Tribocorrosion Behaviour of a Ti–25Nb–3Zr–2Sn–3Mo Alloys Induction Nitride Layer in a Simulated Body Fluid Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemistry Test
2.3. Tribocorrosion Test
2.4. Characterisation
3. Results and Discussion
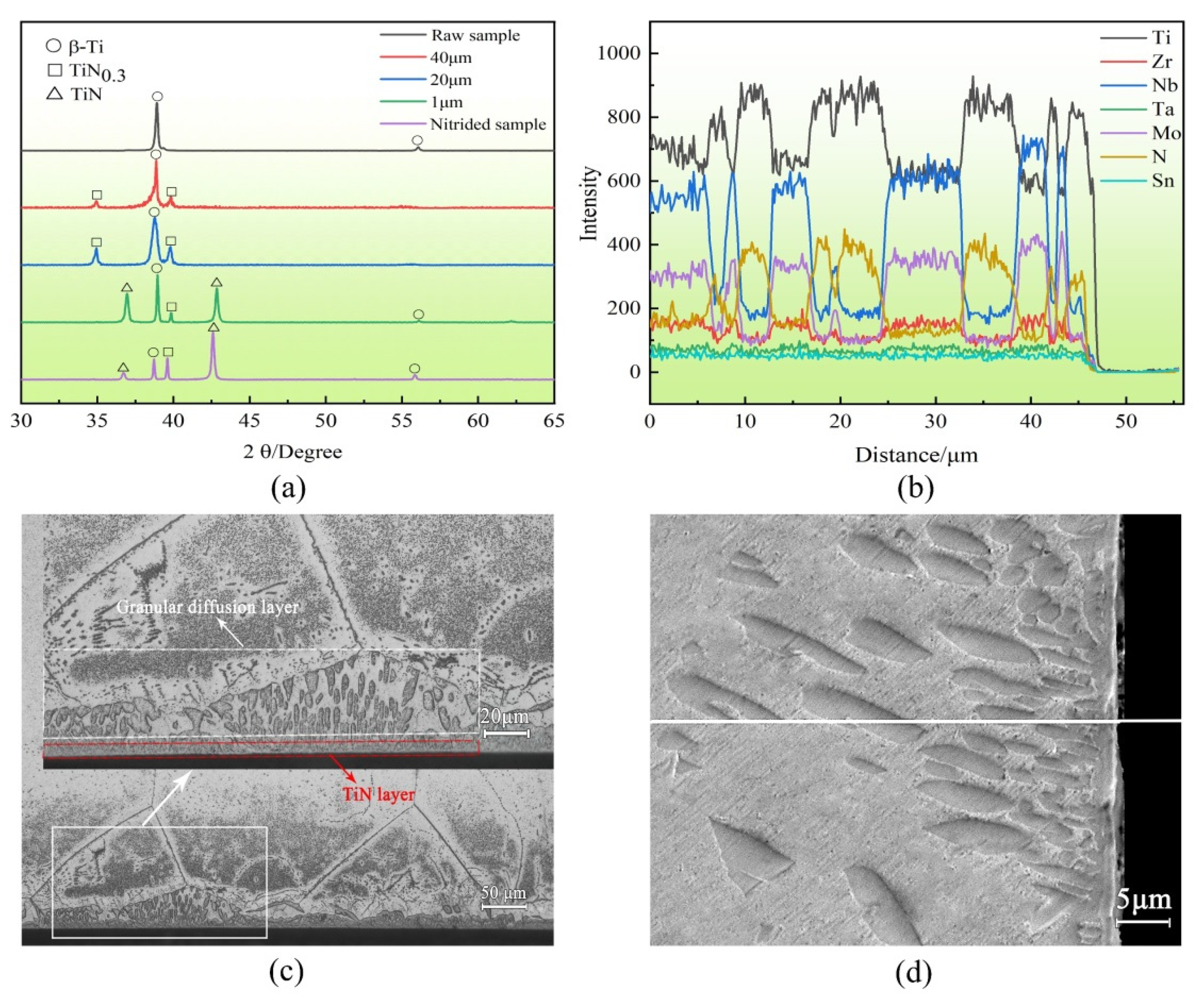
3.1. Characterisation of the Nitride Layer
3.2. Microhardness
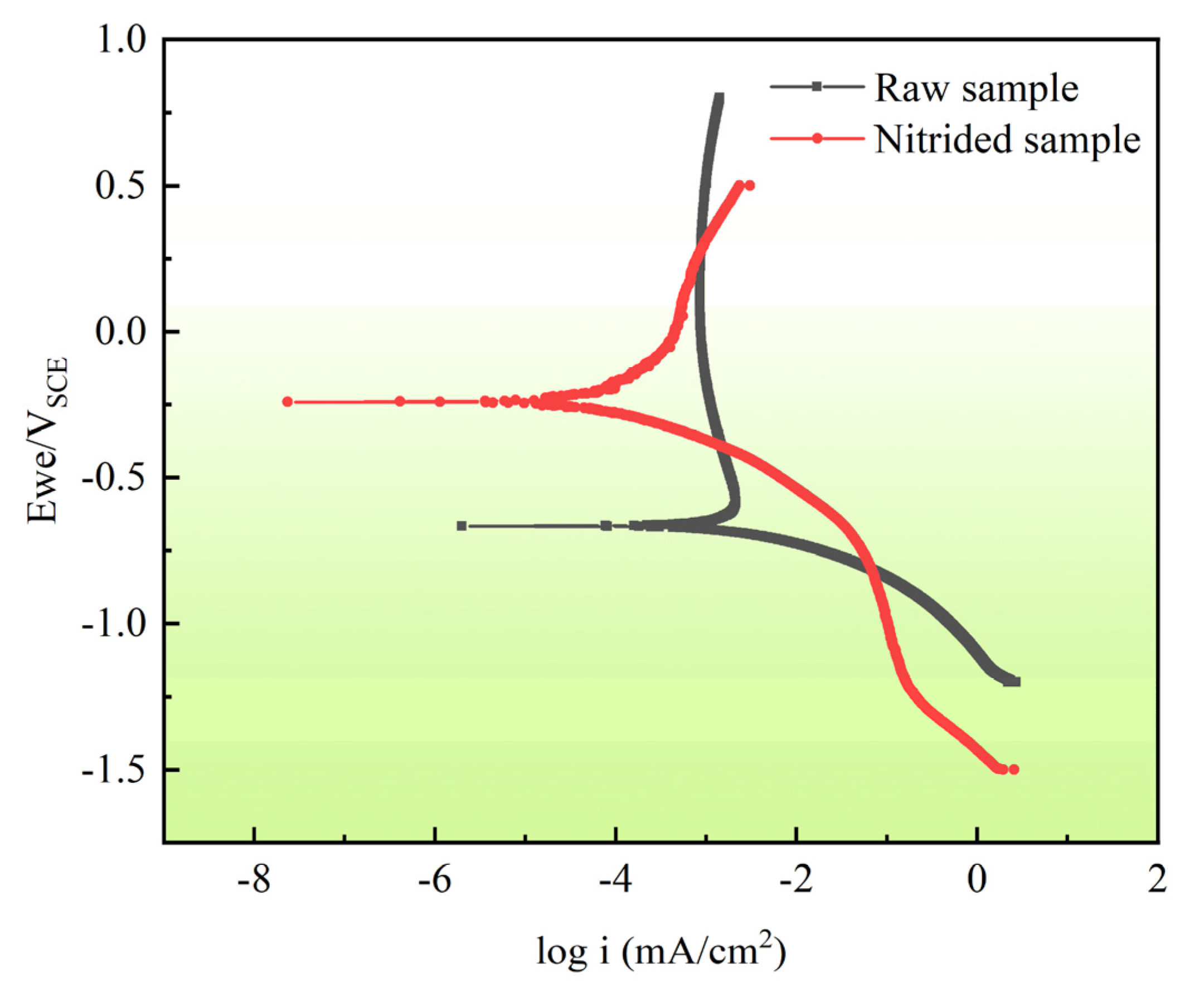
3.3. Electrochemical Tests
3.4. Friction Response and Electrochemical Evolution with Time
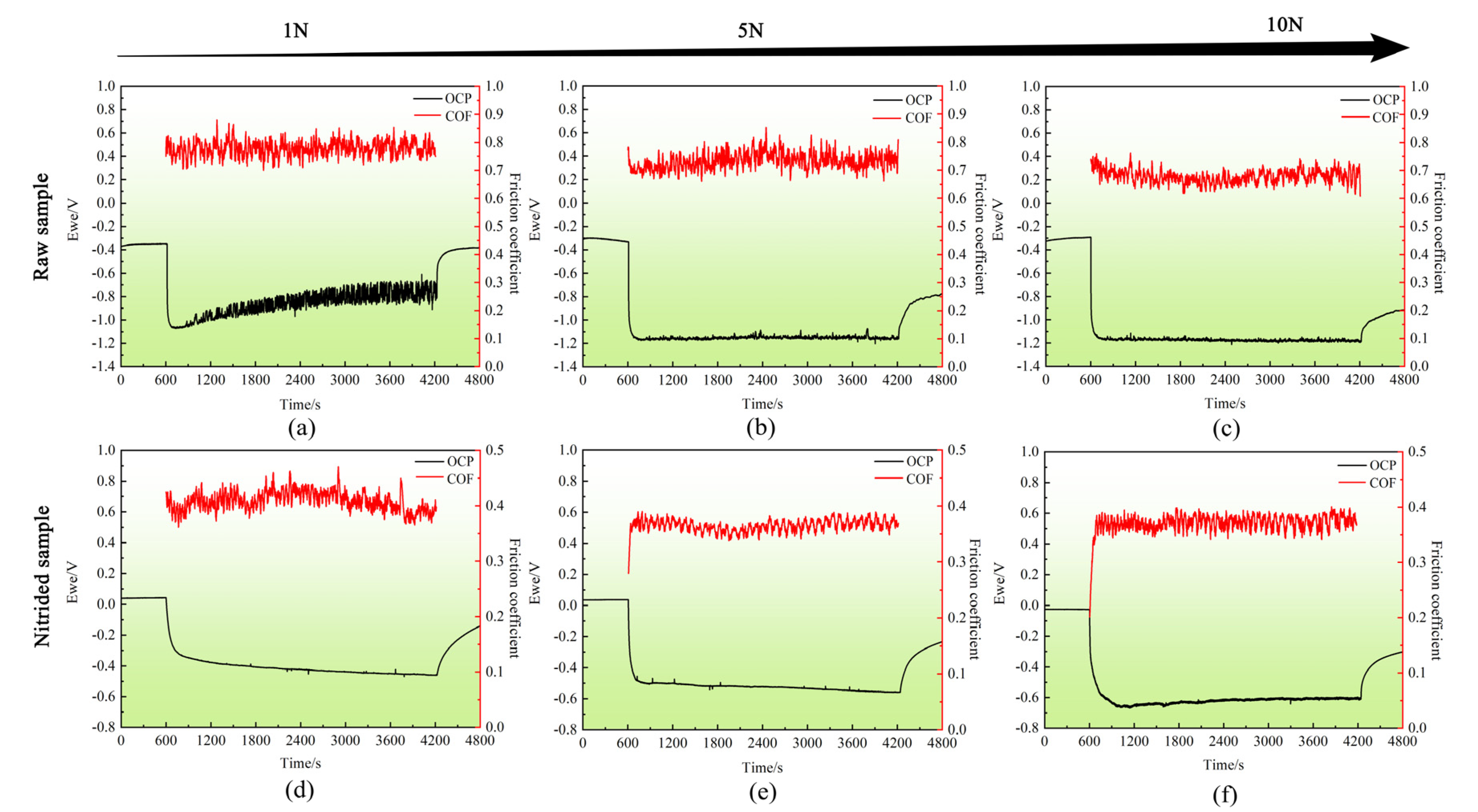
3.4.1. OCP and COF Evolution during Sliding
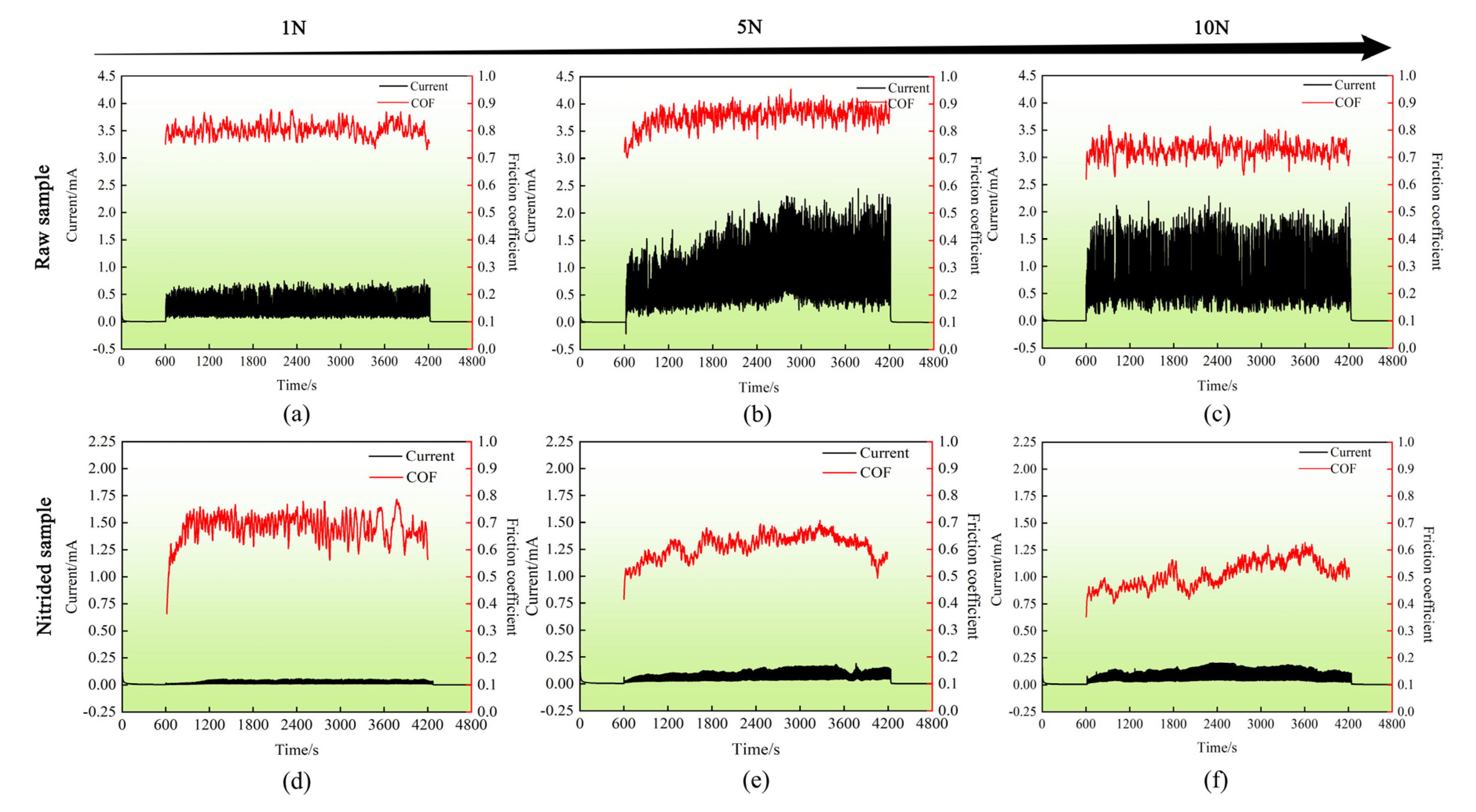
3.4.2. Current Evolution during Sliding
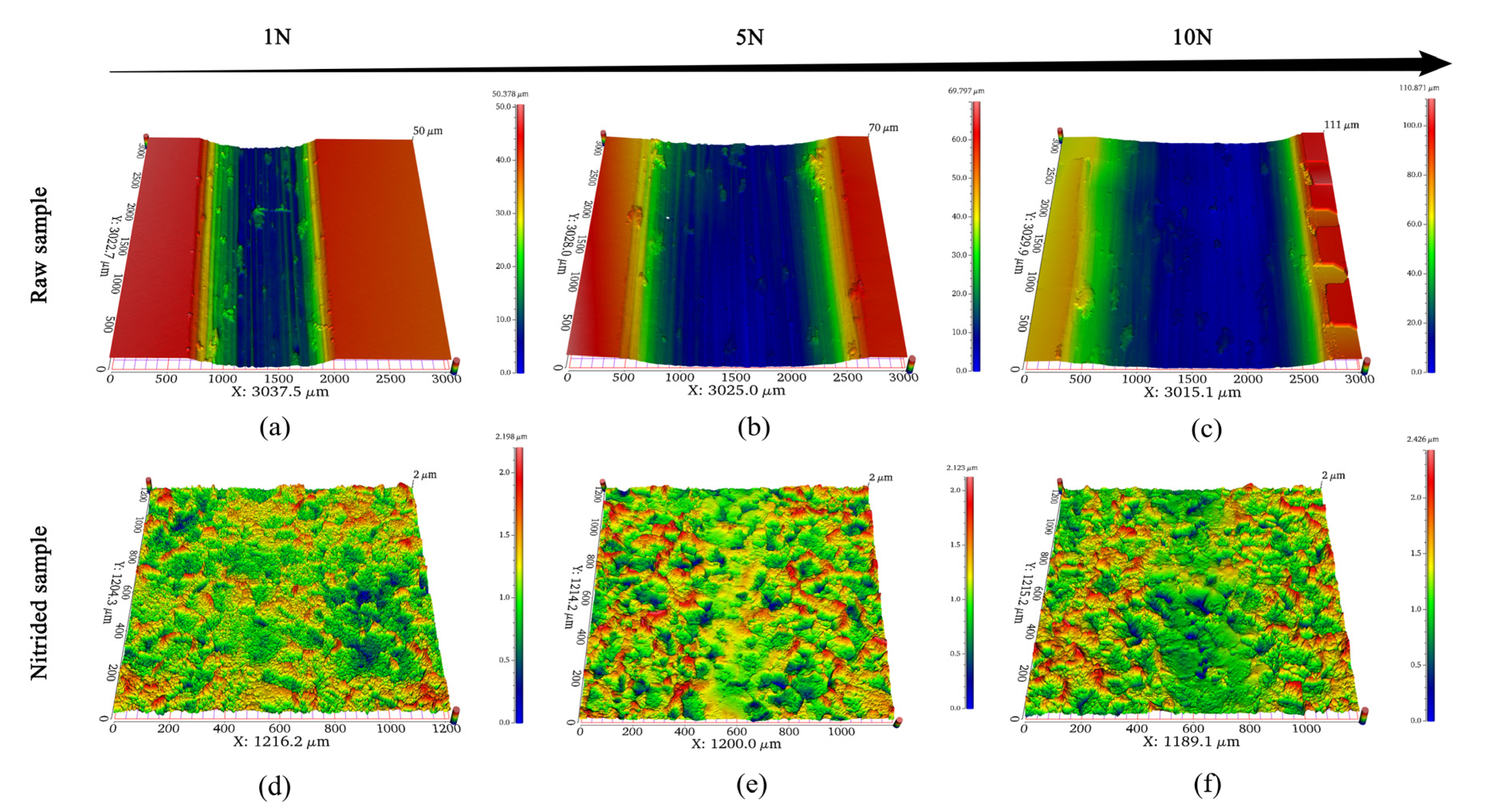
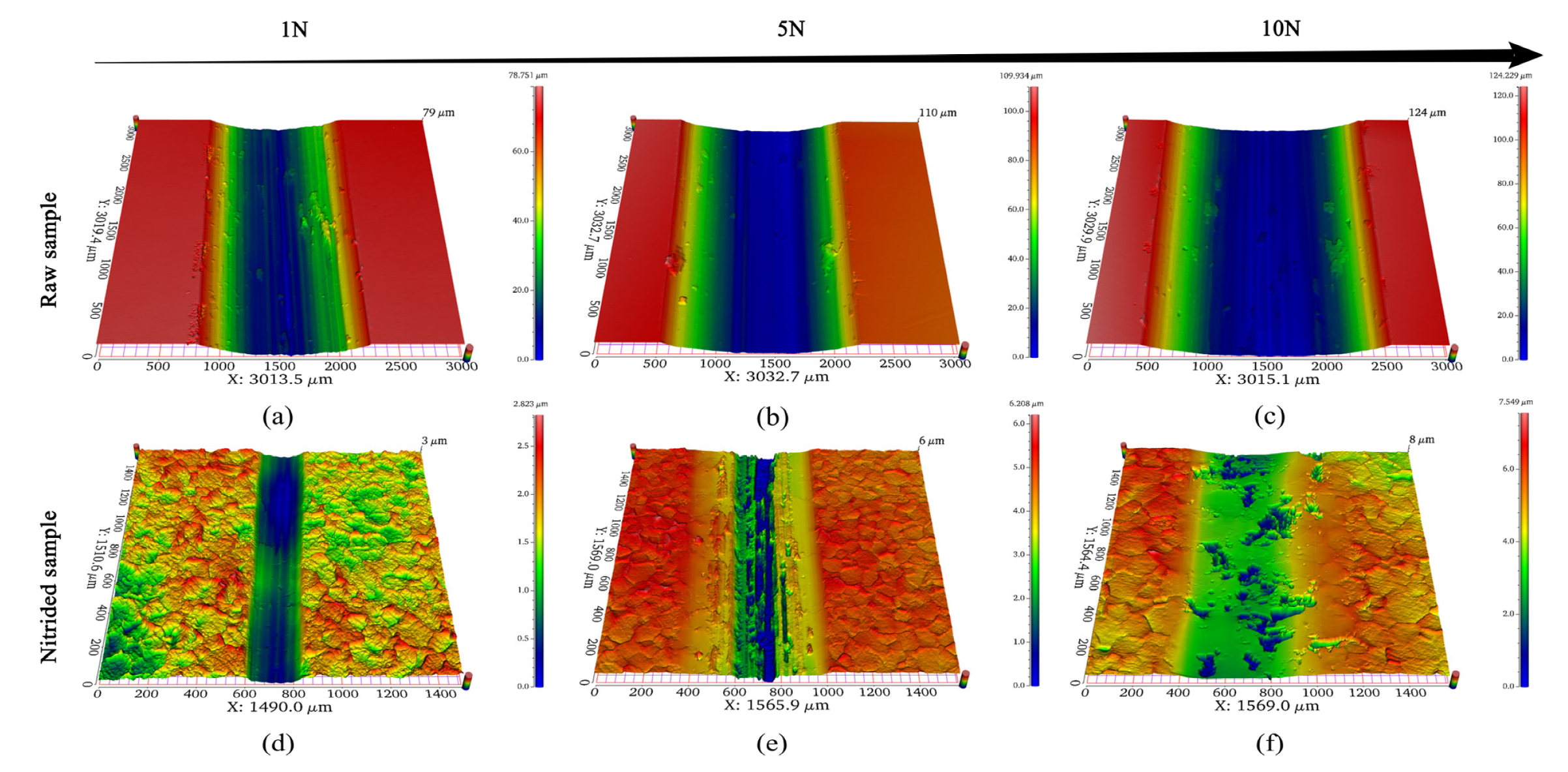
3.5. Wear Morphology and Profile
3.5.1. OCP Condition
3.5.2. Applied Potentials Conditions
3.6. Synergistic Effects of Wear and Corrosion
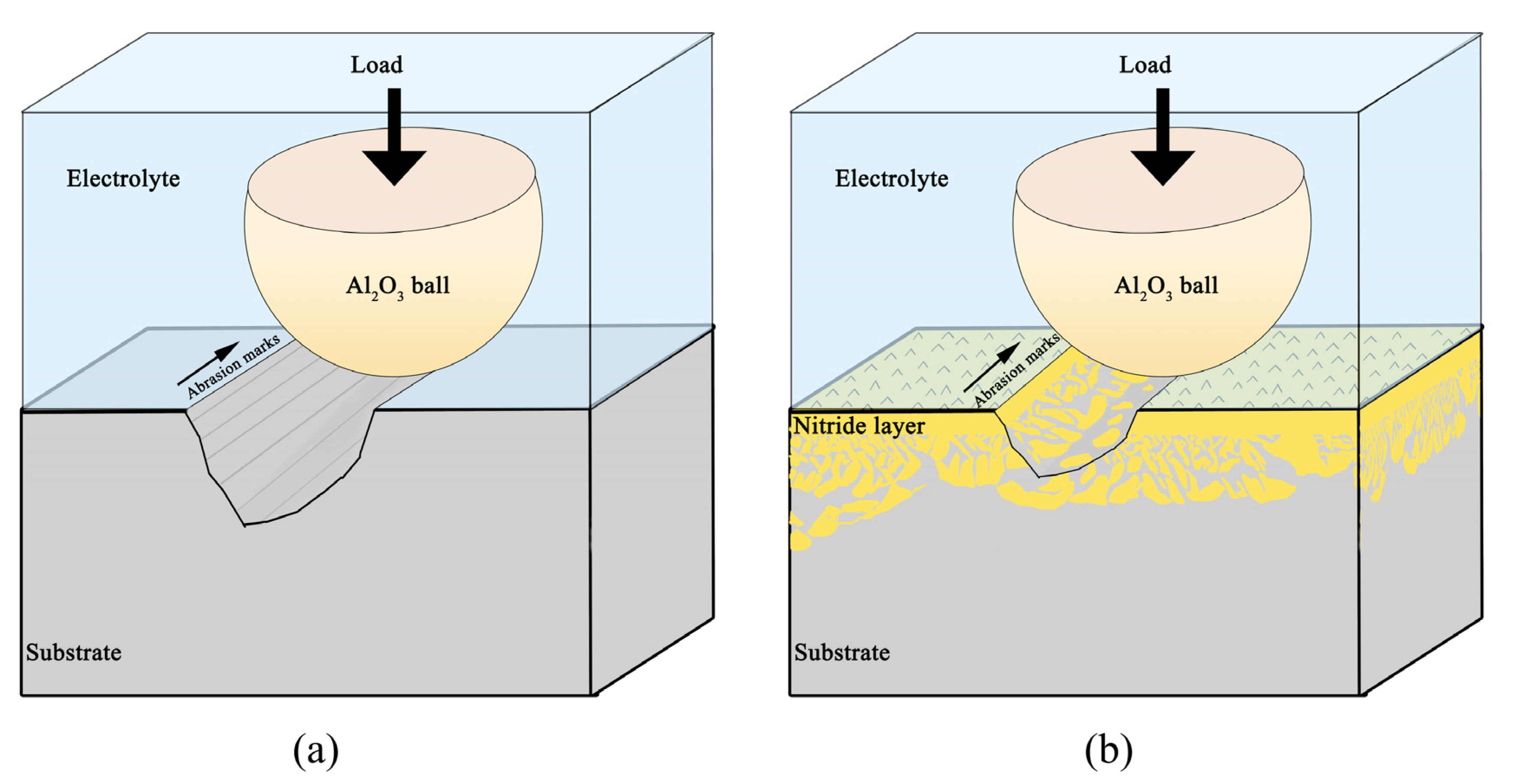
3.7. Tribocorrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gwam, C.; Mistry, J.B.; Mohamed, N.; Thomas, M.; Bigart, K.C.; Mont, M.A.; Delanois, R.E. Current epidemiology of revision total hip arthroplasty in the United States: National inpatient sample 2009 to 2013. Arthroplasty 2017, 32, 2088–2092. [Google Scholar] [CrossRef]
- Xie, K.Y.; Wang, Y.B.; Zhao, Y.; Chang, L.; Wang, G.; Chen, Z.; Cao, Y.; Liao, X.; Lavernia, E.J.; Valiev, R.Z.; et al. Nanocrystalline β-Ti alloy with high hardness, low Young’s modulus and excellent in vitro biocompatibility for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3530–3536. [Google Scholar] [CrossRef]
- Heilshorn, S.C.; Goodman, S.B.; Raphel, J.; Holodniy, M. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Krikke, H.; van der Laan, E. International journal of advanced manufacturing technology introduction to the special issue. Int. J. Adv. Manuf. Technol. 2021, 115, 1605–1620. [Google Scholar] [CrossRef]
- Yetim, T. Corrosion behavior of Ag-doped TiO2 coating on commercially pure titanium in simulate bod fluid solute. J. Bionic. Eng. 2016, 13, 397–405. [Google Scholar] [CrossRef]
- Urena, J.; Tsipas, S.; Pinto, A.M.; Toptan, F.; Gordo, E.; Jimenez-Morales, A.J. Corrosion and tribocorrosion behavior of β-type Ti-Nb and Ti-Mo surfaces designed by diffusion treatments for biomedical applications. Corros. Sci. 2018, 140, 51–60. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In silico contact pressure of metal-on-metal total hip implant with different materials subjected to gait loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Wang, L.Q. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 5, 1901258. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Rościszewska, M.; Majkowska-Marzec, B.; Jażdżewska, M.; Bartmański, M.; Zieliński, A.; Tybuszewska, N.; Samsel, P. Influence of surface modification of titanium and its alloys for medical implants on their corrosion behavior. Materials 2022, 15, 7556. [Google Scholar] [CrossRef]
- Ongtrakulkij, G.; Kajornchaiyakul, J.; Kondoh, K.; Khantachawana, A. Investigation of microstructure, residual stress, and hardness of Ti-6Al-4V after plasma nitriding process with different times and temperatures. Coatings 2022, 12, 1932. [Google Scholar] [CrossRef]
- Kikuchi, S.; Yoshida, S.; Ueno, A. Improvement of fatigue properties of Ti-6Al-4V alloy under four-point bending by low temperature nitriding. Int. J. Fatigue. 2019, 120, 134–140. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Copley, S.M.; Segall, A.E.; Todd, J.A. Laser-sustained plasma (LSP) nitriding of titanium: A review. Coatings 2019, 9, 283. [Google Scholar] [CrossRef]
- Jambagi, S.C.; Malik, V.R. A Review on surface engineering perspective of metallic implants for orthopaedic applications. JOM 2021, 73, 4349–4364. [Google Scholar] [CrossRef]
- Chan, C.W.; Lee, S.; Smith, G.; Sarri, G.; Ng, C.H.; Sharba, A.; Man, H.C. Enhancement of wear and corrosion resistance of beta titanium alloy by laser gas alloying with nitrogen. Appl. Surf. Sci. 2016, 367, 80–90. [Google Scholar] [CrossRef]
- Sathish, S.; Geetha, M.; Pandey, N.D.; Richard, C.; Asokamani, R. Studies on the corrosion and wear behavior of the laser nitrided biomedical titanium and its alloys. Mater. Sci. Eng. C 2010, 30, 376–382. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Vaz, F.; Ariza, E.; Rocha, L.A.; Ribeiro, A.R.L.; Vieira, A.C.; Rivière, J.P.; Pichon, L. Tribocorrosion behaviour of plasma nitrided and plasma nitride + oxidised Ti6Al4V alloy. Surf. Coat. Technol. 2006, 200, 6218–6224. [Google Scholar] [CrossRef]
- Xiang, H.; Ke, F.; Tan, Y.F.; Wang, X.L.; Hua, T. Effects of process parameters on microstructure and wear resistance of TiN coatings deposited on TC11 titanium alloy by electrospark deposition. Trans. Nonferrous Met. Soc. China 2017, 27, 1767–1776. [Google Scholar] [CrossRef]
- Das, S.; Guha, S.; Ghadai, R.K.; Sharma, A. Influence of nitrogen gas over microstructural, vibrational and mechanical properties of CVD Titanium nitride (TiN) thin film coating. Ceram. Int. 2021, 47, 16809–16819. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.S.; Yu, S.; Du, Z.X.; Zhang, X.Y.; Zhang, W.; Gai, J.Y.; Wang, H.C.; Song, H.J.; Yu, Z.T. Cold rolling deformation characteristic of a biomedical beta type Ti-25Nb-3Zr-2Sn-3Mo Alloy plate and its influence on alpha precipitated phases and room temperature mechanical properties during aging treatment. Front. Bioeng. Biotechnol. 2020, 8, 598529. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.; Wang, G.; Yu, Z.; Dargusch, M.S. Pseudoelastic behaviour of a β Ti–25Nb–3Zr–3Mo–2Sn alloy. Mater. Sci. Eng. A 2010, 527, 2246–2252. [Google Scholar] [CrossRef]
- Wang, Z.G.; Li, Y.; Huang, W.J.; Chen, X.L.; He, H. Micro-abrasion-corrosion behaviour of a biomedical Ti–25Nb–3Mo–3Zr–2Sn alloy in simulated physiological fluid. J. Mech. Behav. Biomed. 2016, 63, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Li, K.M.; Song, K.J.; Guan, J.; Yang, F.; Liu, J. Tribocorrosion behavior of a Ti6Al4V alloy electromagnetic induction nitride layer in a fluorine-containing solution. Surf. Coat. Technol. 2020, 386, 125506. [Google Scholar] [CrossRef]
- Guan, J.; Jiang, X.T.; Xiang, Q.; Yang, F.; Liu, J. Corrosion and tribocorrosion behavior of titanium surfaces designed by electromagnetic induction nitriding for biomedical applications. Surf. Coat. Technol. 2021, 409, 126844. [Google Scholar] [CrossRef]
- Jiang, X.T.; Dai, Y.; Xiang, Q.; Liu, J.; Yang, F.; Zhang, D.X. Microstructure and wear behavior of inductive nitriding layer in Ti-25Nb-3Zr-2Sn-3Mo alloys. Surf. Coat. Technol. 2021, 427, 127835. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, S.Z.; Feng, Z.H.; Qi, H.B.; Fu, H.; Liu, R.P. Improving vacuum gas nitriding of a Ti-based alloy via surface solid phase transformation. Vacuum 2022, 197, 110860. [Google Scholar] [CrossRef]
- Barda, H.; Rabkin, E. The role of interface diffusion in solid state dewetting of thin films: The nano-marker experiment. Acta Mater. 2019, 177, 121–130. [Google Scholar] [CrossRef]
- Lin, N.M.; Huang, X.B.; Zhang, X.Y.; Fan, A.; Lin, Q.; Tang, B. In vitro assessments on bacterial adhesion and corrosion performance of TiN coating on Ti6Al4V titanium alloy synthesized by multi-arc ion plating. Appl. Surf. Sci. 2012, 258, 7047–7051. [Google Scholar] [CrossRef]
- Wei, Y.; Pan, Z.M.; Yu, F.; Yu, W.; He, S.; Yuan, Q.; Luo, H.; Li, X. Effect of annealing temperatures on microstructural evolution and corrosion behavior of Ti-Mo titanium alloy in hydrochloric acid. Corros. Sci. 2022, 197, 110079. [Google Scholar] [CrossRef]
- Xu, Y.D.; Qi, J.H.; Nutter, J.; Sharp, J.; Nathet, S. Correlation between the formation of tribofilm and repassivation in biomedical titanium alloys during tribocorrosion. Tribol. Int. 2021, 163, 107147. [Google Scholar] [CrossRef]
- Si, Y.H.; Liu, H.Y.; Yu, H.Y.; Jiang, X.Z.; Sun, D.B. A heterogeneous TiO2/SrTiO3 coating on titanium alloy with excellent photocatalytic antibacterial, osteogenesis and tribocorrosion properties. Surf. Coat. Technol. 2022, 431, 128008. [Google Scholar] [CrossRef]
- Pohrelyuk, L.M.; Fedriko, V.M.; Tkachuk, O.V.; Proskurnyak, R.V. Corrosion resistance of Ti-6Al-4V alloy with nitride coatings in Ringer’s solution. Corros. Sci. 2013, 66, 392–398. [Google Scholar] [CrossRef]
- Milosev, I.; Kosec, T.; Strehblow, H.H. XPS and EIS study of the passive film formed on orthopaedic Ti-6Al-7Nb alloy in Hank’s physiological solution. Electrochim. Acta. 2008, 53, 3547–3558. [Google Scholar] [CrossRef]
- Milosev, I.; Zerjav, G.; Moreno, J.M.C.; Popa, M. Electrochemical properties, chemical composition and thickness of passive film formed on novel Ti-20Nb-10Zr- 5Ta alloy. Electrochim. Acta 2013, 99, 176–189. [Google Scholar] [CrossRef]
- Alkan, S.; Gok, M.S. Effect of sliding wear and electrochemical potential on tribocorrosion behaviour of AISI 316 stainless steel in seawater. Eng. Sci. Technol. 2021, 24, 524–532. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Almeida, S.M.A.; Soares, R.B.; Juliani, L.; Bracarense, A.Q.; Lins, V.D.F.C.; Junqueira, R.M.R. Synergism between mechanical wear and corrosion on tribocorrosion of a titanium alloy in a Ringer solution. J. Mater. Sci. Technol. 2019, 8, 1593–1600. [Google Scholar] [CrossRef]
- Pejakovic, V.; Berger, L.M.; Thiele, S.; Rojacz, H.; Ripoll, M.R. Fine grained titanium carbonitride reinforcements for laser deposition processes of 316L boost tribocorrosion resistance in marine environments. Mater. Design. 2021, 207, 109847. [Google Scholar] [CrossRef]
- Wang, C.T.; Hakala, T.J.; Laukkanen, A.; Ronkainen, H.; Holmberg, K.; Gao, N.; Wood, R.J.K.; Langdon, T.G. An investigation into the effect of substrate on the load-bearing capacity of thin hard coatings. J. Mater. Sci. 2016, 51, 4390–4398. [Google Scholar] [CrossRef]
- Suárez, A.; Veiga, F.; Polvorosa, R.; Artaza, T.; Holmberg, J.; López de Lacalle, L.N.; Wretland, A. Surface integrity and fatigue of non-conventional machined Alloy 718. J. Manuf. Process. 2019, 48, 44–50. [Google Scholar] [CrossRef]
- Bolelli, G.; Vork€otter, C.; Lusvarghi, L.; Morelli, S.; Vaßen, R. Performance of wear resistant MCrAlY coatings with oxide dispersion strengthening. Wear 2020, 444–335, 203116. [Google Scholar] [CrossRef]
- Chen, S.N.; Zhao, Y.M.; Zhang, Y.F.; Chen, L.; Zhang, X. Influence of carbon content on the structure and tribocorrosion properties of TiAlCN/TiAlN/TiAl multilayer composite coatings. Surf. Coat. Technol. 2021, 411, 126886. [Google Scholar] [CrossRef]
- Cao, S.F.; Mischler, S. Modeling tribocorrosion of passive metals-A review. Curr. Opin. Solid State Mater. Sci. 2018, 22, 127–141. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact mechanics and the wear of metals. Wear 1995, 190, 162–170. [Google Scholar] [CrossRef]
- Cui, W.F.; Cheng, J.; Liu, Z.Y. Bio-tribocorrosion behavior of a nanocrystalline TiZrN coating on biomedical titanium alloy. Surf. Coat. Technol. 2019, 369, 79–86. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.C.; Kuroda, P.A.B.; Grandini, C.R.; Pinto, A.M.P.; Rocha, L.A.; Toptan, F. Degradation behavior of Ti-Nb alloys: Corrosion behavior through 21 days of immersion and tribocorrosion behavior against alumina. Corros. Sci. 2020, 167, 108488. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.C.; Chirico, C.; Pinto, A.; Tsipas, S.; Gordo, E.; Toptan, F. Corrosion and tribocorrosion behavior of Ti-40Nb and Ti-25Nb-5Fe alloys processed by powder metallurgy. Metall, Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 3256–3267. [Google Scholar] [CrossRef]
- Guo, X.H.; Du, K.Q.; Guo, Q.Z.; Wang, Y.; Wang, F. Experimental study of corrosion protection of a three-layer film on AZ31B Mg alloy. Corros. Sci. 2012, 65, 367–375. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, Z.; Chen, M.; Zhang, W.; Mao, J.; Zhao, Y.; Maitz, M.F.; Huang, N.; Wan, G. Sandwiched polydopamine (PDA) layer for titanium dioxide (TiO2) coating on magnesium to enhance corrosion protection. Corros. Sci. 2015, 96, 67–73. [Google Scholar] [CrossRef]
- Falodun, O.E.; Obadele, B.A.; Oke, S.R.; Maja, M.E.; Olubambi, P.A. Effect of sintering parameters on densification and microstructural evolution of nano-sized titanium nitride reinforced titanium alloys. J. Alloys Compd. 2018, 736, 202–210. [Google Scholar] [CrossRef]
- Vieira, A.C.; Ribeiro, A.R.; Rocha, L.A.; Celis, J.P. Influence of pH and corrosion inhibitors on the tribocorrosion of titanium in artificial saliva. Wear 2006, 261, 994–1001. [Google Scholar] [CrossRef]





| Ions | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− | Buffer | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| SBF | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 | Tris | 7.4 |
| Sample | Ecorr/mV | icorr/μA·cm−2 | βa | βc |
|---|---|---|---|---|
| Raw | −665.766 | 1.459 | 422.200 | 72.800 |
| Nitrided | −241.003 | 0.036 | 175.200 | 98.400 |
| Sample | Load/N | ic/mA | iw/mA |
|---|---|---|---|
| Raw | 1 | - | 2.80 × 10−1 |
| 5 | 1.01 × 10−2 | 8.64 × 10−1 | |
| 10 | - | 8.96 × 10−1 | |
| Nitrided | 1 | - | 2.72 × 10−2 |
| 5 | 9.98 × 10−3 | 8.63 × 10−2 | |
| 10 | - | 9.52 × 10−2 |
| Sample | Load/N | WT/g | WA/g | WC/g | WS/g | SA/g | SC/g | WS/WT |
|---|---|---|---|---|---|---|---|---|
| Raw | 1 | 3.39 × 10−3 | 1.31 × 10−3 | - | 2.07 × 10−3 | 1.91 × 10−3 | 1.66 × 10−4 | 61.06% |
| 5 | 6.51 × 10−3 | 4.98 × 10−3 | 6.01 × 10−6 | 1.52 × 10−3 | 1.01 × 10−3 | 5.14 × 10−4 | 23.35% | |
| 10 | 8.37 × 10−3 | 7.70 × 10−3 | - | 6.64 × 10−4 | 1.31 × 10−4 | 5.33 × 10−4 | 7.93% | |
| Nitrided | 1 | 2.72 × 10−5 | 1.83 × 10−6 | - | 1.94 × 10−5 | 4.20 × 10−6 | 1.52 × 10−5 | 71.32% |
| 5 | 5.87 × 10−4 | 9.45 × 10−6 | 5.94 × 10−6 | 5.72 × 10−4 | 5.22 × 10−4 | 5.04 × 10−5 | 97.44% | |
| 10 | 1.23 × 10−3 | 1.75 × 10−5 | - | 1.21 × 10−3 | 1.15 × 10−3 | 5.57 × 10−5 | 98.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Jiang, X.; Ou, M.; Li, K.; Xiang, Q.; Yang, F.; Liu, J. Tribocorrosion Behaviour of a Ti–25Nb–3Zr–2Sn–3Mo Alloys Induction Nitride Layer in a Simulated Body Fluid Solution. Coatings 2023, 13, 231. https://doi.org/10.3390/coatings13020231
Dai Y, Jiang X, Ou M, Li K, Xiang Q, Yang F, Liu J. Tribocorrosion Behaviour of a Ti–25Nb–3Zr–2Sn–3Mo Alloys Induction Nitride Layer in a Simulated Body Fluid Solution. Coatings. 2023; 13(2):231. https://doi.org/10.3390/coatings13020231
Chicago/Turabian StyleDai, Yan, Xueting Jiang, Meigui Ou, Kunmao Li, Qing Xiang, Feng Yang, and Jing Liu. 2023. "Tribocorrosion Behaviour of a Ti–25Nb–3Zr–2Sn–3Mo Alloys Induction Nitride Layer in a Simulated Body Fluid Solution" Coatings 13, no. 2: 231. https://doi.org/10.3390/coatings13020231
APA StyleDai, Y., Jiang, X., Ou, M., Li, K., Xiang, Q., Yang, F., & Liu, J. (2023). Tribocorrosion Behaviour of a Ti–25Nb–3Zr–2Sn–3Mo Alloys Induction Nitride Layer in a Simulated Body Fluid Solution. Coatings, 13(2), 231. https://doi.org/10.3390/coatings13020231





