The Stability of the Layer Nitrided in Low-Pressure Nitriding Process
Abstract
1. Introduction
1.1. Fe-N2 Equilibrium
1.2. Nitride Phases
1.3. Stability of Iron Nitrides
2. Materials and Methods
- After the saturation stage, the ammonia dosing valve was closed, the nitrogen valve was opened and the furnace chamber was depressurised to below 0.05 hPa at a decreasing nitrogen flow;
- After the saturation stage, the ammonia dosing valve was closed, the furnace chamber was depressurised to below 0.05 hPa and then the furnace chamber was filled with nitrogen up to 1100 hPa. In both cases, the annealing took place without any gas flow.
3. Results
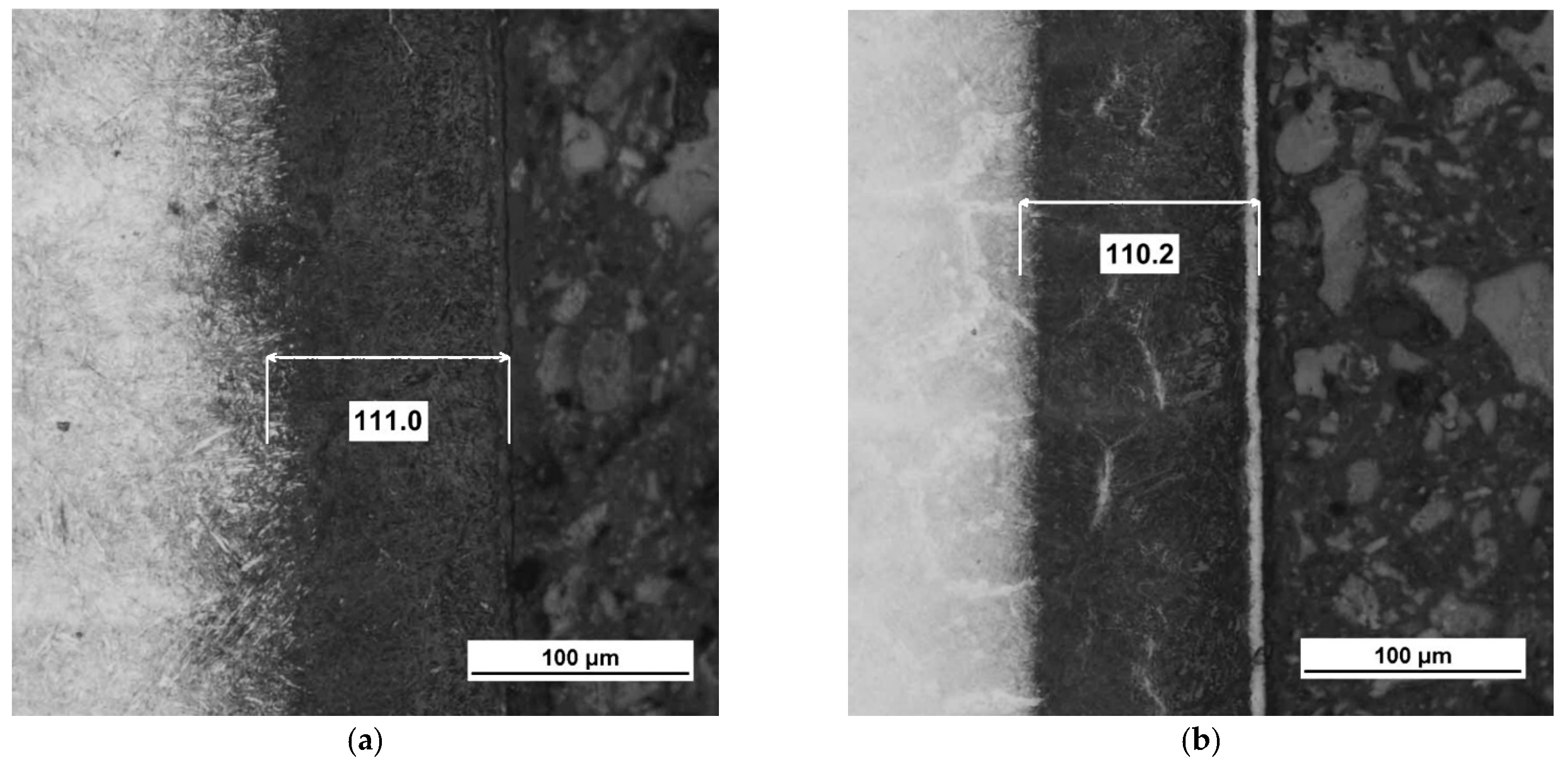
3.1. Thickness of the Iron Nitride Layer
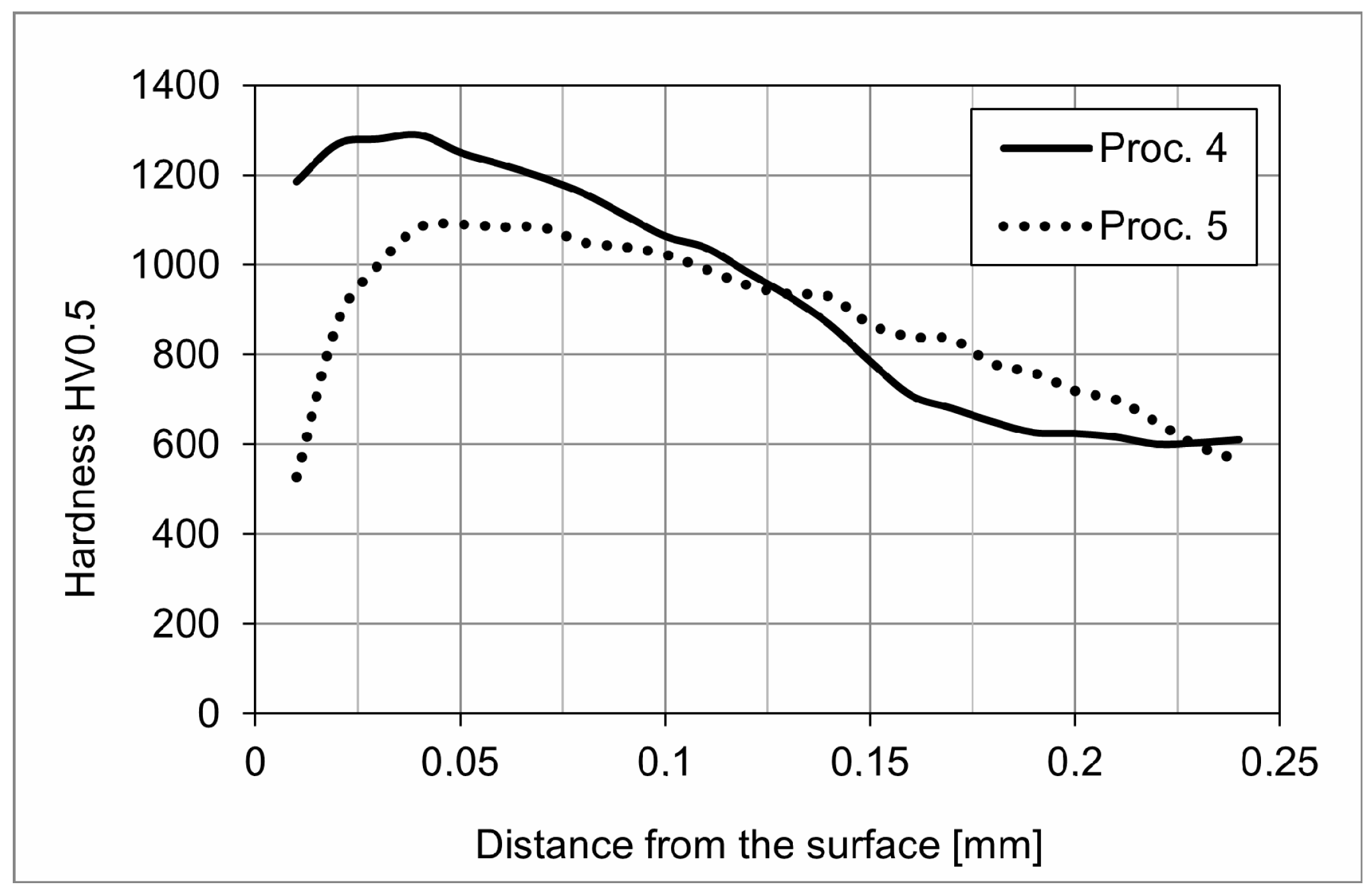
3.2. Hardness
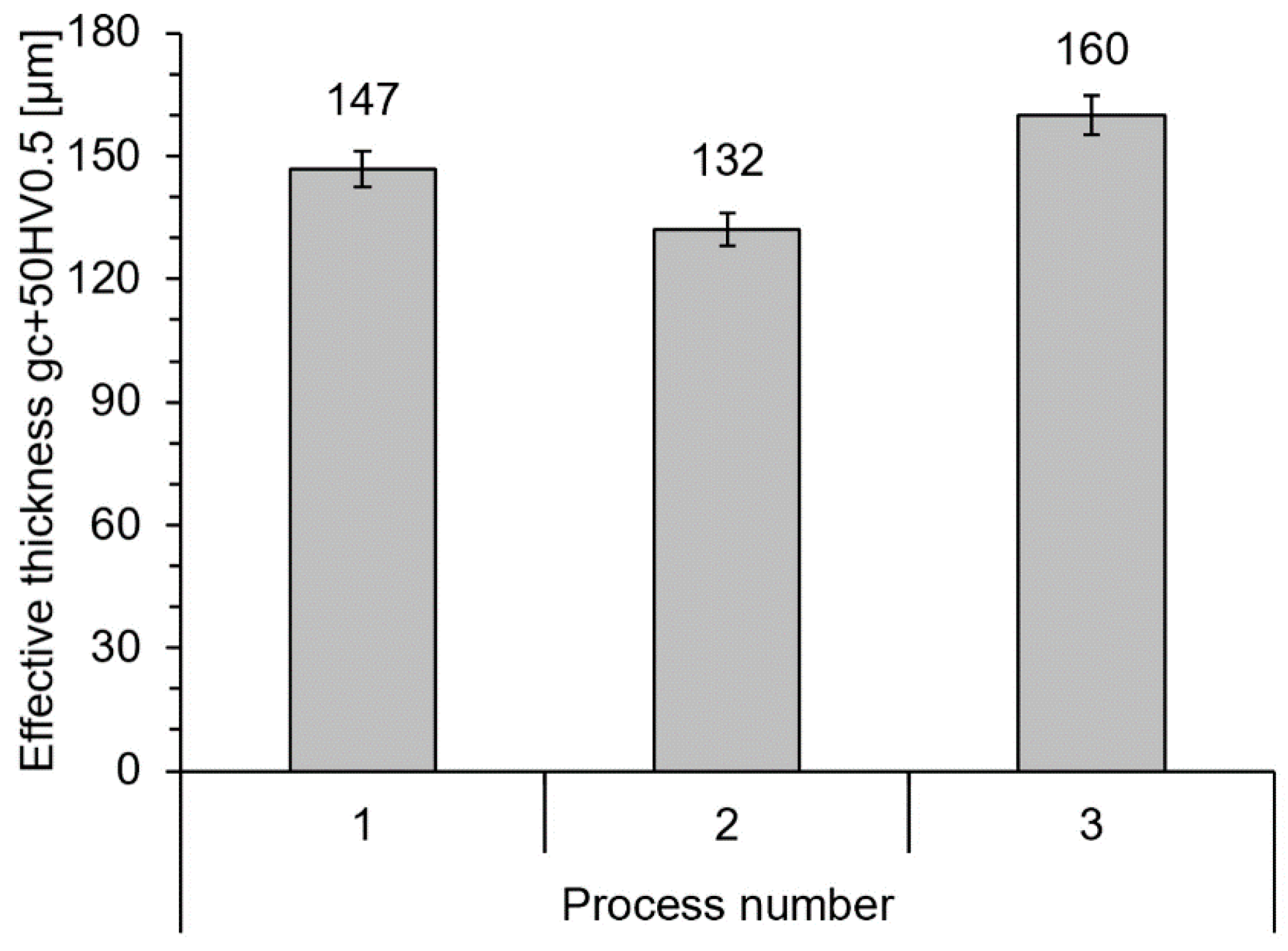
3.3. Thickness of the Diffusion Layer (Solution)
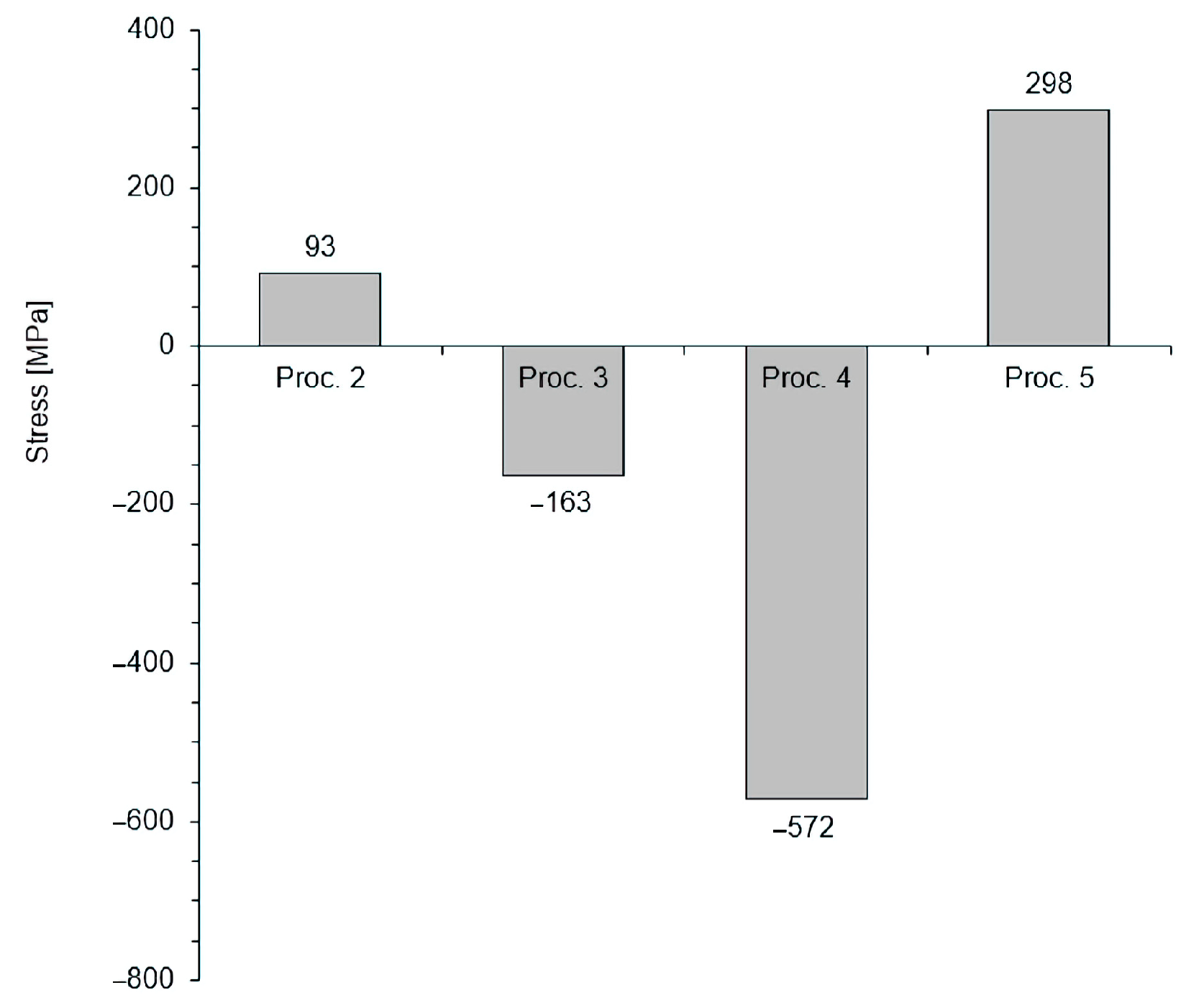
3.4. Stresses
4. Discussion
4.1. Formation of the Iron Nitride Layer
4.2. Decomposition of the Iron Nitride Layer
4.3. Development of the Solution Layer
4.4. Annealing in Inert Gas
5. Conclusions
- In the process of low-pressure nitriding, the stability or decomposition of iron nitrides in the nitrided layer (during the annealing stage) depends on the partial pressure of ammonia in the annealing atmosphere. Lowering this pressure below a critical value (this value has not been defined mathematically in the literature so far) inhibits the growth of the internal nitriding zone thickness and initiates the decomposition of the iron nitride layer formed in the saturation stage.
- The subsurface layer of iron nitrides is not a source of nitrogen for the internal nitriding zone.
- Annealing of the nitrided layer being formed in an inert atmosphere inhibits the growth of the thickness of the iron nitride layer and the internal nitriding zone. Instead, the processes of transformation of the mixture of ε + γ’ phases to a uniform γ’ (ɛ + γ’ → γ’) phase and development of the nitrogen profile in the solution layer caused by the gradient of atomic nitrogen concentration within the solution layer occur in the layer.
- Vacuum annealing during the low-pressure nitriding process leads to the decomposition of the iron nitride layer (formed during the saturation stage) and to unfavourable changes in the structure of the nitrided layer formed. This leads to a change in the type of stress from compressive to tensile and excludes their fitness for practical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somers, M.; Mittemeijer, E. Layer-Growth Kinetics on Gaseous Nitriding of Pure Iron: Evaluation of Diffusion Coefficients for Nitrogen in Iron Nitrides. Metall. Mater. Trans. A 1995, 26, 57–74. [Google Scholar] [CrossRef]
- Somers, M. IFHTSE Global 21: Heat Treatment and Surface Engineering in the Twenty-First Century Part 14—Development of Compound Layer during Nitriding and Nitrocarburising; Current Understanding and Future Challenges. Int. Heat Treat. Surf. Eng. 2011, 5, 7–16. [Google Scholar] [CrossRef]
- Lehrer, E. Über das Eisen-Wasserstoff-Amoniak-Gleichgewicht. Z. Elektrochem. 1930, 36, 383–392. [Google Scholar]
- Kubaszewski, O. Iron-Binary Phase Diagrams; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Michalski, J. Using Nitrogen Availability as a Nitriding Process Parameter. Ind. Heat. 2012, 10, 63–68. [Google Scholar]
- Ratajski, J. Wybrane Aspekty Współczesnego Azotowania Gazowego Pod Kątem Sterowania Procesem; Politechnika Koszalińska: Koszalin, Poland, 2003. [Google Scholar]
- Tacikowski, J.; Zyśk, J.; Sułkowski, I. Opracowanie Procesu Azotowania Stali Węglowych i Stopowych w Atmosferach Zmniejszających Zużycie Deficytowego Amoniaku; Project no 106.00.0051; Instytut Mechaniki Precyzyjnej: Warszawa, Poland, 1984. [Google Scholar]
- Michalski, J.; Burdyński, K.; Wach, P.; Łataś, Z. Nitrogen Availability of Nitriding Atmosphere in Controlled Gas Nitriding Processes. Arch. Metall. Mater. 2015, 60, 747–754. [Google Scholar] [CrossRef]
- Malkiewicz, T. Metaloznawstwo Stopów Żelaza; PWN: Warszawa, Poland, 1976. [Google Scholar]
- Fast, J.; Verrijp, M. Solubility of Nitrogen in Alpha-Iron. J. Iron Steel Inst. 1955, 180, 337–343. [Google Scholar]
- Rawling, R.; Hatherley, P. Iron-Manganese-Nitrogen Ferrite: The Activity of Nitrogen and Solubility of Manganese Nitrides. Met. Sci. 1973, 3, 97–103. [Google Scholar] [CrossRef]
- Grabke, H. Reaktionen von Ammoniak, Stickstoff und Wasserstoff an der Oberfläche von Eisen. Ber. Bunsenges. Phys. Chem. 1968, 4, 533–548. [Google Scholar]
- Smirnov, A.; Kuleshov, Y. Calculations for Nitriding with Diluted Ammonia. Met. Sci. Heat Treat. 1966, 8, 395–403. [Google Scholar] [CrossRef]
- Yurovskikh, A.; Kardonina, N.; Kolpakov, A. Phase Transformations in Nitrided Iron Powders. Met. Sci. Heat Treat. 2015, 57, 507–514. [Google Scholar] [CrossRef]
- Jentzsch, W.; Esser, F.; Bohmer, S. Mathematissches Modell Fur Die Nitrierung von Weicheinsen. Neue Hutte 1981, 1, 19–23. [Google Scholar]
- Kardonina, N.; Yurovskikh, A.; Kolpakov, A. Transformations in the Fe–N System. Met. Sci. Heat Treat. 2010, 52, 457–467. [Google Scholar] [CrossRef]
- Du Marchie van Voorthuysen, E.; Boerma, D.; Chechenin, N. Low-Temperature Extension of the Lehrer Diagram and the Iron-Nitrogen Phase Diagram. Metall. Mater. Trans. A 2002, 33, 2593–2598. [Google Scholar] [CrossRef]
- Lightfoot, B.; Jack, D. Kinetics of Nitriding with and without White Layer Formation. In Heat Treatment’73, Proceedings of a Conference Organized by the Heat Treatment Joint Committee of the Iron and Steel Institute, the Institute of Metals, and the Institution of Metallurgists, Held at the Portman Intercontinental Hotel, London, UK, 12–13 December 1973; The Metals Society: London, UK, 1975; pp. 59–66. [Google Scholar]
- Lakhtin, Y.; Kogan, Y.; Bulgach, A. Calculation of the Effect of Alloying, Elements on the Solubility and Diffusion of Nitrogen in Steel during the Nitriding o the Alpha and Gamma’ Phases. In TRUDY NAMI; Central Scientific Research Automobile and Automotive Engines Institute: Moscow, Russia, 1978; pp. 42–59. [Google Scholar]
- Schwerdtfeger, K.; Grieveson, P.; Turdogan, E. Growth Rate of Fe4N on Alpha. Trans. Metall. Soc. AIME 1969, 245, 2461–2466. [Google Scholar]
- Ratajski, J. Relation between Phase Composition of Compound Zone and Growth Kinetics of Diffusion Zone during Nitriding of Steel. Surf. Coat. Technol. 2009, 203, 2300–2306. [Google Scholar] [CrossRef]
- Ratajski, J. Matematyczne Modelowanie Procesu Azotowania Gazowego; Politechnika Koszalińska: Koszalin, Poland, 2011. [Google Scholar]
- Sugita, Y.; Takahashi, H.; Komuro, M. Magnetic and Mössbauer Studies of Single-crystal Fe16N2 and Fe-N Martensite Films Epitaxially Grown by Molecular Beam Epitaxy. J. Appl. Phys. 1994, 76, 6637–6641. [Google Scholar] [CrossRef]
- Kooi, B.; Somers, M.; Jutte, R.; Mittemeijer, E. On the Oxidation of α-Fe and ε-Fe2N1−z II. Residual Strains and Blisters in the Oxide Layer. Oxid. Met. 1997, 48, 111–128. [Google Scholar] [CrossRef]
- Wołowiec-Korecka, E.; Michalski, J.; Kucharska, B. Kinetic Aspects of Low-Pressure Nitriding Process. Vacuum 2018, 155, 292–299. [Google Scholar] [CrossRef]
- Frączek, T.; Michalski, J.; Kucharska, B.; Opydo, M.; Ogórek, M. Phase Transformations in the Nitrided Layer during Annealing under Reduced Pressure. Arch. Civ. Mech. Eng. 2021, 21, 48. [Google Scholar] [CrossRef]
- Liapina, T.; Leineweber, A.; Mittemeijer, E. Phase Transformations in Iron-Nitride Compound Layers upon Low-Temperature Annealing: Diffusion Kinetics of Nitrogen in ε- and Γ′-Iron Nitrides. Metall. Mater. Trans. A 2006, 37, 319–330. [Google Scholar] [CrossRef]
- Kula, P.; Wołowiec, E.; Pietrasik, R.; Dybowski, K.; Januszewicz, B. Non-Steady State Approach to the Vacuum Nitriding for Tools. Vacuum 2013, 88, 1–7. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Stańczyk-Wołowiec, E. Method of Nitriding Tools Made of Iron. Alloys. Patent PL 219125, 2014. [Google Scholar]
- Liapina, T. Phase Transformations in Interstitial Fe-N Alloys. Ph.D. Thesis, Universitat Stuttgart, Stuttgart, Germany, 2005. [Google Scholar]
- Liapina, T.; Leineweber, A.; Mittemeijer, E. Nitrogen Redistribution in ε/Γ′-Iron Nitride Compound Layers upon Annealing. Scr. Mater. 2003, 48, 1643–1648. [Google Scholar] [CrossRef]
- Liapina, T.; Leineweber, A.; Mittemeijer, E. Phase Transformations in ε-/γ’-Iron Nitride Compound Layers in the Temperature Range of 613–693 K. Defect Diffus. Forum 2005, 237–240, 1147–1152. [Google Scholar] [CrossRef]



| C | Si | Mn | Cr | Mo | Ni | V |
|---|---|---|---|---|---|---|
| 0.37 | 1.00 | 0.38 | 5.15 | 1.30 | 0.00 | 0.40 |
| Process No. | Saturation Time/Atmosphere/Pressure (hPa) | Annealing Time/Atmosphere/Pressure (hPa) |
|---|---|---|
| 1 | 2 h/NH3/24 | None |
| 2 | 2 h/NH3/24 | 2 h/vacuum/<0.05 |
| 3 | 2 h/NH3/24 | 2h/N2/1100 |
| 4 | 6 h/NH3/24 | None |
| 5 | 6 h/NH3/24 | 6 h/vacuum/<0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołowiec-Korecka, E.; Michalski, J.; Januszewicz, B. The Stability of the Layer Nitrided in Low-Pressure Nitriding Process. Coatings 2023, 13, 257. https://doi.org/10.3390/coatings13020257
Wołowiec-Korecka E, Michalski J, Januszewicz B. The Stability of the Layer Nitrided in Low-Pressure Nitriding Process. Coatings. 2023; 13(2):257. https://doi.org/10.3390/coatings13020257
Chicago/Turabian StyleWołowiec-Korecka, Emilia, Jerzy Michalski, and Bartłomiej Januszewicz. 2023. "The Stability of the Layer Nitrided in Low-Pressure Nitriding Process" Coatings 13, no. 2: 257. https://doi.org/10.3390/coatings13020257
APA StyleWołowiec-Korecka, E., Michalski, J., & Januszewicz, B. (2023). The Stability of the Layer Nitrided in Low-Pressure Nitriding Process. Coatings, 13(2), 257. https://doi.org/10.3390/coatings13020257







