Stress Corrosion Cracking of Copper–Nickel Alloys: A Review
Abstract
:1. Introduction
2. Corrosion of Cu-Ni Alloys
2.1. Basic Corrosion Mechanisms
- Cathodic
- Anodic
2.2. Effect of Environments
- Chlorine
- the adsorption of Cl− in the oxide layer will destroy its structure and integrity;
- Cl− may inhibit the formation or passivation of the surface oxide layer;
- Cl−-induced electric field will accelerate the dissolution of the substrate.
- Sulfide
- Ammonium
3. SCC Studies of Cu-Ni Alloys
3.1. SCC Testing Methods
3.1.1. Slow Strain Rate Test
3.1.2. Constant Loading Method
3.1.3. Constant Strain Method
3.2. SCC Performance
3.2.1. Sulfide
3.2.2. Ammonium
3.2.3. Sulfide + Ammonium−
3.2.4. Strain Rate
3.2.5. Temperature
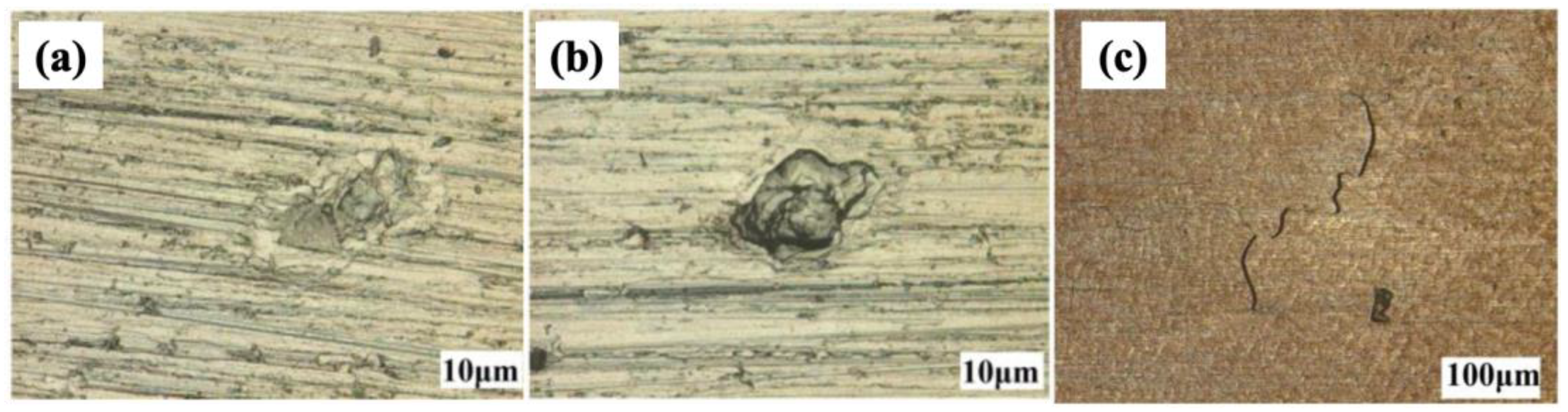
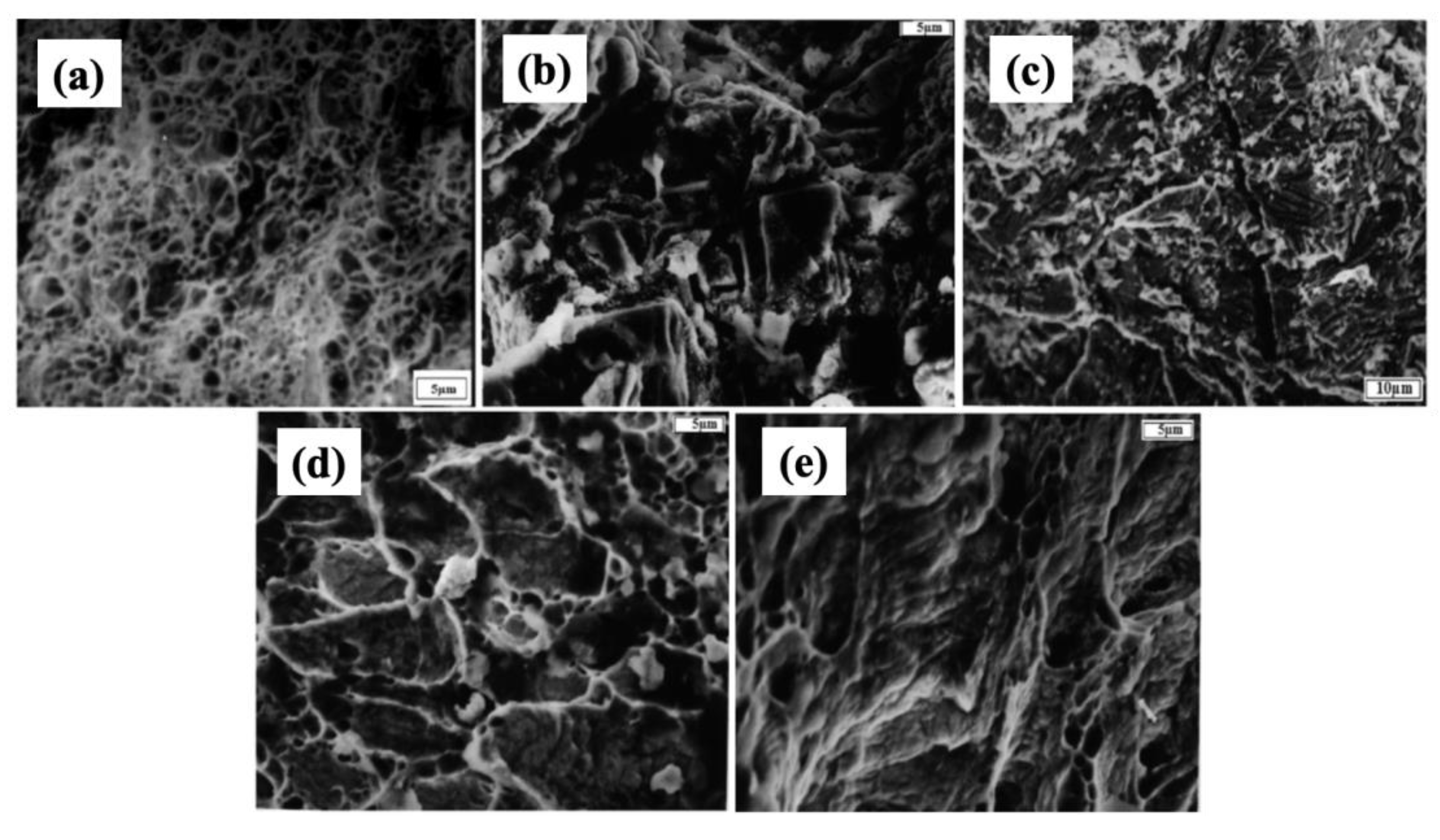
3.3. SCC Mechanisms
4. Corrosion Cracking Mitigation
4.1. Inhibitors
4.2. Alloying Elements
4.3. Remove or Reduce the Stress Level
5. Summary and Future Directions
5.1. Summary
5.2. Future Directions
- Using advanced characterization techniques to characterize the structure degradation mechanisms at the atomic level, especially for the atoms at the crack tip.
- Enlarging the SCC testing environments. More testing factors, such as the presence of NO3− or other ions, a wider range of testing temperature, and flow conditions, should be taken into consideration during the SCC tests.
- Exploring other corrosion protection methods, such as coatings, to reduce SCC susceptibility. The related SCC mechanisms with coatings should also be illustrated. In addition, effective surface modification methods, such as laser processing [40], might also be used to inhibit the initiation of SCC cracks.
- Making modifications to the current operating practices and reducing the possibility of crack growth. In addition, finding effective methods to repair the existing cracks.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. A Summary of the SCC of Cu-Ni Alloys in Different Environments
| Year | Materials | Solution | Temperature | SCC Test Method | Time to Failure | Main Finding- | References |
|---|---|---|---|---|---|---|---|
| 1961 | Cu-Ni | seawater | - | - | - | Immune to SCC | [18] |
| 1968 | α Cu | Cu(NO3)2 | - | - | - | Susceptibility of Cu–Ni alloys to intergranular SCC in Cu(NO3)2 solutions. | [41] |
| 1973 | Cu-10Ni | moist NH3 | - | U-bend | 1000 h | Stress corrosion resistance was improved by solutionized Fe, but damaged by precipitation of Fe. | [35] |
| 1974 | Cu-Zn-Al | pure water/steam | 150–300 °C | - | 1000 h | Low-Zn alloys: intergranular SCC high-Zn: layer corrosion, immune to IGSCC. | [42] |
| Cu-10Ni/30Ni | 300–350 °C | 500 h | Accelerated intergranular SCC due to the equilibrium grain boundary segregation of active metals. | ||||
| Monel metal | 350 °C | No SCC (due to metallurgical factors and service duration). | |||||
| 1975 | 25 copper-base alloys | industrial and marine environments. | - | - | - | Only a few are susceptible to SCC | [19] |
| 1979 | - | - | - | SSRT | - | Strain rates between 10−7 and 10−5 s−1 promote SCC | [43] |
| 1987 | CuZn\CuZnMn\CuZnNi | PH4-14 Mattsson’s solution | RT | U-bend | - | Stress corrosion of copper alloys is controlled by anodic crack tip dissolution | [44] |
| 1991 | Cu-10Ni | Na2S | RT | SSRT | - | Cu-10Ni is susceptible to intergranular SCC in concentrated pure sulfide solutions, but not in dilute solutions | [22] |
| 1992 | Cu-10Ni, Cu-30Ni | chloride + sulfide | - | - | - | No SCC. The passive surface is responsible for the resistance to chloride and sulfide SCC. | [45] |
| 1994 | Cu-30Ni | NaCl + S2− | 25–70 °C | SSRT | - | The most severe SCC on a Cu-30Ni in seawater with 3120 ppm S2− at 25 °C. | [21] |
| Cu-10Ni | SCC of Cu-10Ni is not affected by S2− or T. | ||||||
| 1997 | Cu-10Ni | NaCl + S2− | 20 °C | SSRT | 97 h for 100 ppm; ~41 h for 1000 ppm | Two SCC mechanisms: anodic dissolution-low c(S2−), hydrogen embrittlement-high c(S2−). | [16] |
| 2002 | Cu-5.37Ni | 5% NH3 | 26 ± 1 °C | SSRT | 16–17 days | Brittle transgranular SCC. MgCl2 is beneficial when low nickel copper alloys are exposed to ammonia. | [34] |
| NaCl + NH3 + MgCl2 | 20–21 days | ||||||
| air | 26 days | ||||||
| 2002 | Cu-5.37Ni | air | 28 ± 1 °C | SSRT | 24 days 18 h | NO SCC. | [46] |
| NaCl + MgCl2 | 23 days 0 h | NH3 reacts directly with Cu and Ni resulting in blue–green complexes and shows corrosion-assisted failure. No clear evidence of SCC. | |||||
| NaCl + MgCl2 + NH3 | 17 days 2 h | ||||||
| 2002 | Cu10Ni | NaCl + Na2S | 24 ± 1 °C | SSRT | 0.98/0.70 | Susceptible to SCC, mainly intergranular. Mechanism: sulfide stress corrosion cracking assisted with pitting corrosion for Cu10Ni at slip steps. | [47] |
| 2002 | 90Cu-10Ni | NaCl + S2− | 25/50/70 °C | SSRT | 40.8/27.8/25.0 h | The deterioration percentage of SCC of the Cu-10Ni specimen in polluted seawater was found to range between 19.6 and 39% (200 ppm, 70 °C) | [31] |
| 70Cu-30Ni | 95.3/73.1/74.2 h | 70Cu-30Ni -between 19.4 and 27.3% (1000 ppm, 50 °C; 3120 ppm, 70 °C) | |||||
| 2003 | Cu-5.37% Ni | 3.5 wt.% NaCl + 10.0 wt.% MgCl2 + 1.0 wt.% NH3 | 28 ± 1 °C | SSRT | 62.93 h | 1. With NH3, failure changed from ductile to brittle intergranular SCC. 2. Inhibition of SCC by MgCl2. | [33] |
| 2003 | Cu-5.37Ni | air/5/10% NH3 | 28 + 1 °C | SSRT | 75.54/57.26/52.14 h | Intergranular SCC was observed to occur below the elastic limit in the presence of the superimposed cyclic stress, which propagates by transgranular SCC mechanism. | [25] |
| 2004 | CuAl9Ni3Fe2 | sea water | - | SSRT | Cross-section reduced by 20% in 3 weeks | Intergranular crack; second phases play a key role. | [48] |
| 2005 | Cu-Ni-Al-Mn-Nb | NaCl + acetic acid + hydrogen sulfide | 82 °C | SSRT | 720 h | Brittle intergranular failure. With Ni up to 25%, it is resistant to hydrogen embrittlement, sulfide stress corrosion, and stress corrosion in NH3. | [49] |
| MTA150 (Cu-Ni14.9-Sn7.9-C72900) | 180 h | Susceptibility to stress corrosion; brittle intergranular fracture | |||||
| 2005 | Cu-5.37%Ni | NH3/NH3 + NaCl/NH3 + NaCl + MaCl2 | 28 ± 3 °C | SSRT | 57.26/57.37/62.73 h | Brittle SCC failures. Superimposed cyclic stresses on prestressed specimens accelerate the stress corrosion failures in an ammoniacal environment. | [27] |
| 2005 | Cu-5.37%Ni | 10% NH3 + x% MgCl2 | 28 ± 3 °C | SSRT | 40.91–97.58 h | Intergranular SCC. Beyond 7.5% MgCl2 completely restores the ductility loss that was observed under the influence of ammonia alone. | [28] |
| 2006 | Cu-10Ni | wet H2S | 35–120 °C | - | - | The failure of a Cu-10Ni bundle used in the overhead condenser of a steam cracker unit was attributed to intergranular SCC caused by wet H2S condensed in the overhead column. | [50] |
| 2007 | UNS C72900 | ISO 15156-3 [51] | RT | - | 30 days | Cold-worked and hardened UNS C72900 is more prone to SSC and SCC than hot-worked and hardened, and exhibited intergranular fracture with transgranular branching. | [52] |
| 2007 | Cu-30Ni | 18% monoethanolamine (MEA) | 60/90 °C | constantextension rate test (CERT) technique | 81/65 h | Susceptible to transgranular SCC in the 90 °C aerated MEA solution of pH 9. Cracking mechanism: stress-assisted dissolution of copper from the alloy matrix. | [32] |
| 2009 | Cu-10Ni | 7.5% NH3/Na2S | RT | SSRT | 67.57 h/26.12 h | Crack propagation depends on the concentration and the exposure to the environment post-crack initiation. Better SCC resistance due to higher Ni. | [9] |
| 2011 | Cu-10Ni | NaCl + S2− | RT | 5.0 × 10−6s−1 | 397 min | S2− increases the susceptibility of brittle transgranular SCC at different strain rates. | [30] |
| NaCl + S2− + glycine | 420 min | Glycine is a great SCC inhibitor. Surface adsorption inhibits anodic dissolution | |||||
| NaCl + S2− + glycine + KI | 430 min | Synergistic effect of KI and glycine improves SCC resistance in sulfide-polluted saltwater | |||||
| 2011 | Cupronickel | NH3/S2− | - | - | - | 1. Ni in Cu enhances the SCC resistance, while Zn decreases it. 2. Two SCC mechanisms: Passive-film rupture and de-alloying. 3. Mitigate the SCC: heat treatment and inhibitors. | [8] |
| 2013 | C70600\C76400 | - | - | ISO 6957 [53] | 1 year | NO SCC. Corrosion rates under tensile timevarying load is 39% higher than untensioned fixed configuration. | [4] |
| 2018 | CuNiSn | sour H2S | 150 ± 5 °C | 4PB | 720 h | SCC: cracks up to 3 mm deep, severe depletion of Cu close to the cracks due to selective corrosion | [54] |
| simulated seawater | NO HSC. | ||||||
| 2019 | KS C1220, UNS C12200 | heating water supply system of a residential building | 10 °C | U-bolt | - | Susceptible to SCC and exhibits 4-stage fracture process. | [55] |
| 2021 | Cu-27%Ni | Mattsson’s solution | - | SSRT | 2301/7037 sec without/with BTA | 1. BTA as a SCC inhibitor by forming a protective surface film. 2. Crack propagation regime is controlled by the BTA concentration. 3. Fracture type changes from intergranular brittle to ductile. | [26] |
| 2021 | Cu-10Ni (UNS C70600) | 10% NH3/10% Na2S/mixture of both | RT | C-ring | 15 days | 1. In the incubation stage of SCC with slip dissolution mechanism accompanied by pitting and intergranular corrosion. 2. No well-defined effect of tensile stress on the corrosion rate. | [29] |
| 2023 | Cu-30Ni | NH3 | 65 ± 0.2℃ | C-rings | 7 days | 1. NH4+/stress increases the depth and diameter of the pits and the number of pits in the specimens. 2. Pitting is the synergistic effect of stress and NH4+. | [13] |
| 2023 | Phosphorus deoxidized copper | 3.5 wt%NaCl | RT | SSRT | 2 days (BJ)/4.5 days (MJ) | Worse SCC and poorer fracture toughness on Brazed joint (BJ) than mechanical joint (MJ) due to a brittle surface copper oxide layer | [17] |
References
- ASTM B111-98(2004); Standard Specification for Copper and Copper-Alloy Seamless Condenser Tubes and Ferrule Stock. ASTM International: West Conshohocken, PA, USA, 2004.
- Zhang, P.; Meng, G.; Wang, Y.; Lei, B.; Wang, F. Significantly enhanced corrosion resistance of Ni–Cu coating modified by minor cerium. Corros. Commun. 2021, 2, 72–81. [Google Scholar] [CrossRef]
- Tolulope Loto, R. Correlative investigation of the corrosion susceptibility of C70600 and C26000 copper based alloys for application in seawater environment. Mater. Today Proc. 2022, 65, 2151–2155. [Google Scholar] [CrossRef]
- Drach, A.; Tsukrov, I.; DeCew, J.; Aufrecht, J.; Grohbauer, A.; Hofmann, U. Field studies of corrosion behaviour of copper alloys in natural seawater. Corros. Sci. 2013, 76, 453–464. [Google Scholar] [CrossRef]
- Mathiyarasu, J.; Palaniswamy, N.; Muralidharan, V. Corrosion resistance of cupronickels—An overview. Corros. Rev. 2000, 18, 65–103. [Google Scholar] [CrossRef]
- Shi, R.; Tu, Y.; Gao, K.; Qiao, L.; Pang, X. High stress corrosion cracking resistance of in-situ nanoparticle strengthened steel. Corros. Commun. 2022, 5, 14–24. [Google Scholar] [CrossRef]
- Dong, C.; Ji, Y.; Wei, X.; Xu, A.; Chen, D.; Li, N.; Kong, D.; Luo, X.; Xiao, K.; Li, X. Integrated computation of corrosion: Modelling, simulation and applications. Corros. Commun. 2021, 2, 8–23. [Google Scholar] [CrossRef]
- Kannan, M.B.; Shukla, P.K. Stress Corrosion Cracking (SCC) of Copper and Copper-Based Alloys. In Stress Corrosion Cracking; Woodhead Publishing: Sawston, UK, 2011; pp. 409–426. [Google Scholar]
- Agarwal, D.C.; Bapat, A.M. Effect of Ammonia and Sulphide Environment on 90/10 and 70/30 Cupronickel Alloy. J. Fail. Anal. Prev. 2009, 9, 444–460. [Google Scholar] [CrossRef]
- Li, B.-Z.; Zhang, Z.-Q.; Qiu, Z.-H.; Meng, X.; Zhao, Y.-B.; Zhang, H.-B.; Hou, J.; Sun, J.; Lin, C.-G.; Zeng, R.-C. Insight to corrosion mechanism of 90/10 copper-nickel alloys under different sea depths. Mater. Lett. 2021, 303, 130513. [Google Scholar] [CrossRef]
- Zhu, X.; Lei, T. Characteristics and formation of corrosion product of 70Cu-30Ni alloy in seawater. Corros. Sci. 2002, 44, 67–79. [Google Scholar] [CrossRef]
- Crundwell, F.K. The anodic dissolution of 90% copper-10% nickel alloy in hydrochloric acid solutions. Electrochim. Acta 1991, 36, 2135–2141. [Google Scholar] [CrossRef]
- Xing, H.; Du, M.; Huang, G.; Ma, L. Acceleration of pitting corrosion of 70Cu–30Ni alloy in seawater by NH4+ and stress. J. Mater. Res. Technol. 2023, 23, 221–237. [Google Scholar] [CrossRef]
- Ma, A.L.; Jiang, S.L.; Zheng, Y.G.; Ke, W. Corrosion product film formed on the 90/10 copper–nickel tube in natural seawater: Composition/structure and formation mechanism. Corros. Sci. 2015, 91, 245–261. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Cui, Y.; Li, Y.; Wang, F. Influence of hydrostatic pressure on the corrosion behavior of 90/10 copper-nickel alloy tube under alternating dry and wet condition. Corros. Sci. 2019, 146, 202–212. [Google Scholar] [CrossRef]
- Domiaty, A.E.; Alhajji, J.N. The susceptibility of 90Cu-10Ni alloy to stress corrosion cracking in seawater polluted by sulfide ions. J. Mater. Eng. Perform. 1997, 6, 534–544. [Google Scholar] [CrossRef]
- Anaman, S.Y.; Sung, H.-M.; Yu, H.G.; Rho, N.; Kim, J.; Lee, J.-S.; Han, H.N.; Cho, H.-H. Study on the Microstructure and Corresponding Stress Corrosion Cracking Behavior of Joints of Copper Tubes. Met. Mater. Int. 2023. [Google Scholar] [CrossRef]
- Thompson, D.H. A simple stress-corrosion-cracking test for Copper alloys. Mater. Res. Stand. 1961, 1, 108–111. [Google Scholar]
- Popplewell, J.M.; Gearing, T.V. Stress corrosion resistance of some copper base alloys in natural atmospheres. Corrosion 1975, 31, 279–286. [Google Scholar] [CrossRef]
- ASTM G129-21; Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking. ASTM International: West Conshohocken, PA, USA, 2021.
- Habib, K.; Husain, A. Stress corrosion cracking of copper-nickel alloys in sulplxidepolluted natural seawater at moderate temperatures. Desalination 1994, 97, 29–34. [Google Scholar] [CrossRef]
- Islam, M.; Riad, W.T.; Al-Kharraz, S.; Abo-Namous, S. Stress Corrosion Cracking Behavior of 90/10 Cu-Ni Alloy in Sodium Sulfide Solutions. Corrosion 1991, 47, 260–268. [Google Scholar] [CrossRef]
- LaQue, F.L. Marine Corrosion: Causes and Prevention; Wiley Interscience: Weinheim, Germany, 1975. [Google Scholar]
- Pinchback, T.R.; Wilkinson, G.A.; Heldt, L.A. Stress corrosion cracking of 60/40 cupro-nickel alloy: Fractography and chemical analysis. Corrosion 1975, 31, 197–201. [Google Scholar] [CrossRef]
- Agarwal, D.C. Effect of cyclic stresses on stress corrosion cracking of Cu–Ni alloy. Corros. Eng. Sci. Technol. 2003, 38, 275–285. [Google Scholar] [CrossRef]
- Johar, M.H.; Torbati-Sarraf, H.; Ahangari, M.; Saremi, M. Inhibiting effect of Benzotriazole on the stress corrosion cracking of Cu-27%Ni cupronickel and Cu-30%Zn brass in Mattsson’s solution. Mater. Lett. 2021, 293, 129735. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Sarin, S.; Bapat, A.M.; Seshadari, S.; Vishwakarma, R. Mitigation of ammonia-induced SCC in a cupronickel alloy by additions of MgCl2 Part 1. J. Fail. Anal. Prev. 2005, 5, 64–69. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Sarin, S.; Wadhwa, K.; Vishwakarma, R.; Deshmukh, M.B.; Kurian, S. Mitigation of ammonia-induced SCC in a cupronickel alloy by additions of MgCl2 Part 2. J. Fail. Anal. Prev. 2005, 5, 70–78. [Google Scholar] [CrossRef]
- Ardy, H.; Sasmita, F.; Pradana, E.A.P. The corrosion study of 90Cu-10Ni (UNS C70600) materials in ammonia and sulfide environments. In Proceedings of the 13th Aun/Seed-Net Regional Conference on Materials (RCM 2020) and the 1st International Conference on Materials Engineering and Manufacturing (ICMEM 2020), Yogyakarta, Indonesia, 27–28 January 2021. [Google Scholar]
- Abdel Nazeer, A.; Allam, N.K.; Youssef, G.I.; Ashour, E.A. Effect of Glycine on the Electrochemical and Stress Corrosion Cracking Behavior of Cu10Ni Alloy in Sulfide Polluted Salt Water. Ind. Eng. Chem. Res. 2011, 50, 8796–8802. [Google Scholar] [CrossRef]
- Habib, K.; Almatar, O.; Alfeeli, B. Risk assessment and evaluation of materials commonly used in desalination plants subjected to stress corrosion cracking in polluted marine environment. Desalination 2002, 153, 217–221. [Google Scholar] [CrossRef]
- Shalaby, H.M.; Husain, A.; Hasan, A.A.; Abdullah, A.Y. Electrochemical behaviour and stress corrosion cracking of 70: 30 copper–nickel alloy in monoethanolamine solutions. Corros. Eng. Sci. Technol. 2007, 42, 64–72. [Google Scholar] [CrossRef]
- Agarwal, D.C. Effect of Ammonia Concentration on Environment-Assisted Failures in a Low-Nickel Copper Alloy. Pract. Fail. Anal. 2003, 5, 58–68. [Google Scholar] [CrossRef]
- Agarwal, D.C. Stress corrosion in copper–nickel alloys: Influence of ammonia. Br. Corros. J. 2002, 37, 267–275. [Google Scholar] [CrossRef]
- Popplewell, J.M. The effect of iron on the stress corrosion resistance of 90/10 cupro-nickel in ammoniacal environment. Corros. Sci. 1973, 13, 593–603. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, X.; Wang, H.; Yang, Y.; Baker, I.; Wang, P.; Wu, H. Unraveling the inherent anisotropic properties of in situ alloyed copper-modified titanium alloys produced by laser powder bed fusion. J. Alloys Compd. 2023, 966, 171323. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, W.; Lin, X.; Huang, C.; Liu, F.; Huang, W.; Wang, P.; Li, X. Effect of isothermal temperature on bainite transformation, microstructure and mechanical properties of LSFed 300M steel. Mater. Today Commun. 2020, 25, 101452. [Google Scholar] [CrossRef]
- Gao, H.-J.; Zhang, Y.-D.; Wu, Q.; Song, J. Experimental investigation on the fatigue life of Ti-6Al-4V treated by vibratory stress relief. Metals 2017, 7, 158. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, W.; Lin, X.; Huang, C.; Wang, Z.; Liu, F.; Huang, W.; Wang, P.; Li, X. Achieving superior ductility for laser directed energy deposition 300 M steel through isothermal bainitic transformation. J. Manuf. Process. 2020, 60, 426–434. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, F.; Gao, J.; Liu, F.; Huang, C.; Zheng, H.; Wang, P.; Qiu, H. Effect of different heat input on the microstructure and mechanical properties of laser cladding repaired 300M steel. J. Mater. Res. Technol. 2023, 22, 556–568. [Google Scholar] [CrossRef]
- Craig, J.; Montague, W.; Pugh, E. Factors influencing the path of stress-corrosion cracking in alpha-phase copper alloys exposed to aqueous ammonia environments. ASM TRANS Q 1968, 61, 468–473. [Google Scholar]
- Shiro Sato, K.N. Stress Corrosion Cracking of Copper Alloys in Pure Steam and Water at High Temperatures. Boshoku Gijutsu 1974, 23, 125–133. [Google Scholar]
- Ugiansky, G.; Payer, J. Stress Corrosion Cracking: The Slow Strain-Rate Technique; ASTM International: West Conshohocken, PA, USA, 1979; Volume 665. [Google Scholar]
- Parthasarathi, A.; Polan, N.W. Stress Corrosion Cracking of Copper Alloys: The Effects of Corrosion Potential and Tarnishing Characteristics. Corrosion 1987, 43, 747–755. [Google Scholar] [CrossRef]
- Powell, C.A. Cu-Ni Alloy Resistance to Corrosion and Biofouling; Copper Development Association Inc.: New York, NY, USA, 1992. [Google Scholar]
- Agarwal, D.C. Effect of ammoniacal sea water on material properties of copper–nickel alloy. Br. Corros. J. 2002, 37, 105–113. [Google Scholar] [CrossRef]
- Sayed, S.M.; Ashour, E.A.; Youssef, G.I.; El-Raghy, S.M. Effect of sulfide ions on the stress corrosion behaviour of Al-brass and Cu10Ni alloys in salt water. J. Mater. Sci. 2002, 37, 2267–2272. [Google Scholar] [CrossRef]
- Fonlupt, S.; Bayle, B.; HeuzÈ, J.L.; Delafosse, D. Stress corrosion cracking of CuAl9Ni3Fe2. In Proceedings of the EUROCORR 2004, Nice, France, 12–16 September 2004. [Google Scholar]
- Tuck, C.D.S. The development of very high strength copper alloys with resistance to hydrogen embrittlement and stress corrosion. In Proceedings of the CORROSION 2005, Houston, TX, USA, 3–7 April 2005; p. 05462. [Google Scholar]
- Veld, A. Rapid failure of a Copper/Nickel overhead condenser bundle. Mater. Perform. 2006, 45, 52–54. [Google Scholar]
- ISO 15156-3; Petroleum and Natural Gas Industries: Materials for Use in H2S-Containing Environments in Oil and Gas Production. Part 3: Cracking-Resistant Cras (Corrosion-Resistant Alloys) and Other Alloys. ISO: Genebra, Switzerland, 2015.
- Cribb, W.R. Sour service testing of High strength TS temper CuNiSn alloy (UNS C72900). In Proceedings of the NACE CORROSION 2007, Nashville, TN, USA, 11–15 March 2007; p. NACE-07100. [Google Scholar]
- ISO 6957; Copper Alloys—Ammonia Test for Stress Corrosion Resistance. ISO: Genebra, Switzerland, 1988.
- Johnsen, R.; Lange, T.; Stenerud, G.; Olsen, J.S. Environmentally assisted degradation of spinodal copper alloy C72900. Corros. Sci. 2018, 142, 45–55. [Google Scholar] [CrossRef]
- Chae, H.; Wang, H.; Hong, M.; Kim, W.C.; Kim, J.-G.; Kim, H.; Lee, S.Y. Stress Corrosion Cracking of a Copper Pipe in a Heating Water Supply System. Met. Mater. Int. 2019, 26, 989–997. [Google Scholar] [CrossRef]


| Cu | Ni | Fe | Mn | Sn | C | Pb | S | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Cu-10Ni | Bal. | 9.0–11.0 | 1.2–2.0 | 0.5–1.0 | <0.02 | <0.05 | <0.03 | <0.05 | <0.5 |
| Cu-90Ni | Bal. | 29.0–32.0 | 0.4–1.0 | 0.5–1.5 | <0.02 | <0.06 | <0.03 | <0.06 | <0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, K.; Gao, J.; Liu, Y.; Qin, C.; Li, J.; Li, Y.; Cao, W.; Zhai, Y.; Huang, G. Stress Corrosion Cracking of Copper–Nickel Alloys: A Review. Coatings 2023, 13, 1690. https://doi.org/10.3390/coatings13101690
Li D, Li K, Gao J, Liu Y, Qin C, Li J, Li Y, Cao W, Zhai Y, Huang G. Stress Corrosion Cracking of Copper–Nickel Alloys: A Review. Coatings. 2023; 13(10):1690. https://doi.org/10.3390/coatings13101690
Chicago/Turabian StyleLi, Dandan, Kaiyang Li, Jiajie Gao, Yunfeng Liu, Chao Qin, Jianfeng Li, Yongshuai Li, Wei Cao, Yunlong Zhai, and Guojie Huang. 2023. "Stress Corrosion Cracking of Copper–Nickel Alloys: A Review" Coatings 13, no. 10: 1690. https://doi.org/10.3390/coatings13101690
APA StyleLi, D., Li, K., Gao, J., Liu, Y., Qin, C., Li, J., Li, Y., Cao, W., Zhai, Y., & Huang, G. (2023). Stress Corrosion Cracking of Copper–Nickel Alloys: A Review. Coatings, 13(10), 1690. https://doi.org/10.3390/coatings13101690






