Enhanced Corrosion Resistance of TiZrN-Coated Additively Manufactured 8620 Low-Alloy Steel in Nitrate Salt Solution and Salt Bath
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Selective Laser Melting
2.3. TiZrN Coating
2.4. Material Characterization
2.5. Electrochemical Test
2.6. High-Temperature Oxidation Test
3. Results and Discussion
3.1. Microstructure
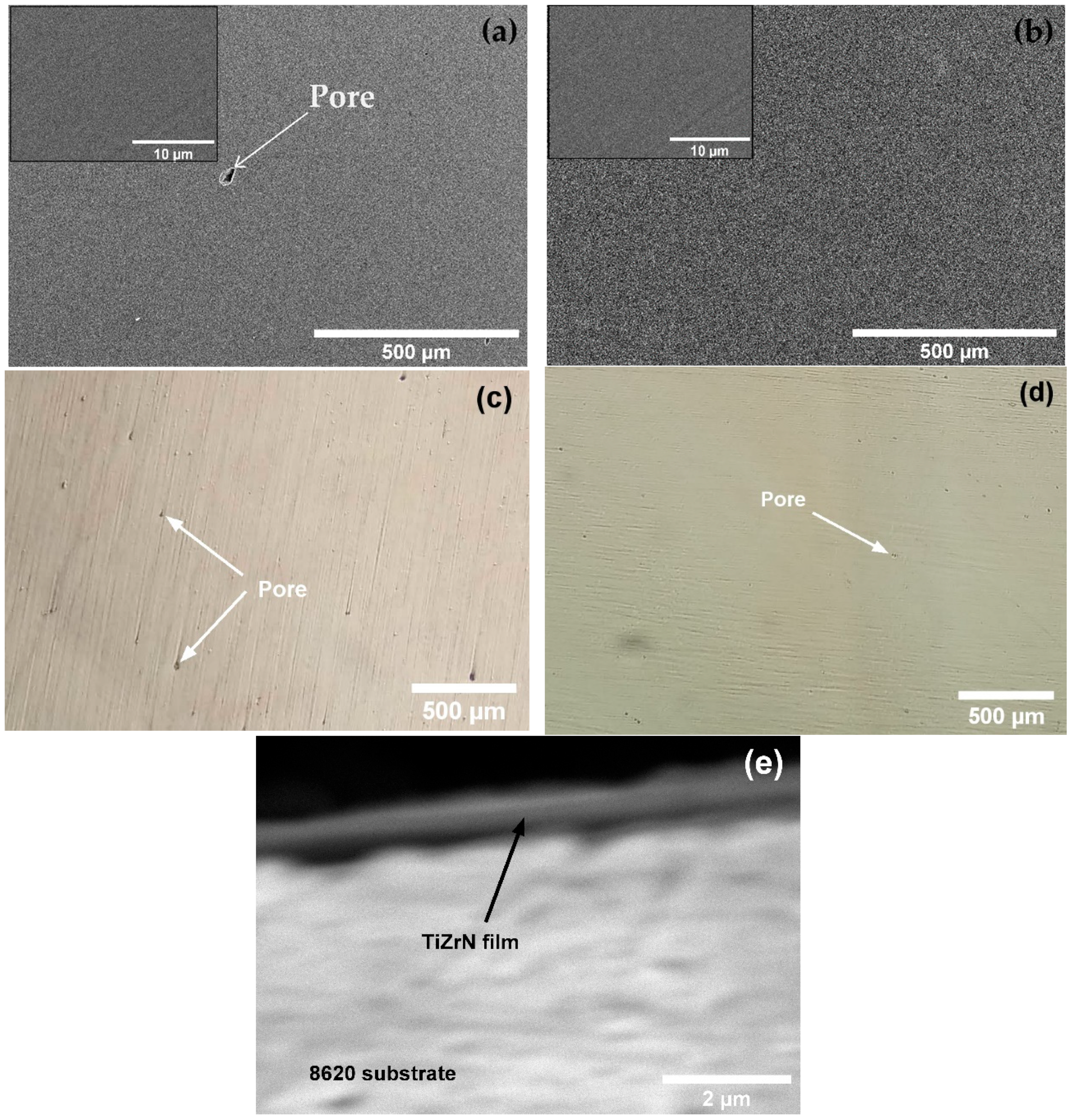
3.2. TiZrN Coating
3.3. Potentiodynamic Polarization
3.4. Electrochemical Impedance Spectroscopy
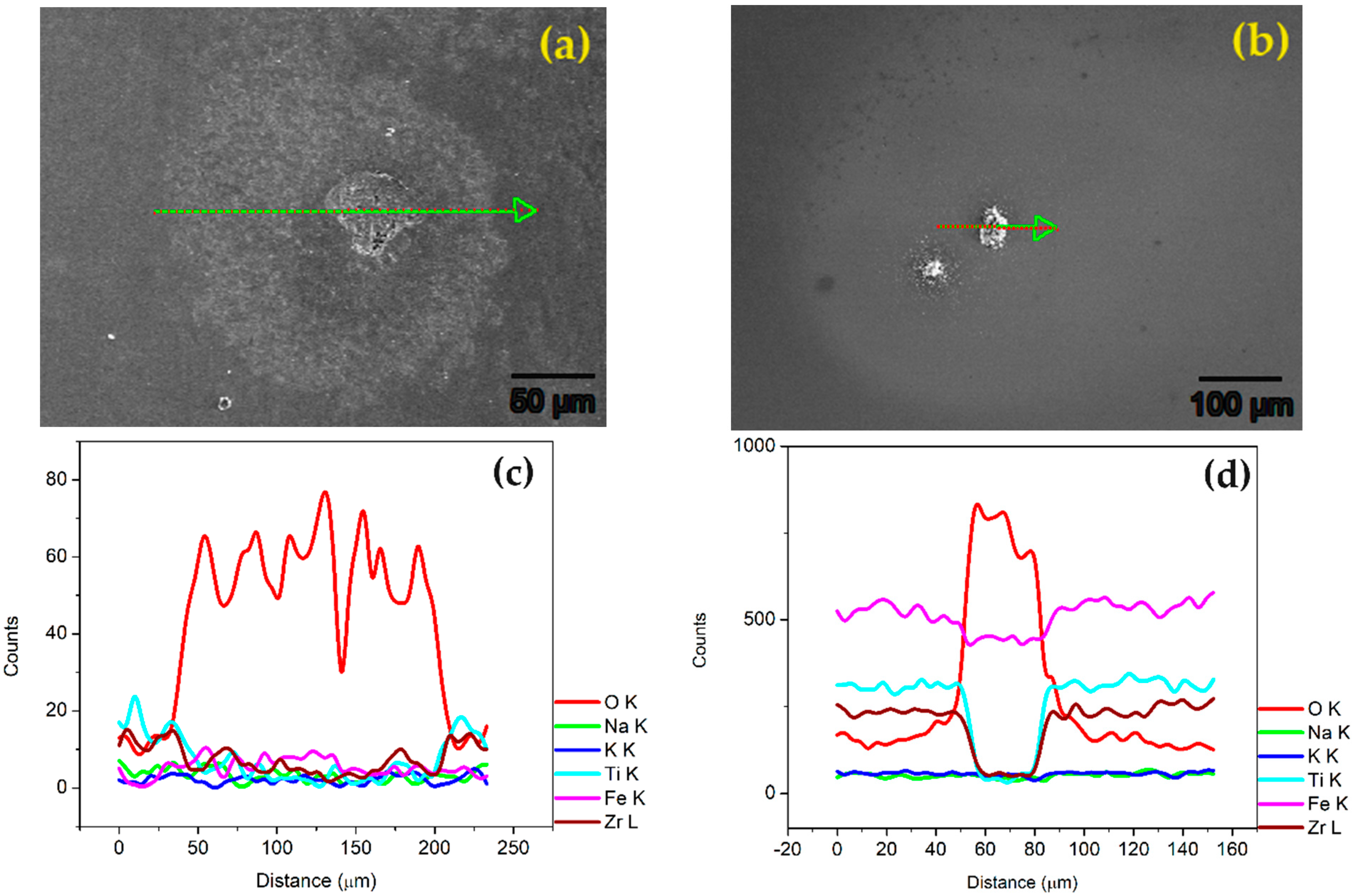
3.5. Surface Analysis after Potentiodynamic Polarization
3.6. High-Temperature Oxidation Analysis
4. Conclusions
- Test specimens of 8620 steel were prepared via selective laser melting. The microstructure of the as-built bare SLMed sample, after etching, showed a tempered martensitic phase. This microstructure resulted from the fast solidification rate of the 3D printing process. The microstructure of wrought 8620, on the other hand, showed a ferritic–pearlitic microstructure.
- Both SLMed and wrought samples were coated via the magnetron-sputtered TiZrN thin film. The TiZrN film exhibited good adhesion to both SLMed and wrought 8620 samples, although they had dissimilar microstructures. However, the presence of pores on the deposited film was observed more on the SLMed sample than the wrought sample due to the existing pores on the surface of the SLMed sample generated during 3D printing. Coated samples were further heat-treated at 270 °C to reduce residual stress of the deposited film and partially oxidize to increase corrosion resistance. EDS analysis of the heat-treated film confirmed the presence of Ti, Zr, N, and O.
- In potentiodynamic polarization and electrochemical impedance spectroscopy tests in an aqueous nitrate salt solution, bare SLMed and wrought 8620 showed similar performance. This signifies that 8620 steel manufactured via SLM is as good as wrought 8620 steel in terms of corrosion resistance in nitrate salt solution. All coated samples (both SLMed and wrought) exhibited better corrosion resistance in nitrate salt solution. In particular, coated samples showed an ~8-fold lower corrosion current density, a~7-fold higher charge transfer resistance, and a ~20-fold lower double layer capacitance than their bare counterparts.
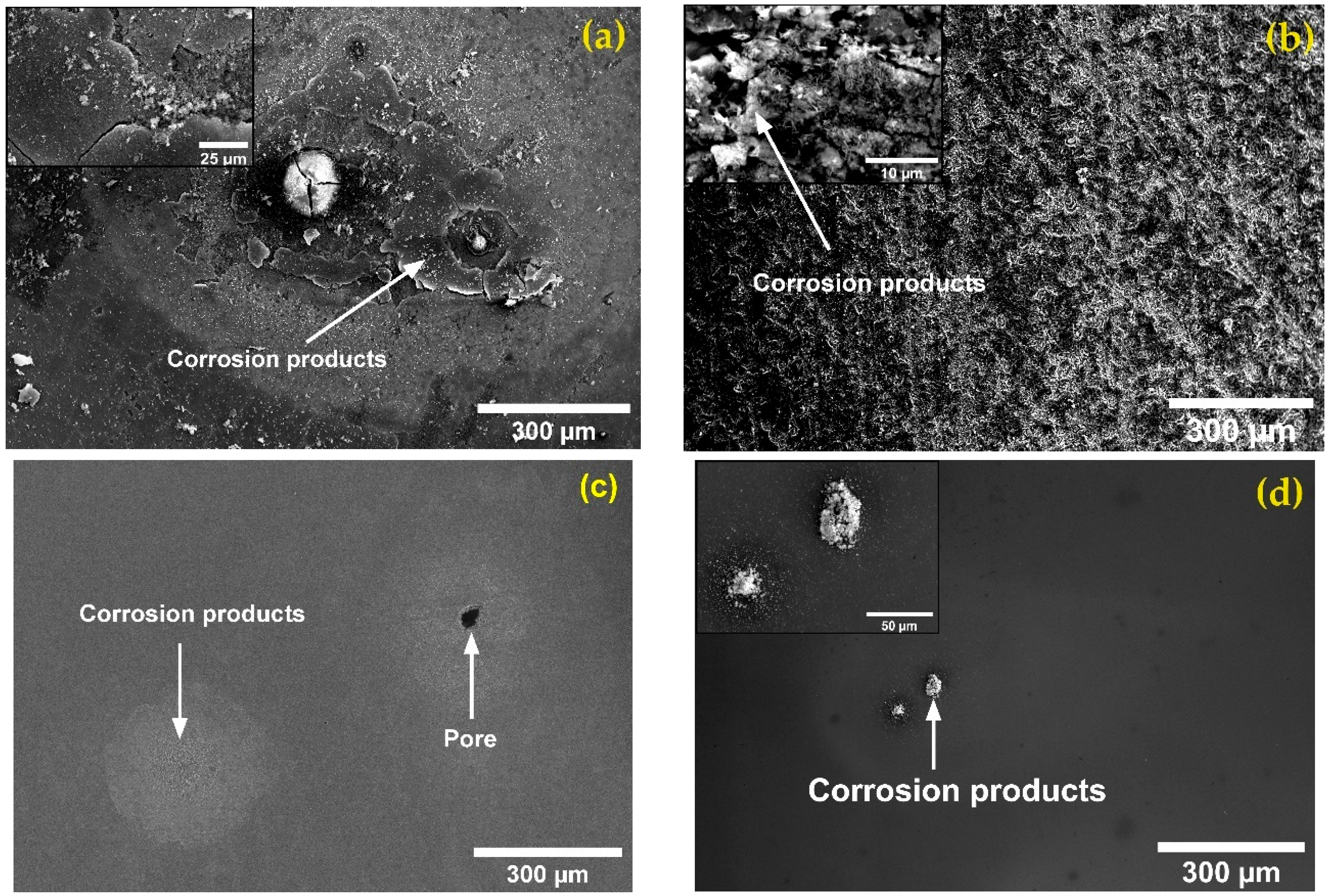
- SEM observation of the corroded samples after the potentiodynamic polarization test showed that the coated SLMed and wrought samples underwent mild corrosion attacks predominantly on the existing pores present on the film. The bare samples, on the other hand, were subjected to significant corrosion attacks, resulting in the deposition of oxides. This signifies the corrosion protection capability of the deposited film.
- The high-temperature corrosion test in molten solar salt revealed that the coated samples did not have any corrosion product, but the bare samples had a thick layer of corrosion products on top of the sample. EDS analysis of the corrosion products on the bare samples revealed the presence of magnetite (Fe3O4) and hematite (Fe2O3). Thus, bare SLMed and wrought 8620 steel are susceptible to significant corrosion in a molten-salt environment which could be reduced via TiZrN thin-film coating.
- The improvement in corrosion resistance of the thin-film-coated sample is attributed to the incorporation of oxygen, the formation of zirconium and titanium oxides, and the lower number of reaction sites in the film.
- Future research endeavors could center around a comprehensive examination of the long-term corrosion resistance exhibited by the TiZrN film. As upcoming concentrated solar power (CSP) technologies are anticipated to operate at elevated temperatures surpassing 600 °C, it is imperative to assess the performance of the TiZrN film under such conditions. Additionally, exploring alternative additive manufacturing techniques like wire arc additive manufacturing (WAAM), known for its rapid and cost-effective metal component printing, presents an intriguing avenue. Investigating the corrosion resistance of WAAM-produced components in molten-salt environments could provide valuable insights.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging Renewable and Sustainable Energy Technologies: State of the Art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of Commercial Thermal Energy Storage in Concentrated Solar Power Plants: Steam vs. Molten Salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Mao, A.; Park, J.H.; Han, G.Y.; Seo, T.; Kang, Y. Heat Transfer Characteristics of High Temperature Molten Salt for Storage of Thermal Energy. Korean J. Chem. Eng. 2010, 27, 1452–1457. [Google Scholar] [CrossRef]
- Madathil, P.K.; Balagi, N.; Saha, P.; Bharali, J.; Rao, P.V.C.; Choudary, N.V.; Ramesh, K. Preparation and Characterization of Molten Salt Based Nanothermic Fluids with Enhanced Thermal Properties for Solar Thermal Applications. Appl. Therm. Eng. 2016, 109, 901–905. [Google Scholar] [CrossRef]
- Pramod, K.M.; Rao, P.V.C.; Choudary, N.V.; Ramesh, K. Novel Methodology to Prepare Homogenous Ternary Molten Salts for Concentrated Solar Power Applications and Their Thermo-Physical Characterization. Appl. Therm. Eng. 2016, 109, 906–910. [Google Scholar] [CrossRef]
- Kim, H.; Jo, B. Anomalous Increase in Specific Heat of Binary Molten Salt-Based Graphite Nanofluids for Thermal Energy Storage. Appl. Sci. 2018, 8, 1305. [Google Scholar] [CrossRef]
- An, X.-H.; Cheng, J.-H.; Su, T.; Zhang, P. Determination of Thermal Physical Properties of Alkali Fluoride/Carbonate Eutectic Molten Salt. AIP Conf. Proc. 2017, 1850, 070001. [Google Scholar]
- Fereres, S.; Prieto, C.; Giménez-Gavarrell, P.; Rodríguez, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Molten Carbonate Salts for Advanced Solar Thermal Energy Power Plants: Cover Gas Effect on Fluid Thermal Stability. Sol. Energy Mater. Sol. Cells 2018, 188, 119–126. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Peng, Q.; Ding, J.; Yang, J. Quaternary Chloride Eutectic Mixture for Thermal Energy Storage at High Temperature. Energy Procedia 2015, 75, 417–422. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Molten Chloride Salts for next Generation CSP Plants: Selection of Promising Chloride Salts & Study on Corrosion of Alloys in Molten Chloride Salts. AIP Conf. Proc. 2019, 2126, 200014. [Google Scholar]
- Li, X.; Li, N.; Liu, W.; Tang, Z.; Wang, J. Unrevealing the Thermophysical Properties and Microstructural Evolution of MgCl2–NaCl–KCl Eutectic: FPMD Simulations and Experimental Measurements. Sol. Energy Mater. Sol. Cells 2020, 210, 110504. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; Iparraguirre-Torres, I.; García-Romero, A.; Sala-Lizarraga, J.M. Molten Salt-Based Nanofluids as Efficient Heat Transfer and Storage Materials at High Temperatures. An Overview of the Literature. Renew. Sustain. Energy Rev. 2018, 82, 3924–3945. [Google Scholar] [CrossRef]
- Aljaerani, H.A.; Samykano, M.; Pandey, A.K.; Kadirgama, K.; Saidur, R. Thermo-Physical Properties and Corrosivity Improvement of Molten Salts by Use of Nanoparticles for Concentrated Solar Power Applications: A Critical Review. J. Mol. Liq. 2020, 314, 113807. [Google Scholar] [CrossRef]
- Kruizenga, A.; Gill, D. Corrosion of Iron Stainless Steels in Molten Nitrate Salt. Energy Procedia 2014, 49, 878–887. [Google Scholar] [CrossRef]
- Fernández, A.G.; Rey, A.; Lasanta, I.; Mato, S.; Brady, M.P.; Pérez, F.J. Corrosion of Alumina-Forming Austenitic Steel in Molten Nitrate Salts by Gravimetric Analysis and Impedance Spectroscopy: Corrosion in Molten Nitrate Salts. Mater. Corros. 2014, 65, 267–275. [Google Scholar] [CrossRef]
- Pumps for Concentrated Solar Power Plants. Available online: https://www.ruhrpumpen.com/en/markets/power-generation/concentrated-solar-power-plants (accessed on 29 January 2022).
- VEY and VNY Molten Salt Pump for Concentrated Solar Power. Available online: https://www.sulzer.com/en/shared/products/vey-molten-salt-for-concentrated-solar-power (accessed on 29 January 2022).
- Gomes, A.; Navas, M.; Uranga, N.; Paiva, T.; Figueira, I.; Diamantino, T.C. High-Temperature Corrosion Performance of Austenitic Stainless Steels Type AISI 316L and AISI 321H, in Molten Solar Salt. Sol. Energy 2019, 177, 408–419. [Google Scholar] [CrossRef]
- Goods, S.H.; Bradshaw, R.W. Corrosion of Stainless Steels and Carbon Steel by Molten Mixtures of Commercial Nitrate Salts. J. Mater. Eng. Perform. 2004, 13, 78–87. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Wu, Y.; Lu, Y. Experimental Research of High Temperature Dynamic Corrosion Characteristic of Stainless Steels in Nitrate Eutectic Molten Salt. Sol. Energy 2020, 209, 618–627. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, D.; Chen, Y.; Wu, Z. Corrosion of Stainless Steel 316 in a Ternary Nitrate Salt and Its Composite with Expanded Graphite for Solar Thermal Applications. Sol. Energy Mater. Sol. Cells 2021, 219, 110823. [Google Scholar] [CrossRef]
- Xie, W.; Ding, J.; Wei, X.; Wang, W.; Xia, G.; Xing, J. Corrosion Resistance of Stainless Steel and Pure Metal in Ternary Molten Nitrate for Thermal Energy Storage. Energy Procedia 2019, 158, 4897–4902. [Google Scholar] [CrossRef]
- Baraka, A.; Abdel-Rohman, A.I.; Hosary, A.A.E. Corrosion of Mild Steel in Molten Sodium Nitrate–Potassium Nitrate Eutectic. Br. Corros. J. 1976, 11, 44–46. [Google Scholar] [CrossRef]
- García-Martín, G.; Lasanta, M.I.; Encinas-Sánchez, V.; de Miguel, M.T.; Pérez, F.J. Evaluation of Corrosion Resistance of A516 Steel in a Molten Nitrate Salt Mixture Using a Pilot Plant Facility for Application in CSP Plants. Sol. Energy Mater. Sol. Cells 2017, 161, 226–231. [Google Scholar] [CrossRef]
- Ruiz-Cabañas, F.J.; Prieto, C.; Osuna, R.; Madina, V.; Fernández, A.I.; Cabeza, L.F. Corrosion Testing Device for In-Situ Corrosion Characterization in Operational Molten Salts Storage Tanks: A516 Gr70 Carbon Steel Performance under Molten Salts Exposure. Sol. Energy Mater. Sol. Cells 2016, 157, 383–392. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Durham, R.N.; Galetz, M.C. High Temperature Corrosion in Molten Solar Salt: The Role of Chloride Impurities. Mater. Corros. 2017, 68, 943–951. [Google Scholar] [CrossRef]
- Fernández, A.G.; Galleguillos, H.; Pérez, F.J. Thermal Influence in Corrosion Properties of Chilean Solar Nitrates. Sol. Energy 2014, 109, 125–134. [Google Scholar] [CrossRef]
- Fernández, A.G.; Cortes, M.; Fuentealba, E.; Pérez, F.J. Corrosion Properties of a Ternary Nitrate/Nitrite Molten Salt in Concentrated Solar Technology. Renew. Energy 2015, 80, 177–183. [Google Scholar] [CrossRef]
- Fernández, A.G.; Muñoz-Sánchez, B.; Nieto-Maestre, J.; García-Romero, A. High Temperature Corrosion Behavior on Molten Nitrate Salt-Based Nanofluids for CSP Plants. Renew. Energy 2019, 130, 902–909. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Grosu, Y.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Faik, A. Development of Molten Nitrate Salt Based Nanofluids for Thermal Energy Storage Application: High Thermal Performance and Long Storage Components Life-Time. AIP Conf. Proc. 2019, 2126, 200025. [Google Scholar]
- Nithiyanantham, U.; Grosu, Y.; Anagnostopoulos, A.; Carbó-Argibay, E.; Bondarchuk, O.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Navarro, M.E.; Ding, Y.; et al. Nanoparticles as a High-Temperature Anticorrosion Additive to Molten Nitrate Salts for Concentrated Solar Power. Sol. Energy Mater. Sol. Cells 2019, 203, 110171. [Google Scholar] [CrossRef]
- Nieto-Maestre, J.; Muñoz-Sánchez, B.; Fernández, A.G.; Faik, A.; Grosu, Y.; García-Romero, A. Compatibility of Container Materials for Concentrated Solar Power with a Solar Salt and Alumina Based Nanofluid: A Study under Dynamic Conditions. Renew. Energy 2020, 146, 384–396. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Grosu, Y.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Faik, A. Corrosion Aspects of Molten Nitrate Salt-Based Nanofluids for Thermal Energy Storage Applications. Sol. Energy 2019, 189, 219–227. [Google Scholar] [CrossRef]
- Gonzalez, M.; Nithiyanantham, U.; Carbó-Argibay, E.; Bondarchuk, O.; Grosu, Y.; Faik, A. Graphitization as Efficient Inhibitor of the Carbon Steel Corrosion by Molten Binary Nitrate Salt for Thermal Energy Storage at Concentrated Solar Power. Sol. Energy Mater. Sol. Cells 2019, 203, 110172. [Google Scholar] [CrossRef]
- Piquot, J.; Nithiyanantham, U.; Grosu, Y.; Faik, A. Spray-Graphitization as a Protection Method against Corrosion by Molten Nitrate Salts and Molten Salts Based Nanofluids for Thermal Energy Storage Applications. Sol. Energy Mater. Sol. Cells 2019, 200, 110024. [Google Scholar] [CrossRef]
- Grosu, Y.; Anagnostopoulos, A.; Navarro, M.E.; Ding, Y.; Faik, A. Inhibiting Hot Corrosion of Molten Li2CO3-Na2CO3-K2CO3 Salt through Graphitization of Construction Materials for Concentrated Solar Power. Sol. Energy Mater. Sol. Cells 2020, 215, 110650. [Google Scholar] [CrossRef]
- Fähsing, D.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion Testing of Diffusion-Coated Steel in Molten Salt for Concentrated Solar Power Tower Systems. Surf. Coat. Technol. 2018, 354, 46–55. [Google Scholar] [CrossRef]
- Meißner, T.M.; Oskay, C.; Bonk, A.; Grégoire, B.; Donchev, A.; Solimani, A.; Galetz, M.C. Improving the Corrosion Resistance of Ferritic-Martensitic Steels at 600 °C in Molten Solar Salt via Diffusion Coatings. Sol. Energy Mater. Sol. Cells 2021, 227, 111105. [Google Scholar] [CrossRef]
- Treewiriyakitja, P.; Joy-A-Ka, S.; Promdirek, P. Study of Corrosion Resistance of Stainless Steel AISI430 Coated by Slurry Aluminizing in Molten Nitrate Salt. Mater. Today Proc. 2018, 5, 9630–9634. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Galetz, M.C. Slurry Aluminizing: A Solution for Molten Nitrate Salt Corrosion in Concentrated Solar Power Plants. Sol. Energy Mater. Sol. Cells 2016, 146, 8–15. [Google Scholar] [CrossRef]
- Kondaiah, P.; Pitchumani, R. Fractal Textured Surfaces for High Temperature Corrosion Mitigation in Molten Salts. Sol. Energy Mater. Sol. Cells 2021, 230, 111281. [Google Scholar] [CrossRef]
- Thirugnanasambantham, K.G.; Ganesh Kumar, A.G. Mechanistic Studies on Degradation in Sliding Wear Behavior of Carburized AISI 8620 Steel at 100 °C under Unlubricated Conditions. Mater. Today Proc. 2018, 5, 6258–6267. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, P.K.; Kumar, S. Thermal and Metallurgical Characteristics of Surface Modification of AISI 8620 Steel Produced by TIG Arcing Process. J. Mater. Process. Technol. 2017, 240, 420–431. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, L.; Zhang, G.; Shi, J.; Wang, M. Rolling Contact Fatigue Properties of SAE 8620 Steel after Case Carburizing. J. Iron Steel Res. Int. 2016, 23, 711–716. [Google Scholar] [CrossRef]
- Asi, O.; Can, A.Ç.; Pineault, J.; Belassel, M. The Effect of High Temperature Gas Carburizing on Bending Fatigue Strength of SAE 8620 Steel. Mater. Des. 2009, 30, 1792–1797. [Google Scholar] [CrossRef]
- Tillmann, W.; Hagen, L.; Stangier, D.; Lopes Dias, N.F.; Görtz, J.; Kensy, M.D. Lapping and Polishing of Additively Manufactured 316L Substrates and Their Effects on the Microstructural Evolution and Adhesion of PVD CrAlN Coatings. Surf. Coat. Technol. 2021, 428, 127905. [Google Scholar] [CrossRef]
- Salhi, F.; Aissani, L.; Fellah, M.; Chadli, A.; Cheriet, A.; Belgroune, A.; Nouveau, C.; Obrosov, A.; Abdul Samad, M.; Alhussein, A. Experimental Investigation of Structural, Wetting, Mechanical and Tribological Properties of TiZrN Thin Films Deposited by Magnetron Sputtering. Surf. Interfaces 2021, 27, 101519. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.; Liu, Z. Bio-Tribocorrosion Behavior of a Nanocrystalline TiZrN Coating on Biomedical Titanium Alloy. Surf. Coat. Technol. 2019, 369, 79–86. [Google Scholar] [CrossRef]
- Donohue, L.A.; Cawley, J.; Brooks, J.S. Deposition and Characterisation of Arc-Bond Sputter TixZryN Coatings from Pure Metallic and Segmented Targets. Surf. Coat. Technol. 1995, 72, 128. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Chen, H.-A.; Yu, G.-P.; Huang, J.-H. Effect of Bias on the Structure and Properties of TiZrN Thin Films Deposited by Unbalanced Magnetron Sputtering. Thin Solid Films 2016, 618, 13–20. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Huang, J.-H.; Yu, G.-P. Effect of Nitrogen Flow Rate on Properties of Nanostructured TiZrN Thin Films Produced by Radio Frequency Magnetron Sputtering. Thin Solid Films 2010, 518, 7308–7311. [Google Scholar] [CrossRef]
- Kang, D.; Kim, Y.S.; Kim, J.N.; Park, I.K. Characteristics of TiN Thin Films Deposited by Substrate Temperature Variables Using Scanning Acoustic Microscopy. Appl. Sci. 2022, 12, 3571. [Google Scholar] [CrossRef]
- Hariharan, R.; Raja, R.; Golden Renjith Nimal, R.J.; Refaai, M.R.A.; Ravi, S.; Allasi, H.L. Characterization of TiZrN and TaZrN Nanocomposite Multilayer Coating Deposited via RF/DC Magnetron Sputtering on AISI4140 Steel. Adv. Mater. Sci. Eng. 2021, 2021, 8273708. [Google Scholar] [CrossRef]
- Ashok, K.; Subramanian, B.; Kuppusami, P.; Jayachandran, M. Effect of Substrate Temperature on Structural and Materials Properties of Zirconium Nitride Films on D9 Steel Substrates. Cryst. Res. Technol. 2009, 44, 511–516. [Google Scholar] [CrossRef]
- Helmersson, U.; Johansson, B.O.; Sundgren, J.E.; Hentzell, H.T.G.; Billgren, P. Adhesion of Titanium Nitride Coatings on Highspeed Steels. J. Vac. Sci. Technol. 1985, 3, 308. [Google Scholar] [CrossRef]
- Xu, X.; Vignarooban, K.; Xu, B.; Hsu, K.; Kannan, A.M. Prospects and Problems of Concentrating Solar Power Technologies for Power Generation in the Desert Regions. Renew. Sustain. Energy Rev. 2016, 53, 1106–1131. [Google Scholar] [CrossRef]
- Ruegamer, T.; Kamp, H.; Kuckelkorn, T.; Schiel, W.; Weinrebe, G.; Nava, P.; Riffelmann, K.; Richert, T. Molten Salt for Parabolic Trough Applications: System Simulation and Scale Effects. Energy Procedia 2014, 49, 1523–1532. [Google Scholar] [CrossRef]
- Giaconia, A.; Iaquaniello, G.; Metwally, A.A.; Caputo, G.; Balog, I. Experimental Demonstration and Analysis of a CSP Plant with Molten Salt Heat Transfer Fluid in Parabolic Troughs. Sol. Energy 2020, 211, 622–632. [Google Scholar] [CrossRef]
- Al-Barqi, A.S.; Bukharin, N.; Zazoum, B.; El Hassan, M. Design of a 100 MW Concentrated Solar Power Linear Fresnel Plant in Riyadh, Saudi Arabia: A Comparison between Molten Salt and Liquid Sodium Thermal Energy Storage. Energy Rep. 2022, 8, 697–704. [Google Scholar] [CrossRef]
- Al-Mamun, N.S.; Haider, W.; Shabib, I. Corrosion Resistance of Additively Manufactured 316L Stainless Steel in Chloride–thiosulfate Environment. Electrochim. Acta 2020, 362, 137039. [Google Scholar] [CrossRef]
- Sabuz, E.H.; Pitts-Reed, D.; Haider, W.; Shabib, I. Electrochemical Performance of Additively Manufactured 8620 Low-Alloy Steel: Effect of Acetic Acid. In Proceedings of the Steel Properties & Applications in Conjunction with Materials Science & Technology 2022, Pittsburgh, PA, USA, 9–12 October 2022; Association for Iron & Steel Technology: Warrendale, PA, USA, 2022; p. 16. [Google Scholar]
- Sabuz, E.H.; Noor-A-Alam, M.; Haider, W.; Shabib, I. Improving the Mechanical and Electrochemical Performance of Additively Manufactured 8620 Low Alloy Steel via Boriding. Corros. Mater. Degrad. 2023, 4, 623–643. [Google Scholar] [CrossRef]
- Binkley, M. Microstructure Development in Multi-Pass Laser Melting of Aisi 8620 Steel; Purdue University: West Lafayette, IN, USA, 2020. [Google Scholar]
- Kazemipour, M.; Mohammadi, M.; Mfoumou, E.; Nasiri, A.M. Microstructure and Corrosion Characteristics of Selective Laser-Melted 316L Stainless Steel: The Impact of Process-Induced Porosities. JOM 2019, 71, 3230–3240. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Bai, Q. Defect Formation Mechanisms in Selective Laser Melting: A Review. Chin. J. Mech. Eng. 2017, 30, 515–527. [Google Scholar] [CrossRef]
- Kempen, K.; Thijs, L.; Van Humbeeck, J.; Kruth, J.-P. Processing AlSi10Mg by Selective Laser Melting: Parameter Optimisation and Material Characterisation. Mater. Sci. Technol. 2015, 31, 917–923. [Google Scholar] [CrossRef]
- Marulanda, D.M.; Cortés, J.G.; Pérez, M.A.; García, G. Microstructure and Mechanical Properties of AISI 8620 Steel Processed by ECAP. MRS Proc. 2014, 1611, 89–94. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Huang, J.-H.; Yu, G.-P. Microstructure and Corrosion Resistance of Nanocrystalline TiZrN Films on AISI 304 Stainless Steel Substrate. J. Vac. Sci. Technol. Vac. Surf. Films 2010, 28, 774–778. [Google Scholar] [CrossRef]
- Valvoda, V.; Kuzĕl, R.; Cĕrný, R.; Dobiásŏvá, L. Detailed X-Ray Diffraction Study of Titanium Nitride Coatings: Some Interpretation Problems. Mater. Sci. Eng. A 1988, 104, 223–234. [Google Scholar] [CrossRef]
- Qi, R.; Pan, L.; Feng, Y.; Wu, J.; Li, W.; Wang, Z. Evolution of Chemical, Structural, and Mechanical Properties of Titanium Nitride Thin Films Deposited under Different Nitrogen Partial Pressure. Results Phys. 2020, 19, 103416. [Google Scholar] [CrossRef]
- Chang, C.-C.; Nogan, J.; Yang, Z.-P.; Kort-Kamp, W.J.M.; Ross, W.; Luk, T.S.; Dalvit, D.A.R.; Azad, A.K.; Chen, H.-T. Highly Plasmonic Titanium Nitride by Room-Temperature Sputtering. Sci. Rep. 2019, 9, 15287. [Google Scholar] [CrossRef]
- Lee, J.-K.; Yang, G.-S. Preparation of TiAlN/ZrN and TiCrN/ZrN Multilayers by RF Magnetron Sputtering. Trans. Nonferrous Met. Soc. China 2009, 19, 795–799. [Google Scholar] [CrossRef]
- Chinsakolthanakorn, S.; Buranawong, A.; Witit-anun, N.; Chaiyakun, S.; Limsuwan, P. Characterization of Nanostructured TiZrN Thin Films Deposited by Reactive DC Magnetron Co-Sputtering. Procedia Eng. 2012, 32, 571–576. [Google Scholar] [CrossRef]
- Phae-ngam, W.; Horprathum, M.; Chananonnawathorn, C.; Lertvanithphol, T.; Samransuksamer, B.; Songsiriritthigul, P.; Nakajima, H.; Chaiyakun, S. Oblique Angle Deposition of Nanocolumnar TiZrN Films via Reactive Magnetron Co-Sputtering Technique: The Influence of the Zr Target Powers. Curr. Appl. Phys. 2019, 19, 894–901. [Google Scholar] [CrossRef]
- Ajenifuja, E.; Popoola, A.P.I.; Popoola, O.M. Thickness Dependent Chemical and Microstructural Properties of DC Reactive Magnetron Sputtered Titanium Nitride Thin Films on Low Carbon Steel Cross-Section. J. Mater. Res. Technol. 2019, 8, 377–384. [Google Scholar] [CrossRef]
- Huang, J.-H.; Kuo, K.-L.; Yu, G.-P. Oxidation Behavior and Corrosion Resistance of Vacuum Annealed ZrN-Coated Stainless Steel. Surf. Coat. Technol. 2019, 358, 308–319. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.-H.; Navinšek, B. Comparison of TiN, ZrN and CrN Hard Nitride Coatings: Electrochemical and Thermal Oxidation. Thin Solid Films 1997, 303, 246–254. [Google Scholar] [CrossRef]
- Bertrand, N.; Desgranges, C.; Poquillon, D.; Lafont, M.C.; Monceau, D. Iron Oxidation at Low Temperature (260–500 °C) in Air and the Effect of Water Vapor. Oxid. Met. 2010, 73, 139–162. [Google Scholar] [CrossRef]
- Bhuvaneswari, H.B.; Rajagopal Reddy, V.; Chandramani, R.; Mohan Rao, G. Annealing Effects on Zirconium Nitride Films. Appl. Surf. Sci. 2004, 230, 88–93. [Google Scholar] [CrossRef]
- Taweesup, K.; Visuttipitukul, P.; Yongvanich, N.; Lothongkum, G. Corrosion Behavior of Ti-Cr-N Coatings on Tool Steel Substrates Prepared Using DC Magnetron Sputtering at Low Growth Temperatures. Surf. Coat. Technol. 2019, 358, 732–740. [Google Scholar] [CrossRef]
- Larose, S.; Rapp, R.A. Review of Low-Temperature Oxidation of Carbon Steels and Low-Alloy Steels for Use as High-Level Radioactive Waste Package Materials; Center for Nuclear Waste Regulatory Analyses: San Antonio, TX, USA, 1997. [Google Scholar]
- Fernández, Á.G.; Cabeza, L.F. Molten Salt Corrosion Mechanisms of Nitrate Based Thermal Energy Storage Materials for Concentrated Solar Power Plants: A Review. Sol. Energy Mater. Sol. Cells 2019, 194, 160–165. [Google Scholar] [CrossRef]
- Massiani, Y.; Medjahed, A.; Gravier, P.; Argème, L.; Fedrizzi, L. Electrochemical Study of Titanium Nitride Films Obtained by Reactive Sputtering. Thin Solid Films 1990, 191, 305–316. [Google Scholar] [CrossRef]
- Brown, R.; Alias, M.N.; Fontana, R. Effect of Composition and Thickness on Corrosion Behavior of TiN and ZrN Thin Films. Surf. Coat. Technol. 1993, 62, 467–473. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.F.; Schultz, L. Effect of Surface Finishing of a Zr-Based Bulk Metallic Glass on Its Corrosion Behaviour. Corros. Sci. 2010, 52, 1711–1720. [Google Scholar] [CrossRef]
- Khan, M.M.; Shabib, I.; Haider, W. A Combinatorially Developed Zr-Ti-Fe-Al Metallic Glass with Outstanding Corrosion Resistance for Implantable Medical Devices. Scr. Mater. 2019, 162, 223–229. [Google Scholar] [CrossRef]
- Khan, M.M.; Deen, K.M.; Haider, W. Combinatorial Development and Assessment of a Zr-Based Metallic Glass for Prospective Biomedical Applications. J. Non-Cryst. Solids 2019, 523, 119544. [Google Scholar] [CrossRef]
- Jabed, A.; Rahman, Z.U.; Khan, M.M.; Haider, W.; Shabib, I. Combinatorial Development and In Vitro Characterization of the Quaternary Zr–Ti–X–Y (X–Y = Cu–Ag/Co–Ni) Metallic Glass for Prospective Bioimplants. Adv. Eng. Mater. 2019, 21, 1900726. [Google Scholar] [CrossRef]
- Jabed, A.; Khan, M.M.; Camiller, J.; Greenlee-Wacker, M.; Haider, W.; Shabib, I. Property Optimization of Zr-Ti-X (X = Ag, Al) Metallic Glass via Combinatorial Development Aimed at Prospective Biomedical Application. Surf. Coat. Technol. 2019, 372, 278–287. [Google Scholar] [CrossRef]
- Igual Muñoz, A.; García Antón, J.; Guiñón, J.L.; Pérez Herranz, V. The Effect of Chromate in the Corrosion Behavior of Duplex Stainless Steel in LiBr Solutions. Corros. Sci. 2006, 48, 4127–4151. [Google Scholar] [CrossRef]
- Maruf, M.A.; Rizvi, S.M.M.; Noor-A-Alam, M.; Shin, D.; Haider, W.; Shabib, I. Corrosion Resistance and Thermal Stability of Sputtered Fe44Al34Ti7N15 and Al61Ti11N28 Thin Films for Prospective Application in Oil and Gas Industry. Prog. Nat. Sci. Mater. Int. 2021, 31, 688–697. [Google Scholar] [CrossRef]
- Ćurković, L.; Ćurković, H.O.; Salopek, S.; Renjo, M.M.; Šegota, S. Enhancement of Corrosion Protection of AISI 304 Stainless Steel by Nanostructured Sol–Gel TiO2 Films. Corros. Sci. 2013, 77, 176–184. [Google Scholar] [CrossRef]
- Pineda, F.; Mallco, A.; De Barbieri, F.; Carrasco, C.; Henriquez, M.; Fuentealba, E.; Fernández, Á.G. Corrosion Evaluation by Electrochemical Real-Time Tracking of VM12 Martensitic Steel in a Ternary Molten Salt Mixture with Lithium Nitrates for CSP Plants. Sol. Energy Mater. Sol. Cells 2021, 231, 111302. [Google Scholar] [CrossRef]
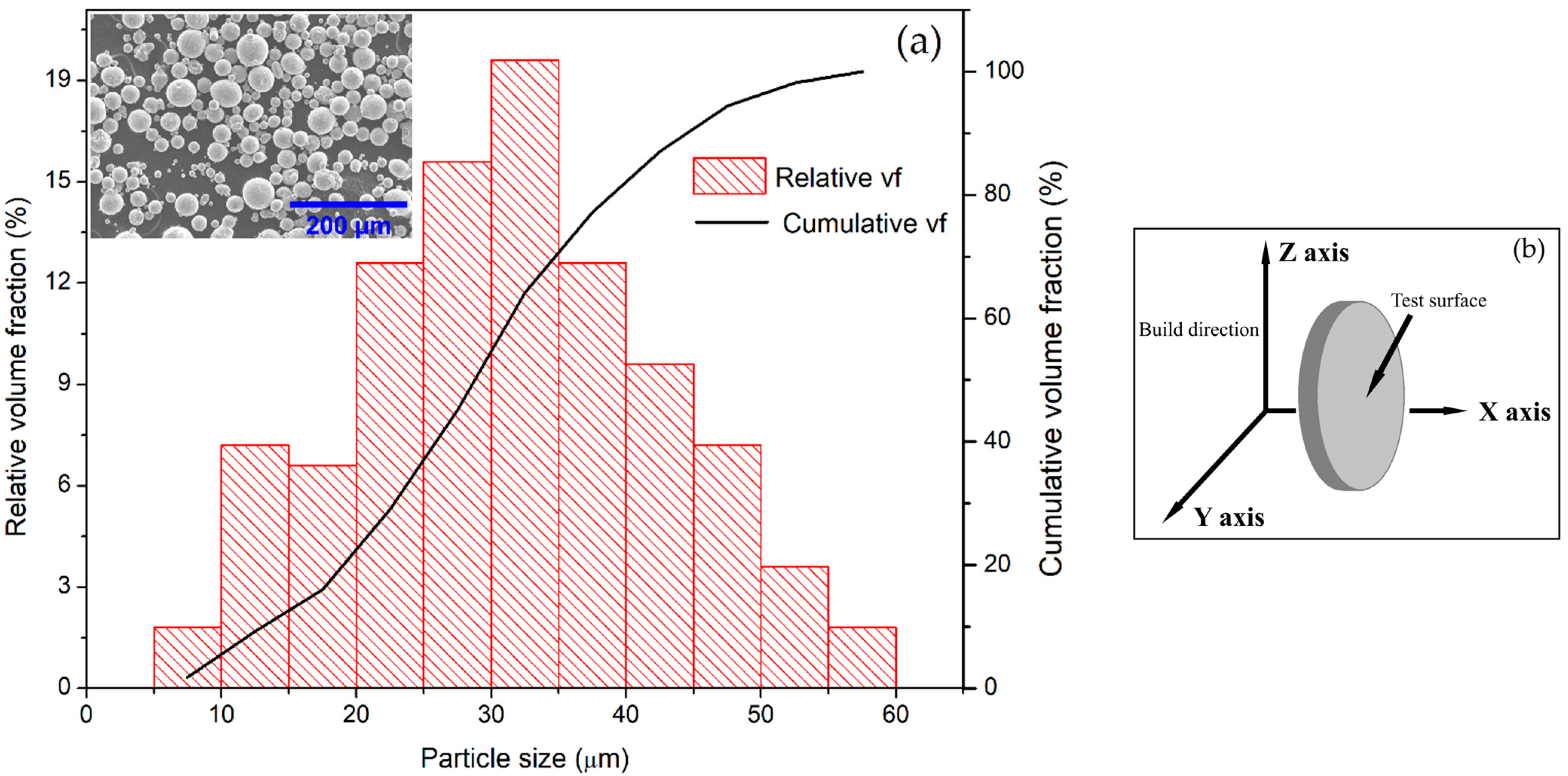
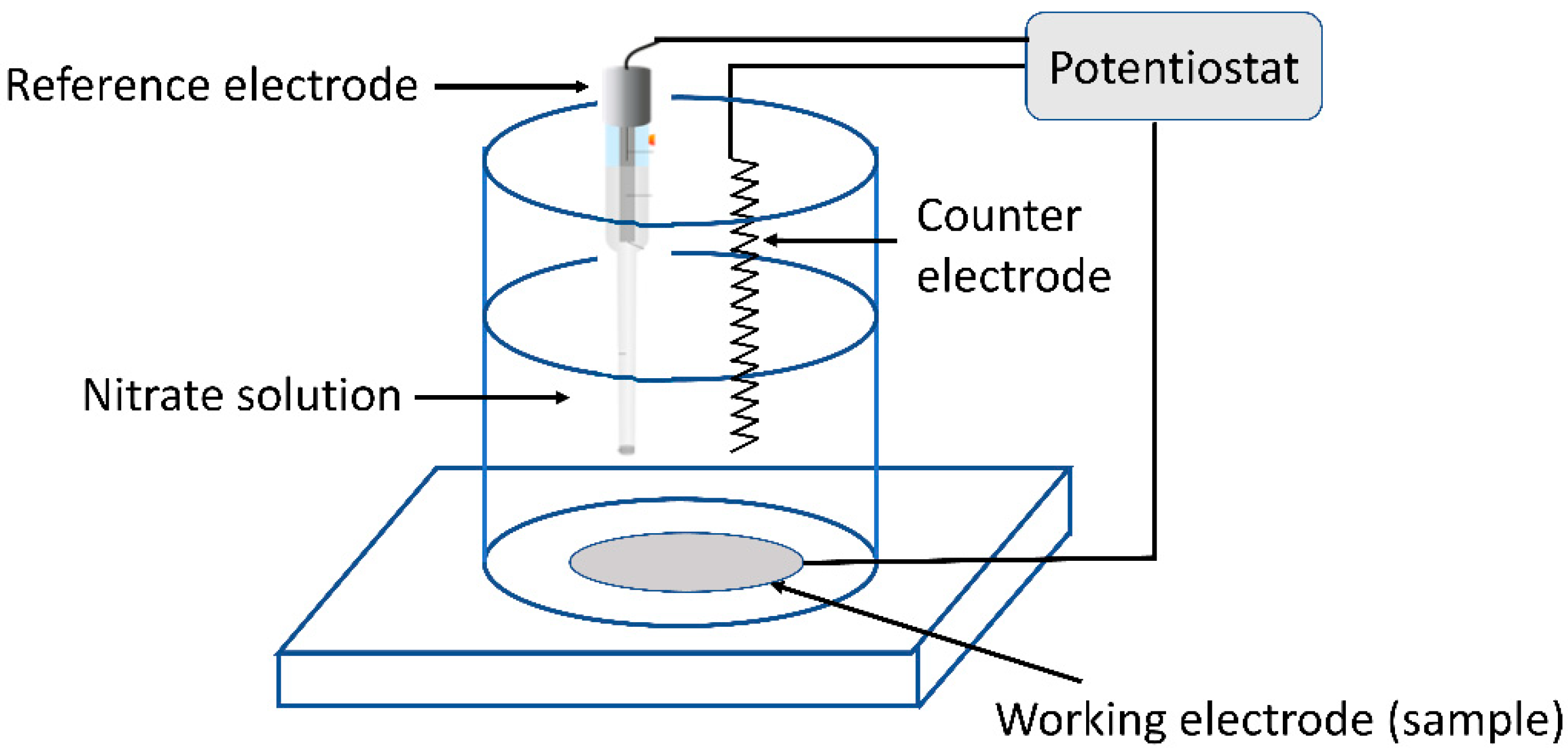
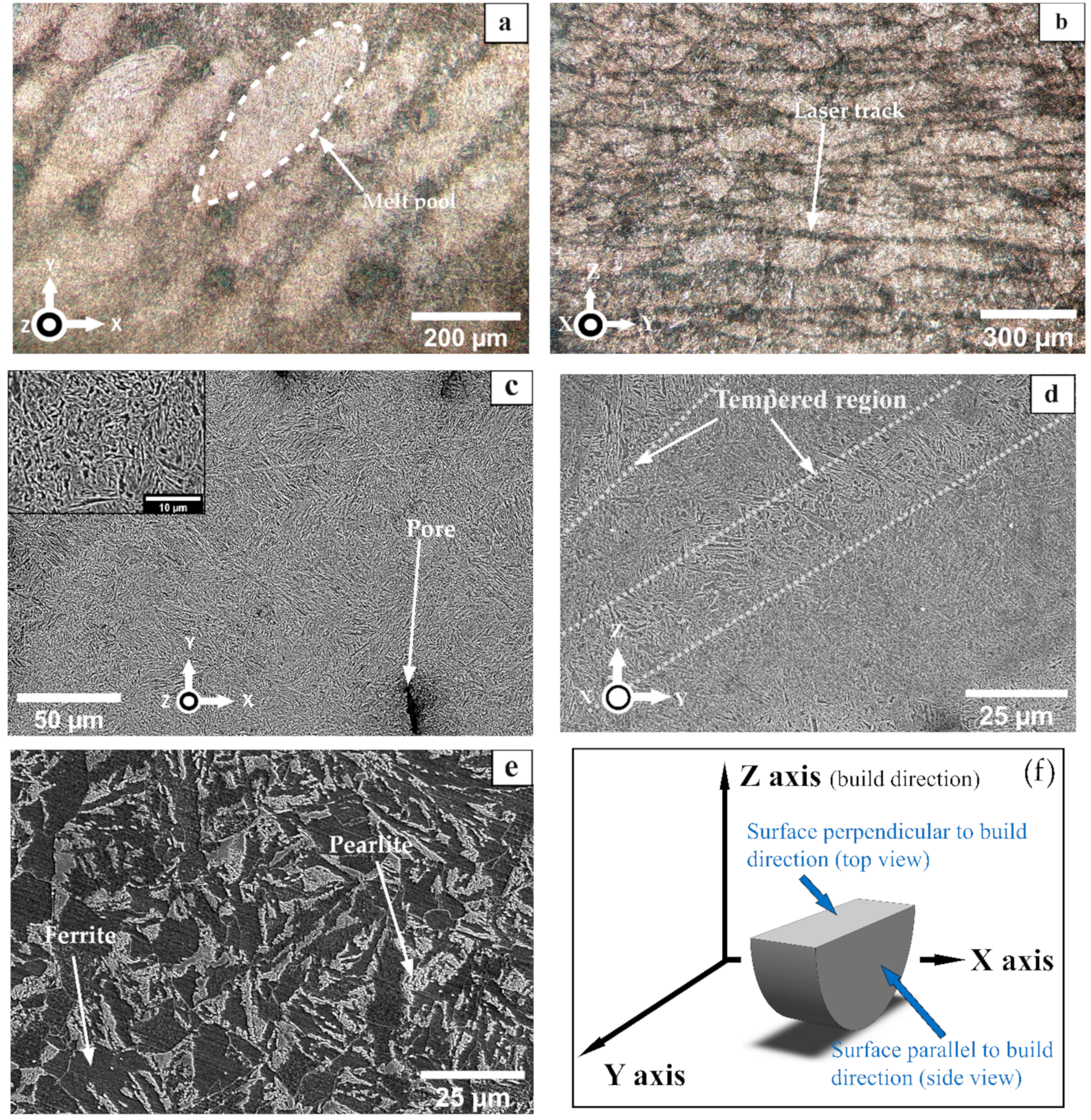
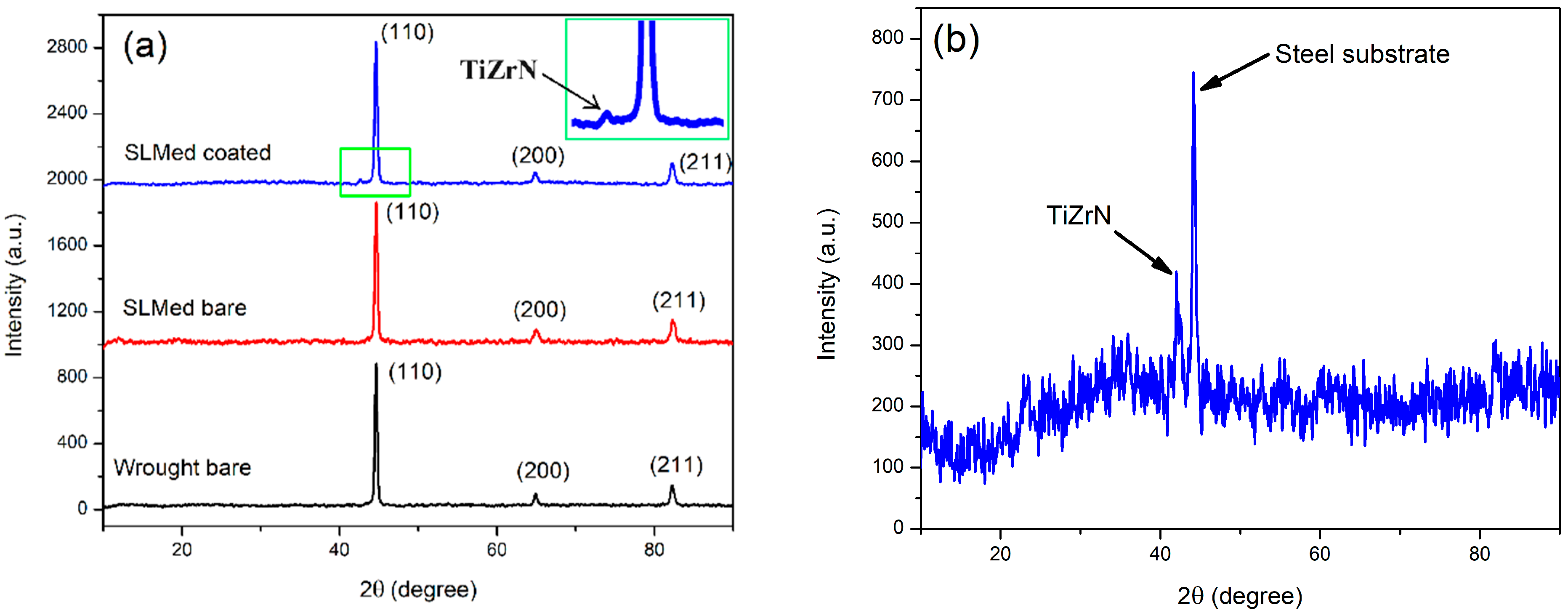



| Element | Cr | Ni | Mn | Mo | Si | C | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Powder | 0.55 | 0.56 | 0.71 | 0.2 | 0.29 | 0.19 | 0.005 | 0.015 | Bal. |
| Wrought | 0.4 | 0.4 | 0.7 | 0.15 | 0.15 | 0.18 | 0.04 | 0.035 | Bal. |
| Element | Ti | Zr | O | N |
|---|---|---|---|---|
| Before heat treatment | 22.7 ± 0.9 | 6.9 ± 0.2 | - | Bal. |
| After heat treatment | 26.6 ± 0.3 | 7.4 ± 0.2 | 31.4 ± 0.8 | Bal. |
| Sample | Ecorr (mV) | Icorr (µA/cm2) | βA (mV/Decade) | βC (mV/Decade) |
|---|---|---|---|---|
| Coated wrought | −276.0 ± 17.1 | 0.59 ± 0.06 | 121.2 ± 35.9 | 176.1 ± 23.6 |
| Coated SLMed | −442.3 ± 13.6 | 0.55 ± 0.07 | 144.3 ± 57.1 | 261.0 ± 96.2 |
| Bare wrought | −665.7 ± 38.1 | 4.69 ± 0.95 | 51.5 ± 12.2 | 206.3 ± 26.9 |
| Bare SLMed | −709.7 ± 46.3 | 4.80 ± 0.32 | 54.0 ± 5.7 | 186.6 ± 14.8 |
| Samples | Rs (Ω·cm2) | Rct (kΩ·cm2) | Qdl μS-sn cm−2 | Rf (Ω·cm2) | Qf μS-sn cm−2 | Rt (kΩ·cm2) | Goodness of Fit (10−3) |
|---|---|---|---|---|---|---|---|
| Coated wrought | 65.9 ± 6.7 | 8.3 ± 3.2 | 64.5 ± 35.4 | 1098 ± 451 | 100.4 ± 39.0 | 9.4 ± 2.8 | 4.2 ± 1.9 |
| Coated SLMed | 61.0 ± 2.4 | 9.8 ± 1.1 | 57.3 ± 24.3 | 3058 ± 923 | 96.9 ± 31.7 | 12.9 ± 1.9 | 2.7 ± 0.7 |
| Bare wrought | 15.7 ± 5.0 | 1.1 ± 0.1 | 853 ± 149 | 46.7 ± 5.8 | 484 ± 113 | 1.2 ± 0.1 | 6.7 ± 0.8 |
| Bare SLMed | 7.2 ± 0.1 | 1.3 ± 0.2 | 1180 ± 170 | 17.5 ± 3.5 | 844 ± 192 | 1.3 ± 0.2 | 7.7 ± 2.0 |
| Element | Coated SLMed (wt%) | Coated Wrought (wt%) | Bare SLMed (wt%) | Bare Wrought (wt%) |
|---|---|---|---|---|
| O | 21.8 ± 0.3 | 20.2 ± 0.9 | 33.7 ± 2.0 | 22.3 ± 1.3 |
| Fe | 46.7 ± 1.8 | 49.0 ± 1.9 | 63.8 ± 2.1 | 76.8 ± 1.5 |
| N | 3.4 ± 0.5 | 2.8 ± 0.3 | 2.5 ± 0.2 | 0.2 ± 0.1 |
| Ti | 13.1 ± 0.7 | 14.5 ± 0.4 | - | - |
| Zr | 7.1 ± 0.9 | 7.5 ± 0.3 | - | - |
| Si | 4.7 ± 0.4 | 3.9 ± 0.1 | - | - |
| Na | 3.2 ± 0.3 | 2.2 ± 0.3 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabuz, E.H.; Maruf, M.A.; Haider, W.; Shabib, I. Enhanced Corrosion Resistance of TiZrN-Coated Additively Manufactured 8620 Low-Alloy Steel in Nitrate Salt Solution and Salt Bath. Coatings 2023, 13, 1998. https://doi.org/10.3390/coatings13121998
Sabuz EH, Maruf MA, Haider W, Shabib I. Enhanced Corrosion Resistance of TiZrN-Coated Additively Manufactured 8620 Low-Alloy Steel in Nitrate Salt Solution and Salt Bath. Coatings. 2023; 13(12):1998. https://doi.org/10.3390/coatings13121998
Chicago/Turabian StyleSabuz, Ezazul Haque, Mahbub Alam Maruf, Waseem Haider, and Ishraq Shabib. 2023. "Enhanced Corrosion Resistance of TiZrN-Coated Additively Manufactured 8620 Low-Alloy Steel in Nitrate Salt Solution and Salt Bath" Coatings 13, no. 12: 1998. https://doi.org/10.3390/coatings13121998
APA StyleSabuz, E. H., Maruf, M. A., Haider, W., & Shabib, I. (2023). Enhanced Corrosion Resistance of TiZrN-Coated Additively Manufactured 8620 Low-Alloy Steel in Nitrate Salt Solution and Salt Bath. Coatings, 13(12), 1998. https://doi.org/10.3390/coatings13121998








