Chromium Diffusion Coatings for Mo-Based Silicides to Improve Their Oxidation Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy Manufacturing
2.2. Coating Manufacturing
2.3. Thermogravimetric Oxidation
2.4. Microstructural Analysis
3. Results and Discussion
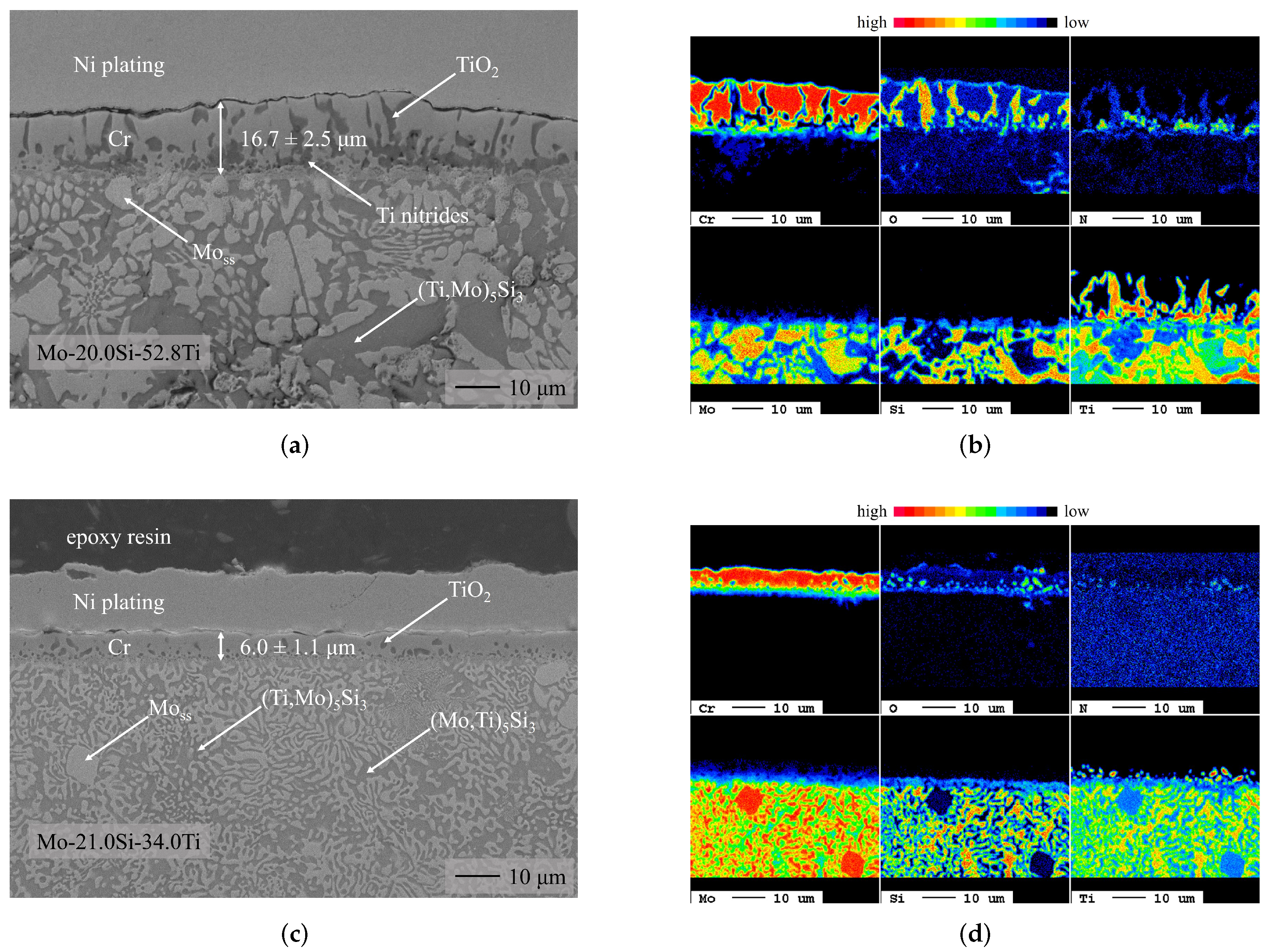
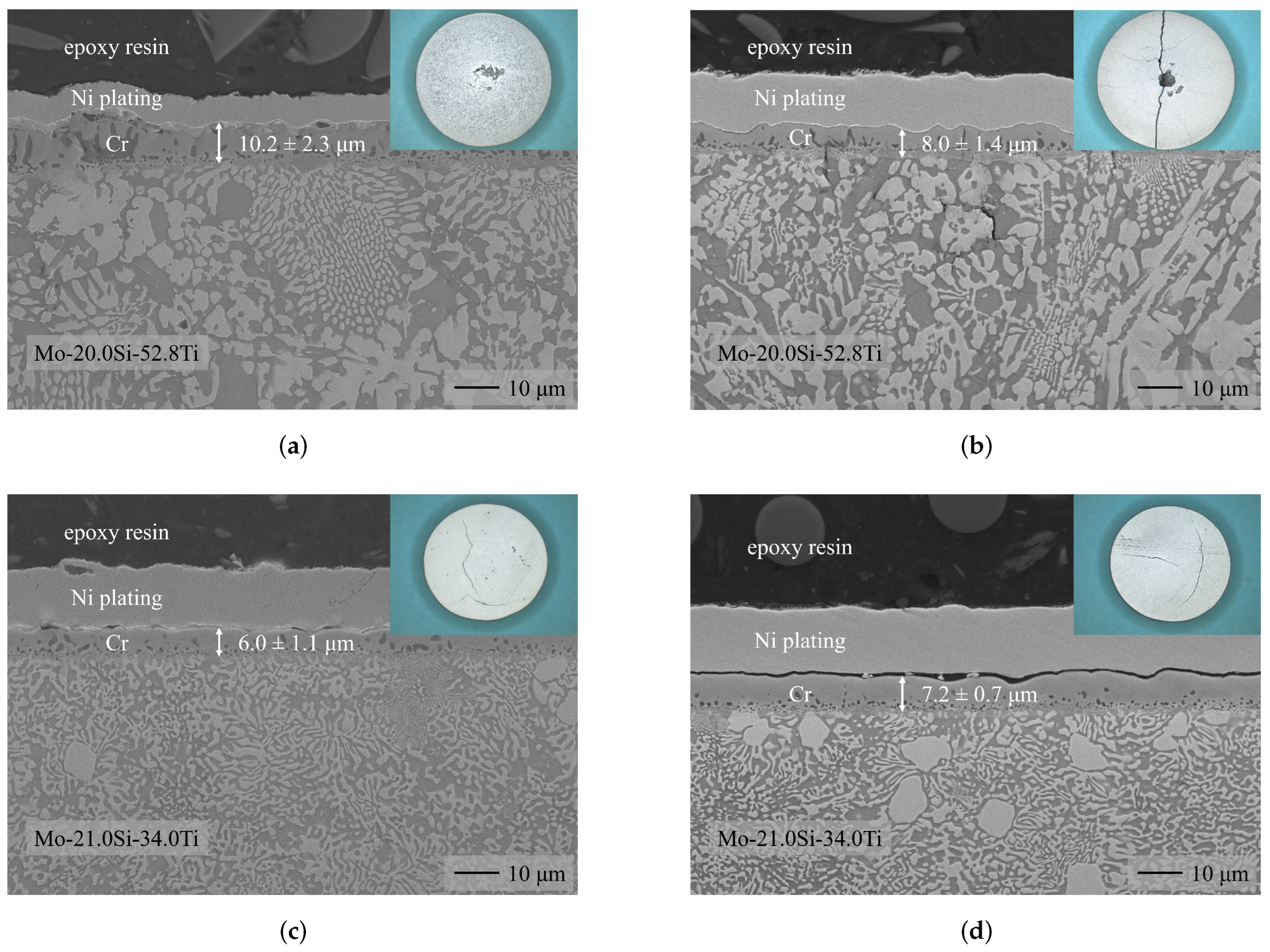
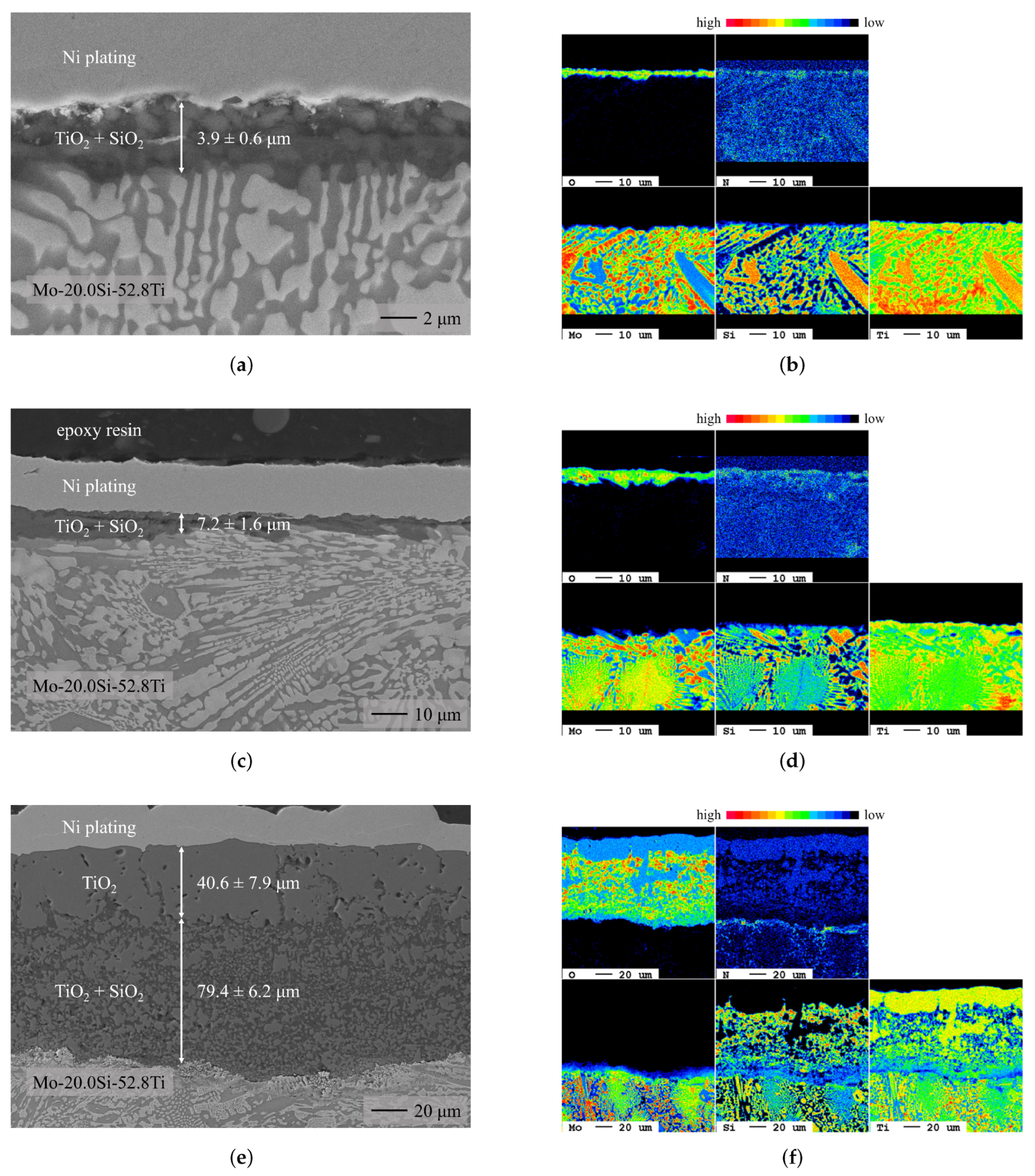
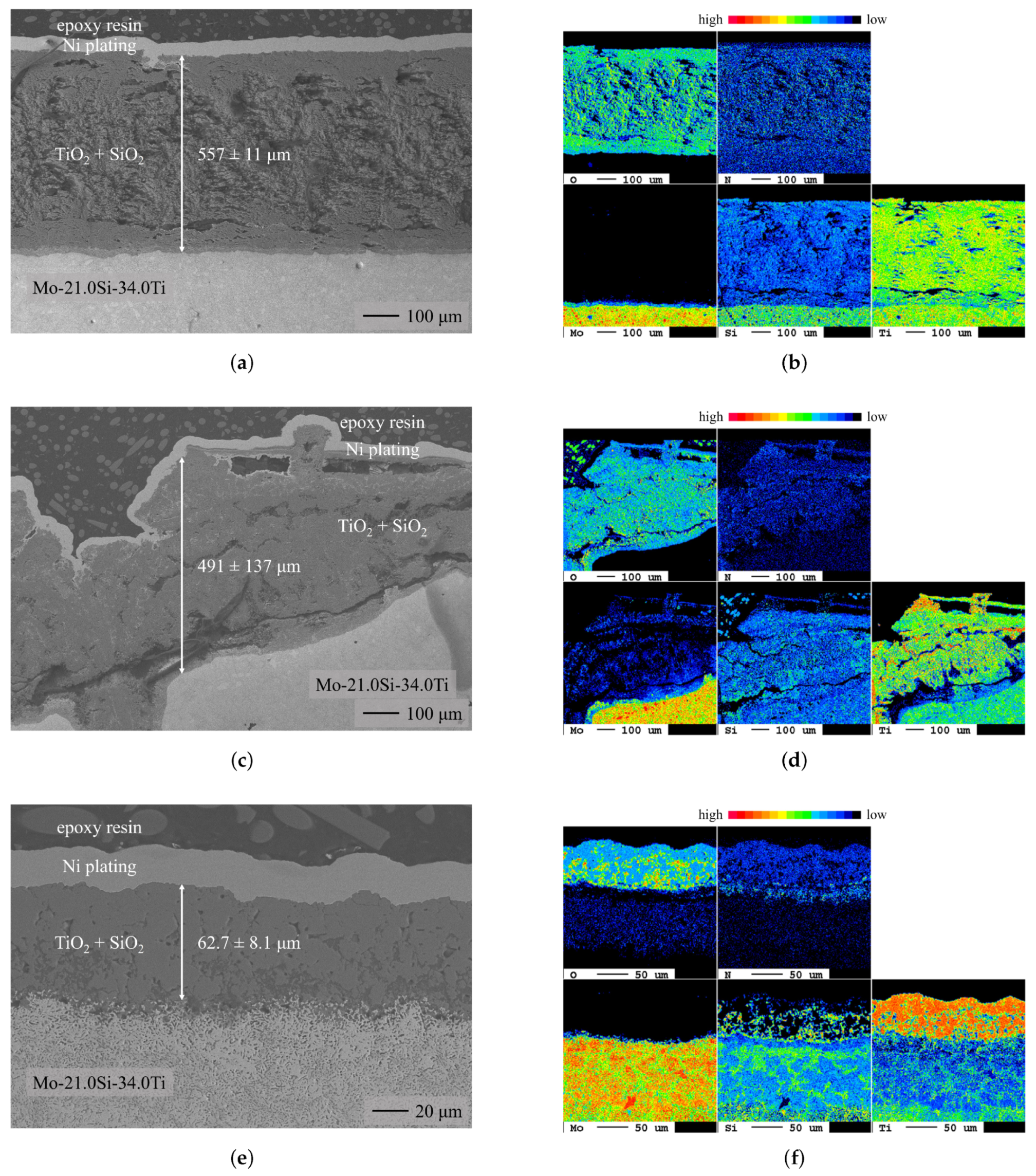
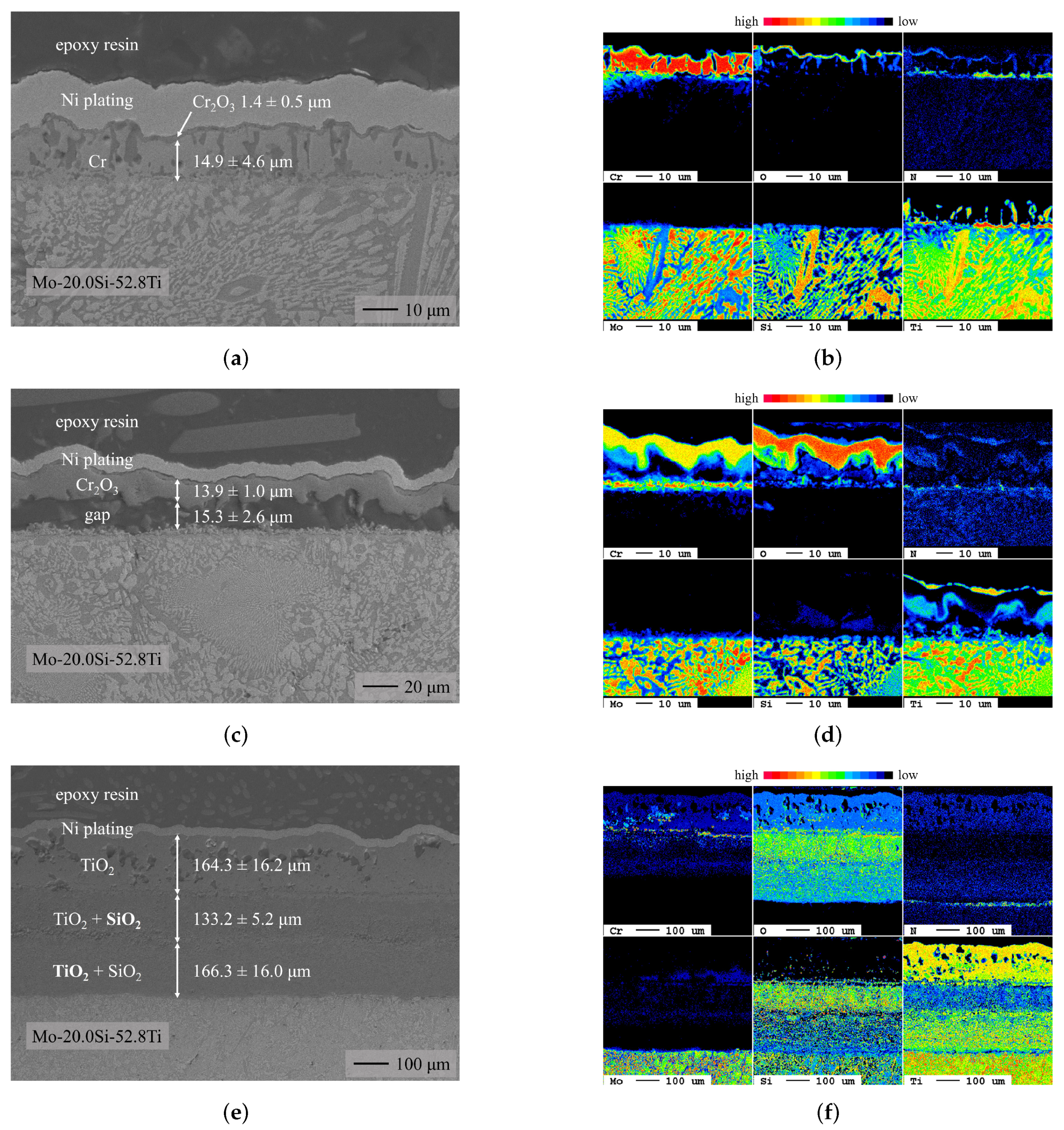
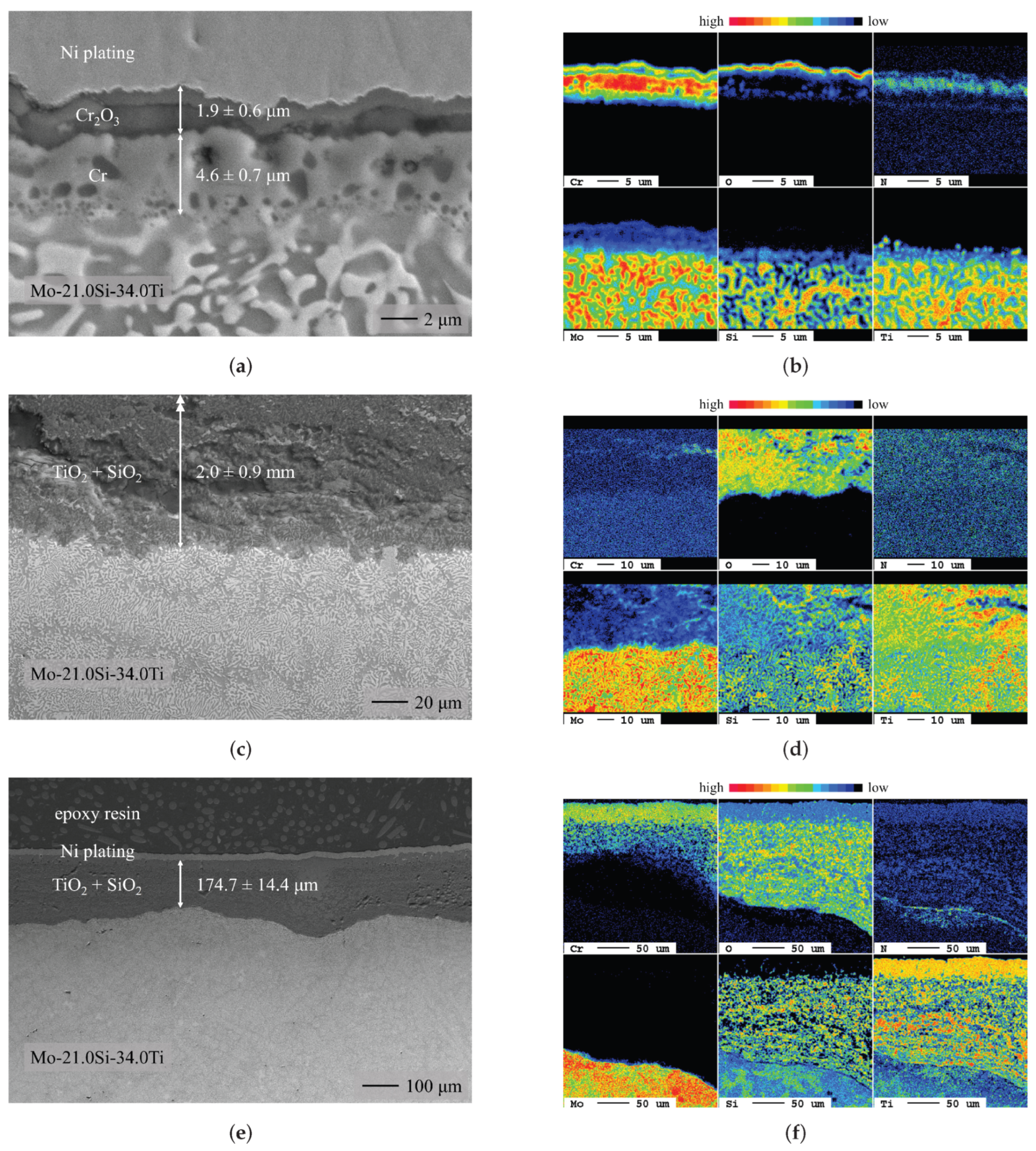
3.1. Chromium Diffusion Coating Investigations
3.2. Oxidation Behavior Investigations
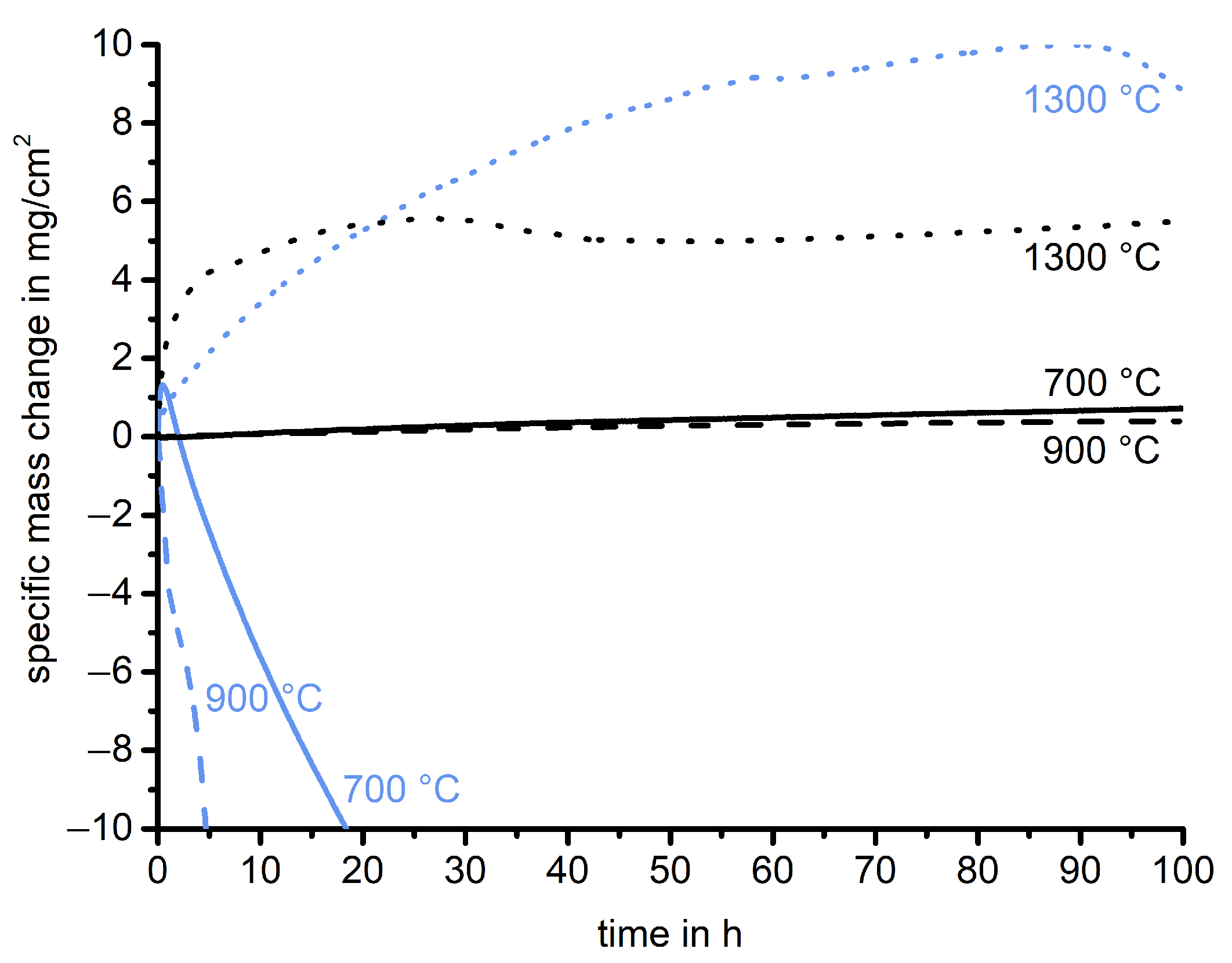
3.2.1. Uncoated Mo-Si-Ti Alloys
3.2.2. Cr-Coated Mo-Si-Ti Alloys
4. Summary and Conclusions
- Chromium diffusion coatings were successfully applied to two ternary Mo-Si-Ti alloys (eutectic Mo-20.0Si-52.8Ti and eutectoid Mo-21.0Si-34.0Ti).
- During oxidation of the uncoated eutectic alloy at 700 and 900 , a thin oxide layer consisting of mixed TiO and SiO formed. At 1300 , a higher mass gain and the formation of a duplex scale, consisting of an outer TiO and an inner TiO/SiO layer, were detected.
- The uncoated eutectoid alloy exhibited catastrophic oxidation behavior during the oxidation tests at 700 and 900 . At 1300 , a dense protective oxide layer, consisting of TiO and a thin continuous SiO layer at the oxide/substrate interface, decelerated oxide growth.
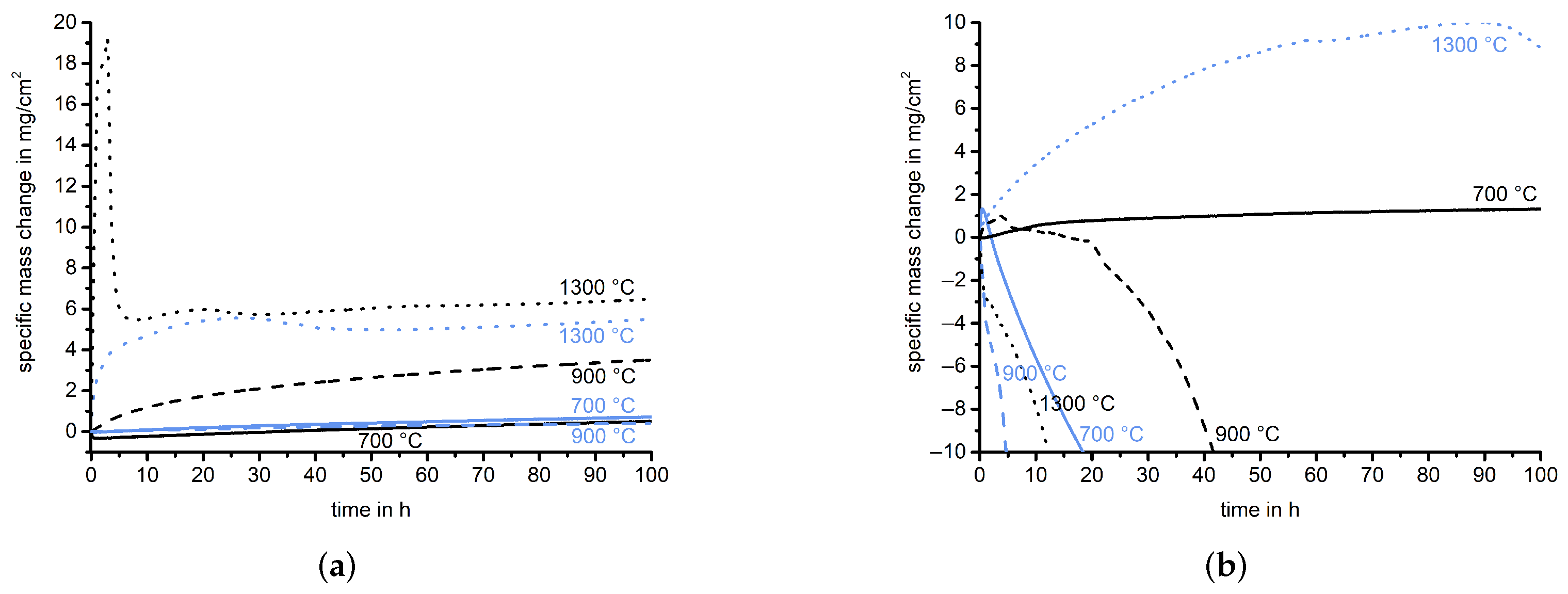
- The oxidation behavior of the Cr-coated eutectic Mo-20.0Si-52.8Ti alloy was comparable to the behavior of the uncoated samples but at 700 and 900 , the evaporation of volatile Mo species was prevented by the CrO formation.
- During the oxidation of the Cr-coated eutectoid Mo-21.0Si-34.0Ti at 700 , a minimal mass gain was observed, which was a significant improvement compared to the catastrophic oxidation observed previously. At higher temperatures, the Cr reservoir was depleted and the CrO failed during the oxidation experiment; therefore, the oxidation behavior was mainly determined by the composition of the underlying substrate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. XRD Analysis
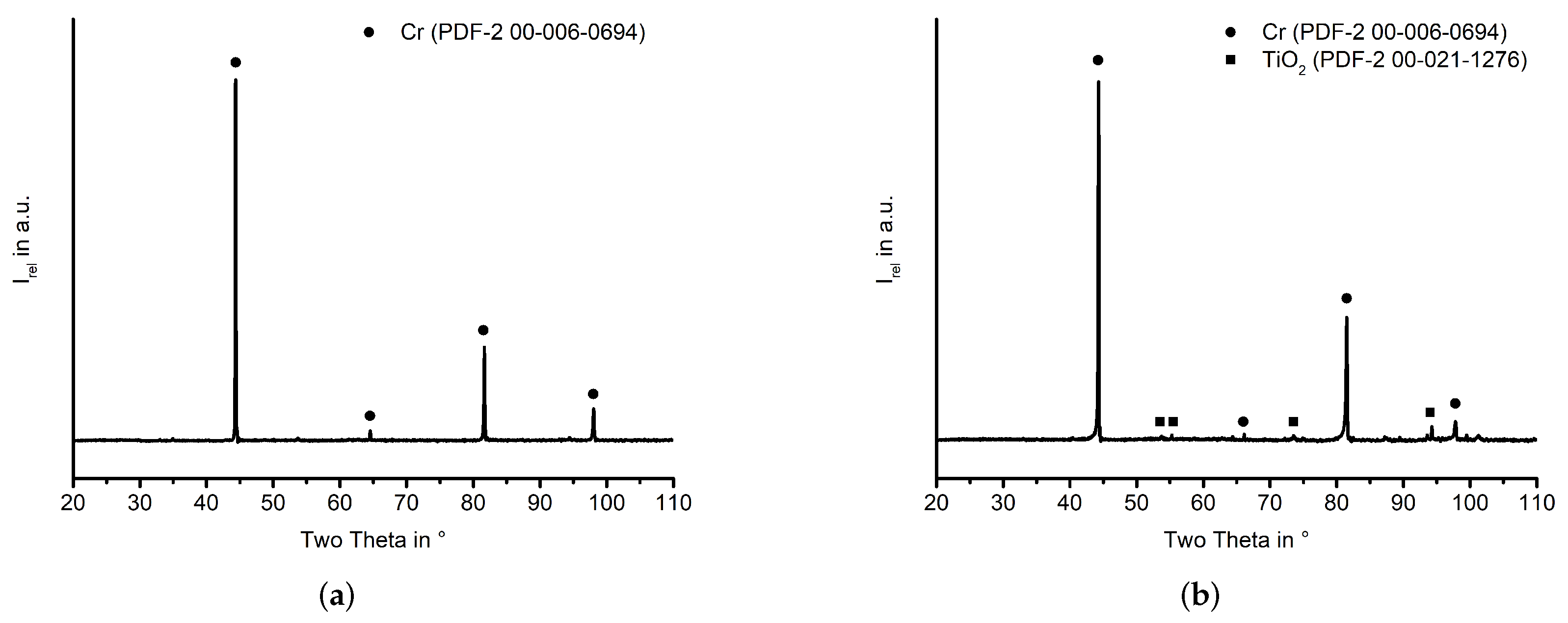

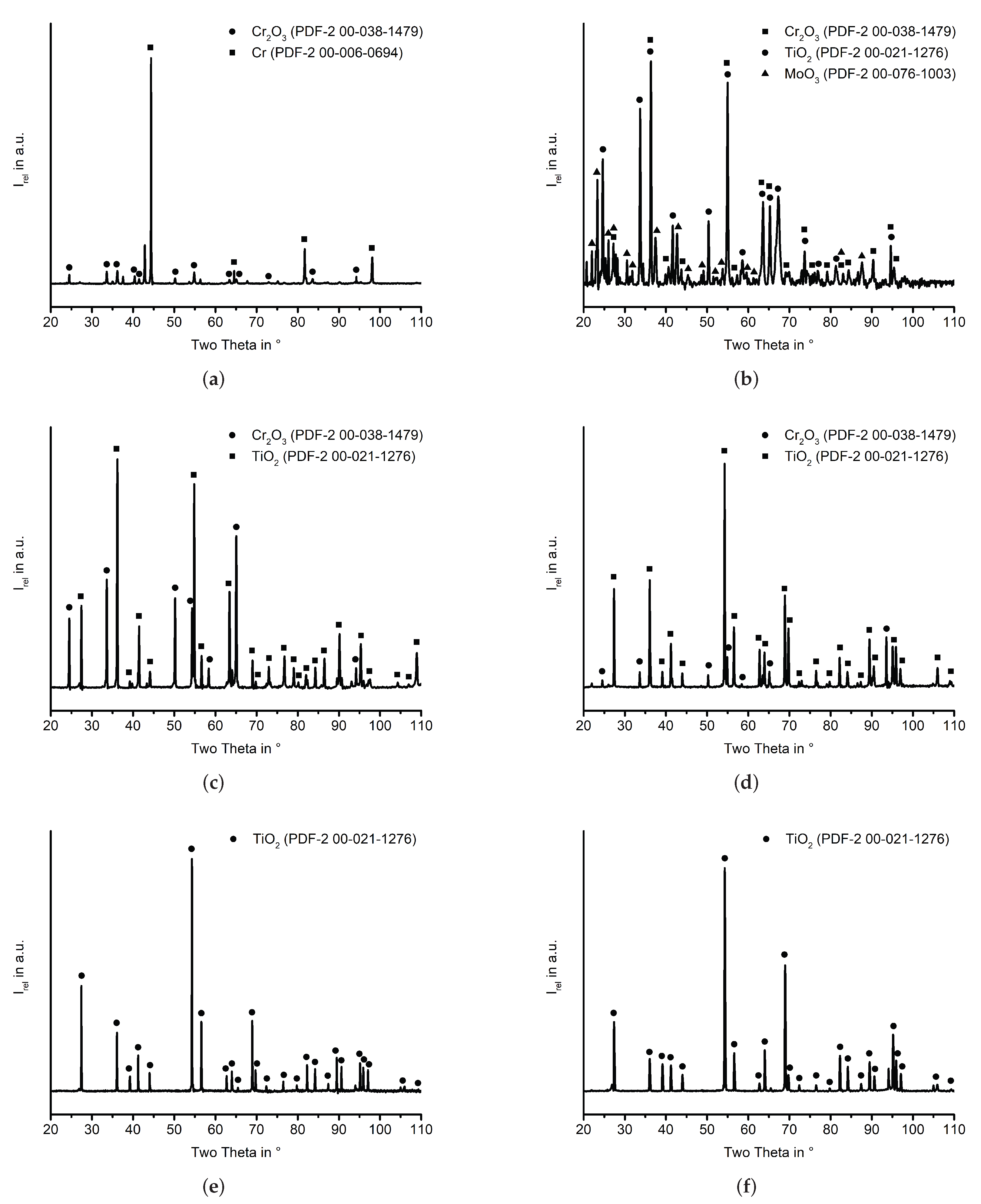
References
- Perepezko, J.H. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef]
- Dimiduk, D.M.; Perepezko, J.H. Mo-Si-B alloys: Developing a revolutionary turbine-engine material. MRS Bull. 2003, 28, 639–645. [Google Scholar] [CrossRef]
- Lemberg, J.A.; Ritchie, R.O. Mo-Si-B alloys for ultrahigh-temperature structural applications. Adv. Mater. 2012, 24, 3445–3480. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, T.; Mendiratta, M.; Dimiduk, D. Oxidation mechanisms in Mo-reinforced Mo5SiB2 (T2)-Mo3Si alloys. Acta Mater. 2002, 50, 1857–1868. [Google Scholar] [CrossRef]
- Bürgel, R.; Maier, H.J.; Niendorf, T. Handbuch Hochtemperatur-Werkstofftechnik: Grundlagen, Werkstoffbeanspruchungen, Hochtemperaturlegierungen und -Beschichtungen; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Reyhani, M.R.; Alizadeh, M.; Fathi, A.; Khaledi, H. Turbine blade temperature calculation and life estimation—A sensitivity analysis. Propuls. Power Res. 2013, 2, 148–161. [Google Scholar] [CrossRef]
- Mazur, Z.; Luna-Ramirez, A.; Juárez-Islas, J.; Campos-Amezcua, A. Failure analysis of a gas turbine blade made of Inconel 738LC alloy. Eng. Fail. Anal. 2005, 12, 474–486. [Google Scholar] [CrossRef]
- Jéhanno, P.; Heilmaier, M.; Kestler, H. Characterization of an industrially processed Mo-based silicide alloy. Intermetallics 2004, 12, 1005–1009. [Google Scholar] [CrossRef]
- Erickson, G.L. A new, third-generation, single-crystal, casting superalloy. JOM 1995, 47, 36–39. [Google Scholar] [CrossRef]
- Azim, M.A.; Burk, S.; Gorr, B.; Christ, H.J.; Schliephake, D.; Heilmaier, M.; Bornemann, R.; Bolívar, P.H. Effect of Ti (macro-)alloying on the high-temperature oxidation behavior of ternary Mo-Si-B alloys at 820–1300 °C. Oxid. Met. 2013, 80, 231–242. [Google Scholar] [CrossRef]
- Schliephake, D.; Azim, M.A.; von Klinski-Wetzel, K.; Gorr, B.; Christ, H.J.; Bei, H.; George, E.P.; Heilmaier, M. High-temperature creep and oxidation behavior of Mo-Si-B alloys with high Ti contents. Metall. Mater. Trans. A 2014, 45, 1102–1111. [Google Scholar]
- Schmitt, A.; Kumar, K.S.; Kauffmann, A.; Li, X.; Stein, F.; Heilmaier, M. Creep of binary Fe-Al alloys with ultrafine lamellar microstructures. Intermetallics 2017, 90, 180–187. [Google Scholar] [CrossRef]
- Rioult, F.; Imhoff, S.; Sakidja, R.; Perepezko, J. Transient oxidation of Mo-Si-B alloys: Effect of the microstructure size scale. Acta Mater. 2009, 57, 4600–4613. [Google Scholar] [CrossRef]
- Maruyama, K.; Yamamoto, R.; Nakakuki, H.; Fujitsuna, N. Effects of lamellar spacing, volume fraction and grain size on creep strength of fully lamellar TiAl alloys. Mater. Sci. Eng. A 1997, 239, 419–428. [Google Scholar] [CrossRef]
- Hasemann, G.; Kaplunenko, D.; Bogomol, I.; Krüger, M. Near-eutectic ternary Mo-Si-B alloys: Microstructures and creep properties. JOM 2016, 68, 2847–2853. [Google Scholar] [CrossRef]
- Schliephake, D.; Kauffmann, A.; Cong, X.; Gombola, C.; Azim, M.; Gorr, B.; Christ, H.J.; Heilmaier, M. Constitution, oxidation and creep of eutectic and eutectoid Mo-Si-Ti alloys. Intermetallics 2019, 104, 133–142. [Google Scholar] [CrossRef]
- Obert, S.; Kauffmann, A.; Seils, S.; Schellert, S.; Weber, M.; Gorr, B.; Christ, H.J.; Heilmaier, M. On the chemical and microstructural requirements for the pesting-resistance of Mo-Si-Ti alloys. J. Mater. Res. Technol. 2020, 9, 8556–8567. [Google Scholar] [CrossRef]
- Unnam, J.; Shenoy, R.; Clark, R. Oxidation of commercial purity titanium. Oxid. Met. 1986, 26, 231–252. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Bianco, R.; Rapp, R.A. Pack cementation diffusion coatings. In Metallurgical and Ceramic Protective Coatings; Springer: Berlin/Heidelberg, Germany, 1996; pp. 236–260. [Google Scholar]
- Ito, K.; Numakura, H.; Hayashi, T.; Yokobayashi, M.; Murakami, T. Oxidation protective silicide coating on Mo-Si-B alloys. Metall. Mater. Trans. A 2005, 36, 627–636. [Google Scholar] [CrossRef]
- Sakidja, R.; Park, J.; Hamann, J.; Perepezko, J. Synthesis of oxidation resistant silicide coatings on Mo-Si-B alloys. Scr. Mater. 2005, 53, 723–728. [Google Scholar] [CrossRef]
- Tang, Z.; Thom, A.J.; Kramer, M.; Akinc, M. Characterization and oxidation behavior of silicide coating on multiphase Mo-Si-B alloy. Intermetallics 2008, 16, 1125–1133. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Sakidja, R. Oxidation-resistant coatings for ultra-high-temperature refractory Mo-based alloys. JOM 2010, 62, 13–19. [Google Scholar] [CrossRef]
- Lange, A.; Heilmaier, M.; Sossamann, T.A.; Perepezko, J.H. Oxidation behavior of pack-cemented Si-B oxidation protection coatings for Mo-Si-B alloys at 1300 °C. Surf. Coat. Technol. 2015, 266, 57–63. [Google Scholar] [CrossRef]
- Schliephake, D.; Gombola, C.; Kauffmann, A.; Heilmaier, M.; Perepezko, J.H. Enhanced oxidation resistance of Mo-Si-B-Ti alloys by pack cementation. Oxid. Met. 2017, 88, 267–277. [Google Scholar] [CrossRef]
- Rioult, F.; Sekido, N.; Sakidja, R.; Perepezko, J.H. Aluminum pack cementation on Mo-Si-B alloys: Kinetics and lifetime prediction. J. Electrochem. Soc. 2007, 154, C692. [Google Scholar] [CrossRef]
- Choi, K.; Yang, W.; Baik, K.H.; Kim, Y.; Lee, S.; Park, J.S. Growth kinetics and isothermal oxidation behavior of aluminide pack coatings on a multiphase Mo-Si-B alloy. Oxid. Met. 2019, 92, 423–437. [Google Scholar] [CrossRef]
- Sakidja, R.; Rioult, F.; Werner, J.; Perepezko, J.H. Aluminum pack cementation of Mo-Si-B alloys. Scr. Mater. 2006, 55, 903–906. [Google Scholar] [CrossRef]
- Trzebiatowski, W.; Ploszek, H.; Lobzowski, J. X-ray analysis of chromium-molybdenum and chromium-tungsten alloys. Anal. Chem. 1947, 19, 93–95. [Google Scholar] [CrossRef]
- Rapp, R.A. Hot corrosion of materials: A fluxing mechanism? Corros. Sci. 2002, 44, 209–221. [Google Scholar] [CrossRef]
- Otsuka, N.; Rapp, R.A. The role of chromium in the hot corrosion of metals. ECS Trans. 2009, 16, 271–282. [Google Scholar] [CrossRef]
- Fauzi, F.; Kurniawan, T.; Salwani, M.; Bin, Y.; Harun, W. Chromium enrichment on P11 ferritic steel by pack cementation. MATEC Web Conf. 2016, 74, 00036. [Google Scholar] [CrossRef]
- Hänni, W.; Hintermann, H. Chemical vapour deposition of chromium. Thin Solid Film. 1977, 40, 107–114. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, Y.; Hu, F.; He, F.; Guo, P. Anti-corrosion performance of chromium-coated steel in a carbon dioxide-saturated simulated oilfield brine. Int. J. Electrochem. Sci. 2017, 12, 5628–5635. [Google Scholar] [CrossRef]
- Leferink, R.; Huijbregts, W. Chromium diffusion coatings for the protection of low-alloy steel in a sulphidizing atmosphere. Corros. Sci. 1993, 35, 1235–1242. [Google Scholar] [CrossRef]
- Lin, N.M.; Xie, F.Q.; Zhou, J.; Zhong, T.; Wu, X.Q.; Tian, W. Microstructures and wear resistance of chromium coatings on P110 steel fabricated by pack cementation. J. Cent. South Univ. Technol. 2010, 17, 1155–1162. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Behnamian, Y.; Luo, H.; Wang, X.Z.; Leitch, M.; Zeng, H.; Luo, J.L. Anticorrosion performance of chromized coating prepared by pack cementation in simulated solution with H2S and CO2. Appl. Surf. Sci. 2017, 419, 197–205. [Google Scholar] [CrossRef]
- Zou, J.; Xie, F.; Lin, N.; Yao, X.; Tian, W.; Tang, B. Formation of chromium coating and comparative examination on corrosion resistance with 13Cr steel in CO2-saturated simulated oilfield brine. Surf. Rev. Lett. 2013, 20, 1350041. [Google Scholar] [CrossRef]
- Gaillard-Allemand, B.; Vilasi, M.; Belmonte, T.; Rives, C.; Czerwiec, T.; Belnet, F.; Kerrec, O.; Michel, H. Passivation of nickel-base superalloy Inconel 690 by pack-cementation chromium coatings. Mater. Sci. Forum 2001, 369, 735–742. [Google Scholar] [CrossRef]
- Ledoux, X.; Vilasi, M.; Mathieu, S.; Pantiex, P.J.; Del-Gallo, P.; Wanger, M. Development of chromium and aluminum coatings on superalloys by pack-cementation technique. Adv. Mater. Res. 2011, 278, 491–496. [Google Scholar] [CrossRef]
- Mazille, H.M. Chemical vapour deposition of chromium onto nickel. Thin Solid Films 1980, 65, 67–74. [Google Scholar] [CrossRef]
- Holleman, A.F. Lehrbuch der Anorganischen Chemie; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2019. [Google Scholar]
- Murray, J. The Cr-Ti (chromium-titanium) system. Bull. Alloy Phase Diagr. 1981, 2, 174–181. [Google Scholar] [CrossRef]
- Yabe, H.; Kuji, T. Mechanically driven bcc TiCr alloy and its hydrogen solubility. J. Alloys Compd. 2005, 404, 533–536. [Google Scholar] [CrossRef]
- Liu, Z.; Welsch, G. Literature survey on diffusivities of oxygen, aluminum, and vanadium in alpha titanium, beta titanium, and in rutile. Metall. Trans. A 1988, 19, 1121–1125. [Google Scholar] [CrossRef]
- Amherd Hidalgo, A.; Frykholm, R.; Ebel, T.; Pyczak, F. Powder metallurgy strategies to improve properties and processing of titanium alloys: A review. Adv. Eng. Mater. 2017, 19, 1600743. [Google Scholar] [CrossRef]
- Maier, K.; Mehrer, H.; Rein, G. Self-diffusion in molybdenum. Int. J. Mater. Res. 1979, 70, 271–276. [Google Scholar] [CrossRef]
- Kroll, S.; Mehrer, H.; Stolwijk, N.; Herzig, C.; Rosenkranz, R.; Frommeyer, G. Titanium self-diffusion in the intermetallic compound γ-TiAl. Int. J. Mater. Res. 1992, 83, 591–595. [Google Scholar] [CrossRef]
- Mortimer, R.G. Physical Chemistry; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Ulrich, A.S.; Pfizenmaier, P.; Solimani, A.; Glatzel, U.; Galetz, M.C. Improving the oxidation resistance of Cr-Si-based alloys by ternary alloying. Corros. Sci. 2020, 165, 108376. [Google Scholar] [CrossRef]
- Briant, C.; Wang, Z.; Chollocoop, N. Hydrogen embrittlement of commercial purity titanium. Corros. Sci. 2002, 44, 1875–1888. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D. The hydrogen embrittlement of titanium-based alloys. JOM 2005, 57, 46–49. [Google Scholar] [CrossRef]
- Madina, V.; Azkarate, I. Compatibility of materials with hydrogen. Particular case: Hydrogen embrittlement of titanium alloys. Int. J. Hydrogen Energy 2009, 34, 5976–5980. [Google Scholar] [CrossRef]
- Gomes, J.; Huntz, A. Comparison of the kinetics and morphologic properties of titanium, Ti-1.5 Ni and Ti-2.5 Cu during oxidation in pure oxygen between 600 and 820 °C. Oxid. Met. 1980, 14, 471–498. [Google Scholar] [CrossRef]
- Epifano, E.; Monceau, D. Ellingham diagram: A new look at an old tool. Corros. Sci. 2023, 217, 111113. [Google Scholar] [CrossRef]
- Soleimani-Dorcheh, A.; Galetz, M. Oxidation and nitridation behavior of Cr-Si alloys in air at 1473 K. Oxid. Met. 2015, 84, 73–90. [Google Scholar] [CrossRef]
- Solimani, A.; Nguyen, T.; Zhang, J.; Young, D.J.; Schütze, M.; Galetz, M.C. Morphology of oxide scales formed on chromium-silicon alloys at high temperatures. Corros. Sci. 2020, 176, 109023. [Google Scholar] [CrossRef]
- Royer, L.; Ledoux, X.; Mathieu, S.; Steinmetz, P. On the oxidation and nitridation of chromium at 1300 °C. Oxid. Met. 2010, 74, 79–92. [Google Scholar] [CrossRef]
- Caplan, D.; Cohen, M. The volatilization of chromium oxide. J. Electrochem. Soc. 1961, 108, 438. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Andrew, K.F. Kinetics of the oxidation of chromium. J. Electrochem. Soc. 1957, 104, 334. [Google Scholar] [CrossRef]
- Lillerud, K.; Kofstad, P. On high temperature oxidation of chromium: I. Oxidation of annealed, thermally etched chromium at 800–1100 °C. J. Electrochem. Soc. 1980, 127, 2397. [Google Scholar] [CrossRef]
- Schütze, M. Stress effects in high temperature oxidation. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar]
- Hagel, W.C. Factors controlling the high-temperature oxidation of chromium. J. Electrochem. Soc. 1962, 109, C78. [Google Scholar]
- Grimley, R.; Burns, R.; Inghram, M.G. Thermodynamics of the vaporization of Cr2O3: Dissociation energies of CrO, CrO2, and CrO3. J. Chem. Phys. 1961, 34, 664–667. [Google Scholar] [CrossRef]
- Graham, H.; Davis, H. Oxidation/Vaporization kinetics of Cr2O3. J. Am. Ceram. Soc. 1971, 54, 89–93. [Google Scholar] [CrossRef]
- Kofstad, P.; Lillerud, K. Chromium transport through Cr2O3 scales I. On lattice diffusion of chromium. Oxid. Met. 1982, 17, 177–194. [Google Scholar] [CrossRef]
- Lillerud, K.; Kofstad, P. Chromium transport through Cr2O3 scales II. Changes in scale morphology during high vacuum treatment of oxidized chromium specimens. Oxid. Met. 1982, 17, 195–203. [Google Scholar] [CrossRef]
- Hinrichs, F.; Kauffmann, A.; Tirunilai, A.S.; Schliephake, D.; Beichert, B.; Winkens, G.; Beck, K.; Ulrich, A.S.; Galetz, M.C.; Long, Z.; et al. A novel nitridation-and pesting-resistant Cr-Si-Mo alloy. Corros. Sci. 2022, 207, 110566. [Google Scholar] [CrossRef]

| Substrate | Powder Composition | Temperature |
|---|---|---|
| Mo-20.0Si-52.8Ti | 50 Cr, NHBr, AlO | 1100 °C |
| Mo-21.0Si-34.0Ti | 15 Cr, CrCl, AlO | 1100 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, K.; Hinrichs, F.; Oskay, C.; Ulrich, A.S.; Heilmaier, M.; Galetz, M.C. Chromium Diffusion Coatings for Mo-Based Silicides to Improve Their Oxidation Resistance. Coatings 2023, 13, 1712. https://doi.org/10.3390/coatings13101712
Beck K, Hinrichs F, Oskay C, Ulrich AS, Heilmaier M, Galetz MC. Chromium Diffusion Coatings for Mo-Based Silicides to Improve Their Oxidation Resistance. Coatings. 2023; 13(10):1712. https://doi.org/10.3390/coatings13101712
Chicago/Turabian StyleBeck, Katharina, Frauke Hinrichs, Ceyhun Oskay, Anke S. Ulrich, Martin Heilmaier, and Mathias C. Galetz. 2023. "Chromium Diffusion Coatings for Mo-Based Silicides to Improve Their Oxidation Resistance" Coatings 13, no. 10: 1712. https://doi.org/10.3390/coatings13101712
APA StyleBeck, K., Hinrichs, F., Oskay, C., Ulrich, A. S., Heilmaier, M., & Galetz, M. C. (2023). Chromium Diffusion Coatings for Mo-Based Silicides to Improve Their Oxidation Resistance. Coatings, 13(10), 1712. https://doi.org/10.3390/coatings13101712






