Abstract
All-inorganic CsPbX3 perovskite material not only has the benefits of advanced light absorption coefficient, long carrier lifetime, and simple preparation process of organic–inorganic perovskite materials but it also maintains excellent stability under the erosion of damp heat. Stability is the premise of its industrialization, so all-inorganic perovskite is undoubtedly a very competitive direction for the development of perovskite materials. However, there are still many defects in the all-inorganic perovskite thin films, and it is difficult to obtain high power conversion efficiency (PCE). This review systematically summarizes additive engineering, solvent engineering, and interface engineering methods to promote the thin film property for a high PCE in recent years.
1. Introduction
Perovskite materials have attracted widespread attention in recent years due to their high light absorption coefficient, long carrier lifetime, low cost, simple preparation process [1,2,3,4], and ability for large-scale preparation [5,6,7,8]. The organic–inorganic hybrid perovskite solar cells (PSCs) have rapidly increased their power conversion efficiency (PCE) from 9.7% to over 25.5% [9,10,11,12]. However, in organic–inorganic hybrid perovskite devices, their organic parts, such as MA+ (CH3NH3+), FA+ ((CH(NH)2)2+), are easily affected and decomposed by the external temperature, illumination, and humidity [1,13,14,15,16,17], which leads to a serious decline in the PCE and stability [2,18]. For example, Juarez-Perez et al. [19] reported the decomposition temperature of MA+ was 80 °C, which was in the operating temperature range of PSCs. In 2015, Eperon et al. [20] for the first time prepared the all-inorganic CsPbI3 cell and the PCE was 2.9%. This historic breakthrough provided a new idea to develop the PSCs. Liu et al. [21] revealed the PSCs based on Cs-doped MAPbI3 can work steadily at 85 °C for 1000 h. They have manufactured modules (6.5 cm × 7 cm) with an advanced PCE of 21.08%. Steele et al. [22] found black CsPbI3 was stable even at a high temperature of 330 °C. All-inorganic perovskite also showed smaller exciton binding energy, higher defect tolerance, and better photoelectric property [23,24,25]. In particular, a CsPbI3 battery with an appropriate forbidden bandwidth of ~1.73 eV for the roof of a battery with silicon or other narrow band gap formation series battery [26,27]. All-inorganic perovskite solar cells have aroused wide public concern, as the PCE went to 21.0% in just a few years [28,29,30,31,32,33]. Although all-inorganic PSCs exhibited many excellent properties, their long-term development also faces many challenges, such as poor phase stability, high-density defects, and lower PCE. The property of the all-inorganic perovskite film affects the capability of the solar cells directly [34,35]. This review summarizes the main methods to obtain high-property all-inorganic perovskite films, including additive engineering, solvent engineering, and interface engineering. Then, we prospected the research direction and development prospect of all-inorganic PSCs in the future.
2. Film Quality Improvement
2.1. Additive Engineering
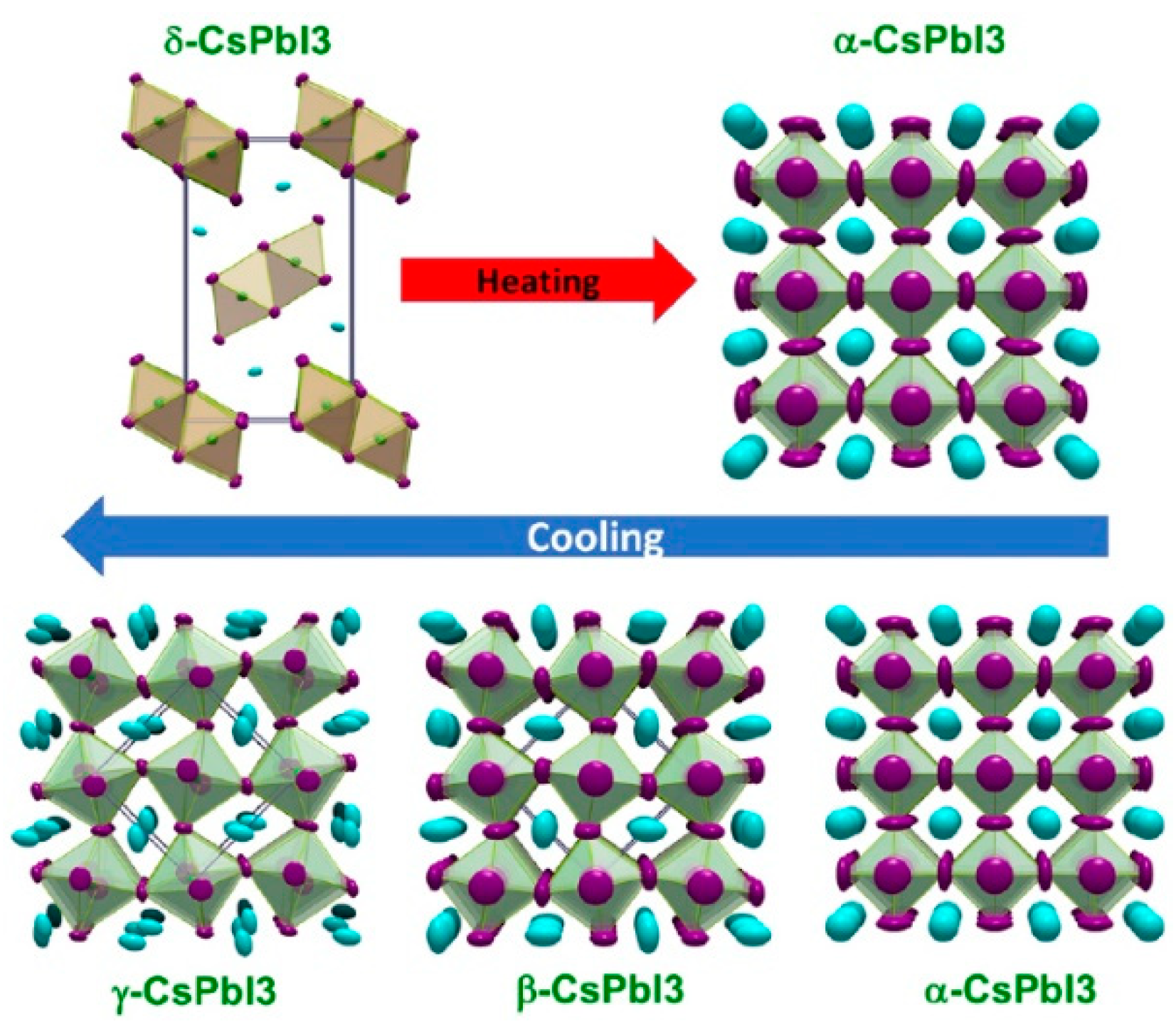
ABX3 (A = Cs+, B = Pb2+, X = I−, Br−, Cl−) is the general formula crystal structure for all-inorganic perovskite materials [1,14,36,37,38]. As shown in Figure 1, there are four common crystal structures: the cubic phase (α-, Pm3m), the tetragonal phase (β-, P4/mbm), the orthorhombic phase (γ-, Pbnm), and the non-perovskite phase (δ-, Pnma) [39,40]. α, β, and γ phases are perovskite phases, and have a photovoltaic effect. They are also called black phases. However, they are only stable at high temperatures. At room temperature, these black perovskite phases will naturally transform into the yellow non-perovskite phase, which will seriously affect the performance of the PSCs [5,41]. The perovskite crystal structure stability can be calculated by the tolerance factor t: t = (RA + RB)/(RB + RX), where RA, RB, and RX refer to the ionic radii of A, B, and X site ions, separately. Under normal conditions, if the crystal wants to maintain stability, the tolerance factor t needs to be in the range of 0.9–1. If the t value is less than this range, the crystal symmetry will be reduced and the entire structure stability will be affected. If the t value is more than this range, the system will occur a certain phase transition [38,42,43,44]. Therefore, in the research of all-inorganic perovskites, there is a certain theoretical basis for the selection of ions.
Figure 1.
Four different structural phases of CsPbI3 and their phase transitions reprinted with permission from Ref. [39] 2018 American Chemical Society.
The method of additive engineering is an easy way to raise the stability of CsPbX3 perovskite solar cells. Based on the tolerance factor t, lesser X-site anions mean bigger t values. The Br− (1.96 Å) and Cl− (1.81 Å) both have a smaller radius than I− (2.2 Å), so some groups have added them into CsPbI3. The CsPbI3 structural stability increased with the bigger t values [9,25,26,45,46,47,48,49,50,51]. The SCN− ion, a kind of pseudohalide ion, has a smaller radius, which could also strengthen the crystallization behavior of the CsPbI3 film, and promoted the PCE to 17.03% [52]. Wang et al. [53] resoundingly synthesized high-property CsPbBr3 films by adding 1.5% of NH4SCN into the precursor solution, which contained NH4+ and pseudohalide ion SCN-.
The lesser B-site cations are also good for phase stability [54]. Sn2+ and Pb2+ are both from group IVA and have similar physicochemical properties. The smaller Sn2+ ionic radius means a bigger t value, giving higher phase stability. Thus, Sn2+ is the most hopeful candidate to replace Pb2+. Murugadoss et al. [55] used different concentrations of Sn2+ doping to synthesize highly stable black phase CsPbI3 films, improving the PCE to 5.12%. The alkali metal ions from the group ⅡA, such as Ca2+ [56], Sr2+ [57], and Ba2+ [58,59], have the same valence state as Pb2+, which are also potential competitors. In addition, Mn2+ could improve the stability of CsPbI3 perovskite effectively [60,61,62]. Xu et al. found the appropriate amount of Cd2+ (1.0% mol of Pb2+) could improve the crystallinity of CsPbIBr2, reduce its trap density, and suppress its photo-generated carrier recombination [63]. The trivalent smaller cations could also replace the B-site. Typical trivalent ions are Bi3+ [64], Sb3+ [65], Yb3+ [66], Eu3+ [67], In3+ [18], Er3+ [68], and Gd3+ [69]. Faheem et al. [68] deposited a series of different concentrations of trivalent anion Er3- into CsPbIBr2 perovskite. They found CsI-(PbBr2)0.97 (ErCl3)0.03 was the optimal ratio. The PCE of this solar cell was increased by about 60% (7.28%). Pu et al. [69] used different molar ratios of gadolinium chloride (GdCl3) to increase the tolerance coefficient of CsPbI2Br. The results showed that the 0.4% GdCl3-incorporated CsPbI2Br film had the highest PCE of 16.24%.
Since Eperon et al. [20] first prepared stable cubic CsPbI3 films at room temperature by adding HI into its precursor solution, HI became the most common additive in CsPbI3 PSCs. Some groups [70,71] suggested that the precursor solution with HI was a polar solvent, which could cause the α-CsPbI3 to convert into the γ-CsPbI3. Yu et al. [72] used 35 μL HI and 10 μL H2O as additives to prepare high-quality CsPbI3 films by one-step spin coating at 100 °C. In addition, the perovskite films containing micro-water had a smoother surface with bigger grain sizes. The grain interface was repaired by dissolution and recrystallization to obtain a smooth film after removing the water. The fill factor (FF) was increased from 62% to around 69% and the PCE was improved from 10.34% to 11.42%. However, some researchers reported that the water in the HI acid may give a passive effect to form perovskite. They used DMF instead of water. Zhang et al. [73] first used HPbI3 to synthesize black CsPbI3 perovskite films at 150 °C. Then, many groups added HPbI3 in the precursor to prepare stable CsPbI3 films [23,47,52,74,75,76,77,78]. The PSCs with the HPbI3-treated CsPbI3 have a PCE of over 18 % [48,78]. Xi et al. [79] found that adding more HI could get a new compound of H2PbI4. H2PbI4 could also increase the phase stability.
However, the mechanism of improving CsPbI3 stability by HPbI3 treatment is controversial. Some groups [80,81,82] found that the really useful material is DMAPbI3, which was produced by the acid hydrolysis of DMF. DMA+ has a bigger radius than Cs+. When DMA+ partially replaced Cs+, it is easy to form a stable black phase. Some groups [78,83,84] had different opinions. They reported that annealing could remove most of the organic matter (DMA+), so only CsPbI3 was left. Some groups [29,85] even added DMAI into the CsPbI3 precursor immediately and got the best PCE of 19.03% [29].
Adding precursor material itself into the perovskite is also a good choice. Xiang et al. [86] added extra CsI into the precursor solution to synthesize black CsPbI3 films in an air atmosphere at 110 °C. Becker et al. [87] showed that more CsI in the precursor could directly obtain the γ-CsPbI3 perovskite phase. Their CsI-rich device gave a PCE of 12.5%. Bai et al. [88] controlled the ratio of CsI and PbI2 in the precursor precisely. The best PCE based on the obtained 0D Cs4PbI6 and γ-CsPbI3 heterojunction was 16.39%. Fu et al. [89] introduced excess CsBr into the CsPbI3 precursor to prepare a more stable CsPbI3 phase at low temperatures.
Fu et al. [90] added 2-hydroxyethyl methacrylate (HEMA) into the perovskite precursor to simultaneously increase the crystallization and decrease the deficiencies. The performance was best when 3 mg/mL HEMA was added. Yoon et al. [91] controlled the crystal growth procedure by adding a methylammonium chloride SDMS solution in continuous drops. Then, they used octyl ammonium iodides to passivate the surface in ambient air. CsPbI3 with a 45 mM MACl film was highly uniform and pinhole-minimized. Li et al. [92] introduced 16.90 mg·mL−1 CsPbBr3 quantum dots on the surface of the CsPbI3 film to passivate the deficiencies and improve the PCE to 16%. Zhang et al. [93] used the Zn2+ ions from zinc oxalate to recover the vacancies of Pb and Sn; then, they obtained perfect CsPb0.7Sn0.3IBr2 films with high crystallinity and lower defect density. Wang et al. [94] improved the properties of CsPbIBr2 films by bringing in a 3% molar ratio of guanidinium thiocyanate (GuaSCN), which was beneficial to optimize the PCE and stability of the PSCs. The various inorganic PSC performances using additive engineering are summarized in Table 1. Additive engineering was helpful for PSC performances. In addition to choosing the radius of the ions to add, the amount of material to add was also precisely controlled, as too many additions could have negative effects. Further, uncontrollable ionic components also greatly reduced the repeatability of the preparation process.

Table 1.
Summary of various inorganic PSCs’ performance using additive engineering.
2.2. Solvent Engineering
Solvent engineering is a valid strategy to enhance the properties of perovskite for excellent PSCs [95]. Snaith et al. [25] prepared high-property CsPbI2Br film for the first time by precisely controlling the dissolvability of CsPbI2Br in DMF. McGehee et al. [27] used DMSO as the solvent to get high-property CsPbI2Br films. Some groups [57,96,97,98,99] used dimethyl sulfoxide (DMSO) and dimethyl formamide (DMF) as co-solvents to obtain a CsPbI2Br film, which could promote the solubility of CsPbI2Br. Luo et al. [100] first obtained stable CsPbI3 in fully open-air conditions by treating it with hydroiodic acid (HI) and isopropanol (IPA). Meng et al. [101] dissolved CsBr with ethylene glycol monomethyl ether (EGME) and IPA to control the Ostwald ripening. They got high crystalline and favorable CsPbBr3 films. The EGME/IPA bi-solvent treatment increased the PCE from 3.57% to 7.29%.
With the increase of people’s awareness of environmental protection, green solvents are becoming more and more popular. He et al. [102] proved that green antisolvent IPA and thiourea can optimize the quality of CsPbIBr2 films. The carbon-based CsPbIBr2 PSCs have a PCE of 6.79%, which was almost 30% higher than the untreated PSCs. Wang et al. [103] replaced the noxious methanol with the environmentally friendly bi-component solvent (water and ethylene glycol monomethyl ether) to prepare high-property CsPbBr3 films. The CsPbBr3 PSC showed an optimal PCE of 9.55% with remarkable humidity stability. The eco-friendly ethyl acetate (EA) is another kind of solvent used in solvent engineering. Dong et al. [104] showed that the green EA antisolvent can optimize the properties of the CsPbI2Br films. The EA-engineered CsPbI2Br helped the PCE reach 10.0%. Han et al. [105] showed that the green EA antisolvent was able to promote better growth of the CsPbI3 films. Saparbaev et al. [106] used eco-friendly methylammonium acetate liquid to assemble CsPbI3 PSCs with a PCE of 14.4%.
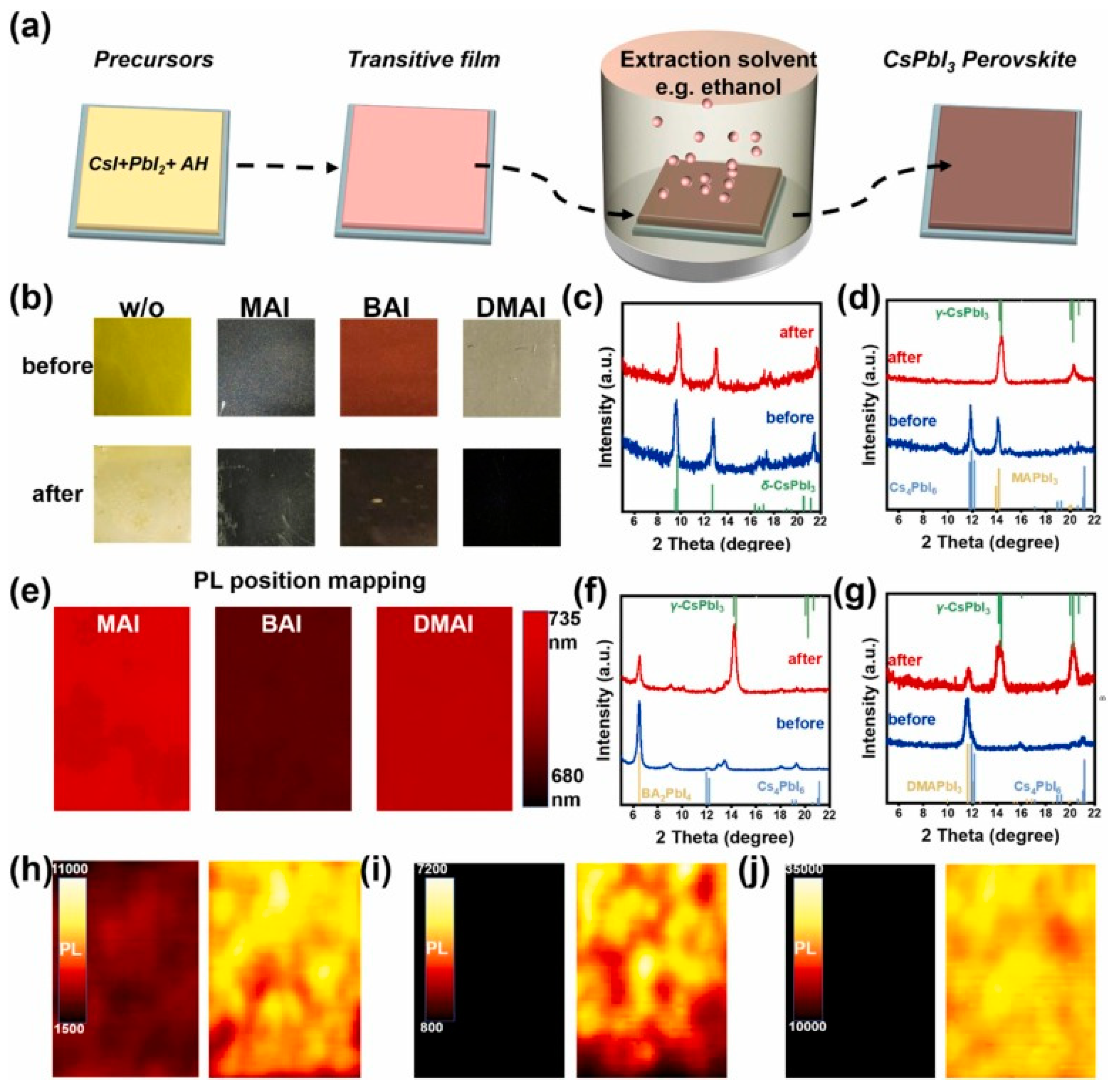
Regulating the preparation process with solvent engineering is also a valid method to optimize the quality of the all-inorganic perovskite films. Wang et al. [24] adopted a solvent-controlled growth method to obtain a stable α-CsPbI3 film. The CsPbI3 solar cells showed a 15.7% PCE. Teng et al. [107] reported that the decomposition rate of the CsPbBr3 precursor in a CsBr and methanol solution was faster, and CsPbBr3 films synthesized by the traditional two-step approach had low quality. Zai et al. [108] developed an easy low-temperature solution process to carefully regulate the CsPbI2Br crystallization kinetics, which increased PCE to 14.31%. The thermal and light stability was also perfect. Liu et al. [109] and Gao et al. [110] both applied a multistep spin-coating method to obtain the brilliant property of CsPbBr3 films, which could improve the uniformity of nucleation. Wang et al. [111] found alcohol could cause a quick crystallization process of the CsPbIBr2 films, which was very beneficial for improving its crystallinity. The obtained PSCs showed a high PCE of 11.49%. Wang et al. [112] adopted EAH-S solvent to extract the ammonium halide (Figure 2). This new strategy could solve the phase instability problems of CsPbI3. The carbon-based CsPbI3 PSCs attained a PCE of 15.35%. Zhong et al. [113] regulated the crystalline quality of CsPbBr3 films by adding 2-phenethylamine bromide (PEABr) to the PbBr2 DMF solution. The PCE increased to 8.25%. The PSCs’ performances related to solvent engineering are summarized in Table 2.
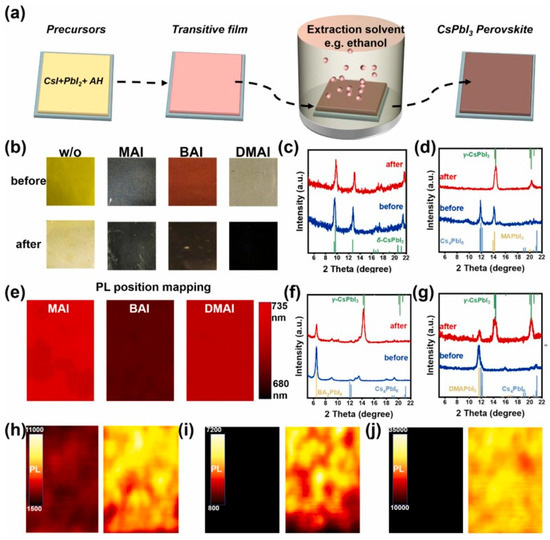
Figure 2.
EAH-S method to grow CsPbI3 perovskite. (a) Schematic diagram of EAH-S method. AH: ammonium halide. (b) Photographs of the transitive films prepared from the precursor solutions with/without ammonium iodides and the corresponding films after soaking in EtOH. XRD patterns of the transitive films and the corresponding films after soaking in EtOH: (c) without ammonium iodides, (d) MAI, (f) BAI, and (g) DMAI. (e) PL position mapping of the transitive films after soaking in EtOH. PL intensity mapping of the transitive films and the corresponding films after soaking in EtOH: (h) MAI, (i) BAI, and (j) DMAI reprinted with permission from Ref. [112] 2022 Elsevier.

Table 2.
Summary of various inorganic PSCs’ performance using solvent engineering.
2.3. Interface Engineering
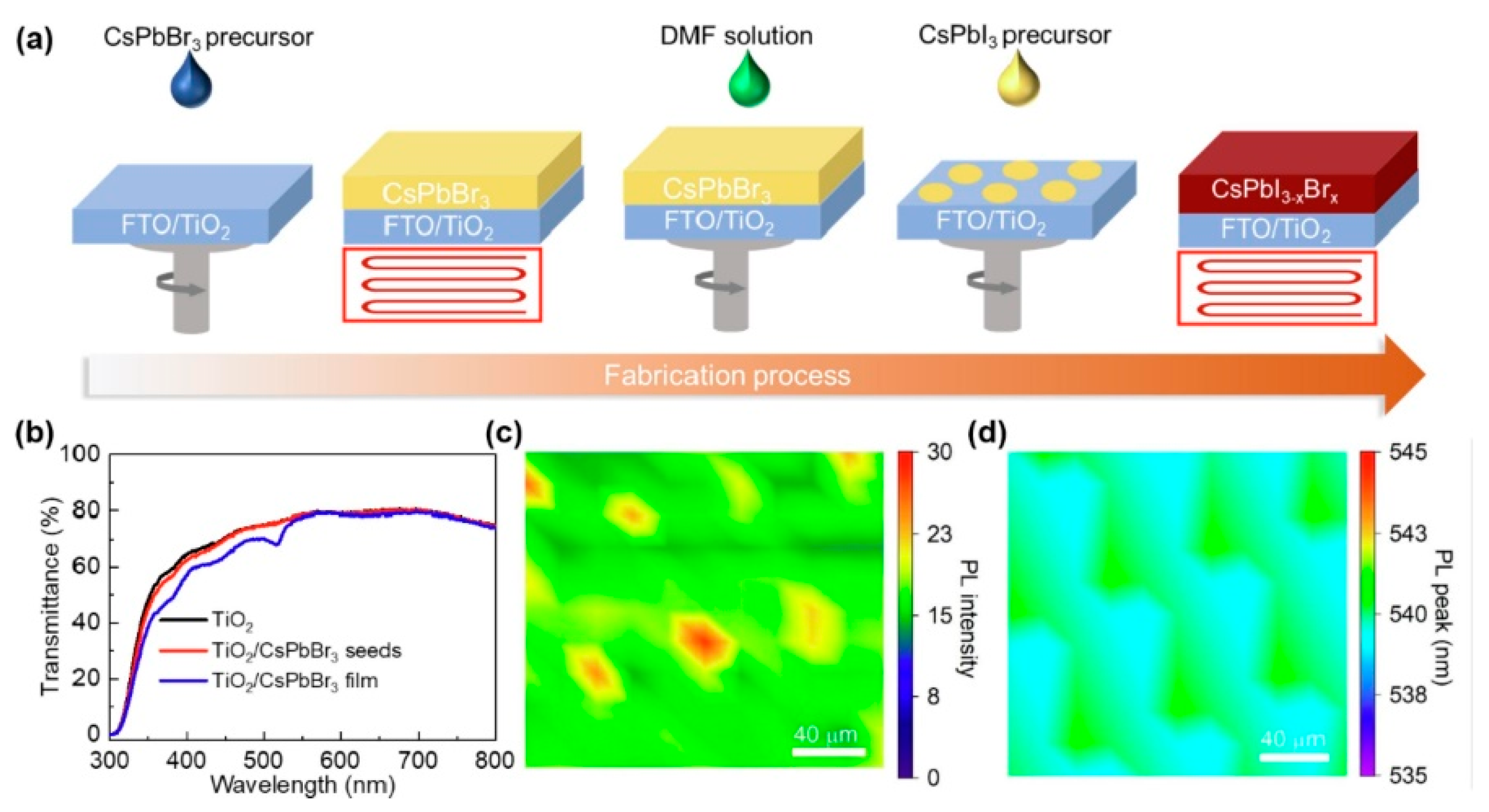
Interface engineering is also commonly used to modulate the morphology of perovskite films, stabilize the perovskite phase, and enhance operational stability [114,115]. Choosing contact materials and surface modification of the chosen contact materials are the main focuses of interface engineering research. For example, new materials are used in the electron transport layer (ETL). Yan et al. [116] introduced the SnO2/ZnO bilayer ETL to obtain regular crystal grains and full coverage CsPbI2Br films. Yue et al. [117] used the ZnO-doped TiO2 buffer layer to improve the PCE to 21.06%, and the Voc and FF were 1.31 V and 74.1%. Yang et al. [118] explored a better wettability surface of the In2S3 ETL to increase the rate of CsPbIBr2 nucleation. Zhu et al. [119] presented a TiO2/SnO2 ETL that could control the CsPbIBr2 crystallization. Li et al. [120] adopted a strategy of interface engineering by depositing the CsPbI2Br perovskite film on a ZnO/MgxZn1-xO bilayer ETL, which could alleviate energy loss in the PSCs. The best PCE was 16.04%. Pan et al. [121] chose well-arranged TiO2 nanopillar arrays (TiO2 NaPAs) to boost the interface of CsPbI2Br and ETL, which resulted in continuously dense CsPbI2Br films, with few defects. Guo et al. [122] improved the perovskite quality by the passivation of SnO2 ETL. It could enhance the carrier transport across the ETL/CsPbIBr2 boundary surface, raise the charge reorganization resistance, and reduce the Eloss for the ultimate PSCs. Lu et al. [123] took TiCl4 treatment to enhance the perovskite film property for better PSCs. The PCE of TiCl4-TiO2- and TiCl4-ZnO-based PSCs increased to 16.5% and 17.0%, respectively. Wang et al. [124] adopted the amino group from polyethyleneimine ethoxylated (PEIE) to inactivate the defects of CsPbIBr2 and increase the PCE from 8.7% to 11.2%. Wang et al. [125] modified the interface of TiO2/perovskite with CsAc, which enhanced the CsPbI2.25Br0.75 film morphology, crystallinity, and electrochemical properties. The PSCs based on the CsAc-modified method showed an optimized PCE of 13.81%. Chai et al. [126] added CsPbBr3 seeds into the TiO2 ETL to regulate the CsPbI3 crystal growth and promote the PCE (Figure 3). This new method provided a new idea for the commercial application of PSCs.
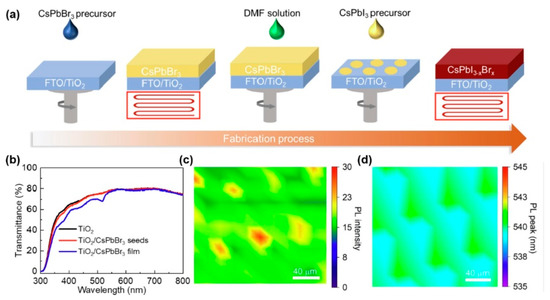
Figure 3.
(a) Schematic of preparation process of CsPbI3 films with CsPbBr3 pieces. (b) Transmittance spectra of TiO2, TiO2/CsPbBr3 seeds, and TiO2/CsPbBr3 film. (c) Confocal PL intensity and (d) PL peak position maps of TiO2/CsPbBr3 pieces, respectively reprinted with permission from Ref. [126] 2023 Elsevier.
The boundary surface between the perovskite and the hole transport layer (HTL) or electrode (without HTL) is another target to optimize perovskite film properties. Liu et al. [127] used a subtle MoOx cushion coat to raise the carrier injection, and reduce the trap state and the contact resistance. Zhou et al. [128] introduced an interfacial layer of MoO3 to allow for highly competent charge separation and to suppress carrier reorganization. The PSCs exhibited a fairly high PCE of 14.05% with an FF of 81.5%. Zong et al. [129] introduced a simple synthetic and efficient MoO2/N-doped carbon nanospheres complex inorganic HTL to establish a high capability and steady CsPbBr3 PSCs. Xue et al. [130] used dopamine (DA) doping to improve the HTL work function and increase the perovskite film crystallinity. Ding et al. [131] modified a polyvinyl acetate with a carbonyl group at the boundary surface of CsPbBr3/carbon to inactivate the perovskite surface defect states and intensify the energy level alignment between the CsPbBr3 valence band and the carbon work function. Yang et al. [132] found that tris(N,N,N-tributyl-1-butanaminium)[[2,2′′6′ ,2′′-terpyridine] -4,4′,4 ′′—tricarboxylato(3−)-N1,N1′,N1′′]tris(thiocyanato-N)hydrogen ruthenate(4−) (N749) between the Cs-NiOx and CsPbIBr2 can inhibit the phase separation and block moisture penetration. The inverted CsPbIBr2 PSCs treated by N749 had amazing humidity stability. Zhu et al. [133] found that the diazonium atoms embracing the unpaired electrons in N,N’-Dicyclohexylcarbodiimide (DCC) have a strong passivation effect on the unpaired Pb2+ and Cs+ ion drawbacks, which greatly reduced the drawback state of CsPbBr3 films and caused the non-radiation reorganization. Du et al. [134] drafted (R)-(-)-1-cyclohexylethylamine iodide (R-CEAI) to inactivate the deficiencies of perovskite, and grew a quasi-2D Ruddlesden–Popper perovskite on the 3D perovskite. The R-CEAI-served 2D/3D PSCs had a PCE of 22.52%. Xu et al. [135] constructed a PbS/CdS heterojunction in CsPbI1.5Br1.5 and the carbon electrode to perfect the PSCs stability of the device. Zou et al. [136] passivated the CsPbBr3/carbon interface by using NiO nanocrystals. The obtained CsPbBr3 films showed prominent physical and chemical properties.
Some groups even considered both the perovskite/ETL and perovskite/HTL interfaces. Liao et al. [137] added carbon quantum dots to the CsPbBr3/TiO2 interfaces and red phosphorus quantum dots to the CsPbBr3/Carbon interfaces, which accelerated both electron and hole transfer into the interface layer. As a result, the Voc was increased by about 0.2 V. CsPbBr3/CsSnBr2I QDs bilayers [138], CsPbBr3/CdZnSe@ZnSe QDs bilayers [139], and CsPbI2Br/CsPbI3 QDs bilayers [140] were also used to reduce the Eloss. Chen et al. [141] enhanced the morphology of CsPbIBr2, PCE, and stability by introducing rubidium acetate (RbAc) to the ETL/perovskite/HTL interfaces. Shi et al. [142] synthesized two kinds of C3N4 materials (w-CN and y-CN) simultaneously and employed them in the two-sided boundary surface of the perovskite film. Table 3 summarizes the performances of PSCs based on interface engineering. The properties of the perovskite film were optimized, and the PCE was increased. Interface engineering can improve the transmission of charge between the interface of PSCs, reduce recombination loss, and improve the stability, which is an effective method to promote the photoelectric performance and long-term stability at the same time [143].

Table 3.
Summary of various inorganic PSCs’ performance using interface engineering.
3. Results
Maximizing the PCE and improving the stability of all-inorganic CsPbX3 perovskite solar cells is still a major challenge. We summarized some methods here. First, introducing additives such as X-site anions, B-site cations, HI, HPbI3, precursor materials, and quantum dots could improve the crystallinity and morphology of the perovskite film. Choosing the appropriate radius for the addition was important, as was the molar ratio of the addition. Second, the proper solvent was helpful to enhance the properties of perovskite for excellent PSCs. To adapt to the future market demand, green solvents were popular. Third, interface modification could improve the film surface morphology and crystallinity. The charge transfer between layers was directly related to the PSCs’ performance. The heterojunction will be one of the future trends. PCE, long-term stability, and preparation cost are important evaluation indices of solar cells. There is no doubt that all-inorganic PSCs have infinite potential and broad application prospects. However, compared with organic–inorganic hybrid PSCs, the PCE of all-inorganic PSCs still has a certain gap. Some existing problems and research prospects of all-inorganic PSCs are summarized as follows: (1) the mechanism of all-inorganic PSCs have not been studied thoroughly until now. It was difficult to optimize the PSCs’ performance, ensure repeatability, and prepare them on a large scale. (2) There were inconsistencies in the evaluation of all-inorganic PSCs’ stability in reports. It may be necessary in the future to develop uniform evaluation criteria to better adapt to commercial applications. (3) The toxicity of lead was an unavoidable problem and may be studied in terms of lead leakage or replacement.
Author Contributions
Conceptualization, Y.C. and F.L.; validation, Y.C., F.L., M.Z. and Z.Y.; writing—original draft preparation, Y.C. and F.L.; writing—review and editing, Y.C.; visualization, Y.C.; supervision, Y.C.; project administration, Y.C.; funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21701041; the Open Foundation of Hubei Collaborative Innovation Center for High-Efficiency Utilization of Solar Energy, grant number HBSKFQN2017001; and the Talents of the High-Level Scientific Research Foundation of the Hubei University of Technology, grant number BSQD2017010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, L.; Yuan, S.; Ma, L.; Zhang, B.; Fang, T.; Li, X.; Song, J. All-inorganic perovskite quantum dots as light-harvesting, interfacial, and light-converting layers toward solar cells. J. Mater. Chem. A 2021, 9, 18947–18973. [Google Scholar] [CrossRef]
- Xiang, W.; Tress, W. Review on Recent Progress of All-Inorganic Metal Halide Perovskites and Solar Cells. Adv. Mater. 2019, 31, 1902851. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, H. Spacer Organic Cation Engineering for Quasi-2D Metal Halide Perovskites and the Optoelectronic Application. Small Struct. 2022, 3, 2100232. [Google Scholar] [CrossRef]
- Zhu, W.K.; Wang, S.R.; Zhang, X.; Wang, A.L.; Wu, C.; Hao, F. Ion Migration in Organic-Inorganic Hybrid Perovskite Solar Cells: Current Understanding and Perspectives. Small 2022, 18, 2105783. [Google Scholar] [CrossRef]
- Ghosh, P.; Bruckbauer, J.; Trager-Cowan, C.; Krishnan Jagadamma, L. Crystalline grain engineered CsPbIBr2 films for indoor photovoltaics. Appl. Surf. Sci. 2022, 592, 152865. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The Main Progress of Perovskite Solar Cells in 2020–2021. Nano Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Lai, C.S.; Jia, Y.; Lai, L.L.; Xu, Z.; McCulloch, M.D.; Wong, K.P. A comprehensive review on large-scale photovoltaic system with applications of electrical energy storage. Renew. Sustain.Energy Rev. 2017, 78, 439–451. [Google Scholar] [CrossRef]
- Ma, X.; Yang, L.; Zheng, S.; Dai, Q.; Chen, C.; Song, H. All-Inorganic Perovskite Solar Cells: Status and Future. Prog. Chem. 2020, 32, 1608–1632. [Google Scholar] [CrossRef]
- Wang, K.L.; Wang, R.; Wang, Z.K.; Li, M.; Zhang, Y.; Ma, H.; Liao, L.S.; Yang, Y. Tailored Phase Transformation of CsPbI2Br Films by Copper(II) Bromide for High-Performance All-Inorganic Perovskite Solar Cells. Nano Lett. 2019, 19, 5176–5184. [Google Scholar] [CrossRef]
- Yao, X.; He, B.; Zhu, J.; Ti, J.; Cui, L.; Tui, R.; Wei, M.; Chen, H.; Duan, J.; Duan, Y.; et al. Tailoring type-II all-in-one buried interface for 1.635V-voltage, all-inorganic CsPbBr3 perovskite solar cells. Nano Energy 2022, 96, 107138. [Google Scholar] [CrossRef]
- Faheem, M.B.; Khan, B.; Feng, C.; Farooq, M.U.; Raziq, F.; Xiao, Y.; Li, Y. All-Inorganic Perovskite Solar Cells: Energetics, Key Challenges, and Strategies toward Commercialization. ACS Energy Lett. 2020, 5, 290–320. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, J.F.; Tian, J.J. All-inorganic perovskite solar cells with efficiency >20%. Sci. China Mater. 2021, 64, 2624–2626. [Google Scholar] [CrossRef]
- Dong, C.; Xu, B.; Liu, D.; Moloney, E.G.; Tan, F.; Yue, G.; Liu, R.; Zhang, D.; Zhang, W.; Saidaminov, M.I. Carbon-based all-inorganic perovskite solar cells: Progress, challenges and strategies toward 20% efficiency. Mater. Today 2021, 50, 239–258. [Google Scholar] [CrossRef]
- Maafa, I.M. All-Inorganic Perovskite Solar Cells: Recent Advancements and Challenges. Nanomaterials 2022, 12, 1651. [Google Scholar] [CrossRef]
- Brunetti, B.; Cavallo, C.; Ciccioli, A.; Gigli, G.; Latini, A. On the Thermal and Thermodynamic (In)Stability of Methylammonium Lead Halide Perovskites. Sci. Rep. 2016, 6, 31896. [Google Scholar] [CrossRef]
- Liang, J.; Han, X.; Yang, J.H.; Zhang, B.Y.; Fang, Q.Y.; Zhang, J.; Ai, Q.; Ogle, M.M.; Terlier, T.; Marti, A.A.; et al. Defect-Engineering-Enabled High-Efficiency All-Inorganic Perovskite Solar Cells. Adv. Mater. 2019, 31, 1903448. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paterno, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Zhang, Y.; Xia, J.; Yin, J.; Li, M.; Brooks, K.G.; Hu, R.; Gao, X.; Kim, Y.H.; et al. High-efficiency perovskite photovoltaic modules achieved via cesium doping. Chem. Eng. J. 2022, 431, 133713. [Google Scholar] [CrossRef]
- Steele, J.A.; Jin, H.; Dovgaliuk, I.; Berger, R.F.; Braeckevelt, T.; Yuan, H.; Martin, C.; Solano, E.; Lejaeghere, K.; Rogge, S.M.J.; et al. Thermal unequilibrium of strained black CsPbI3 thin films. Science 2019, 365, 679–684. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Kan, M.; Zhao, Y. Bifunctional Stabilization of All-Inorganic alpha-CsPbI3 Perovskite for 17% Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 12345–12348. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Horantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, J.; Niu, G.; Tang, J. Inorganic CsPbI3 Perovskite-Based Solar Cells: A Choice for a Tandem Device. Sol. RRL 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Ding, X.; Chen, H.; Wu, Y.; Ma, S.; Dai, S.; Yang, S.; Zhu, J. Triple cation additive NH3+C2H4NH2+C2H4NH3+-induced phase-stable inorganic α-CsPbI3 perovskite films for use in solar cells. J. Mater. Chem. A 2018, 6, 18258–18266. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, T.; Wang, X.; Kan, M.; Shi, J.; Zhao, Y. The Role of Dimethylammonium Iodide in CsPbI3 Perovskite Fabrication: Additive or Dopant? Angew. Chem. Int. Ed. 2019, 58, 16691–16696. [Google Scholar] [CrossRef]
- Yang, S.; Duan, Y.; Liu, Z.; Liu, S. Recent Advances in CsPbX3 Perovskite Solar Cells: Focus on Crystallization Characteristics and Controlling Strategies. Adv. Energy Mater. 2022, 2201733. [Google Scholar] [CrossRef]
- Wang, L.; Fan, B.; Zheng, B.; Yang, Z.; Yin, P.; Huo, L. Organic functional materials: Recent advances in all-inorganic perovskite solar cells. Sustain. Energy Fuels 2020, 4, 2134–2148. [Google Scholar] [CrossRef]
- Duan, J.; Xu, H.; Sha, W.E.I.; Zhao, Y.; Wang, Y.; Yang, X.; Tang, Q. Inorganic perovskite solar cells: An emerging member of the photovoltaic community. J. Mater. Chem. A 2019, 7, 21036–21068. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, W.; Ren, F.; Wang, J.; Wang, H.; Chen, R.; Zhang, S.; Zhang, Y.; Liu, Z.; Chen, W. Strategies for highly efficient and stable cesium lead iodide perovskite photovoltaics: Mechanisms and processes. J. Mater. Chem. C 2022, 10, 4999–5023. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, J.M.; Yang, P.X.; Liu, L.L.; Yang, S.E.; Xia, T.Y.; Guo, H.Z.; Chen, Y.S. All-inorganic CsPbBr3 perovskite: A promising choice for photovoltaics. Mater. Adv. 2021, 2, 646–683. [Google Scholar] [CrossRef]
- Tai, Q.; Tang, K.-C.; Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 2019, 12, 2375–2405. [Google Scholar] [CrossRef]
- Duan, C.P.; Zhao, Z.C.; Yuan, L.J. Lead-Free Cesium-Containing Halide Perovskite and Its Application in Solar Cells. IEEE J. Photovol. 2021, 11, 1126–1135. [Google Scholar] [CrossRef]
- Wu, H.; Pi, J.; Zhou, D.; Wang, Q.; Long, Z.; Qiu, J. Effect of cation vacancy on lattice and luminescence properties in CsPbBr3 quantum dots. Ceram. Int. 2022, 48, 3383–3389. [Google Scholar] [CrossRef]
- Aftab, A.; Ahmad, M.I. A review of stability and progress in tin halide perovskite solar cell. Sol. Energy 2021, 216, 26–47. [Google Scholar] [CrossRef]
- Marronnier, A.; Roma, G.; Boyer-Richard, S.; Pedesseau, L.; Jancu, J.-M.; Bonnassieux, Y.; Katan, C.; Stoumpos, C.C.; Kanatzidis, M.G.; Even, J. Anharmonicity and Disorder in the Black Phases of Cesium Lead Iodide Used for Stable Inorganic Perovskite Solar Cells. ACS Nano 2018, 12, 3477–3486. [Google Scholar] [CrossRef]
- Montecucco, R.; Quadrivi, E.; Po, R.; Grancini, G. All-Inorganic Cesium-Based Hybrid Perovskites for Efficient and Stable Solar Cells and Modules. Adv. Energy Mater. 2021, 11, 2100672. [Google Scholar] [CrossRef]
- Ahmed, Y.; Khan, B.; Faheem, M.B.; Huang, K.Q.; Gao, Y.J.; Yang, J.L. Organic additives in all-inorganic perovskite solar cells and modules: From moisture endurance to enhanced efficiency and operational stability. J. Energy Chem. 2022, 67, 361–390. [Google Scholar] [CrossRef]
- Deng, J.; Li, J.; Yang, Z.; Wang, M. All-inorganic lead halide perovskites: A promising choice for photovoltaics and detectors. J. Mater. Chem. C 2019, 7, 12415–12440. [Google Scholar] [CrossRef]
- Bensouda, Y.; Barrit, D. Mini-review on all-inorganic lead-based perovskite solar cells: Challenges and opportunities for production and upscaling. Emergent Mater. 2022, 5, 207–225. [Google Scholar] [CrossRef]
- Ouedraogo, N.A.N.; Chen, Y.; Xiao, Y.Y.; Meng, Q.; Han, C.B.; Yan, H.; Zhang, Y. Stability of all-inorganic perovskite solar cells. Nano Energy 2020, 67, 104249. [Google Scholar] [CrossRef]
- Zhang, C.L.; Arumugam, G.M.; Liu, C.; Hu, J.L.; Yang, Y.Z.; Schropp, R.E.I.; Mai, Y.H. Inorganic halide perovskite materials and solar cells. APL Mater. 2019, 7, 120702. [Google Scholar] [CrossRef]
- Dastidar, S.; Egger, D.A.; Tan, L.Z.; Cromer, S.B.; Dillon, A.D.; Liu, S.; Kronik, L.; Rappe, A.M.; Fafarman, A.T. High Chloride Doping Levels Stabilize the Perovskite Phase of Cesium Lead Iodide. Nano Lett. 2016, 16, 3563–3570. [Google Scholar] [CrossRef]
- Wang, H.; Bian, H.; Jin, Z.; Zhang, H.; Liang, L.; Wen, J.; Wang, Q.; Ding, L.; Liu, S.F. Cesium Lead Mixed-Halide Perovskites for Low-Energy Loss Solar Cells with Efficiency Beyond 17%. Chem. Mater. 2019, 31, 6231–6238. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, Y.; Mu, S.; Ma, F.; Gao, F.; Chu, Z.; Yin, Z.; Gao, P.; Zhang, X.; You, J. Cesium Lead Inorganic Solar Cell with Efficiency beyond 18% via Reduced Charge Recombination. Adv. Mater. 2019, 31, e1905143. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Yang, X.Y.; Su, R.; Wu, P.; Gong, Q.H.; Zhu, R. High-Performance CsPbIxBr3−x All-Inorganic Perovskite Solar Cells with Efficiency over 18% via Spontaneous Interfacial Manipulation. Adv. Funct. Mater. 2020, 30, 2000457. [Google Scholar] [CrossRef]
- Ke, W.; Yang, X.; Liu, T. Resistance Switching Effect of Memory Device Based on All-Inorganic Cspbbri2 Perovskite. Materials 2021, 14, 6629. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Z.; Liang, L.; Bian, H.; Wang, H.; Feng, J.; Wang, Q.; Liu, S. Chlorine doping for black gamma-CsPbI3 solar cells with stabilized efficiency beyond 16%. Nano Energy 2019, 58, 175–182. [Google Scholar] [CrossRef]
- Yao, Z.; Jin, Z.W.; Zhang, X.R.; Wang, Q.; Zhang, H.; Xu, Z.; Ding, L.M.; Liu, S.Z. Pseudohalide (SCN−)-doped CsPbI3 for high-performance solar cells. J. Mater. Chem. C 2019, 7, 13736–13742. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.J.; Du, Z.B.; Li, G.D.; Sun, W.H.; Wu, J.H.; Lan, Z. Highly Efficient CsPbBr3 Planar Perovskite Solar Cells via Additive Engineering with NH4SCN. ACS Appl. Mater. Interfaces 2020, 12, 10579–10587. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Mir, W.J.; Nag, A. Can B-Site Doping or Alloying Improve Thermal- and Phase-Stability of All-Inorganic CsPbX3 (X = Cl, Br, I) Perovskites? ACS Energy Lett. 2018, 3, 286–289. [Google Scholar] [CrossRef]
- Murugadoss, G.; Thangamuthu, R. Metals doped cesium based all inorganic perovskite solar cells: Investigations on Structural, morphological and optical properties. Sol. Energy 2019, 179, 151–163. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Zheng, J.; Kim, J.; Zhang, Z.; Zhang, M.; Bing, J.; Wilkinson, B.; Hu, L.; Patterson, R.; et al. Enhanced performance via partial lead replacement with calcium for a CsPbl3 perovskite solar cell exceeding 13% power conversion efficiency. J. Mater. Chem. A 2018, 6, 5580–5586. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Zhang, M.; Deng, X.; Zheng, J.; Bing, J.; Ma, Q.; Kim, J.; Hu, L.; Green, M.A.; Huang, S.; et al. Strontium-Doped Low-Temperature-Processed CsPbI2Br Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2319–2325. [Google Scholar] [CrossRef]
- Xue-Ting, W.; Yu-Hao, F.; Guang-Ren, N.; Hong-Dong, L.; Li-Jun, Z. Barium as doping element tuning both toxicity and optoelectric properties of lead-based halide perovskites. Acta Phys. Sin. 2019, 68, 157101. [Google Scholar] [CrossRef]
- Kajal, S.; Kim, G.-H.; Myung, C.W.; Shin, Y.S.; Kim, J.; Jeong, J.; Jana, A.; Kim, J.Y.; Kim, K.S. A thermally stable, barium-stabilized α-CsPbI3 perovskite for optoelectronic devices. J. Mater. Chem. A 2019, 7, 21740–21746. [Google Scholar] [CrossRef]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; De Angelis, F.; Manna, L. Fluorescent Alloy CsPbxMn1−xI3 Perovskite Nanocrystals with High Structural and Optical Stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhao, W.; Chen, S.; Jin, Z.; Liu, S.F. Mn Doping of CsPbI3 Film Towards High-Efficiency Solar Cell. ACS Appl. Energy Mater. 2020, 3, 5190–5197. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.D.; Jing, Y.; Zhang, H.Y.; Wang, X.; Lu, Y.; Wu, J.H.; Lan, Z. n-type absorber by Cd2+ doping achieves high-performance carbon-based CsPbIBr2 perovskite solar cells. J. Colloid Interface Sci. 2022, 608, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth Incorporation Stabilized alpha-CsPbI3 for Fully Inorganic Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, D.; Dutta, A.; Bhattacharyya, S.; Chakraborty, S.; Pradhan, N. Limiting Heterovalent B-Site Doping in CsPbI3 Nanocrystals: Phase and Optical Stability. ACS Energy Lett. 2019, 4, 1364–1369. [Google Scholar] [CrossRef]
- Wang, M.; Deng, K.M.; Meng, L.X.; Li, L. Bifunctional Ytterbium (III) Chloride Driven Low-Temperature Synthesis of Stable alpha-CsPbI3 for High-Efficiency Inorganic Perovskite Solar Cells. Small Methods 2020, 4, 1900652. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Sanehira, Y.; Ikegami, M.; Miyasaka, T. Stabilization of alpha-CsPbI3 in Ambient Room Temperature Conditions by Incorporating Eu into CsPbI3. Chem. Mater. 2018, 30, 6668–6674. [Google Scholar] [CrossRef]
- Faheem, M.B.; Khan, B.; Feng, C.; Ahmed, S.B.; Jiang, J.X.; Rehman, M.U.; Subhani, W.S.; Farooq, M.U.; Nie, J.L.; Makhlouf, M.M.; et al. Synergistic Approach toward Erbium-Passivated Triple-Anion Organic-Free Perovskite Solar Cells with Excellent Performance for Agrivoltaics Application. ACS Appl. Mater. Interfaces 2022, 14, 6894–6905. [Google Scholar] [CrossRef]
- Pu, X.; Yang, J.; Wang, T.; Cheng, S.; Cao, Q.; Zhao, J.; Chen, H.; Zhang, Y.; Xu, T.; Tojiboyev, I.; et al. Gadolinium-incorporated CsPbI2Br for boosting efficiency and long-term stability of all-inorganic perovskite solar cells. J. Energy Chem. 2022, 70, 9–17. [Google Scholar] [CrossRef]
- Sun, J.-K.; Huang, S.; Liu, X.-Z.; Xu, Q.; Zhang, Q.-H.; Jiang, W.-J.; Xue, D.-J.; Xu, J.-C.; Ma, J.-Y.; Ding, J.; et al. Polar Solvent Induced Lattice Distortion of Cubic CsPbI3 Nanocubes and Hierarchical Self-Assembly into Orthorhombic Single-Crystalline Nanowires. J. Am. Chem. Soc. 2018, 140, 11705–11715. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Jin, S.F.; Huang, S.; Liu, N.; Ma, J.Y.; Xue, D.J.; Han, Q.W.; Ding, J.; Ge, Q.Q.; Feng, Y.Q.; et al. Thermodynamically Stable Orthorhombic gamma-CsPbI3 Thin Films for High-Performance Photovoltaics. J. Am. Chem. Soc. 2018, 140, 11716–11725. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, W.; He, F.; Fan, T.; Cai, W.; Zhang, X.; Wei, G. Boosting Performance of CsPbI3 Perovskite Solar Cells via the Synergy of Hydroiodic Acid and Deionized Water. Adv. Energy Sustain. Res. 2022, 3, 2100149. [Google Scholar] [CrossRef]
- Zhang, T.; Dar, M.I.; Li, G.; Xu, F.; Guo, N.; Gratzel, M.; Zhao, Y. Bication lead iodide 2D perovskite component to stabilize inorganic alpha-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017, 3, 1700841. [Google Scholar] [CrossRef]
- Xiang, S.; Fu, Z.; Li, W.; Wei, Y.; Liu, J.; Liu, H.; Zhu, L.; Zhang, R.; Chen, H. Highly Air-Stable Carbon-Based α-CsPbI3 Perovskite Solar Cells with a Broadened Optical Spectrum. ACS Energy Lett. 2018, 3, 1824–1831. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, J.; Ni, Y.; Yang, J.; Wang, Y.; Jiu, T.; Yuan, M.; Chen, J. Reduced-Dimensional alpha-CsPbX3 Perovskites for Efficient and Stable Photovoltaics. Joule 2018, 2, 1356–1368. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Z.; Liang, L.; Bian, H.; Bai, D.; Wang, H.; Zhang, J.; Wang, Q.; Liu, S. All-inorganic cesium lead iodide perovskite solar cells with stabilized efficiency beyond 15%. Nat. Commun. 2018, 9, 4544. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.H.; Liu, Y.; Li, F.M.; Bai, S.; Jian, X.; Liu, M.Z. Unveiling Property of Hydrolysis-Derived DMAPbI(3) for Perovskite Devices: Composition Engineering, Defect Mitigation, and Stability Optimization. iScience 2019, 15, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dar, M.I.; Ono, L.K.; Zhang, T.; Kan, M.; Li, Y.; Zhang, L.; Wang, X.; Yang, Y.; Gao, X.; et al. Thermodynamically stabilized beta-CsPbI3-based perovskite solar cells with efficiencies >18%. Science 2019, 365, 591–595. [Google Scholar] [CrossRef]
- Xi, J.; Piao, C.; Byeon, J.; Yoon, J.; Wu, Z.; Choi, M. Rational Core–Shell Design of Open Air Low Temperature In Situ Processable CsPbI3 Quasi-Nanocrystals for Stabilized p-i-n Solar Cells. Adv. Energy Mater. 2019, 9, 1901787. [Google Scholar] [CrossRef]
- Ke, W.J.; Spanopoulos, I.; Stoumpos, C.C.; Kanatzidis, M.G. Myths and reality of HPbI3 in halide perovskite solar cells. Nat. Commun. 2018, 9, 4785. [Google Scholar] [CrossRef]
- Daub, M.; Hillebrecht, H. On the Demystification of “HPbI3” and the Peculiarities of the Non-innocent Solvents H2O and DMF. Z. Anorg. Allg. Chem 2018, 644, 1393–1400. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Zhong, Y.; Hao, H.; Yang, M.; Liu, R. Improved phase stability of the CsPbI3 perovskite via organic cation doping. Phys. Chem. Chem. Phys. 2019, 21, 11175–11180. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Wang, H.; Li, Z.; Zhou, F.; Xu, Y.; Zhang, H.; Wang, Q.; Ding, L.; Liu, S.; Jin, Z. Unveiling the Effects of Hydrolysis-Derived DMAI/DMAPbI(x) Intermediate Compound on the Performance of CsPbI3 Solar Cells. Adv. Sci. 2020, 7, 1902868. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Xiang, S.S.; Li, W.P.; Liu, H.C.; Zhu, L.Q.; Xiao, S.; Yang, S.H.; Chen, H.N. Skillfully deflecting the question: A small amount of piperazine-1,4-diium iodide radically enhances the thermal stability of CsPbI3 perovskite. J. Mater. Chem. C 2019, 7, 11757–11763. [Google Scholar] [CrossRef]
- Meng, H.G.; Shao, Z.P.; Wang, L.; Li, Z.P.; Liu, R.R.; Fan, Y.P.; Cui, G.L.; Pang, S.P. Chemical Composition and Phase Evolution in DMAI-Derived Inorganic Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 263–270. [Google Scholar] [CrossRef]
- Xiang, S.; Li, W.; Wei, Y.; Liu, J.; Liu, H.; Zhu, L.; Chen, H. The synergistic effect of non-stoichiometry and Sb-doping on air-stable alpha-CsPbI3 for efficient carbon-based perovskite solar cells. Nanoscale 2018, 10, 9996–10004. [Google Scholar] [CrossRef]
- Becker, P.; Márquez, J.A.; Just, J.; Al-Ashouri, A.; Hages, C.; Hempel, H.; Jošt, M.; Albrecht, S.; Frahm, R.; Unold, T. Low Temperature Synthesis of Stable γ-CsPbI3 Perovskite Layers for Solar Cells Obtained by High Throughput Experimentation. Adv. Energy Mater. 2019, 9, 1900555. [Google Scholar] [CrossRef]
- Bai, F.J.; Zhang, J.; Yuan, Y.F.; Liu, H.B.; Li, X.S.; Chueh, C.C.; Yan, H.; Zhu, Z.L.; Jen, A.K.Y. A 0D/3D Heterostructured All-Inorganic Halide Perovskite Solar Cell with High Performance and Enhanced Phase Stability. Adv. Mater. 2019, 31, 1904735. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, J.; Li, Y.H.; Gong, L.; He, H.P.; Fang, Z.S.; Zhou, C.H.; Chen, J.L.; Fan, J.C. A facile interface engineering method to improve the performance of FTO/ZnO/CsPbI3−xBrx (x < 1)/C solar cells. J. Mater. Sci. Mater. Electron. 2022, 33, 3711–3725. [Google Scholar] [CrossRef]
- Fu, S.Q.; Wang, J.H.; Liu, X.H.; Yuan, H.B.; Xu, Z.X.; Long, Y.J.; Zhang, J.; Huang, L.K.; Hu, Z.Y.; Zhu, Y.J. Multifunctional liquid additive strategy for highly efficient and stable CsPbI2Br all-inorganic perovskite solar cells. Chem. Eng. J. 2021, 422, 130572. [Google Scholar] [CrossRef]
- Yoon, S.M.; Min, H.; Kim, J.B.; Kim, G.; Lee, K.S.; Seok, S.I. Surface Engineering of Ambient-Air-Processed Cesium Lead Triiodide Layers for Efficient Solar Cells. Joule 2021, 5, 183–196. [Google Scholar] [CrossRef]
- Li, Y.; Duan, L.; Zhang, Z.; Wang, H.; Chen, T.; Luo, J. Healing the defects in CsPbI3 solar cells by CsPbBr3 quantum dots. Nano Res. 2021. [Google Scholar] [CrossRef]
- Zhang, W.H.; Liu, H.; Qi, X.N.; Yu, Y.Y.; Zhou, Y.C.; Xia, Y.; Cui, J.S.; Shi, Y.Q.; Chen, R.; Wang, H.L. Oxalate Pushes Efficiency of CsPb0.7Sn0.3IBr2 Based All-Inorganic Perovskite Solar Cells to over 14%. Adv. Sci. 2022, 9, 2106054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Zhang, L.; Yang, A.; Bai, T.; Liu, F.; Lyu, M.; Zhu, J. Guanidinium Thiocyanate Additive Engineering for High-Performance CsPbIBr2 Solar Cells with an Efficiency of 10.90%. ACS Appl. Energy Mater. 2022, 5, 3110–3118. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Wang, X.; Yang, D. Inorganic lead-based halide perovskites: From fundamental properties to photovoltaic applications. Mater. Today 2022, 61, 191–217. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zhang, C.; Ma, Y.; Fan, J.; Mai, Y. All-Inorganic CsPbI2Br Perovskite Solar Cells with High Efficiency Exceeding 13%. J. Am. Chem. Soc. 2018, 140, 3825–3828. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, S.; Hutter, O.S.; Phillips, L.J.; Yates, P.J.; Kundu, B.; Durose, K. Stability and Performance of CsPbI2Br Thin Films and Solar Cell Devices. ACS Appl. Mater. Interfaces 2018, 10, 3750–3760. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wu, S.H.; Chen, W.T.; Zhu, H.M.; Xiong, Z.Z.; Yang, Z.C.; Chen, C.L.; Chen, R.; Han, L.Y.; Chen, W. Solvent engineering for efficient inverted perovskite solar cells based on inorganic CsPbI2Br light absorber. Mater. Today Energy 2018, 8, 125–133. [Google Scholar] [CrossRef]
- Yin, G.; Zhao, H.; Jiang, H.; Yuan, S.; Niu, T.; Zhao, K.; Liu, Z.; Liu, S. Precursor Engineering for All-Inorganic CsPbI2Br Perovskite Solar Cells with 14.78% Efficiency. Adv. Funct. Mater. 2018, 28, 1803269. [Google Scholar] [CrossRef]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Solvent Engineering for Ambient-Air-Processed, Phase-Stable CsPbI3 in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef]
- Meng, Q.X.; Feng, J.Y.; Huang, H.T.; Han, X.P.; Zhu, Z.; Yu, T.; Li, Z.S.; Zou, Z.G. Simultaneous Optimization of Phase and Morphology of CsPbBr3 Films via Controllable Ostwald Ripening by Ethylene Glycol Monomethylether/Isopropanol Bi-Solvent Engineering. Adv. Eng. Mater. 2020, 22, 2000162. [Google Scholar] [CrossRef]
- He, Q.; Zhang, H.; Han, S.; Xing, Y.; Li, Y.; Zhang, X.; Wang, R. Improvement of green antisolvent-isopropanol and additive-thiourea on carbon based CsPbIBr2 perovskite solar cells. Mater. Sci. Semicond. Process. 2022, 150, 106940. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Sun, W.; Wang, C.; Yan, Z.; Wang, N.; Lan, Z.; Wu, J. A green Bi-Solvent system for processing high-quality CsPbBr3 films in efficient all-inorganic perovskite solar cells. Mater. Today Phys. 2022, 22, 100614. [Google Scholar] [CrossRef]
- Dong, C.; Han, X.X.; Li, W.H.; Qiu, Q.Q.; Wang, J.Q. Anti-solvent assisted multi-step deposition for efficient and stable carbon-based CsPbI2Br all-inorganic perovskite solar cell. Nano Energy 2019, 59, 553–559. [Google Scholar] [CrossRef]
- Han, B.Q.; Zhang, L.; Cao, Y.W.; Li, B.Y.; Liu, Z.Y.; Xu, L.B.; Wang, P.; Lin, P.; Wu, X.P.; Cui, C. Antisolvent engineering on low-temperature processed CsPbI3 inorganic perovskites for improved performances of solar cells. Nanotechnology 2021, 32, 185402. [Google Scholar] [CrossRef] [PubMed]
- Saparbaev, A.; Zhang, M.L.; Kuvondikov, V.; Nurumbetova, L.; Raji, I.O.; Tajibaev, I.; Zakhidov, E.; Bao, X.C.; Yang, R.Q. High-performance CsPbI3 perovskite solar cells without additives in air condition. Sol. Energy 2021, 228, 405–412. [Google Scholar] [CrossRef]
- Teng, P.P.; Han, X.P.; Li, J.W.; Xu, Y.; Kang, L.; Wang, Y.R.Q.; Yang, Y.; Yu, T. Elegant Face-Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-Based All-Inorganic Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 9541–9546. [Google Scholar] [CrossRef]
- Zai, H.C.; Zhang, D.L.; Li, L.; Zhu, C.; Ma, S.; Zhao, Y.Z.; Zhao, Z.G.; Chen, C.F.; Zhou, H.P.; Li, Y.J.; et al. Low-temperature-processed inorganic perovskite solar cells via solvent engineering with enhanced mass transport. J. Mater. Chem. A 2018, 6, 23602–23609. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X.; Liu, Z.; Ye, H.; Sun, B.; Shi, T.; Tang, Z.; Liao, G. Boosting the efficiency of carbon-based planar CsPbBr3 perovskite solar cells by a modified multistep spin-coating technique and interface engineering. Nano Energy 2019, 56, 184–195. [Google Scholar] [CrossRef]
- Gao, B.W.; Meng, J. High efficiently CsPbBr3 perovskite solar cells fabricated by multi-step spin coating method. Sol. Energy 2020, 211, 1223–1229. [Google Scholar] [CrossRef]
- Wang, H.S.; Sun, J.; Gu, Y.S.; Xu, C.Q.; Lu, Y.W.; Hu, J.T.; Chen, T.; Zhu, C.F.; Luo, P.F. Solvent-engineering-processed CsPbIBr2 inorganic perovskite solar cells with efficiency of similar to 11%. Sol. Energy Mater. Sol. Cells 2022, 238, 111640. [Google Scholar] [CrossRef]
- Wang, H.L.; Liu, H.C.; Dong, Z.J.; Wei, X.Y.; Song, Y.F.; Li, W.P.; Zhu, L.Q.; Bai, Y.; Chen, H.N. Extracting ammonium halides by solvent from the hybrid perovskites with various dimensions to promote the crystallization of CsPbI3 perovskite. Nano Energy 2022, 94, 106925. [Google Scholar] [CrossRef]
- Zhong, T.T.; Zhang, C.; Hamukwaya, S.L.; Xu, W.S.; Tang, K.P.; Xu, X.; Sun, W.T.; Hao, H.Y.; Dong, J.J.; Liu, H.; et al. Efficient and stable carbon-based CsPbBr3 solar cells added with PEABr additive. Acta Phys. Sin. 2022, 71, 028101. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Causes and Solutions of Recombination in Perovskite Solar Cells. Adv. Mater. 2019, 31, e1803019. [Google Scholar] [CrossRef]
- Wang, J.-K.; Hou, H.-Y.; Li, Y.-Q.; Tang, J.-X. Recent advances in interface engineering of all-inorganic perovskite solar cells. Nanoscale 2020, 12, 17149–17164. [Google Scholar] [CrossRef]
- Yan, L.; Xue, Q.F.; Liu, M.Y.; Zhu, Z.L.; Tian, J.J.; Li, Z.C.; Chen, Z.; Chen, Z.M.; Yan, H.; Yip, H.L.; et al. Interface Engineering for All-Inorganic CsPbI2Br Perovskite Solar Cells with Efficiency over 14%. Adv. Mater. 2018, 30, 1802509. [Google Scholar] [CrossRef]
- Yue, M.; Su, J.; Zhao, P.; Lin, Z.H.; Zhang, J.C.; Chang, J.J.; Hao, Y. Optimizing the Performance of CsPbI3-Based Perovskite Solar Cells via Doping a ZnO Electron Transport Layer Coupled with Interface Engineering. Nano Micro Lett. 2019, 11, 91. [Google Scholar] [CrossRef]
- Yang, B.; Wang, M.; Hu, X.; Zhou, T.; Zang, Z. Highly efficient semitransparent CsPbIBr2 perovskite solar cells via low-temperature processed In2S3 as electron-transport-layer. Nano Energy 2019, 57, 718–727. [Google Scholar] [CrossRef]
- Zhu, W.; Chai, W.; Zhang, Z.; Chen, D.; Chang, J.; Liu, S.; Zhang, J.; Zhang, C.; Hao, Y. Interfacial TiO2 atomic layer deposition triggers simultaneous crystallization control and band alignment for efficient CsPbIBr2 perovskite solar cell. Org. Electron. 2019, 74, 103–109. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Ma, J.; Liang, J.; Liu, Y.; Hu, X.; Chen, C.; Yang, W.; Min, J.; Bao, Q.; et al. Minimizing Open-Circuit voltage deficit via interface engineering for highly efficient CsPbI2Br perovskite solar cells. Chem. Eng. J. 2021, 417, 129247. [Google Scholar] [CrossRef]
- Pan, B.K.; Gu, J.H.; Xu, X.L.; Xiao, L.B.; Zhao, J.; Zou, G.F. Interface engineering of high performance all-inorganic perovskite solar cells via low-temperature processed TiO2 nanopillar arrays. Nano Res. 2021, 14, 3431–3438. [Google Scholar] [CrossRef]
- Guo, Z.; Teo, S.; Xu, Z.; Zhang, C.; Kamata, Y.; Hayase, S.; Ma, T. Achievable high Voc of carbon based all-inorganic CsPbIBr2 perovskite solar cells through interface engineering. J. Mater. Chem. A 2019, 7, 1227–1232. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.H.; Du, J.; Zhang, M.; Gao, Y.L.; Liu, Y.B.; Ma, J.; Lin, Z.H. Enhancing Perovskite Solar Cell Performance through Surface Engineering of Metal Oxide Electron-Transporting Layer. Coatings 2020, 10, 46. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Liu, Y.Z.; Xue, Q.F.; Yip, H.L.; Jen, A.K.Y.; Zhu, Z.L. Interface Engineering for All-Inorganic CsPbIBr2 Perovskite Solar Cells with Enhanced Power Conversion Efficiency over 11%. Energy Technol. 2021, 9, 2100562. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Wang, L.; Chen, K.; Tao, L.; Zhang, Y.; Zhou, X. Commercial Carbon-Based all-Inorganic Perovskite Solar Cells with a High Efficiency of 13.81%: Interface Engineering and Photovoltaic Performance. ACS Appl. Energy Mater. 2021, 4, 3255–3264. [Google Scholar] [CrossRef]
- Chai, W.; Zhu, W.; Zhang, Z.; Liu, D.; Ni, Y.; Song, Z.; Dong, P.; Chen, D.; Zhang, J.; Zhang, C.; et al. CsPbBr3 seeds improve crystallization and energy level alignment for highly efficient CsPbI3 perovskite solar cells. Chem. Eng. J. 2023, 452, 139292. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.Z.; Chen, J.H.; Fan, J.D.; Mai, Y.H.; Schropp, R.E.I. Ultra-thin MoOx as cathode buffer layer for the improvement of all-inorganic CsPbIBr2 perovskite solar cells. Nano Energy 2017, 41, 75–83. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, X.; Lin, Z.H.; Ma, J.; Su, J.; Hu, Z.S.; Zhang, C.F.; Liu, S.Z.; Chang, J.J.; Hao, Y. Interface engineering of low temperature processed all-inorganic CsPbI2Br perovskite solar cells toward PCE exceeding 14%. Nano Energy 2019, 60, 583–590. [Google Scholar] [CrossRef]
- Zong, Z.H.; He, B.L.; Zhu, J.W.; Ding, Y.; Zhang, W.Y.; Duan, J.L.; Zhao, Y.Y.; Chen, H.Y.; Tang, Q.W. Boosted hole extraction in all-inorganic CsPbBr3 perovskite solar cells by interface engineering using MoO2/N-doped carbon nanospheres composite. Sol. Energy Mater. Sol. Cells 2020, 209, 110460. [Google Scholar] [CrossRef]
- Xue, Q.F.; Liu, M.Y.; Li, Z.C.; Yan, L.; Hu, Z.C.; Zhou, J.W.; Li, W.Q.; Jiang, X.F.; Xu, B.M.; Huang, F.; et al. Efficient and Stable Perovskite Solar Cells via Dual Functionalization of Dopamine Semiquinone Radical with Improved Trap Passivation Capabilities. Adv. Funct. Mater. 2018, 28, 1707444. [Google Scholar] [CrossRef]
- Ding, Y.; He, B.L.; Zhu, J.W.; Zhang, W.Y.; Su, G.D.; Duan, J.L.; Zhao, Y.Y.; Chen, H.Y.; Tang, Q.W. Advanced Modification of Perovskite Surfaces for Defect Passivation and Efficient Charge Extraction in Air-Stable CsPbBr3 Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 19286–19294. [Google Scholar] [CrossRef]
- Yang, S.Z.; Wang, L.; Gao, L.G.; Cao, J.M.; Han, Q.J.; Yu, F.Y.; Kamata, Y.; Zhang, C.; Fan, M.Q.; Wei, G.Y.; et al. Excellent Moisture Stability and Efficiency of Inverted All-Inorganic CsPbIBr2 Perovskite Solar Cells through Molecule Interface Engineering. ACS Appl. Mater. Interfaces 2020, 12, 13931–13940. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Liu, Y.; He, B.L.; Zhang, W.Y.; Cui, L.F.; Wang, S.D.; Chen, H.Y.; Duan, Y.Y.; Tang, Q.W. Efficient interface engineering of N, N′-Dicyclohexylcarbodiimide for stable HTMs-free CsPbBr3 perovskite solar cells with 10.16%-efficiency. Chem. Eng. J. 2022, 428, 131950. [Google Scholar] [CrossRef]
- Du, Y.T.; Wu, J.H.; Li, G.D.; Wang, X.B.; Song, Z.Y.; Deng, C.Y.; Chen, Q.; Zou, Y.; Sun, W.H.; Lan, Z. Bulky ammonium iodide and in-situ formed 2D Ruddlesden-Popper layer enhances the stability and efficiency of perovskite solar cells. J. Colloid Interface Sci. 2022, 614, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, G.; Li, R.; Jing, Y.; Zhang, H.; Wang, X.; Du, Z.; Wu, J.; Lan, Z. PbS/CdS heterojunction thin layer affords high-performance carbon-based all-inorganic solar cells. Nano Energy 2022, 95, 106973. [Google Scholar] [CrossRef]
- Zou, Y.; Cao, F.; Chen, P.; He, R.; Tong, A.; Yin, C.; Lan, Z.; Sun, W.; Wu, J. Stable and highly efficient all-inorganic CsPbBr3 perovskite solar cells by interface engineering with NiO NCs modification. Electrochim. Acta 2022, 435, 1065–1074. [Google Scholar] [CrossRef]
- Liao, G.Q.; Duan, J.L.; Zhao, Y.Y.; Tang, Q.W. Toward fast charge extraction in all-inorganic CsPbBr3 perovskite solar cells by setting intermediate energy levels. Sol. Energy 2018, 171, 279–285. [Google Scholar] [CrossRef]
- Xu, H.Z.; Duan, J.L.; Zhao, Y.Y.; Jiao, Z.B.; He, B.L.; Tang, Q.W. 9.13%-Efficiency and stable inorganic CsPbBr3 solar cells. Lead-free CsSnBr3−xIx quantum dots promote charge extraction. J. Power Sources 2018, 399, 76–82. [Google Scholar] [CrossRef]
- Li, Q.H.; Bai, J.K.; Zhang, T.T.; Nie, C.; Duan, J.L.; Tang, Q.W. CdZnSe@ZnSe colloidal alloy quantum dots for high-efficiency all-inorganic perovskite solar cells. Chem. Commun. 2018, 54, 9575–9578. [Google Scholar] [CrossRef]
- Bian, H.; Bai, D.; Jin, Z.; Wang, K.; Liang, L.; Wang, H.; Zhang, J.; Wang, Q.; Liu, S. Graded Bandgap CsPbI2+xBr1−x Perovskite Solar Cells with a Stabilized Efficiency of 14.4%. Joule 2018, 2, 1500–1510. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, M.; Li, R.; Zang, Z.; Wang, H. Double-Side Interface Engineering Synergistically Boosts the Efficiency of Inorganic CsPbIBr2 Perovskite Solar Cells Over 12%. Adv. Opt. Mater. 2022, 10, 2200802. [Google Scholar] [CrossRef]
- Shi, L.; Yuan, H.; Zhang, Y.; Sun, X.; Duan, L.; Li, Q.; Huang, Z.; Ban, X.; Zhang, D. Novel C3N4-Assisted Bilateral Interface Engineering for Efficient and Stable Perovskite Solar Cells. Langmuir 2022, 38, 12390–12398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Zhou, Y.; Liu, H.; Xue, Q.; Li, X.; Chueh, C.-C.; Yip, H.-L.; Zhu, Z.; Jen, A.K.Y. Highly efficient all-inorganic perovskite solar cells with suppressed non-radiative recombination by a Lewis base. Nat. Commun. 2020, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).