
3.1.1. Viscosity, Glass Transition Temperature, Reaction Enthalpy, and Shore D Hardness of Gel Coatings
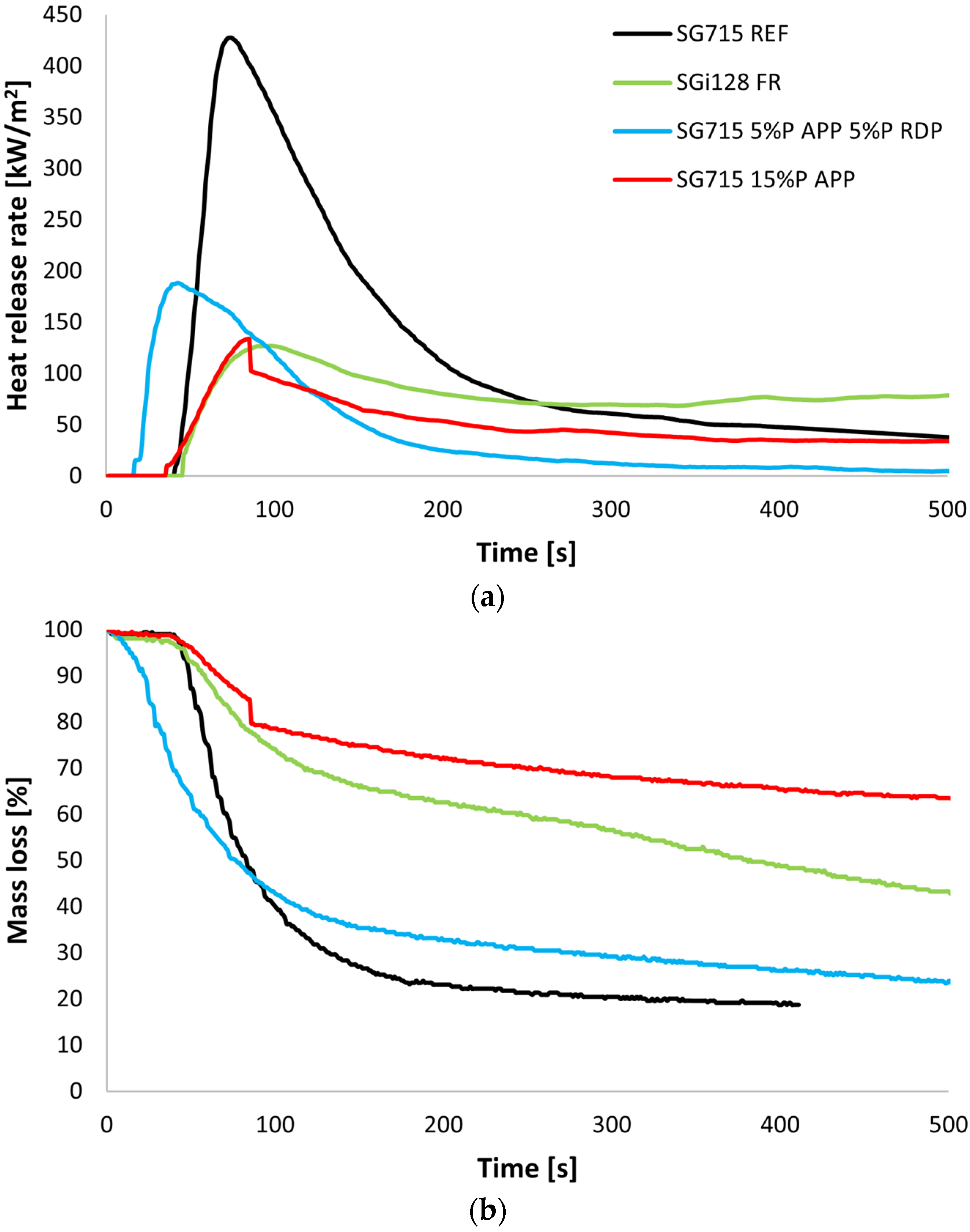
3.1.2. Flammability of Gel Coatings
3.2. Results of Coated PP Samples
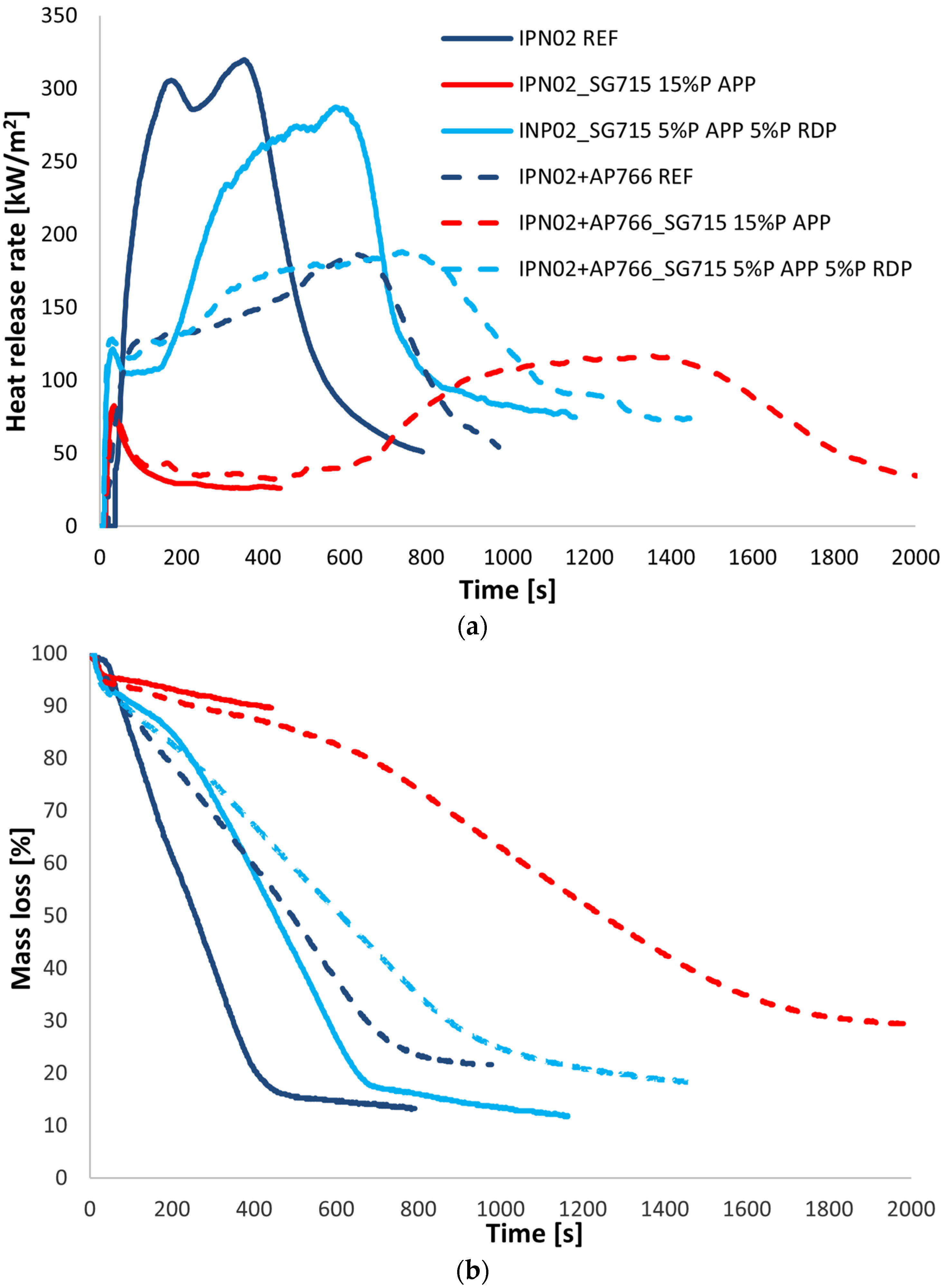
3.3. Results of Coated PUR Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krawczak, P. Automotive Plastics: What Future Is There for Polymers in Tomorrow’s Electric and Autonomous Vehicles? Express Polym. Lett. 2021, 15, 288. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Schartel, B. It Takes Two to Tango: Synergistic Expandable Graphite–Phosphorus Flame Retardant Combinations in Polyurethane Foams. Polymers 2022, 14, 2562. [Google Scholar] [CrossRef] [PubMed]
- Hull, T.R.; Law, R.J.; Bergman, Å. Environmental Drivers for Replacement of Halogenated Flame Retardants. In Polymer Green Flame Retardants; Papaspyrides, C.D., Pantelis, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 119–179. [Google Scholar]
- Toldy, A. Recyclable-by-Design Thermoset Polymers and Composites. Express Polym. Lett. 2021, 15, 1113. [Google Scholar] [CrossRef]
- Marosi, G.; Szolnoki, B.; Bocz, K.; Toldy, A. Fire Retardant Recyclable and Bio-Based Polymer Composites. In Novel Fire Retardant Polymers and Composite Materials: Technological Advances and Commercial Applications; Wang, D.-Y., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2016; pp. 117–146. [Google Scholar]
- Cardamone, G.F.; Ardolino, F.; Arena, U. Can Plastics from End-of-Life Vehicles Be Managed in a Sustainable Way? Sustain. Prod. Consum. 2022, 29, 115–127. [Google Scholar] [CrossRef]
- Pomázi, Á.; Krecz, M.; Toldy, A. Thermal Behaviour and Fire and Mechanical Performance of Carbon Fibre-Reinforced Epoxy Composites Coated with Flame-Retardant Epoxy Gelcoats. J. Therm. Anal. Calorim. 2022. [Google Scholar] [CrossRef]
- Yardimci, A.I.; Tanoglu, M.; Selamet, Y. Development of Electrically Conductive and Anisotropic Gel-Coat Systems Using CNTs. Prog. Org. Coat. 2013, 76, 963–965. [Google Scholar] [CrossRef]
- Sudha, G.S.; Arun, K.V. Effect of Gel Coat on Moisture Absorption and Mechanical Behavior of Jute-Epoxy Laminated Composites. J. Eng. Res. Appl. 2018, 52–59. [Google Scholar]
- Firdosh, S.; Murthy, H.N.N.; Angadi, G.; Raghavendra, N. Investigation of Water Absorption Characteristics of Nano-Gelcoat for Marine Application. Prog. Org. Coat. 2018, 114, 173–187. [Google Scholar] [CrossRef]
- Pomázi, Á.; Toldy, A. Multifunctional Gelcoats for Fiber Reinforced Composites. Coatings 2019, 9, 173. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Flame Retardant Polymer Composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Gu, J.-W.; Zhang, G.; Dong, S.; Zhang, Q.; Kong, J. Study on Preparation and Fire-Retardant Mechanism Analysis of Intumescent Flame-Retardant Coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings—A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259. [Google Scholar] [CrossRef]
- Pomázi, Á.; Toldy, A. Development of Fire Retardant Epoxy-Based Gelcoats for Carbon Fibre Reinforced Epoxy Resin Composites. Prog. Org. Coat. 2021, 151, 106015. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, J.; Liu, S.; Wang, S.; Gong, X. A Novel Anti-Impact and Flame Retardant Gel towards Human Protection and High-Temperature Alarm. Compos. Part A Appl. Sci. Manuf. 2022, 158, 106994. [Google Scholar] [CrossRef]
- Bifulco, A.; Malucelli, G.; Costantini, A.; Venezia, V.; Branda, F.; Gaan, S.; Vitiello, G.; Lehner, S.; Luciani, G.; Matta, S. Detailed Thermal, Fire, and Mechanical Study of Silicon-Modified Epoxy Resin Containing Humic Acid and Other Additives. ACS Appl. Polym. Mater. 2021, 3, 5969–5981. [Google Scholar] [CrossRef]
- Jain, P.; Choudhary, V.; Varma, I.K. Flame Retarding Epoxies with Phosphorous. J. Macromol. Sci. Part C 2002, 42, 139–183. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal Decomposition, Combustion and Flame-Retardancy of Epoxy Resins—A Review of the Recent Literature. Polym. Int. 2004, 53, 1901–1929. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Phosphorus Flame Retardants. In Krik-Othmer Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wang, J.; Su, X.; Mao, Z. The Flame Retardancy and Thermal Property of Poly(Ethylene Terephthalate)/Cyclotriphosphazene Modified Montmorillonite System. Polym. Degrad. Stab. 2014, 109, 154–161. [Google Scholar] [CrossRef]
- Zhang, R.C.; Hong, S.M.; Koo, C. Flame Retardancy and Mechanical Properties of Polyamide 6 with Melamine Polyphosphate and Ionic Liquid Surfactant-Treated Montmorillonite. J. Appl. Polym. Sci. 2014, 131, 40648. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A.E. On Flame Retardancy in Polycaprolactam Composites by Aluminim Diethylphosphinate and Melamine Polyphosphate in Conjunction with Organically Modified Montmorillonite Nanoclay. Polym. Degrad. Stab. 2014, 105, 1–11. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, N.; Li, Y. Functionalized Multi-Wall Carbon Nanotube for Improving the Flame Retardancy of Ramie/Poly(Lactic Acid) Composite. Compos. Sci. Technol. 2014, 104, 26–33. [Google Scholar] [CrossRef]
- Pandey, P.; Mohanty, S.; Nayak, S.K. Improved Flame Retardancy and Thermal Stability of Polymer/Clay Nanocomposites, with the Incorporation of Multiwalled Carbon Nanotube as Secondary Filler: Evaluation of Hybrid Effect of Nanofillers. High Perform. Polym. 2014, 26, 826–836. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Liang, G.; Gu, A.; Qiang, Z.; Chen, X. Unique Hybridized Carbon Nanotubes and Their High Performance Flame Retarding Composites with High Smoke Suppression, Good Toughness and Low Curing Temperature. J. Mater. Chem. A 2014, 2, 4975–4988. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.M.; Hu, Y. Enhanced Thermal and Flame Retardant Properties of Flame-Retardant-Wrapped Graphene/Epoxy Resin Nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Mülhaup, R.; Shartel, B. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polymer 2014, 6, 2875–2895. [Google Scholar] [CrossRef]
- Ran, S.; Chen, C.; Guo, Z.; Fang, Z. Char Barrier Effect of Graphene Nanoplatelets on the Flame Retardancy and Thermal Stability of High-Density Polyethylene Flame-Retarded by Brominated Polystyrene. J. Appl. Polym. Sci. 2014, 131, 40520. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Shen, M.; Huang, X.; Zhu, J.; Zhang, X. Preparation and Properties of Polystyrene Nanocomposites with Graphite Oxide and Graphene as Flame Retardants. J. Mater. Sci. 2013, 48, 4214–4222. [Google Scholar] [CrossRef]
- Knop, S.; Krieger, W. Flame retardant gelcoats on composite laminates. In Proceedings of the Composites in Fire, Newcastle upon Tyne, UK, 9–10 September 2003; pp. 29–34. [Google Scholar]
- Yuhazri, M.Y.; Haeryip, S.; Zaimi, Z.A.M.; Nilson, G.C. A Review on Gelcoat Used in Laminated Composite Structure. Int. J. Res. Eng. Technol. 2015, 4, 49–58. [Google Scholar] [CrossRef]
- Rogers, W.; Hoppins, C.; Gombos, Z.J.; Summerscales, J. In-Mould Gel-Coating of Polymer Composites: A Review. J. Clean. Prod. 2014, 70, 282–291. [Google Scholar] [CrossRef]
- Pomázi, Á.; Szolnoki, B.; Toldy, A. Flame Retardancy of Low-Viscosity Epoxy Resins and Their Carbon Fibre Reinforced Composites via a Combined Solid and Gas Phase Mechanism. Polymers 2018, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Pomázi, Á.; Krecz, M.; Toldy, A. The Effect of the Combined Application of Solid- and Gas-Phase Flame Retardants in Epoxy Gelcoats on the Thermal Stability, Fire Performance and Adhesion of Coated Carbon Fibre–Reinforced Epoxy Composites. J. Therm. Anal. Calorim. 2022, 148, 257–270. [Google Scholar] [CrossRef]



| Samples | Mixing Ratio (Epoxy Resin: Hardener) | Gelcoat (%) | Hardener (%) | APP (%) | RDP (%) | P-Content (%) |
|---|---|---|---|---|---|---|
| SG715 REF | 100:27 | 79 | 21 | 0 | 0 | 0 |
| SGi128 FR | 100:70 | 59 | 41 | 0 | 0 | 0 |
| SG715 5%P APP | 100:27 | 66 | 18 | 16 | 0 | 5 |
| SG715 5%P RDP | 100:27 | 43 | 11 | 0 | 46 | 5 |
| SG715 5%P APP 5%P RDP | 100:27 | 30 | 8 | 16 | 46 | 10 |
| SG715 10%P APP | 100:27 | 53 | 15 | 32 | 0 | 10 |
| SG715 15%P APP | 100:27 | 41 | 11 | 48 | 0 | 15 |
| Samples | Viscosity at 25 °C (Pa·s) | Glass Transition Temperature (°C) | Reaction Enthalpy Epoxy (J/g) | Shore D Hardness (-) |
|---|---|---|---|---|
| SG715 REF | 461 | 97 | 188 | 82 |
| SGi128 FR | 9 | 108 | 198 | 80 |
| SG715 5%P APP | 491 | 105 | 221 | 81 |
| SG715 5%P RDP | 24 | 98 | 177 | 29 |
| SG715 5%P APP 5%P RDP | 10 | 89 | 135 | 11 |
| SG715 10%P APP | 643 | 120 | 255 | 85 |
| SG715 15%P APP | 1963 | 123 | 212 | 79 |
| Samples | LOI | UL-94 1 (Horizontal Flame Spread Rate) | TTI 2 (s) | pHRR 3 (kW/m2) | Time to pHRR (s) | THR 4 (MJ/m2) | Residual Mass (%) |
|---|---|---|---|---|---|---|---|
| SG715 REF | 21 | HB (23 mm/min) | 41 | 428 | 74 | 60.0 | 18.7 |
| SGi128 FR | 42 | V-0 | 46 | 127 | 97 | 45.0 | 39.6 |
| SG715 5%P APP | 33 | V-0 | 29 | 200 | 74 | 42.1 | 32.1 |
| SG715 5%P RDP | 25 | V-0 | 14 | 352 | 37 | 28.3 | 17.7 |
| SG715 5%P APP 5%P RDP | 35 | V-0 | 17 | 189 | 42 | 21.8 | 27.8 |
| SG715 10%P APP | 62 | V-0 | 41 | 158 | 82 | 35 | 48.9 |
| SG715 15%P APP | >85 | V-0 | 36 | 133 | 84 | 27.3 | 62.1 |
| Sample | Shore D Hardness (-) | Pull-Off Adhesion Strength (MPa) |
|---|---|---|
| PUR | 75 ± 1 | n.a. |
| PUR SG715 | n.a. | 1.442 ± 0.157 |
| PUR SG715 15%P APP | 79 ± 1 | 1.535 ± 0.158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, Z.; Pomázi, Á.; Toldy, A. Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications. Coatings 2023, 13, 345. https://doi.org/10.3390/coatings13020345
Kovács Z, Pomázi Á, Toldy A. Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications. Coatings. 2023; 13(2):345. https://doi.org/10.3390/coatings13020345
Chicago/Turabian StyleKovács, Zsófia, Ákos Pomázi, and Andrea Toldy. 2023. "Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications" Coatings 13, no. 2: 345. https://doi.org/10.3390/coatings13020345
APA StyleKovács, Z., Pomázi, Á., & Toldy, A. (2023). Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications. Coatings, 13(2), 345. https://doi.org/10.3390/coatings13020345