Modification of Electrochemical Exfoliation of Graphene Oxide with Dopamine and Tannic to Enhance Anticorrosion Performance of Epoxy Coatings
Abstract
:1. Introduction
2. Materials and Experiments
3. Results and Discussions
3.1. Morphologies and Dispersibility
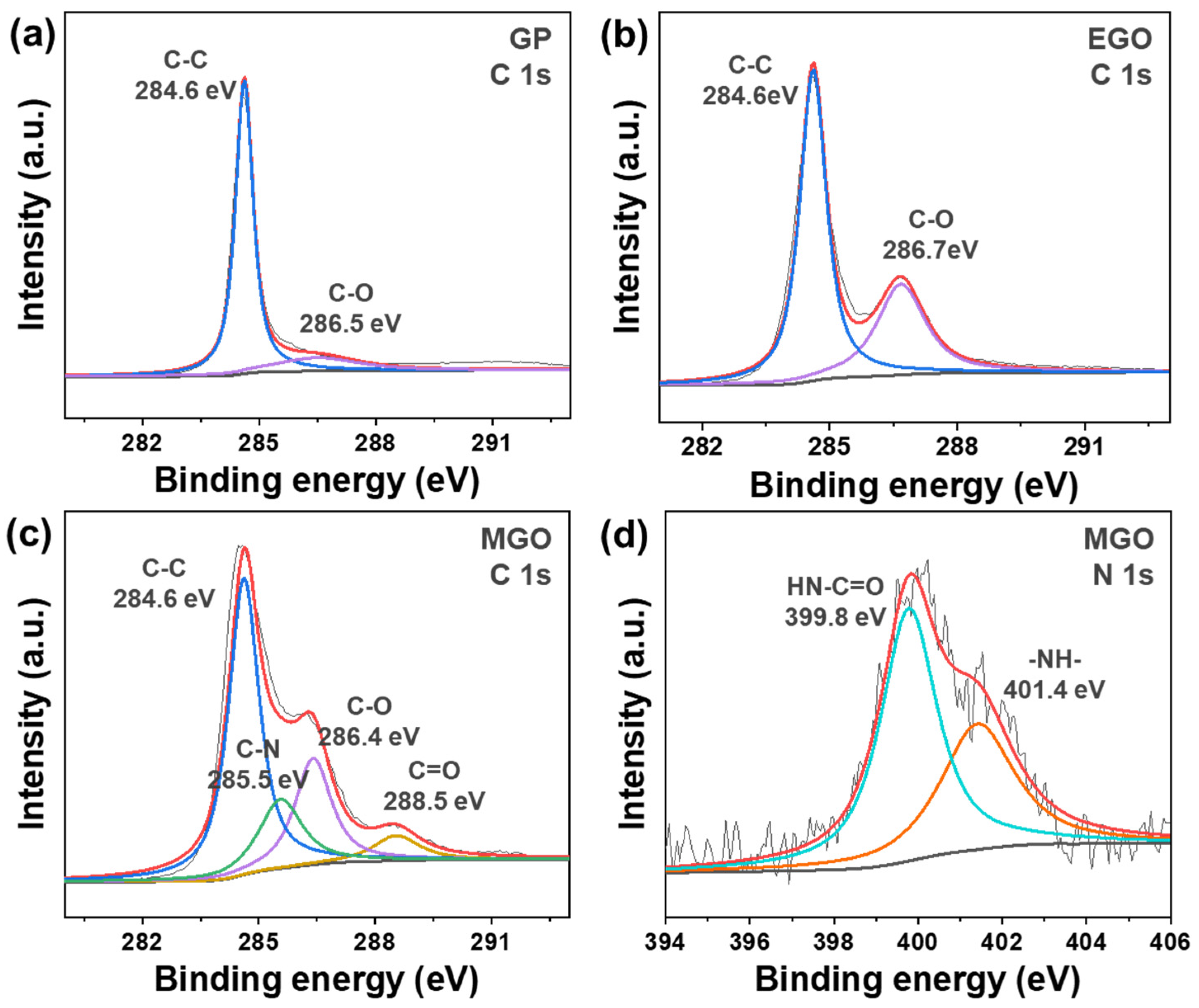
3.2. Structure and Composition
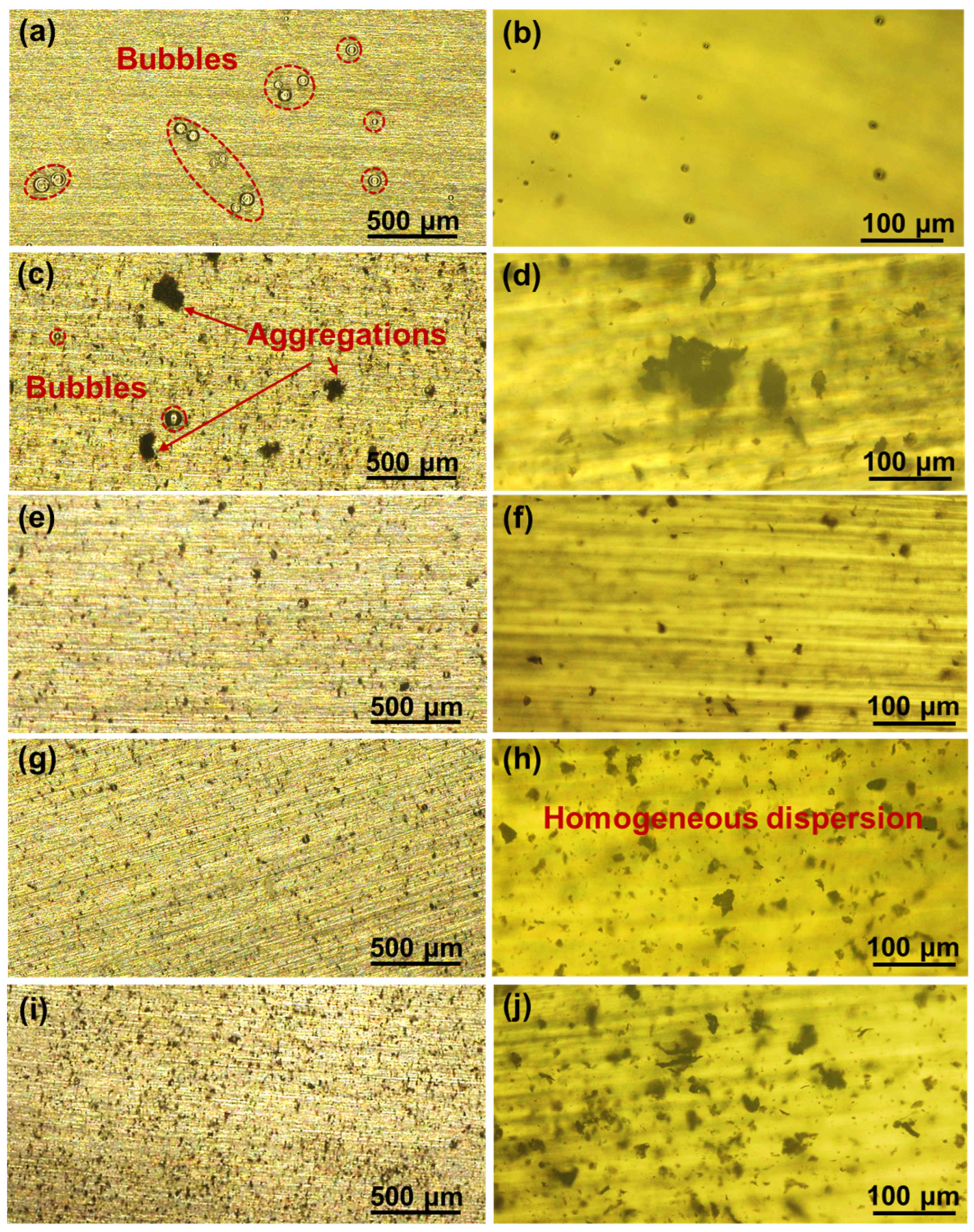
3.3. Surface Morphologies of Coatings
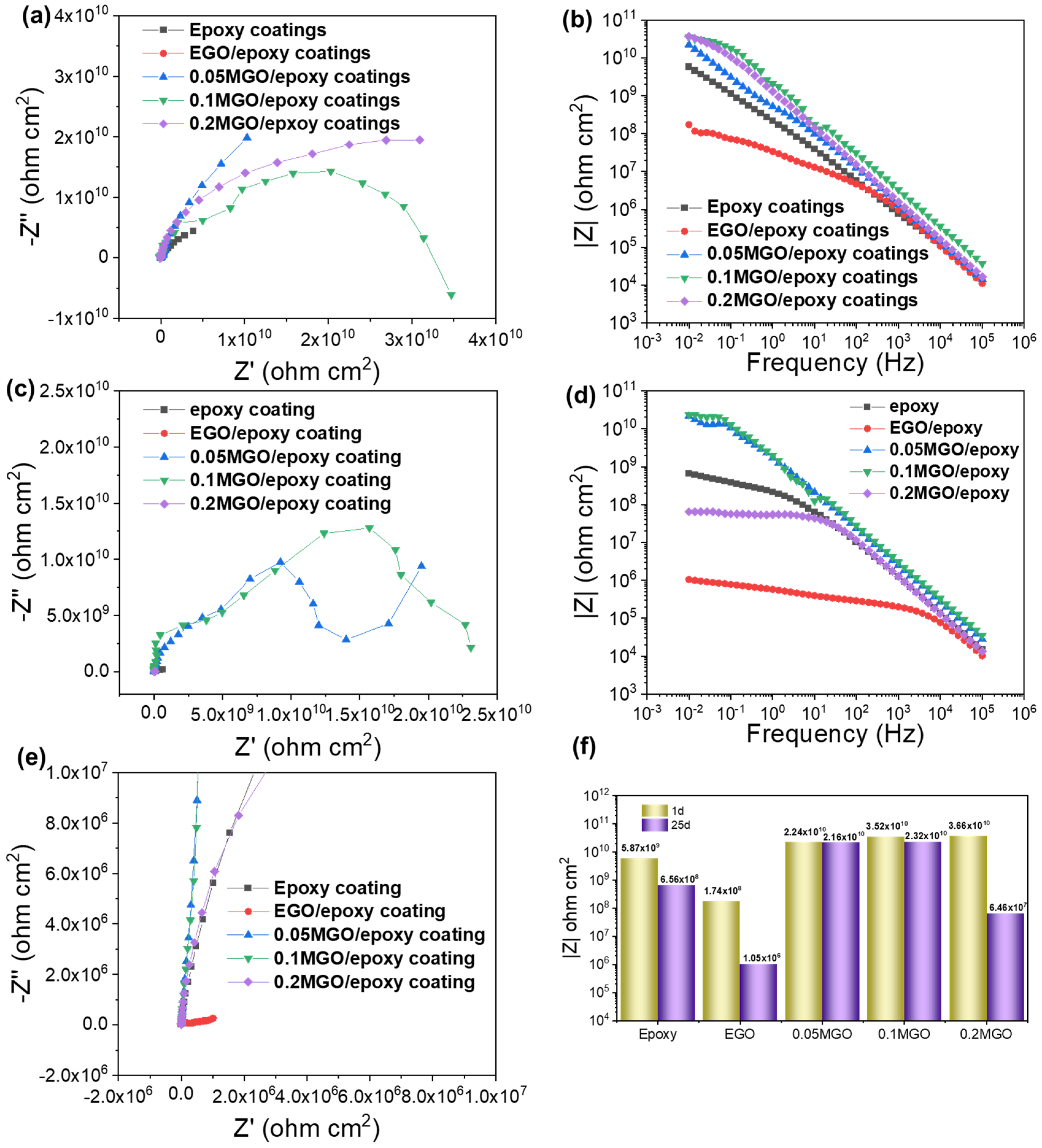
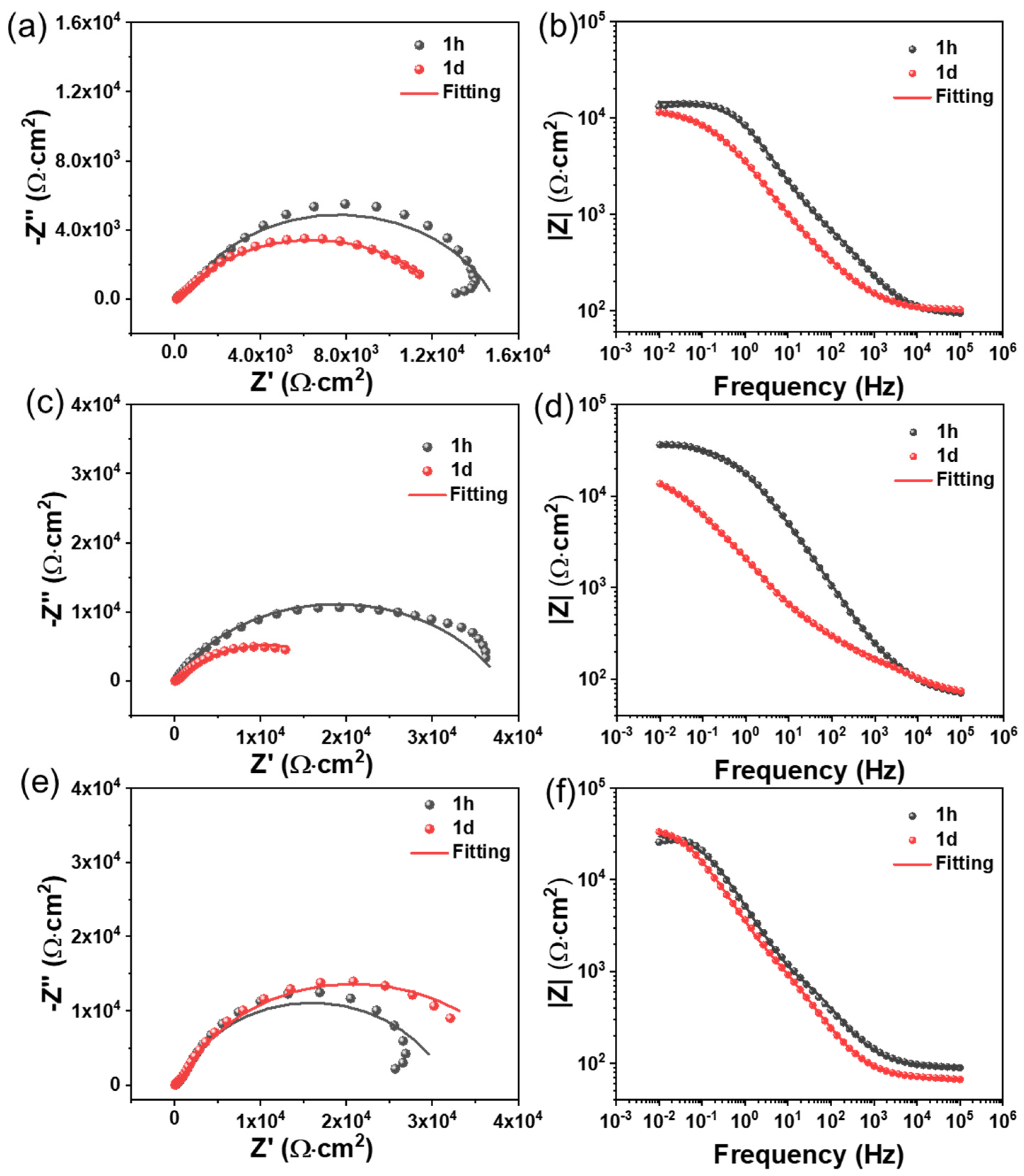
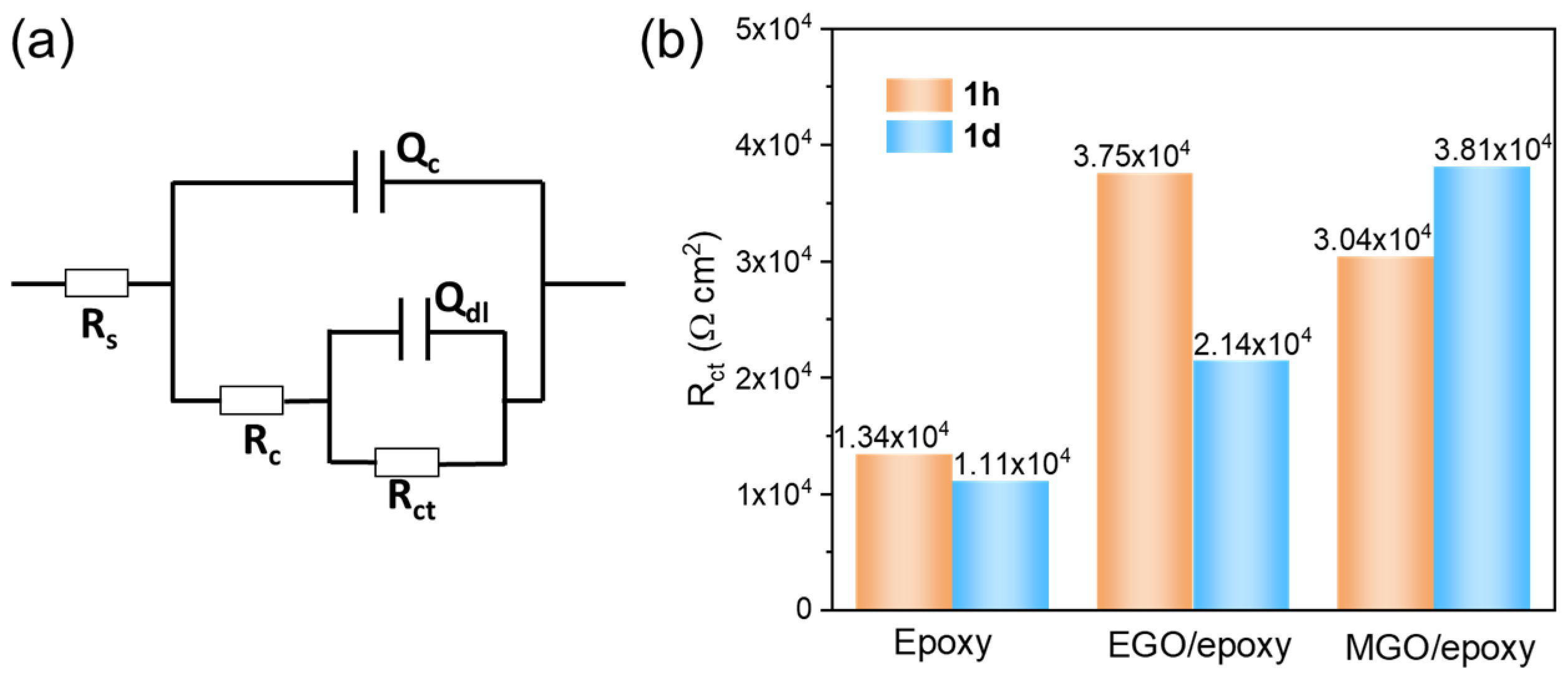
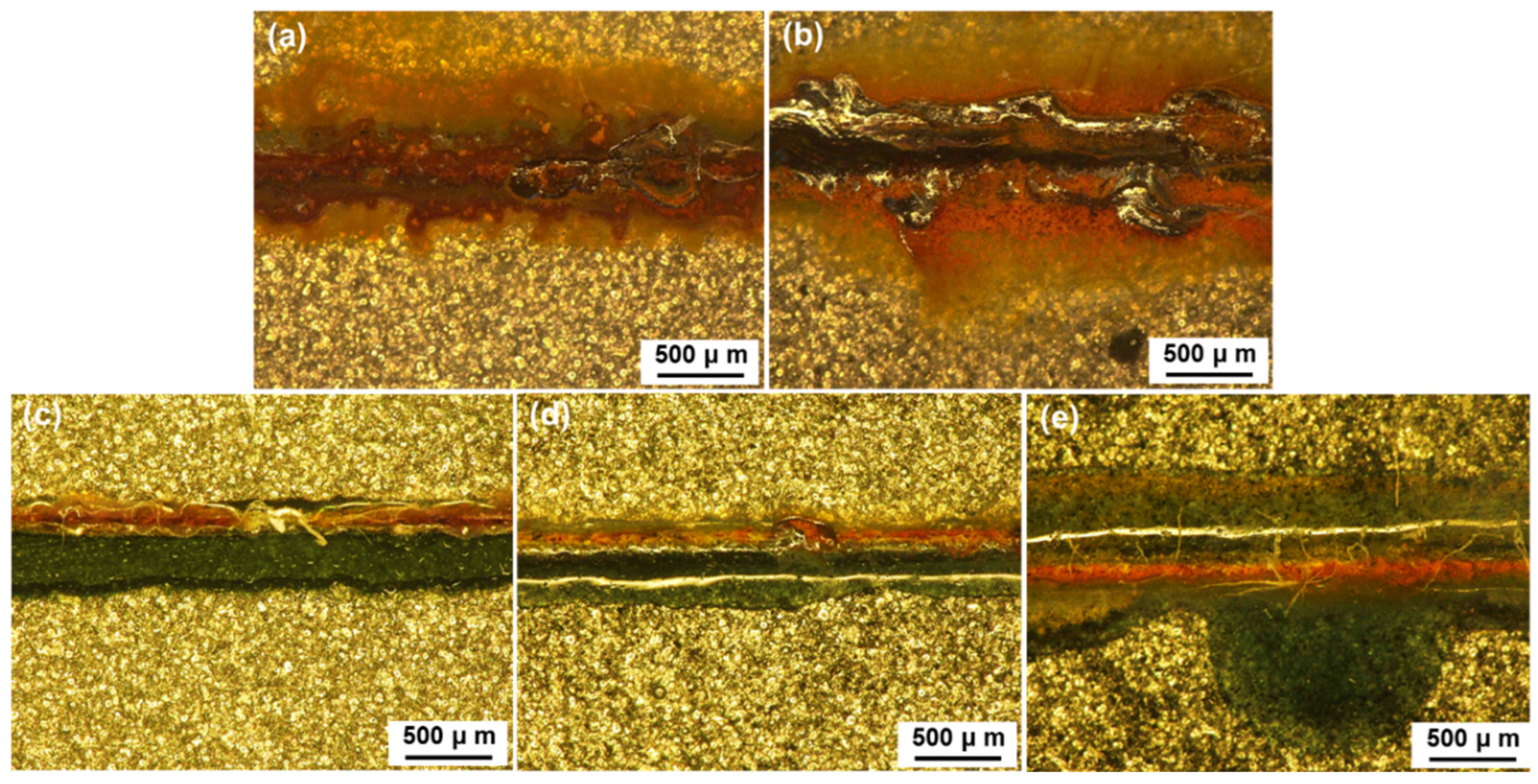
3.4. Anticorrosion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahali, E.; Alves, M.M.; El-Bassi, L.; Bousselmi, L.; de Fátima Montemor, M.; Akrout, H. Enhanced protection of hybrid polyetherimide-ZnO or CuO bilayer composite coatings against mild steel corrosion in chloride media. Prog. Org. Coat. 2022, 163, 106602. [Google Scholar] [CrossRef]
- Wang, Y.; You, Z.; Ma, K.; Dai, C.; Wang, D.; Wang, J. Corrosion resistance of a superhydrophobic calcium carbonate coating on magnesium alloy by ultrasonic cavitation-assisted chemical conversion. Corros. Sci. 2023, 211, 110841. [Google Scholar] [CrossRef]
- Cui, H.; Tang, F.J.; Li, B.; Lin, Z.B. Microstructure and corrosion resistance of quartz sand-modified enamel-coated steel plates. Coatings 2023, 13, 1704. [Google Scholar] [CrossRef]
- Zhang, S.D.; Xu, Y.H.; Liu, L.K.; Lei, Q.D.; Dong, J.L.; Zhang, T. Preparation of Conductive and Corrosion Resistant Phosphate Conversion Coating on AZ91D Magnesium Alloy. Coatings 2023, 13, 1706. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.-D.; Liu, J.; Wang, X.; Gui, T.-J.; Li, B.-J.; Tang, Y.-Z.; et al. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, Y.K.; Chen, C.; Tian, Y.K.; Wang, Y.L.; Cao, S.Y.; Ma, J. Corrosion-resistant SiO2-graphene oxide/epoxy coating reinforced by effective electron beam curing. Prog. Org. Coat. 2023, 184, 107855. [Google Scholar] [CrossRef]
- Li, H.; Xue, C.; Gao, L.; Wang, X.; Wei, H.; Nan, H.; Wang, G.; Lin, H. “Labyrinthine structure” anticorrosive water-based composite coatings. Prog. Org. Coat. 2021, 150, 105974. [Google Scholar] [CrossRef]
- Zhou, X.N.; Huang, H.W.; Zhu, R.; Chen, R.J.; Sheng, X.X.; Xie, D.L.; Mei, Y. Green modification of graphene oxide with phytic acid and its application in anticorrosive water-borne epoxy coatings. Prog. Org. Coat. 2020, 143, 105601. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Lin, Y.; Wu, Y.; Chen, J. Self-assembled graphene oxide-graphene hybrids for enhancing the corrosion resistance of waterborne epoxy coating. Appl. Surf. Sci. 2019, 488, 801–812. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yin, Q.; Xiang, X.; Fu, X.-Z.; Wang, X.-Z.; Luo, J.-L. A facile approach to fabricating graphene/waterborne epoxy coatings with dual functionalities of barrier and corrosion inhibitor. J. Mater. Sci. Technol. 2022, 112, 263–276. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Muellen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Muellen, K. Electrochemically Exfoliated Graphene as Solution-Processable, Highly Conductive Electrodes for Organic Electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Komba, N.; Wei, Q.; Zhang, G.; Rosei, F.; Sun, S. Controlled synthesis of graphene via electrochemical route and its use as efficient metal-free catalyst for oxygen reduction. Appl. Catal. B-Environ. 2019, 243, 373–380. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Wahby, M.H.; Abdallah, M.M.S. Superhydrophobic organic and inorganic clay nanocomposites for epoxy steel coatings. Prog. Org. Coat. 2020, 140, 105502. [Google Scholar] [CrossRef]
- Haeri, S.Z.; Ramezanzadeh, B.; Asghari, M. A novel fabrication of a high performance SiO2-graphene oxide (GO) nanohybrids: Characterization of thermal properties of epoxy nanocomposites filled with SiO2-GO nanohybrids. J. Colloid. Interf. Sci. 2017, 493, 111–122. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Zhang, M.; Dou, W.; Zhang, X.; Chen, S. The effects of interfacial water and SiO2 surface wettability on the adhesion properties of SiO2 in epoxy nanocomposites. Appl. Surf. Sci. 2020, 502, 144151. [Google Scholar] [CrossRef]
- Ye, Y.W.; Yang, D.P.; Zhang, D.W.; Chen, H.; Zhao, H.C.; Li, X.G.; Wang, L.P. POSS-tetraaniline modified graphene for active corrosion protection of epoxy-based organic coating. Chem. Eng. J. 2020, 383, 123160. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel Check. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Prabhu, R.; Roopashree, B.; Jeevananda, T.; Rao, S.; Reddy, K.R.; Raghu, A.V. Synthesis and corrosion resistance properties of novel conjugated polymer-Cu2Cl4L3 composites. Mater. Sci. Energy Technol. 2021, 4, 92–99. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhao, J.; Wang, X.; Xiong, C.; Song, L.; Ding, R.; Han, P.; Li, W. Self-healing performance and corrosion resistance of graphene oxide-mesoporous silicon layer-nanosphere structure coating under marine alternating hydrostatic pressure. Chem. Eng. J. 2019, 361, 792–804. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, P. Corrosion protection mechanism of aluminum triphosphate modified by organic acids as a rust converter. Prog. Org. Coat. 2020, 140, 105508. [Google Scholar] [CrossRef]
- Du, P.; Wang, J.; Liu, G.; Zhao, H.; Wang, L. Facile synthesis of intelligent nanocomposites as encapsulation for materials protection. Mater. Chem. Front. 2019, 3, 321–330. [Google Scholar] [CrossRef]
- Lee, J.; Berman, D. Inhibitor or promoter: Insights on the corrosion evolution in a graphene protected surface. Carbon 2018, 126, 225–231. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Wu, Y.; Liu, G.; Dong, J.; Zhou, K. A polyethyleneimine-grafted graphene oxide hybrid nanomaterial: Synthesis and anti-corrosion applications. Appl. Surf. Sci. 2019, 479, 963–973. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.U.; Jung, B.M.; Byun, J.-H.; Yi, J.-W.; Lee, S.-B.; Kim, B.-S. Simultaneous enhancement of mechanical, electrical and thermal properties of graphene oxide paper by embedding dopamine. Carbon 2013, 65, 296–304. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Al-Hetlani, E.; Amin, M.O.; Quinones-Ruiz, T.; Lednev, I.K. A poly(butyl methacrylate)/graphene oxide/TiO2 nanocomposite coating with superior corrosion protection for AZ31 alloy in chloride solution. Chem. Eng. J. 2019, 361, 485–498. [Google Scholar] [CrossRef]
- Wang, N.; Diao, X.; Zhang, J.; Kang, P. Corrosion resistance of waterborne epoxy coatings by incorporation of dopamine treated mesoporous-TiO2 particles. Coatings 2018, 8, 209. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Zhang, Y.; Zhang, Z.; Li, J.; Sui, G. (3-aminopropyl) triethoxysilane grafted poly(dopamine)@Fe3O4 nanoparticles and their epoxy composites for functional application. Compos. Part. B-Eng. 2019, 169, 148–156. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Ozkan, D.; Erarslan, Y.; Kincal, C.; Gurlu, O.; Yagci, M.B. Wear and corrosion resistance enhancement of chromium surfaces through graphene oxide coating. Surf. Coat. Technol. 2020, 391, 125595. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, W.J.; Miao, L.J. Designed a novel EP plus GO/ZRC plus GO coating with bilayered structure for enhancing corrosion resistance of steel substrate. J. Hazard. Mater. 2021, 403, 123670. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, Q.; Cheng, L.; Wu, H.; Zhao, H.; Wang, L. Self-alignment of cationic graphene oxide nanosheets for anticorrosive reinforcement of epoxy coatings. Chem. Eng. J. 2020, 389, 124435. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhao, W.J.; Qiang, Y.J.; Chen, Z.J.; Wang, L.P.; Gao, X.L.; Fang, Z.W. pi-pi interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: Synthesis and anti-corrosion application. Carbon 2020, 159, 292–302. [Google Scholar] [CrossRef]
- Zheng, H.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Reinforcing the corrosion protection property of epoxy coating by using graphene oxide-poly(urea-formaldehyde) composites. Corros. Sci. 2017, 123, 267–277. [Google Scholar] [CrossRef]
- Bhaskara, S.; Fakrudeen, S.P.; Raju, V.B.; Murthy, H.A.; Raghu, A.V. Comparative studies of inhibitive effects of diamines on corrosion of aluminum alloy in presence of acid media. Rasayan J. Chem. 2021, 72–78. [Google Scholar] [CrossRef]
- Meng, F.D.; Liu, L.; Tian, W.L.; Wu, H.; Li, Y.; Zhang, T.; Wang, F.H. The influence of the chemically bonded interface between fillers and binder on the failure behaviour of an epoxy coating under marine alternating hydrostatic pressure. Corros. Sci. 2015, 101, 139–154. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, H.; Wang, L.; Xue, Q. Bioinspired ultrathin graphene nanosheets sandwiched between epoxy layers for high performance of anticorrosion coatings. Chem. Eng. J. 2021, 410, 128301. [Google Scholar] [CrossRef]
- Zhou, C.; Hong, M.; Yang, Y.; Hu, N.; Zhou, Z.; Zhang, L.; Zhang, Y. Engineering sulfonated polyaniline molecules on reduced graphene oxide nanosheets for high-performance corrosion protective coatings. Appl. Surf. Sci. 2019, 484, 663–675. [Google Scholar] [CrossRef]
- Necolau, M.-I.; Pandele, A.-M. Recent advances in graphene oxide-based anticorrosive coatings: An overview. Coatings 2020, 10, 1149. [Google Scholar] [CrossRef]
- Yazdani, S.; Prince, L.; Vitry, V. Optimization of electroless Ni-B-nanodiamond coating corrosion resistance and understanding the nanodiamonds role on pitting corrosion behavior using shot noise theory and molecular dynamic simulation. Diam. Relat. Mater. 2023, 134, 109793. [Google Scholar] [CrossRef]
- Xu, W.H.; Han, E.H.; Wang, Z.Y. Effect of tannic acid on corrosion behavior of carbon steel in NaCl solution. J. Mater. Sci. Technol. 2019, 35, 64–75. [Google Scholar] [CrossRef]
- Chang, J.W.; Wang, Z.Y.; Han, E.H.; Liang, X.L.; Wang, G.; Yi, Z.Y.; Li, N.Y. Corrosion resistance of tannic acid, D-limonene and nano-ZrO2 modified epoxy coatings in acid corrosion environments. J. Mater. Sci. Technol. 2021, 65, 137–150. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, H.; Shao, N.; Dong, Z. Modification of Electrochemical Exfoliation of Graphene Oxide with Dopamine and Tannic to Enhance Anticorrosion Performance of Epoxy Coatings. Coatings 2023, 13, 1809. https://doi.org/10.3390/coatings13101809
Liu S, Liu H, Shao N, Dong Z. Modification of Electrochemical Exfoliation of Graphene Oxide with Dopamine and Tannic to Enhance Anticorrosion Performance of Epoxy Coatings. Coatings. 2023; 13(10):1809. https://doi.org/10.3390/coatings13101809
Chicago/Turabian StyleLiu, Suyun, Hu Liu, Ningning Shao, and Zhijun Dong. 2023. "Modification of Electrochemical Exfoliation of Graphene Oxide with Dopamine and Tannic to Enhance Anticorrosion Performance of Epoxy Coatings" Coatings 13, no. 10: 1809. https://doi.org/10.3390/coatings13101809
APA StyleLiu, S., Liu, H., Shao, N., & Dong, Z. (2023). Modification of Electrochemical Exfoliation of Graphene Oxide with Dopamine and Tannic to Enhance Anticorrosion Performance of Epoxy Coatings. Coatings, 13(10), 1809. https://doi.org/10.3390/coatings13101809





