Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel
Abstract
1. Introduction
2. Materials and Methods
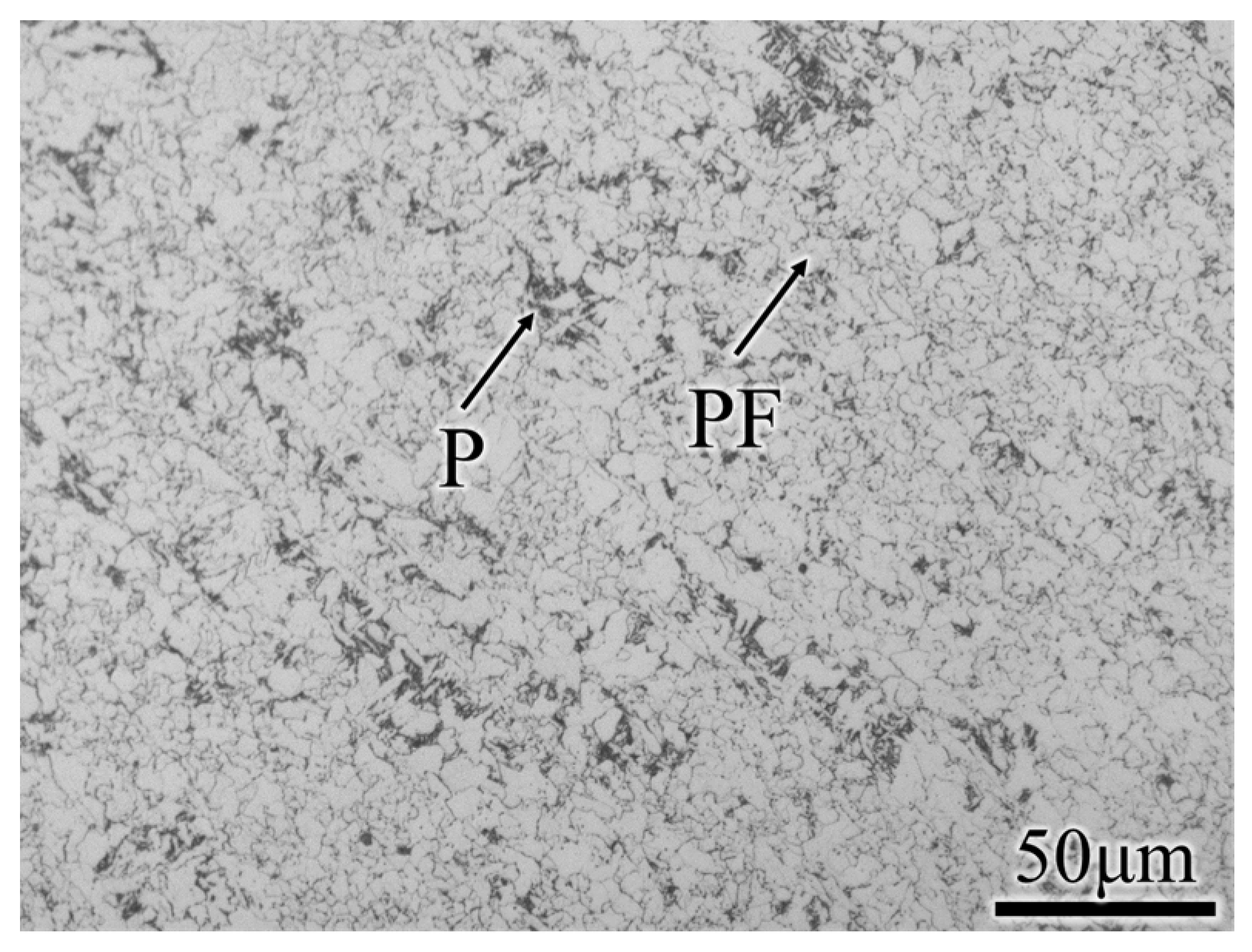
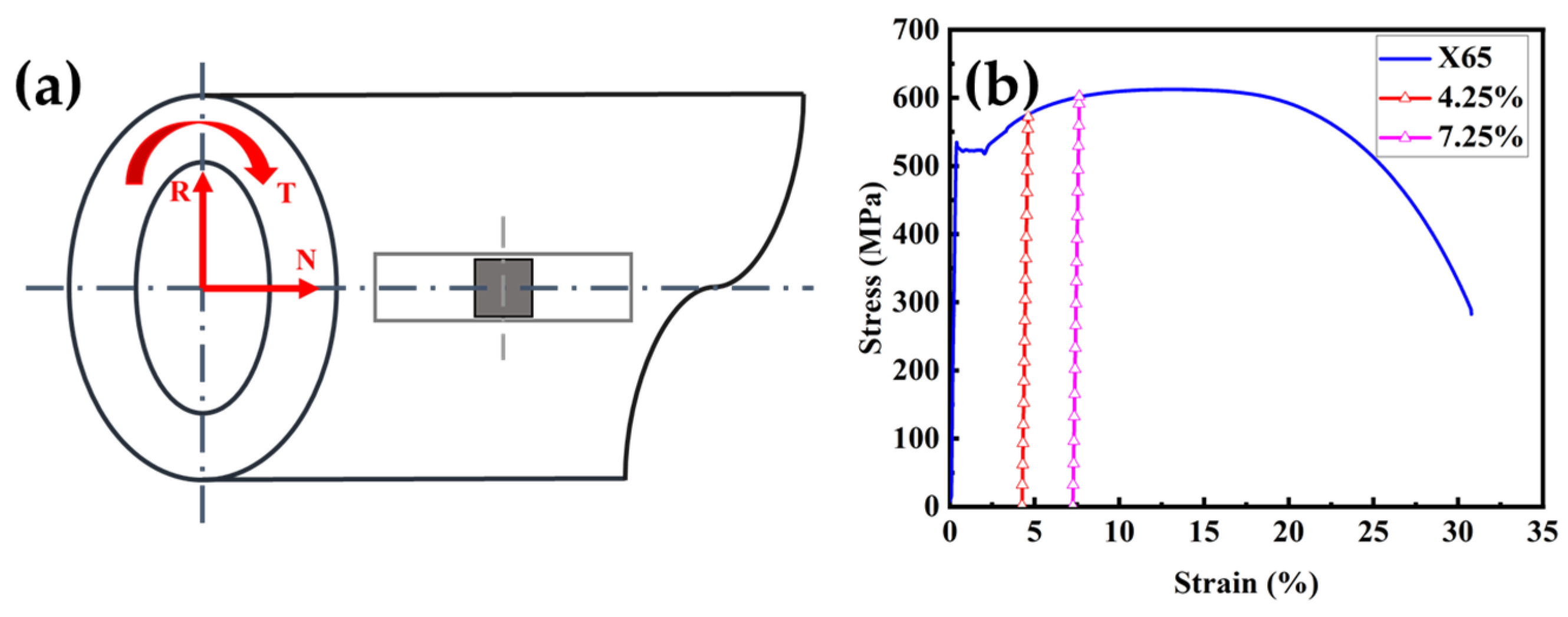
2.1. Materials
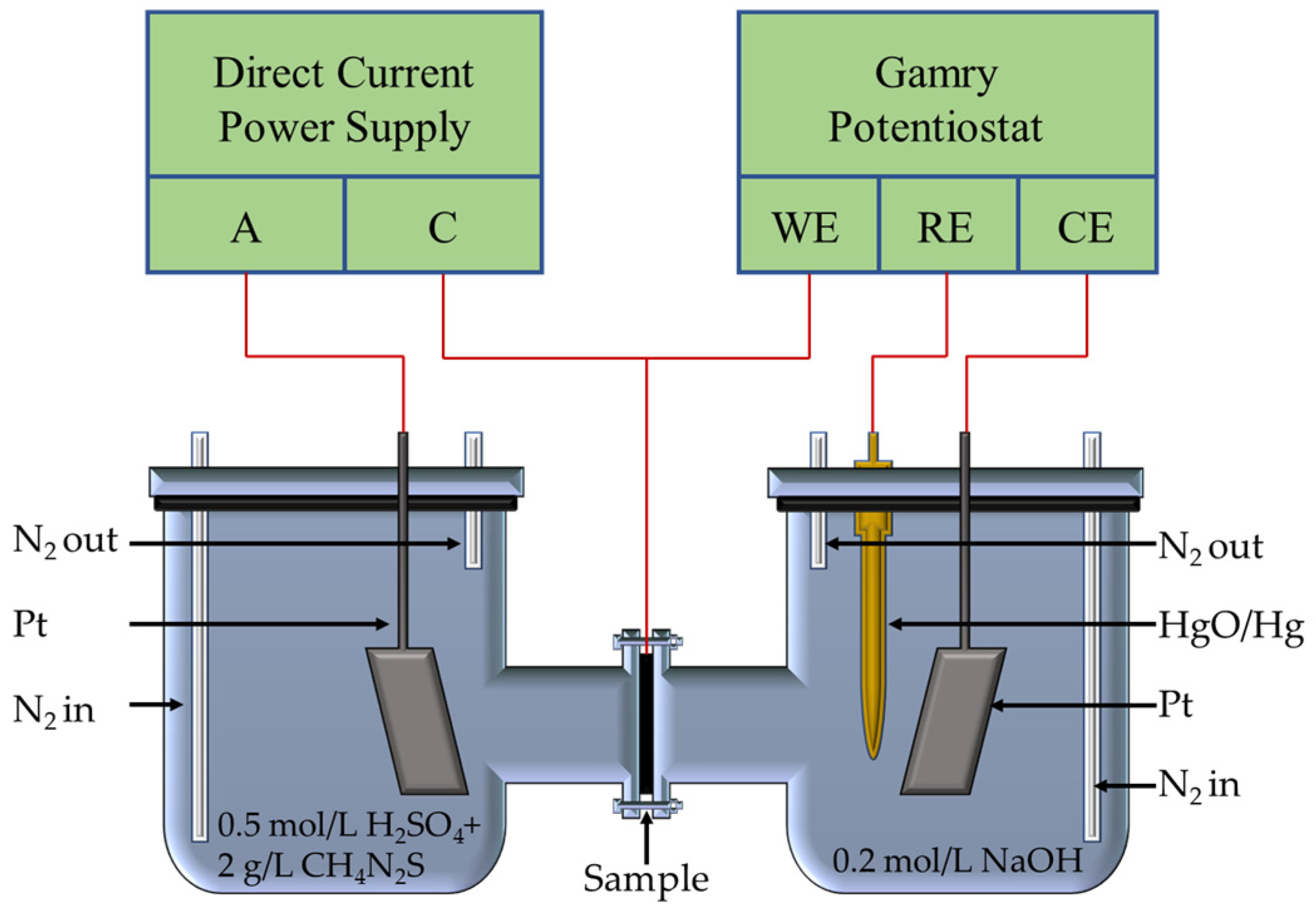
2.2. Hydrogen Permeation Experiment
2.3. Hydrogen Microprint Test
2.4. Hydrogen Desorption Spectroscopy
3. Results and Discussion
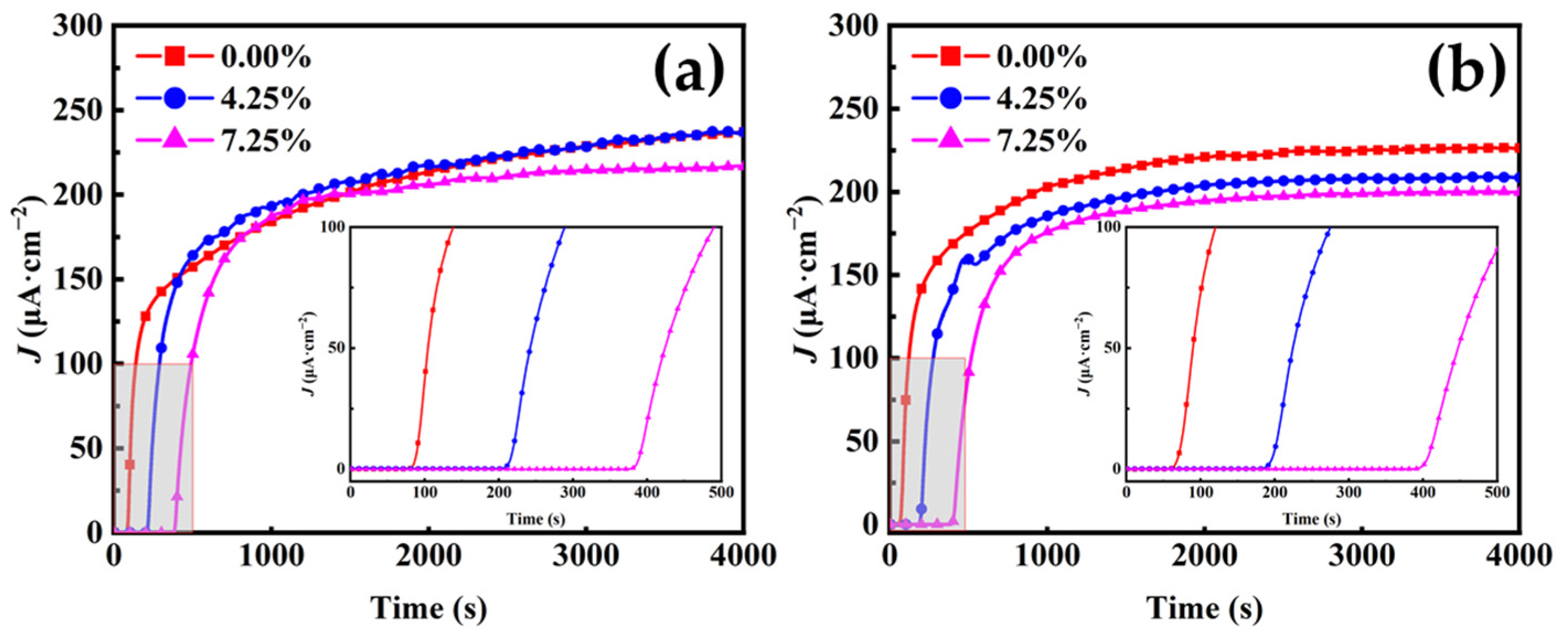
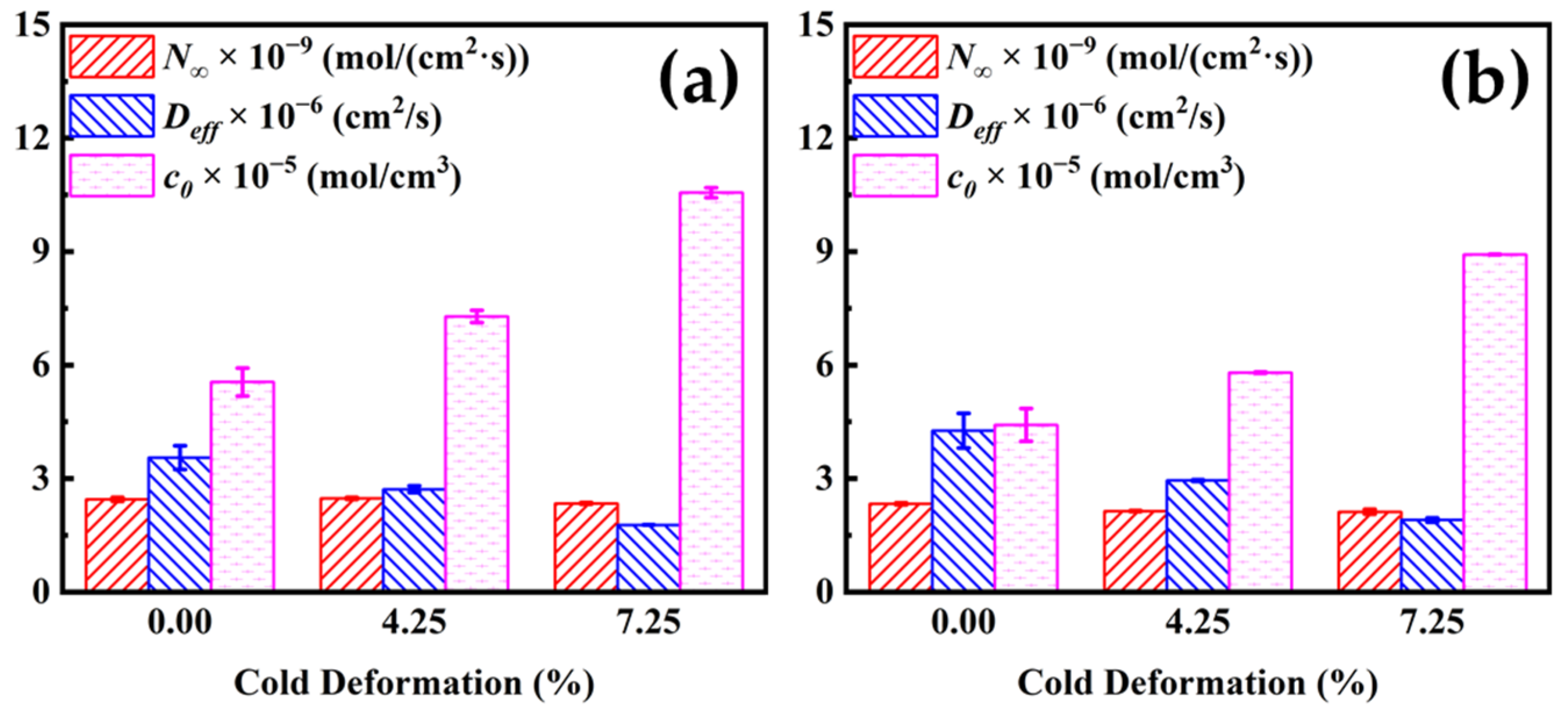
3.1. Hydrogen Permeation
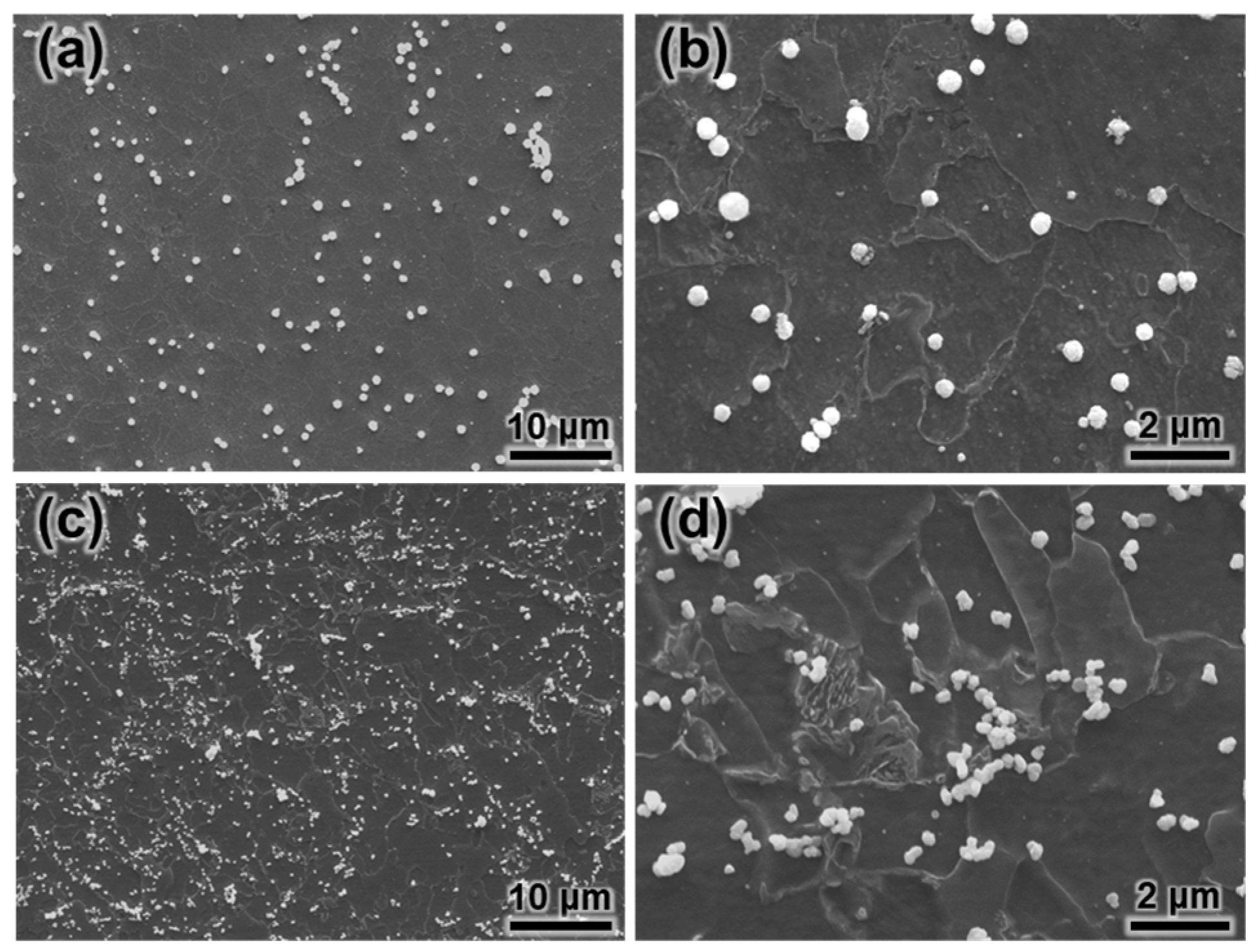
3.2. Hydrogen Microprinting
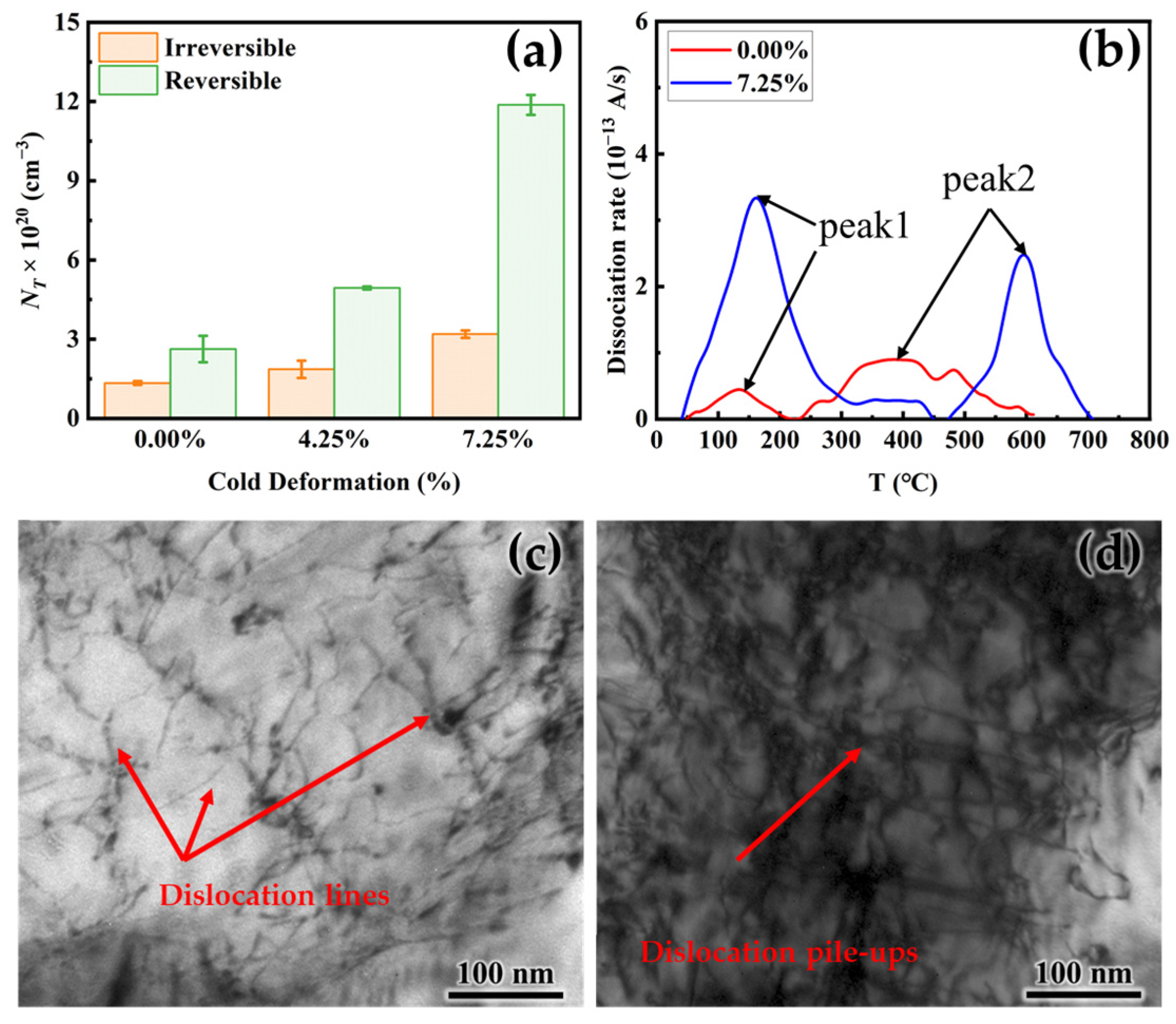
3.3. Effect of Cold Deformation on Hydrogen Permeation
4. Conclusions
- (1)
- The Deff decreases with increasing cold deformation (from 0.00% to 7.25%), from 3.553 × 10−6 to 1.775 × 10−6 cm2/s for the first penetration test and from 4.265 × 10−6 to 1.903 × 10−6 cm2/s for the second one, while c0 increases with increasing cold deformation. The increase in cold deformation level inhibits hydrogen permeation.
- (2)
- In the HMT tests, the enrichment of silver particles on the surface increases with the cold deformation level and particles are more likely to be enriched at the grain boundaries and phase boundaries.
- (3)
- Both NT and NRT increase with increasing cold deformation which is proved by hydrogen penetration tests as well as by TDS. The dislocation density increases significantly after cold deformation and is accompanied by dislocation entanglement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameters | Abbreviations |
|---|---|
| Hydrogen diffusion flux | N∞ |
| Steady current density | J∞ |
| Effective hydrogen diffusion coefficient | Deff |
| Apparent solubility | c0 |
| Specimen thickness | d |
| Lag time | tL |
| Faraday constant | F |
| Trap free bcc iron | DL |
| Hydrogen trap density | NT |
| Reversible hydrogen trap density | NRT |
| Irreversible hydrogen trap density | NIRT |
References
- Khalaj, G.; Azimzadegan, T.; Khoeini, M.; Etaat, M. Artificial neural networks application to predict the ultimate tensile strength of X70 pipeline steels. Neural Comput. Appl. 2013, 23, 2301–2308. [Google Scholar] [CrossRef]
- Khalaj, G.; Pouraliakbar, H.; Arab, N.; Nazerfakhari, M. Correlation of passivation current density and potential by using chemical composition and corrosion cell characteristics in HSLA steels. Measurement 2015, 75, 5–11. [Google Scholar] [CrossRef]
- Gholamreza, K.; Ali, N.; Hesam, P. Prediction of martensite fraction of microalloyed steel by artificial neural networks. Artif. Neural Netw. 2013, 23, 117–130. [Google Scholar]
- Huang, F.; Cheng, P.; Zhao, X.Y.; Liu, J.; Hu, Q.; Cheng, Y.F. Effect of sulfide films formed on X65 steel surface on hydrogen permeation in H2S environments. Int. J. Hydrogen Energy 2017, 42, 4561–4570. [Google Scholar] [CrossRef]
- Khalaj, G.; Pouraliakbar, H.; Mamaghani, K.R.; Khalaj, M.-J. Modeling the correlation between heat treatment, chemical composition and bainite and bainite fraction of pipelines steels by means of artificial neural networks. Artif. Neural Netw. 2013, 23, 351–367. [Google Scholar] [CrossRef]
- You, Y.; Shang, C.; Wenjin, N.; Subramanian, S. Investigation on the microstructure and toughness of coarse grained heat affected zone in X-100 multi-phase pipeline steel with high Nb content. Mater. Sci. Eng. A 2012, 558, 692–701. [Google Scholar] [CrossRef]
- Tiwari, G.P.; Bose, A.; Chakravartty, J.K.; Wadekar, S.L.; Totlani, M.K.; Arya, R.N.; Fotedar, R.K. A study of internal hydrogen embrittlement of steels. Mater. Sci. Eng. A 2000, 286, 269–281. [Google Scholar] [CrossRef]
- Zheng, C.B.; Jiang, H.K.; Huang, Y.L. Hydrogen permeation behaviour of X56 steel in simulated atmospheric environment under loading. Corros. Eng. Sci. Technol. 2013, 46, 365–367. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, X.; Tao, X.; Su, Y.; Qiao, L. Effect of dislocation cell walls on hydrogen adsorption, hydrogen trapping and hydrogen embrittlement resistance. Corrosion Sci. 2020, 166, 108428. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, M.-G. Analysis of hydrogen trapping behaviour in plastically deformed quenching and partitioning steel in relation to microstructure evolution by phase transformation. J. Alloys Compd. 2022, 904, 164018. [Google Scholar] [CrossRef]
- Hutchings, R.B.; Turnbull, A. The effect of prior mechanical deformation on hydrogen transport through 13% chromium martensitic stainless steel. Corros. Sci. 1992, 33, 713–728. [Google Scholar] [CrossRef]
- Turnbull, A. Perspectives on hydrogen uptake, diffusion and trapping. Int. J. Hydrogen Energy 2015, 40, 16961–16970. [Google Scholar] [CrossRef]
- Xiukui, S.; Xu, X.J. Permeation of hydrogen in 21-6-9 austenitic stainless steel. J. Chin. Soc. Corros. Prot. 1987, 7, 274–280. [Google Scholar]
- Jian, X.; Xiukui, S. Hydrogen permeation and diffusion in low-carbon steels and 16Mn steel. J. Mater. Sci. Technol. 1994, 10, 92–96. [Google Scholar]
- Xu, J.; Xiukui, S.; Qingquan, L.; Wenxiu, X. Characteristics of hydrogen permeation in GH718 alloy. J. Chin. Soc. Corros. Prot. 1994, 14, 151–155. [Google Scholar]
- Stachurski, M. The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. R. Soc. Ser. A-Math. Phys. Eng. Sci. 1962, 270, 90–102. [Google Scholar]
- Brass, A.M.; Chêne, J. Influence of tensile straining on the permeation of hydrogen in low alloy Cr–Mo steels. Corros. Sci. 2006, 48, 481–497. [Google Scholar] [CrossRef]
- Cabrini, M.; Coppola, L.; Lorenzi, S.; Testa, C.; Carugo, F.; Bucella, D.P.; Pastore, T. Hydrogen Permeation in X65 Steel under Cyclic Loading. Materials 2020, 13, 2309. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Hui, W.; Xie, Z.; Wang, Z.; Zhao, X. Hydrogen embrittlement of a cold-rolled Al-containing medium-Mn steel: Effect of pre-strain. Int. J. Hydrogen Energy 2020, 45, 22080–22093. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; He, Z.; Sun, J.; Wang, Y. Determination of the critical plastic strain-induced stress of X80 steel through an electrochemical hydrogen permeation method. Electrochim. Acta 2016, 214, 336–344. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Y.F. Hydrogen permeation and distribution at a high-strength X80 steel weld under stressing conditions and the implication on pipeline failure. Int. J. Hydrog. Energy 2021, 46, 23100–23112. [Google Scholar] [CrossRef]
- Li, H.; Venezuela, J.; Zhou, Q.; Shi, Z.; Dong, F.; Yan, M.; Knibbe, R.; Zhang, M.; Atrens, A. Effect of cold deformation on the hydrogen permeation in a dual-phase advanced high-strength steel. Electrochim. Acta 2022, 424, 140619. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Li, T.; Zhao, Y.; Deng, Q.; Wang, Y.; Jiang, W. Comparison of hydrogen embrittlement susceptibility of three cathodic protected subsea pipeline steels from a point of view of hydrogen permeation. Corros. Sci. 2018, 131, 104–115. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; An, T.; Zheng, S.; Yang, K.; Lv, L.; Xie, C.; Chen, L.; Zhang, L. Investigating the influence mechanism of hydrogen partial pressure on fracture toughness and fatigue life by in-situ hydrogen permeation. Int. J. Hydrogen Energy 2021, 46, 20621–20629. [Google Scholar] [CrossRef]
- Jack, T.A.; Pourazizi, R.; Ohaeri, E.; Szpunar, J.; Zhang, J.; Qu, J. Investigation of the hydrogen induced cracking behaviour of API 5L X65 pipeline steel. Int. J. Hydrogen Energy 2020, 45, 17671–17684. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Characterization of inclusions of X80 pipeline steel and its correlation with hydrogen-induced cracking. Corros. Sci. 2011, 53, 1201–1208. [Google Scholar] [CrossRef]
- Cheng, Y. Analysis of electrochemical hydrogen permeation through X-65 pipeline steel and its implications on pipeline stress corrosion cracking. Int. J. Hydrogen Energy 2007, 32, 1269–1276. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; Zhao, Y.; Sun, J.; Wang, Y. Hydrogen permeation and embrittlement susceptibility of X80 welded joint under high-pressure coal gas environment. Corros. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Hydrogen embrittlement behavior at different strain rates in low-carbon martensitic steel. Mater. Today Proc. 2015, 2, S735–S738. [Google Scholar] [CrossRef]
- Koyama, M.; Yamasaki, D.; Nagashima, T.; Tasan, C.C.; Tsuzaki, K. In situ observations of silver-decoration evolution under hydrogen permeation: Effects of grain boundary misorientation on hydrogen flux in pure iron. Scr. Mater. 2017, 129, 48–51. [Google Scholar] [CrossRef]
- Liu, M.A.; Rivera-Diaz-del-Castillo, P.E.J.; Barraza-Fierro, J.I.; Castaneda, H.; Srivastava, A. Microstructural influence on hydrogen permeation and trapping in steels. Mater. Des. 2019, 167, 107605. [Google Scholar] [CrossRef]
- Ghosh, G.; Rostron, P.; Garg, R.; Panday, A. Hydrogen induced cracking of pipeline and pressure vessel steels: A review. Eng. Fract. Mech. 2018, 199, 609–618. [Google Scholar] [CrossRef]
- Sun, Y.; Frank Cheng, Y. Hydrogen-induced degradation of high-strength steel pipeline welds: A critical review. Eng. Fail. Anal. 2022, 133, 105985. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Razavi-Tousi, S.S. Hydrogen induced cracking susceptibility in different layers of a hot rolled X70 pipeline steel. Int. J. Hydrogen Energy 2013, 38, 13831–13841. [Google Scholar] [CrossRef]
- Yu, S.-H.; Lee, S.-M.; Lee, S.; Nam, J.-H.; Lee, J.-S.; Bae, C.-M.; Lee, Y.-K. Effects of lamellar structure on tensile properties and resistance to hydrogen embrittlement of pearlitic steel. Acta Mater. 2019, 172, 92–101. [Google Scholar] [CrossRef]
| Steel | C | Si | P | S | Cr | Mn | Ni | Cu | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| X65 | 0.081 | 0.190 | 0.014 | <0.001 | 0.050 | 1.320 | <0.030 | <0.030 | <0.030 | Bal. |
| Cold Deformation (%) | N∞ × 10−9 (mol/(cm2·s)) | Deff × 10−6 (cm2/s) | c0 × 10−5 (mol/cm3) |
|---|---|---|---|
| 0.00 | 2.451 ± 0.052 | 3.553 ± 0.311 | 5.551 ± 0.368 |
| 4.25 | 2.473 ± 0.026 | 2.717 ± 0.090 | 7.288 ± 0.163 |
| 7.25 | 2.342 ± 0.022 | 1.775 ± 0.006 | 10.557 ± 0.133 |
| Cold Deformation (%) | N∞ × 10−9 (mol/(cm2·s)) | Deff ×10−6 (cm2/s) | c0 × 10−5 (mol/cm3) |
|---|---|---|---|
| 0.00 | 2.331 ± 0.022 | 4.265 ± 0.455 | 4.418 ± 0.431 |
| 4.25 | 2.137 ± 0.013 | 2.947 ± 0.024 | 5.801 ± 0.013 |
| 7.25 | 2.122 ± 0.062 | 1.903 ± 0.058 | 8.922 ± 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Ming, H.; Chen, J.; Wang, J.; Han, E.-H. Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel. Coatings 2023, 13, 280. https://doi.org/10.3390/coatings13020280
Yao C, Ming H, Chen J, Wang J, Han E-H. Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel. Coatings. 2023; 13(2):280. https://doi.org/10.3390/coatings13020280
Chicago/Turabian StyleYao, Chan, Hongliang Ming, Jian Chen, Jianqiu Wang, and En-Hou Han. 2023. "Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel" Coatings 13, no. 2: 280. https://doi.org/10.3390/coatings13020280
APA StyleYao, C., Ming, H., Chen, J., Wang, J., & Han, E.-H. (2023). Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel. Coatings, 13(2), 280. https://doi.org/10.3390/coatings13020280







