Non-Precious Metals Catalysts for Hydrogen Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Catalysts
2.3. Characterization of Catalysts
2.4. Measurements of Hydrolysis of NaBH4
3. Results and Discussions
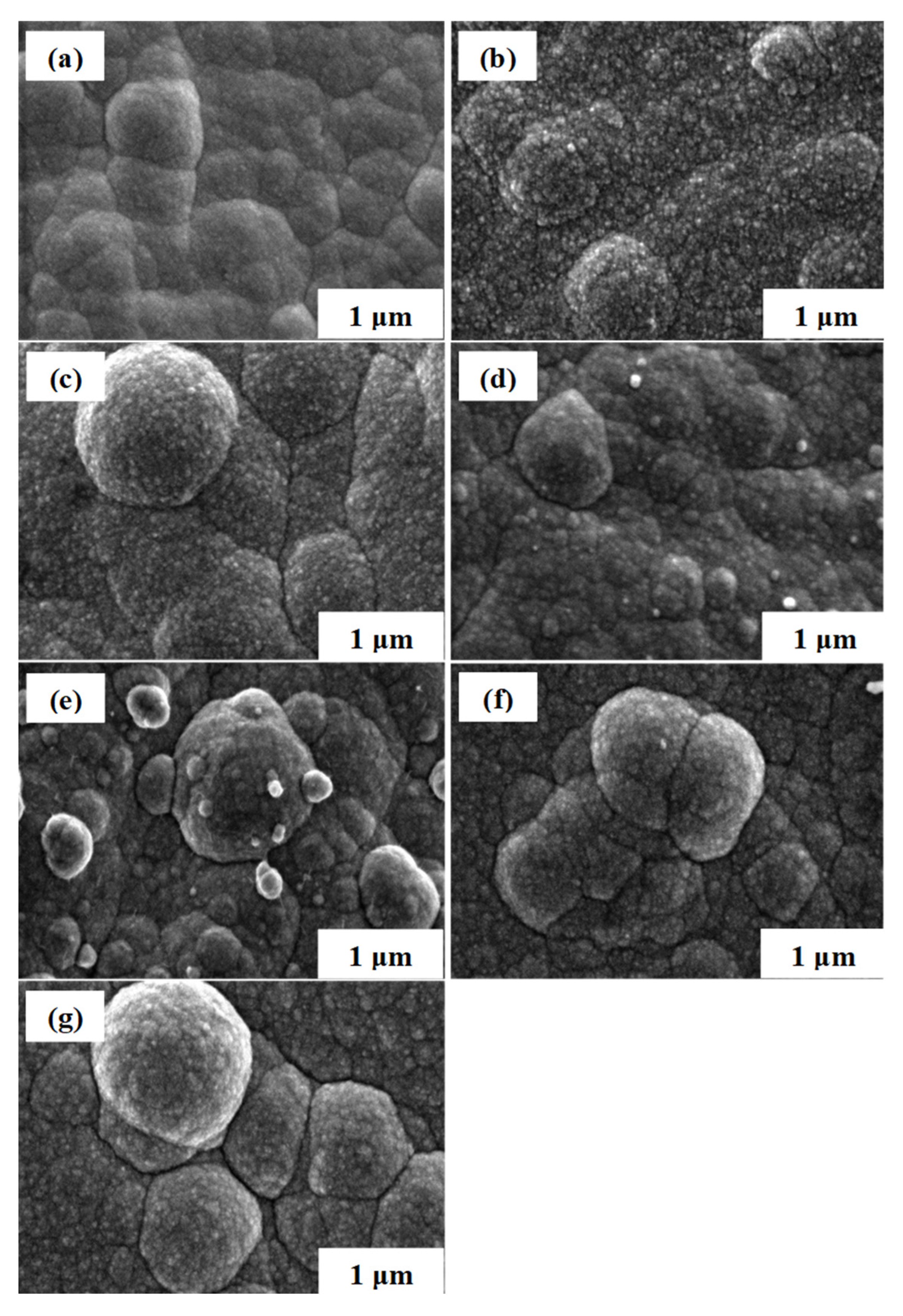
3.1. Microstructure and Morphology Studies
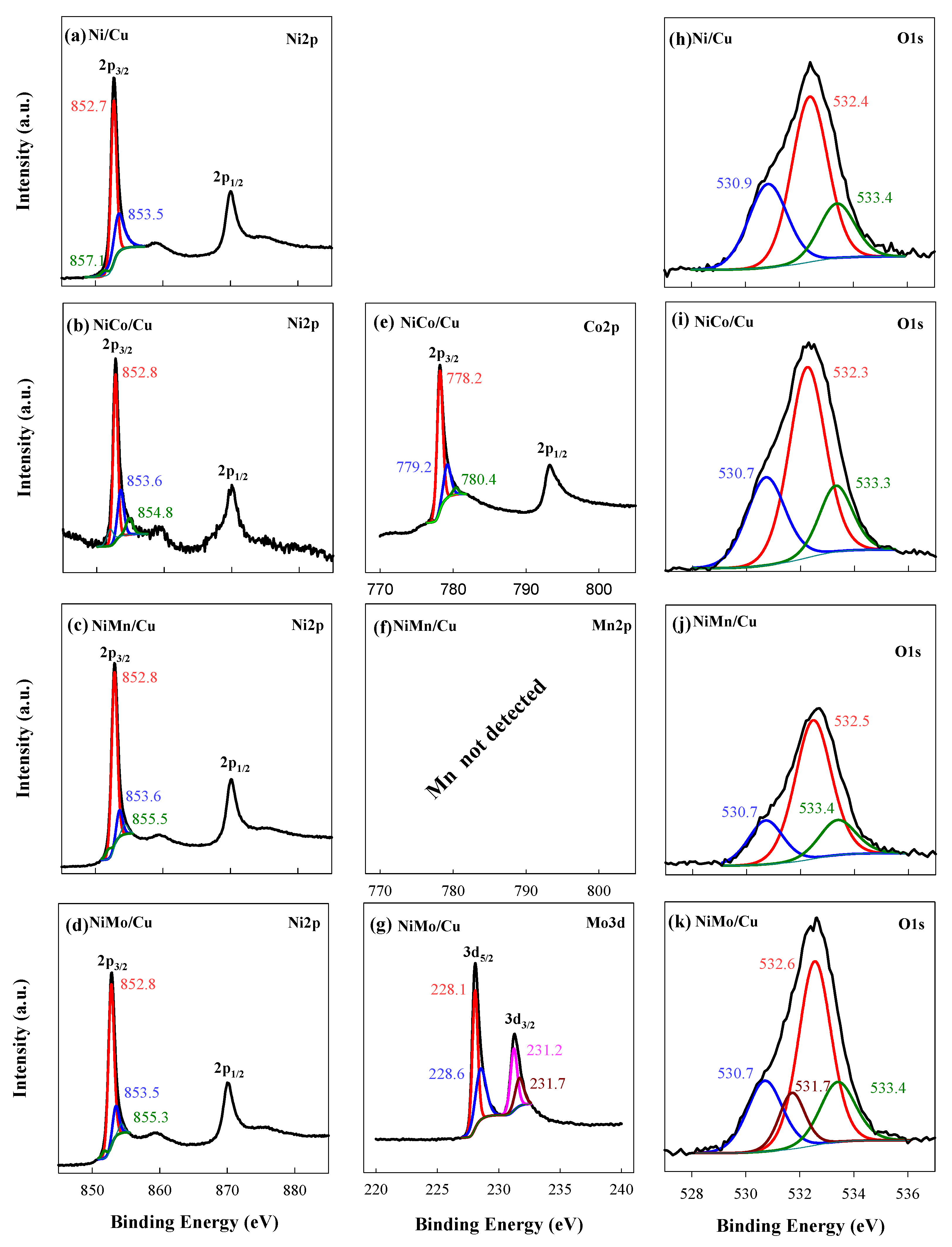
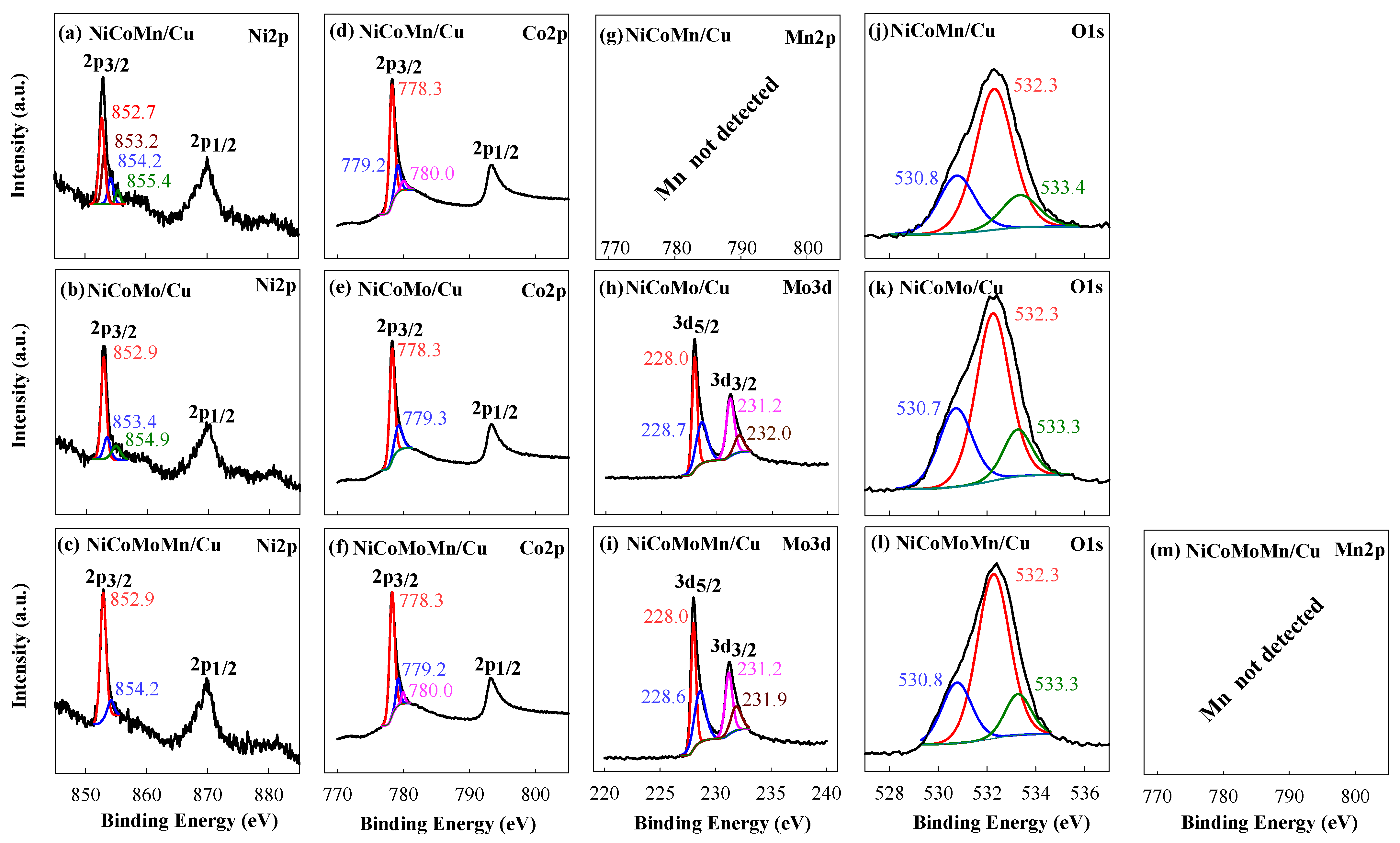
3.2. XPS Analysis of Prepared Catalysts
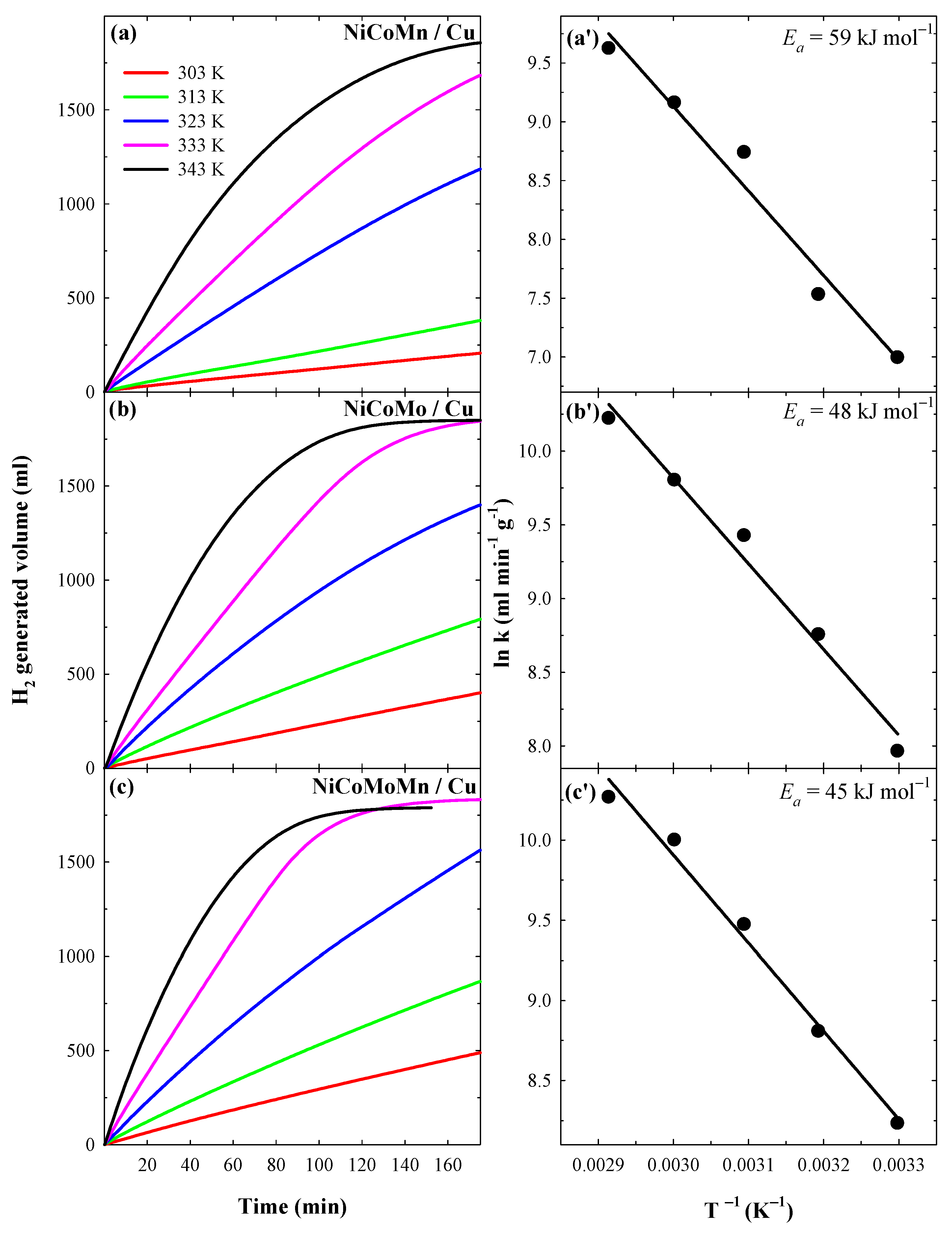
3.3. Catalytic Activity towards NaBH4 Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarhan, C.; Cil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An overview of hydrogen production: Current status, potential, and challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous materials for hydrogen storage. Chemisty 2022, 8, 693. [Google Scholar] [CrossRef]
- Yao, Q.; Ding, Y.; Lu, Z.H. Noble-metal-free nanocatalysts for hydrogen generation from boron- and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837. [Google Scholar] [CrossRef]
- Ekinci, A. Hydrogen generation by hydrolysis of NaBH4 with efficient Co–La–Mo–B catalyst for PEM fuel cells. Kinet. Catal. 2020, 61, 589. [Google Scholar] [CrossRef]
- Bozkurt, G.; Ozer, A.; Yurtcan, A.B. Development of effective catalysts for hydrogen generation from sodium borohydride: Ru, Pt, Pd nanoparticles supported on Co3O4. Energy 2019, 180, 702. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review; License: CC BY 4.0; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 15 August 2023).
- Hydrogen to the rescue. Nat. Mater. 2018, 17, 565. [CrossRef]
- Hydrogen Insights: A Perspective on Hydrogen Investment, Market Development and Cost Competitiveness; Hydrogen Council and McKinsey & Company: Brussels, Belgium, 2021.
- Altaf, C.T.; Colak, T.O.; Minkina, V.G.; Shabunya, S.I.; Sankir, M.; Sankir, N.D.; Kalinin, V.I. Effect of titanium dioxide support for cobalt nanoparticle catalysts for hydrogen generation from sodium borohydride hydrolysis. Catal. Lett. 2022, 287. [Google Scholar] [CrossRef]
- Yang, F.; Ruan, J.; Li, T.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Hydrolysis of sodium borohydride using a highly stable catalyst of ruthenium nanoparticles supported by cobalt–nickel hydroxide-coated nickel foam. J. Alloys Compd. 2022, 926, 166902. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, M.; Chen, K.; Liu, J.; Wang, H.; Zhu, M.; Yartys, V. Recent progress on hydrogen generation from the hydrolysis of light metals and hydrides. J. Alloys Compd. 2022, 910, 164831. [Google Scholar] [CrossRef]
- Comanescu, C. Paving the way to the fuel of the future—Nanostructured complex hydrides. Int. J. Mol. Sci. 2023, 24, 143. [Google Scholar] [CrossRef]
- Dragan, M. Hydrogen storage in complex metal hydrides NaBH4: Hydrolysis reaction and experimental strategies. Catalysts 2022, 12, 356. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726. [Google Scholar] [CrossRef]
- Hansu, T.A.; Sahin, O.; Caglar, A.; Kivrak, H. A remarkable Mo doped Ru catalyst for hydrogen generation from sodium borohydride: The effect of Mo addition and estimation of kinetic parameters. React. Kinet. Mech. Catal. 2020, 131, 661. [Google Scholar] [CrossRef]
- Yu, Y.; Kang, L.; Sun, L.; Xu, F.; Pan, H.; Sang, Z.; Zhang, C.; Jis, X.; Sui, Q.; Bu, Y.; et al. Bimetallic Pt-Ni nanoparticles confined in porous titanium oxide cage for hydrogen generation from NaBH4 hydrolysis. Nanomaterials 2022, 12, 2550. [Google Scholar] [CrossRef]
- Hashimi, A.S.; Nohan, M.A.N.M.; Chin, S.X.; Khiew, P.S.; Zakaria, S.; Chia, C.H. Copper nanowires as highly efficient and recyclable catalyst for rapid hydrogen generation from hydrolysis of sodium borohydride. Nanomaterials 2020, 10, 1153. [Google Scholar] [CrossRef]
- Ruslan, N.; Yahya, M.S.; Siddique, M.N.I.; Yengantiwar, A.P.; Ismail, M.; Awal, M.R.; Yusoff, M.Z.M.; Yap, M.F.A.A.H.; Mustafa, N.S. Review on magnesium hydride and sodium borohydride hydrolysis for hydrogen production. Crystals 2022, 12, 1376. [Google Scholar] [CrossRef]
- Markovic, N.; Gasteiger, H.; Ross, P.N. Kinetics of oxygen reduction on Pt(hkl) electrodes: Implications for the crystallite size effect with supported Pt electrocatalysts. J. Electrochem. Soc. 1997, 144, 1591. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, H.B.; Ma, L.P.; Wang, P.; Cheng, H.M. Hydrogen generation from sodium borohydride solution using a ruthenium supported on graphite catalyst. Int. J. Hydrogen Energy 2010, 35, 3023. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Huang, C.L.; Chen, Y.A.; Lu, S.Y. NiFeMo alloy inverse-opals on Ni foam as outstanding bifunctional catalysts for electrolytic water splitting of ultra-low cell voltages at high current densities. Appl. Catal. B Environ. 2020, 267, 118376. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, W.; Wang, Y.; Gao, Y.; Qi, K.; Zhang, K. Fast hydrogen generation from NaBH4 hydrolysis catalyzed by nanostructured Co-Ni-B catalysts. Int. J. Hydrogen Energy 2017, 42, 6072. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wu, S.; Wei, Y.; Meng, W.; Xie, Y.; Cui, Y.; Lian, X.; Chen, Y.; Zhang, X. Hydrogen generation from alkaline NaBH4 solution using nanostructured Co-Ni-P catalysts. Int. J. Hydrogen Energy 2017, 42, 16529. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, R.; Meng, L.; Wang, Y.; Li, G.; Xin, S.; Zhao, X.; Zhang, K. Hydrogen generation from alkaline NaBH4 solution using a dandelion-like Co-Mo-B catalyst supported on carbon cloth. Int. J. Hydrogen Energy 2017, 42, 9945. [Google Scholar] [CrossRef]
- Akkus, M.S. Examination of the catalytic effect of Ni, NiCr, and NiV catalysts prepared as thin films by magnetron sputtering process in the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2023, 48, 23055. [Google Scholar] [CrossRef]
- Keskin, M.S.; Ağırtaş, M.S. Hydrogen production performance and kinetic behavior from sodium borohydride hydrolysis with TiO2-supported Co-Mo-B catalyst. Ionics 2023, 29, 3713. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Fu, W.; Si, S.; Wei, L.; Zhao, X.; Wang, Y. Mn doped CoP/Ni foam catalyst for hydrogen generation from hydrolysis of sodium borohydride. Mater. Lett. 2022, 308, 131166. [Google Scholar] [CrossRef]
- Liang, Z.; Li, Q.; Li, F.; Zhao, S.; Xia, X. Hydrogen generation from hydrolysis of NaBH4 based on high stable NiB/NiFe2O4 catalyst. Int. J. Hydrogen Energy 2017, 42, 3971. [Google Scholar] [CrossRef]
- Ingersoll, J.C.; Mani, N.; Thenmozhiyal, J.C.; Muthaiah, A. Catalytic hydrolysis of sodium borohydride by a novel nickel–cobalt–boride catalyst. J. Power Sources 2007, 173, 450. [Google Scholar] [CrossRef]
- Zhang, P.; Lv, Z.; Liu, X.; Xie, G.; Zhang, B. Electroless nickel plating on alumina ceramic activated by metallic nickel as electrocatalyst for oxygen evolution reaction. Catal. Commun. 2021, 149, 106238. [Google Scholar] [CrossRef]
- Sabeen, A.H.; Kamaruddin, S.N.B.; Noor, Z.Z. Environmental impacts assessment of industrial wastewater treatment system using electroless nickel plating and life cycle assessment approaches. Int. J. Environ. Sci. Technol. 2019, 16, 3171. [Google Scholar] [CrossRef]
- Baboria, M.; Pathania, A.S. An analytical investigation pertaining to autocatalytic plating of chromium nickel carbide powder on copper alloys using electroless deposition method. World J. Adv. Res. Rev. 2023, 17, 291. [Google Scholar] [CrossRef]
- Takeoka, H.; Seike, M.; Nakamura, Y.; Imai, H.; Oaki, Y.; Fujii, S. Electroless nickel plating on a biomineral-based sponge structure. Mater. Adv. 2022, 3, 931. [Google Scholar] [CrossRef]
- Thakur, A.; Gharde, S.; Kandasubramanian, B. Electroless nickel fabrication on surface modified magnesium substrates. Def. Technol. 2019, 15, 636. [Google Scholar] [CrossRef]
- Huang, Z.; Nguyen, T.T.; Zhou, Y.; Qi, G. A low temperature electroless nickel plating chemistry. Surf. Coat. Technol. 2019, 372, 160. [Google Scholar] [CrossRef]
- Izgia, M.S.; Onata, E.; Kazicib, H.C.; Sahin, O. Hydrogen production through the cooperation of a catalystsynthesized in ethanol medium and the effect of the plasma. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 45, 8271. [Google Scholar] [CrossRef]
- Sukackienė, Z.; Jasulaitienė, V.; Naujokaitis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Electroless deposition of CoBW coatings using morpholine borane as a reducing agent. Thin Solid Film. 2017, 636, 425. [Google Scholar] [CrossRef]
- Kumar, M.; Bhavani, T.; Rawal, S.; Sidpara, A. Magnetorheological finishing of chemically treated electroless nickel plating. Magnetochemistry 2022, 8, 184. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Lei, M.; Li, X.; Ouyang, H. Enhancing the properties of the SAC305-soldered joint: Heat treatment of the nickel-plated copper substrate before reflow soldering. J. Mater. Sci. Mater. Electron. 2022, 33, 3535. [Google Scholar] [CrossRef]
- Gomes, N.; Gonzalez-Estrada, O.A.; Pertuz, A. Electroless nickel phosphorous: A global vision. Rev. UIS Ing. 2019, 18, 173. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, H.; Yu, J.; Lai, J.; He, H.; Zhang, Y.; Qi, H.; Wang, D. Mechanical and tribological properties of Ni-B and Ni-B-W coatings prepared by electroless plating. Lubricants 2023, 11, 42. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chen, T.C.; Juang, Y.; Hua, L.C.; Huang, C. High catalytic performance of CuCo/nickel foam electrode for ammonia electrooxidation. Electrochem. Commun. 2020, 121, 106875. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Zhang, Z.; Li, Y.; Liu, J.; Yang, Y.; Xue, B.; Li, F. Self-adaptively electrochemical reconstruction of NiFe-layered double hydroxide on Ni foam for high-performance water splitting. J. Alloys Compd. 2023, 934, 167846. [Google Scholar] [CrossRef]
- Kytsya, A.; Berezovets, V.; Verbovytskyy, Y.; Bazylyak, L.; Kordan, V.; Zavaliy, I.; Yartys, V.A. Bimetallic Ni-Co nanoparticles as an efficient catalyst of hydrogen generation via hydrolysis of NaBH4. J. Alloys Compd. 2022, 908, 164484. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.W.; Wang, Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. R. Soc. Chem. 2019, 9, 31563. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, W.; Lai, S.; Chen, X. Integral structured Co–Mn composite oxides grown on interconnected Ni foam for catalytic toluene oxidation. R. Soc. Chem. 2019, 9, 6533. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, P.; Wang, G.; Xie, G. Hydrogen generation from alkaline NaBH4 solution using CoNiMoP/g-Al2O3 catalysts. Int. J. Hydrogen Energy 2016, 41, 1468. [Google Scholar] [CrossRef]
- Sukackienė, Z.; Antanavičiūtė, K.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Naujokaitis, A.; Norkus, E. Electroless deposition of nickel-boron coatings. Chemija 2020, 31, 1. [Google Scholar] [CrossRef]
- Tarozaitė, R.; Sudavičius, A.; Sukackienė, Z.; Norkus, E. Electroless cobalt deposition in the glycinate soliution using morpholine borane as a reducing agen. Trans. Inst. Mater. Finish. 2014, 92, 146. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com/search/label/Nickel (accessed on 16 August 2023).
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com/search/label/Oxygen (accessed on 17 August 2023).
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com/search/label/Cobalt (accessed on 16 August 2023).
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111. [Google Scholar] [CrossRef]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.S.; Wang, C.M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2008, 90, 317. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com/search/label/Molybdenum (accessed on 16 August 2023).
- NIST X-ray Photoelectron Spectroscopy Database. Available online: http://srdata.nist.gov/xps/ (accessed on 16 August 2023).
- Sanchez, F.; Motta, D.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Hydrogen Generation from Additive-Free Formic Acid Decomposition Under Mild Conditions by Pd/C: Experimental and DFT Studies. Top. Catal. 2018, 61, 254. [Google Scholar] [CrossRef]
- Soltani, M.; Zabihi, M. Hydrogen generation by catalytic hydrolysis of sodium borohydride using the nano-bimetallic catalysts supported on the core-shell magnetic nanocomposite of activated carbon. Int. J. Hydrogen Energy 2020, 45, 12331. [Google Scholar] [CrossRef]
- Rakapa, M.; Kalua, E.E.; Özkar, S. Cobalt–nickel–phosphorus supported on Pd-activated TiO2 (Co–Ni–P/Pd-TiO2) as cost-effective and reusable catalyst for hydrogen generation from hydrolysis of alkaline sodium borohydride solution. J. Alloys Compd. 2011, 509, 7016. [Google Scholar] [CrossRef]
- Eom, K.; Cho, K.; Kwon, H. Effects of electroless deposition conditions on microstructures of cobalt–phosphorous catalysts and their hydrogen generation properties in alkaline sodium borohydride solution. J. Power Sources 2008, 180, 484. [Google Scholar] [CrossRef]
- Jeong, S.U.; Kima, R.K.; Cho, E.A.; Kimb, H.J.; Namb, S.W.; Oh, I.H.; Hong, S.A.; Kim, S.H. A study on hydrogen generation from NaBH4 solution using the high-performance Co-B catalyst. J. Power Sources 2005, 144, 129. [Google Scholar] [CrossRef]

| Catalysts | Plating Solution Composition (mol L−1) and Plating Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NiSO4 | CH3COONa | MB | Glycine | (CH3COO)2Mn | Na2MoO4 | CoSO4 | pH | T, °C | t, min | |
| Ni/Cu | 0.05 | 0.04 | 0.05 | 0.4 | - | - | - | 7 | 40 | 50 |
| NiMn/Cu | 0.1 | 0.04 | 0.2 | - | 0.07 | - | - | 40 | 30 | |
| NiMo/Cu | 0.1 | 0.04 | 0.2 | - | - | 0.001 | - | 50 | 12 | |
| NiCo/Cu | 0.1 | - | 0.2 | 0.3 | - | - | 0.1 | 50 | 5 | |
| NiCoMn/Cu | 0.1 | 0.04 | 0.2 | - | 0.07 | - | 0.1 | 60 | 10 | |
| NiCoMo/Cu | 0.1 | 0.04 | 0.2 | - | 0.07 | 0.001 | 0.1 | 50 | 10 | |
| NiCoMoMn/Cu | 0.1 | 0.04 | 0.2 | - | 0.07 | 0.001 | 0.1 | 60 | 6 | |
| Catalysts | Element, wt% | Element Loadings, µg cm−2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ni | Mn | Mo | Co | Ni | Mn | Mo | Co | |
| Ni/Cu | 100 | - | - | - | 373.7 | - | - | - |
| NiCo/Cu | 22.61 | - | - | 77.39 | 179.35 | - | - | 614 |
| NiMn/Cu | 99.84 | 0.16 | - | - | 475.15 | 0.745 | - | - |
| NiMo/Cu | 85.46 | - | 14.54 | - | 419.05 | - | 71.3 | - |
| NiCoMn/Cu | 9.81 | 0.01 | - | 90.18 | 59.75 | 0.06 | - | 549.5 |
| NiCoMo/Cu | 8.01 | - | 10.1 | 81.89 | 37.18 | - | 46.875 | 380.1 |
| NiCoMoMn/Cu | 7.95 | 0.01 | 7.33 | 84.71 | 32.515 | 0.05 | 29.985 | 346.65 |
| Catalysts | Ea, kJ mol−1 | T, K | v, mL min−1 |
|---|---|---|---|
| Ni/Cu | 67.9 | 303 K | 0.18 |
| 313 K | 0.39 | ||
| 323 K | 0.77 | ||
| 333 K | 1.64 | ||
| 343 K | 3.99 | ||
| NiCo/Cu | 56.4 | 303 K | 1.24 |
| 313 K | 2.13 | ||
| 323 K | 5.77 | ||
| 333 K | 10.66 | ||
| 343 K | 14.59 | ||
| NiMn/Cu | 66.1 | 303 K | 0.22 |
| 313 K | 0.66 | ||
| 323 K | 1.38 | ||
| 333 K | 2.53 | ||
| 343 K | 5.03 | ||
| NiMo/Cu | 65.5 | 303 K | 0.26 |
| 313 K | 0.49 | ||
| 323 K | 1.16 | ||
| 333 K | 2.00 | ||
| 343 K | 5.45 | ||
| NiCoMn/Cu | 58.8 | 303 K | 1.33 |
| 313 K | 2.30 | ||
| 323 K | 5.05 | ||
| 333 K | 11.6 | ||
| 343 K | 18.41 | ||
| NiCoMo/Cu | 48.3 | 303 K | 2.35 |
| 313 K | 2.84 | ||
| 323 K | 6.51 | ||
| 333 K | 14.80 | ||
| 343 K | 22.47 | ||
| NiCoMoMn/Cu | 45.3 | 303 K | 3.08 |
| 313 K | 5.47 | ||
| 323 K | 8.79 | ||
| 333 K | 18.03 | ||
| 343 K | 23.57 |
| Catalysts | NaBH4 Solution | Ea, kJ mol−1 | References |
|---|---|---|---|
| Ni | 5 wt% NaBH4 + 0.1 M NaOH | 67.90 | in this work |
| Ni | 5 wt% NaBH4 + 10 wt% NaOH | 72.52 | [30] |
| NiCo | 5 wt% NaBH4 + 0.1 M NaOH | 56.40 | in this work |
| NiCo | 2.7 wt% NaBH4 + 15 wt% NaOH | 62.00 | [31] |
| NiCoP | 0.3 M NaBH4 + 10 wt% NaOH | 57.00 | [63] |
| CoMo | 5 wt% NaBH4 + 7 wt% NaOH | 51.00 | [26] |
| Co | 10 wt% NaBH4 + 1 wt% NaOH | 60.20 | [64] |
| Co | 20 wt% NaBH4 + 5 wt% NaOH | 64.90 | [65] |
| NiMn | 5 wt% NaBH4 + 0.1 M NaOH | 66.10 | in this work |
| NiMo | 5 wt% NaBH4 + 0.1 M NaOH | 65.50 | in this work |
| NiCoMn | 5 wt% NaBH4 + 0.1 M NaOH | 58.80 | in this work |
| NiCoMo | 5 wt% NaBH4 + 0.1 M NaOH | 48.30 | in this work |
| NiCoMo | 7 wt% NaBH4 + 10 wt% NaOH | 52.43 | [49] |
| NiCoMoMn | 5 wt% NaBH4 + 0.1 M NaOH | 45.30 | in this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukackienė, Z.; Valeckytė, G.; Kepenienė, V.; Stalnionienė, I.; Jasulaitienė, V.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Stalnionis, G.; Norkus, E. Non-Precious Metals Catalysts for Hydrogen Generation. Coatings 2023, 13, 1740. https://doi.org/10.3390/coatings13101740
Sukackienė Z, Valeckytė G, Kepenienė V, Stalnionienė I, Jasulaitienė V, Vaičiūnienė J, Tamašauskaitė-Tamašiūnaitė L, Stalnionis G, Norkus E. Non-Precious Metals Catalysts for Hydrogen Generation. Coatings. 2023; 13(10):1740. https://doi.org/10.3390/coatings13101740
Chicago/Turabian StyleSukackienė, Zita, Gitana Valeckytė, Virginija Kepenienė, Irena Stalnionienė, Vitalija Jasulaitienė, Jūratė Vaičiūnienė, Loreta Tamašauskaitė-Tamašiūnaitė, Giedrius Stalnionis, and Eugenijus Norkus. 2023. "Non-Precious Metals Catalysts for Hydrogen Generation" Coatings 13, no. 10: 1740. https://doi.org/10.3390/coatings13101740
APA StyleSukackienė, Z., Valeckytė, G., Kepenienė, V., Stalnionienė, I., Jasulaitienė, V., Vaičiūnienė, J., Tamašauskaitė-Tamašiūnaitė, L., Stalnionis, G., & Norkus, E. (2023). Non-Precious Metals Catalysts for Hydrogen Generation. Coatings, 13(10), 1740. https://doi.org/10.3390/coatings13101740









