A Facile Two-Step PVP-Assisted Deposition of Co-Activated Nanosized Nickel Hydroxide Directly on a Substrate for Large-Scale Production of Supercapacitor Electrodes
Abstract
1. Introduction
solid-state reaction
summary reaction
2. Materials and Methods
2.1. Reactants
2.2. Structural Characterization
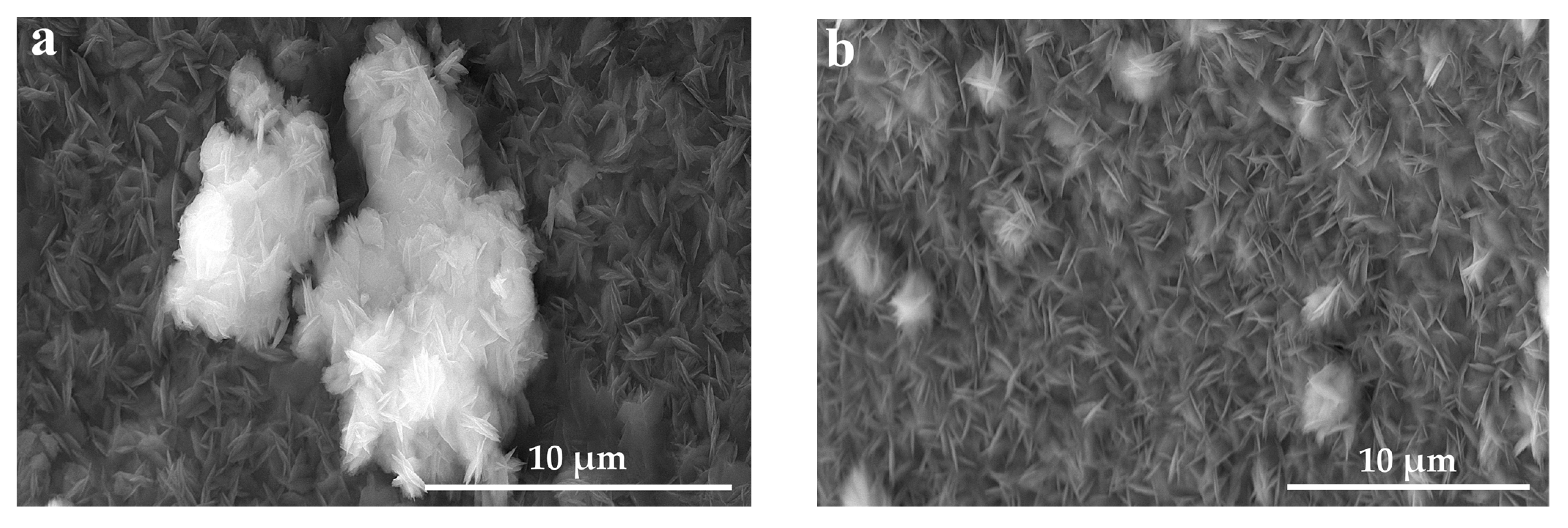
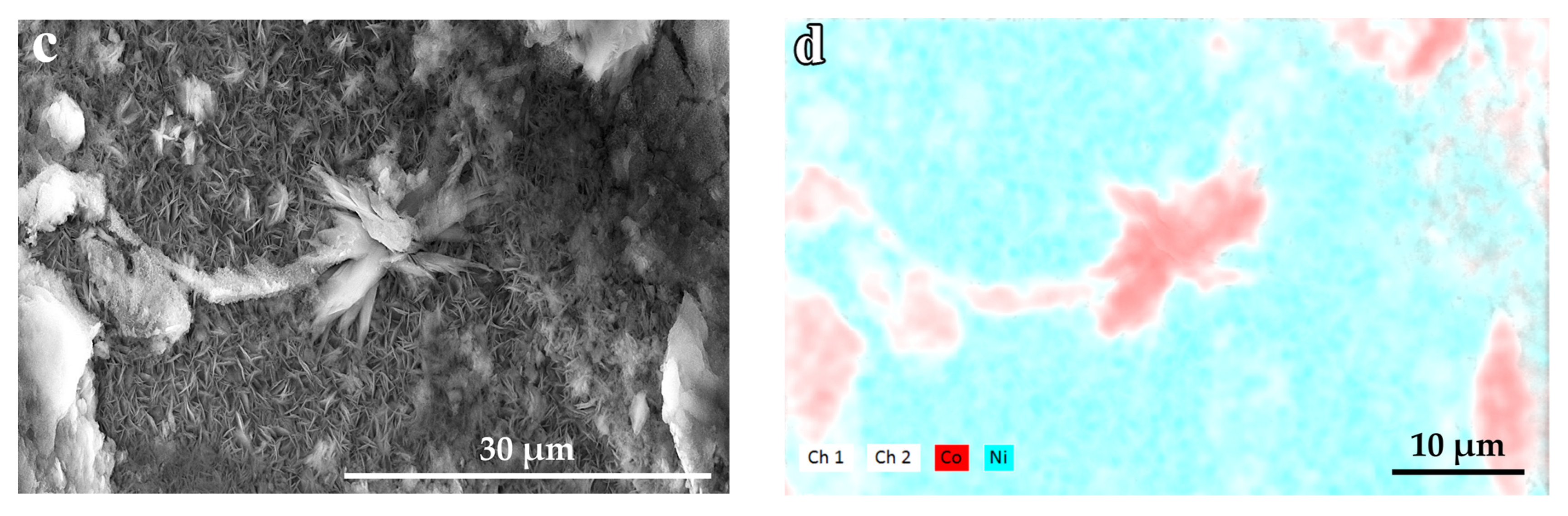
2.3. Morphology and Composition
2.4. Electrochemical Characterization
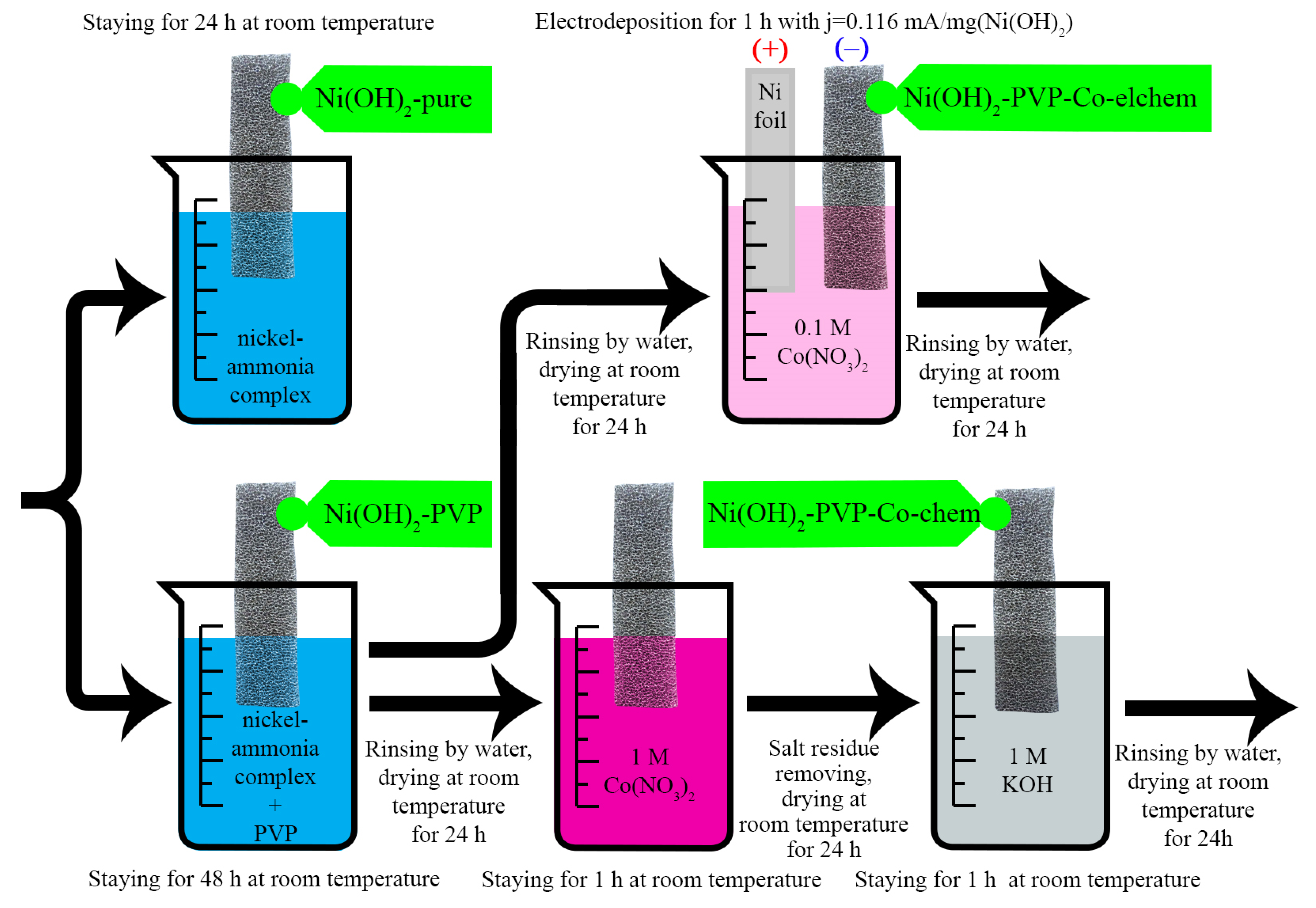
2.5. Formation of Electrodes
2.5.1. Ni(OH)2-Pure Samples
2.5.2. Ni(OH)2-PVP Samples
2.5.3. Ni(OH)2-PVP-Co-Elchem Samples
2.5.4. Ni(OH)2-PVP-Co-Chem Samples
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cui, Z.; Kang, L.; Li, L.; Wang, L.; Wang, K. A hybrid neural network model with improved input for state of charge estimation of lithium-ion battery at low temperatures. Renew. Energy 2022, 198, 1328–1340. [Google Scholar] [CrossRef]
- Li, D.; Yang, D.; Li, L.; Wang, L.; Wang, K. Electrochemical impedance spectroscopy based on the state of health estimation for lithium-ion batteries. Energies 2022, 15, 6665. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V. Definition of effectiveness of β-Ni(OH)2 application in the alkaline secondary cells and hybrid supercapacitors. East.-Eur. J. Enterp. Technol. 2017, 5, 17–22. [Google Scholar] [CrossRef][Green Version]
- Djafri, D.; Henni, A.; Zerrouki, D. Electrochemical synthesis of highly stable and rapid switching electrochromic Ni(OH)2 nanoflake array films as low-cost method. Mater. Chem. Phys. 2022, 279, 125704. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Yu, Z.; Su, G. The stabilization of Ni(OH)2 by In2O3 Rods and the electrochromic performance of Ni(OH)2/In2O3-rod composite porous film. Thin Solid Film. 2021, 734, 138839. [Google Scholar] [CrossRef]
- Kotok, V.; Kovalenko, V.; Nafeev, R.; Melnyk, O. Investigation of the characteristics of sulfurized electrochromic Ni(OH)2-PVA films deposited on transparent substrates. East.-Eur. J. Enterp. Technol. 2022, 1, 24–30. [Google Scholar] [CrossRef]
- Abd-Elsabour, M.; Alhamzani, A.; Abou-Krisha, M. Fabrication of novel nickel-modified electrodes and their application for methanol oxidation in fuel cell. Ionics 2022, 28, 1915–1925. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, J.; An, Y.; Han, D.; Chang, S.; Liu, Y.; Yang, R. Enhanced electrochemical performance by nickel-iron layered double hydroxides (LDH) coated on Fe3O4 as a cathode catalyst for single-chamber microbial fuel cells. Sci. Total Environ. 2020, 745, 141163. [Google Scholar] [CrossRef]
- Djellali, M.; Kameche, M.; Kebaili, H.; Bouhent, M.; Benhamou, A. Synthesis of nickel-based layered double hydroxide (LDH) and their adsorption on carbon felt fibres: Application as low cost cathode catalyst in Microbial Fuel Cell (MFC). Environ. Technol. 2019, 42, 492–504. [Google Scholar] [CrossRef]
- Zhang, R.; Ran, T.; Cao, Y.; Zhang, Q.; Dong, F.; Yang, G.; Zhou, Y. Surface hydrogen atoms promote oxygen activation for solar light-driven no oxidization over monolithic α-Ni(OH)2/Ni foam. Environ. Sci. Technol. 2020, 54, 16221–16230. [Google Scholar] [CrossRef]
- Qin, H.; Wei, X.; Ye, Z.; Liu, X.; Mao, S. Promotion of phenol electro-oxidation by oxygen evolution reaction on an active electrode for efficient pollution control and hydrogen evolution. Environ. Sci. Technol. 2022, 56, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Shi, Y.; Shang, H.; Zhang, L.; Wang, J. Vacancy-rich and porous NiFe-layered double hydroxide ultrathin nanosheets for efficient photocatalytic no oxidation and storage. Environ. Sci. Technol. 2022, 56, 1771–1779. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, X.X.; Zhang, H.Y.; Huang, M.; Yin, S.; Zhang, Y.F.; Wang, J. How to fit a response current-concentration curve? part (II): Synergy of heterogeneous PANI@NI(OH)2/NF towards high performance glucose sensing and a general semi-empirical model. J. Electroanal. Chem. 2022, 909, 116164. [Google Scholar] [CrossRef]
- Manjushree, S.; Adarakatti, P.; Udayakumar, V.; Almalki, A. Hexagonal cerium oxide decorated on β-Ni(OH)2 nanosheets stabilized by reduced graphene oxide for effective sensing of H2O2. Carbon Lett. 2021, 32, 591–604. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, W.; Wu, J.; Zhao, K.; Liu, S.; Zhao, Y. Tailored design of Ni(OH)2 nanocages internally decorated with CuS nanocages to mutually ameliorate electrocatalytic dynamics for highly sensitive glucose detection. J. Electroanal. Chem. 2022, 907, 115893. [Google Scholar] [CrossRef]
- Cagnetta, G.; Zhang, K.; Zhang, Q.; Huang, J.; Yu, G. Augmented hydrogen production by gasification of ball milled polyethylene with Ca(OH)2 and Ni(OH)2. Front. Environ. Sci. Eng. 2019, 13, 11. [Google Scholar] [CrossRef]
- Hayek, N.; Landman, A.; Halpern, Y.; Slobodkin, I.; Davydova, E.; Rothschild, A. Carbon-cloth-supported nickel hydroxide anodes for electrochemical–thermally-activated chemical (E-TAC) water splitting. J. Mater. Chem. A 2022, 10, 726–739. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Wu, H.; Wang, H.; Wang, F. Nickel hydroxide array coated with NiFe alloy nanosheets for overall mixed water splitting. J. Alloys Compd. 2022, 918, 165564. [Google Scholar] [CrossRef]
- Paul, T.; Mesbahi, T.; Durand, S.; Flieller, D.; Uhring, W. Sizing of lithium-ion battery/supercapacitor hybrid energy storage system for forklift vehicle. Energies 2020, 13, 4518. [Google Scholar] [CrossRef]
- Babar, P.; Unde, M.G.; Patil, R.J. Hybrid Energy Control for an Electric Vehicle Using Super Capacitor and Battery. In Proceedings of the 2022 3rd International Conference on Electronics and Sustainable Communication Systems (ICESC), Coimbatore, India, 17–19 August 2022. [Google Scholar] [CrossRef]
- Mishra, D.; Zhou, R.; Hassan, M.; Hu, J.; Gates, I.; Mahinpey, N.; Lu, Q. Bitumen and asphaltene derived nanoporous carbon and nickel oxide/carbon composites for supercapacitor electrodes. Sci. Rep. 2022, 12, 4095. [Google Scholar] [CrossRef]
- Yu, X.-H.; Zhao, Z.-Y.; Yi, J.-L.; Liu, S.; Zhang, R.-L.; Wang, F.-Y.; Liu, L. Facile assembly of cobalt-nickel double hydroxide nanoflakes on nitrogen-doped hollow carbon spheres for high performance asymmetric supercapacitors. J. Alloys Compd. 2022, 918, 165551. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Dang, V.D.; Pandit, B.; Bui, T.A.N.; Tran, H.L.; Doong, R.-A. Flower-like nickel hydroxide@tea leaf-derived biochar composite for high-performance supercapacitor application. J. Colloid Interface Sci. 2022, 623, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Li, X.; Kang, Y.; RehmanLashari, N.U.; Zhang, X.; Zhao, Y.; Wang, H.; Miao, Z.; Fu, C. Ni-doped tin disulfide@Nickel hydroxide as robust cathode toward durable supercapacitor and aqueous Ni-Zn battery. J. Power Sources 2022, 535, 231486. [Google Scholar] [CrossRef]
- Kachina, E.; Ivanova, N.; Zakharov, Y.; Simenyuk, G.; Ismagilov, Z.; Lomakin, M. Electrochemical Properties of the Composites Based on Multiwall Carbon Nanotubes Modified with Nanoparticles of Mixed Cobalt and Nickel Hydroxides. Eurasian Chem. J. 2022, 24, 115–121. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, R.; Zhang, M.; Liu, R.; Zhu, X.; Li, X.; Cui, H.; Zhu, H. Promoting the cyclic and rate performance of nickel hydroxide with ZnO via electrodeposition for supercapacitor. J. Alloys Compd. 2022, 911, 164865. [Google Scholar] [CrossRef]
- Wiston, B.R.; Dhivyaprasath, K.; Tewatia, S.; Ashok, M. Optimization of nickel manganese ratio to attain highly efficient electroactive composite for supercapacitors. J. Alloys Compd. 2023, 935, 167982. [Google Scholar] [CrossRef]
- Erdemutu, E.; Bai, C.; Ding, L. Electrospun Ni-Ni(OH)2/Carbon Nanofibers as Flexible Binder-Free Supercapacitor Electrode with Enhanced Specific Capacitance. J. Electron. Mater. 2020, 49, 7211–7218. [Google Scholar] [CrossRef]
- Nath, A.R.; Madhu, B.; Mohan, A.; Sandhyarani, N. Enhancing the stability of electrochemical asymmetric supercapacitor by incorporating thiophene-pyrrole copolymer with nickel sulfide/nickel hydroxide composite. J. Energy Storage 2021, 46, 103833. [Google Scholar] [CrossRef]
- Boruah, B.D.; Misra, A. Nickel hydroxide coated carbon nanoparticles mediated hybrid three-dimensional graphene foam assembly for supercapacitor. RSC Adv. 2016, 6, 36307–36313. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y.; Zhang, Y.; Li, S.; Wu, M.; Xue, L.; Zhao, J.; Zhang, W.; Ge, M.; Lai, Y.; et al. Ion regulation of hollow nickel cobalt layered double hydroxide nanocages derived from ZIF-67 for High-Performance supercapacitors. Appl. Surf. Sci. 2022, 596, 153582. [Google Scholar] [CrossRef]
- Kapu, S.G.; Guddeti, P.R.; Sambasivam, S.; Yakkate, S.R.; Joo, S.W.; Pallavolu, M.R. Facile fabrication of hexagonal Ni(OH)2 nanoparticles anchored g-C3N4 layered nanocomposite electrode material for energy storage applications. Diam. Relat. Mater. 2022, 129, 109376. [Google Scholar] [CrossRef]
- Wang, G.; Qi, K.; Yan, Z.; Yue, L.; Ding, Y.; Li, W.; Xu, Z. Microwave hydrothermal synthesis of La decorated Ni(OH)2 nanosheets for performance-enhanced hybrid supercapacitor. Appl. Surf. Sci. 2022, 592, 153293. [Google Scholar] [CrossRef]
- Liu, J.; Han, E.; He, Y.; Tong, X.; Guo, S. Effect of soft template on nickel-cobalt layered double hydroxides grown on nickel foam as battery-type electrodes for hybrid supercapacitors. Ionics 2021, 27, 3129–3141. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, L.; Shang, M.; Han, E.; Song, M. Effect of soft templating agent on NiCoAl-LDHs grown in situ on foamed nickel for high-performance asymmetric supercapacitors. Ionics 2019, 26, 1431–1442. [Google Scholar] [CrossRef]
- Liu, J.; Han, E.; He, Y.; Yang, X.; Qiao, S.; Tong, X.; Tian, Y.; Gao, L. Effect of soft template on NiMn-LDH grown on nickel foam for battery-type electrode materials. Ionics 2021, 27, 1451–1463. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Arbi, H.M.; Yadav, A.A.; Kumar, Y.A.; Moniruzzaman; Alzahmi, S.; Obaidat, I.M. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials 2022, 12, 3982. [Google Scholar] [CrossRef]
- Neto, N.A.; Silva, J.; Tranquilin, R.; Longo, E.; Bomio, M.; Motta, F. Stabilization of the γ-Ag2WO4 metastable pure phase by coprecipitation method using polyvinylpyrrolidone as surfactant: Photocatalytic property. Ceram. Int. 2020, 46, 14864–14871. [Google Scholar] [CrossRef]
- Lam, S.-M.; Kee, M.-W.; Sin, J.-C. Influence of PVP surfactant on the morphology and properties of ZnO micro/nanoflowers for dye mixtures and textile wastewater degradation. Mater. Chem. Phys. 2018, 212, 35–43. [Google Scholar] [CrossRef]
- Kotok, V.; Kovalenko, V.; Mikolasek, M.; Ondrejka, P.; Zima, O.; Anataichuk, I.; Vodopyan, D.; Sukhyy, K. Characteristics investigation of composite electrochromic films based on Ni(Oh)2, polyvinyl alcohol, and polyvinylpyrrolidone. East.-Eur. J. Enterp. Technol. 2022, 3, 58–65. [Google Scholar] [CrossRef]
- Kotok, V.; Kovalenko, V. A study of the influence of polyvinyl pyrrolidone concentration in the deposition electrolyte on the properties of electrochromic Ni(OH)2 films. East.-Eur. J. Enterp. Technol. 2020, 4, 31–37. [Google Scholar] [CrossRef]
- Snook, G.A.; Duffy, N.W.; Pandolfo, A.G. Evaluation of the effects of oxygen evolution on the capacity and cycle life of nickel hydroxide electrode materials. J. Power Sources 2007, 168, 513–521. [Google Scholar] [CrossRef]
- Kotok, V.; Kovalenko, V.; Malyshev, V. Comparison of oxygen evolution parameters on different types of nickel hydroxide. East.-Eur. J. Enterp. Technol. 2017, 5, 12–19. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries. J. Electrochem. Soc. 1999, 146, 3606–3612. [Google Scholar] [CrossRef]
- Oshitani, M.; Takayama, T.; Takashima, K.; Tsuji, S. A study on the swelling of a sintered nickel hydroxide electrode. J. Appl. Electrochem. 1986, 16, 403–412. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V. Anionic carbonate activation of layered (α + β) nickel hydroxide. East.-Eur. J. Enterp. Technol. 2019, 3, 44–52. [Google Scholar] [CrossRef]
- Chang, Z.; Tang, H.; Chen, J.G. Surface modification of spherical nickel hydroxide for nickel electrodes. Electrochem. Commun. 1999, 1, 513–516. [Google Scholar] [CrossRef]
- Pralong, V.; Delahaye-Vidal, A.; Beaudoin, B.; Leriche, J.; Tarascon, J. Electrochemical Behavior of Cobalt Hydroxide Used as Additive in the Nickel Hydroxide Electrode. J. Electrochem. Soc. 2000, 147, 1306–1313. [Google Scholar] [CrossRef]
- Andrade, T.M.; Danczuk, M.; Anaissi, F.J. Effect of Precipitating Agents on the Structural, Morphological, and Colorimetric Characteristics of Nickel Hydroxide Particles. Colloid Interface Sci. Commun. 2018, 23, 6–13. [Google Scholar] [CrossRef]
- Hu, C.-W.; Yamada, Y.; Yoshimura, K. Fabrication of nickel oxyhydroxide/palladium (NiOOH/Pd) nanocomposite for gasochromic application. Sol. Energy Mater. Sol. Cells 2018, 177, 120–127. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Das, H.T.; Guddeti, P.R.; Nallapureddy, R.R.; Pallavolu, M.R.; Alzahmi, S.; Obaidat, I.M. Self-Supported Co3O4@Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors. Nanomaterials 2022, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, B.; Madhu, B.; Raju, V.B.; Vynatheya, S.; Devi, B.; Jayashree, G.; Ravikumar, C. Synthesis, spectroscopic analysis and electrochemical performance of modified β-nickel hydroxide electrode with CuO. J. Sci. Adv. Mater. Devices 2017, 2, 93–98. [Google Scholar] [CrossRef]
- Lyons, M.; Doyle, R.L.; Godwin, I.; O’Brien, M.; Russell, L. Hydrous Nickel Oxide: Redox Switching and the Oxygen Evolution Reaction in Aqueous Alkaline Solution. J. Electrochem. Soc. 2012, 159, H932–H944. [Google Scholar] [CrossRef]
- Qin, R.; Pan, Y.; Duan, Z.; Su, H.; Ren, K.; Wang, W.; Li, Y.; Xi, N.; Wang, Y.; Zhang, L.; et al. Achieving High Stability and Rate Performance Using Spherical Nickel-Zinc Layered Double Hydroxide in Alkaline Solution. J. Electrochem. Soc. 2021, 168, 070539. [Google Scholar] [CrossRef]
- Kotok, V.; Kovalenko, V. Definition of the influence of obtaining method on physical and chemical characteristics of Ni (OH)2 powders. East.-Eur. J. Enterp. Technol. 2019, 1, 21–27. [Google Scholar] [CrossRef]
- Conway, B.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Wang, D.; Wei, A.; Tian, L.; Mensah, A.; Li, D.; Xu, Y.; Wei, Q. Nickel-cobalt layered double hydroxide nanosheets with reduced graphene oxide grown on carbon cloth for symmetric supercapacitor. Appl. Surf. Sci. 2019, 483, 593–600. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, B.; Lu, C.; Cai, Z.; Li, L. Hierarchical hollow nanocages of Ni–Co amorphous double hydroxides for high-performance asymmetric supercapacitors. J. Alloys Compd. 2020, 833, 155130. [Google Scholar] [CrossRef]
- Bao, Y.; Deng, Y.; Wang, M.; Xiao, Z.; Wang, M.; Fu, Y.; Guo, Z.; Yang, Y.; Wang, L. A controllable top-down etching and in-situ oxidizing strategy: Metal-organic frameworks derived α-Co/Ni(OH)2@Co3O4 hollow nanocages for enhanced supercapacitor performance. Appl. Surf. Sci. 2019, 504, 144395. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.; Tong, X.; Chen, Y.; Duan, M.; Shi, J.; Jiang, C.; Hu, L.; Kong, Q.; Zhang, J. High-performance battery-type supercapacitor based on porous biocarbon and biocarbon supported Ni–Co layered double hydroxide. J. Alloys Compd. 2020, 837, 155529. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Hasanin, M.S. Green and facile synthesis of nickel oxide-porous carbon composite as improved electrochemical electrodes for supercapacitor application from banana peel waste. Environ. Sci. Pollut. Res. 2021, 28, 66888–66900. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, H.; Meng, C.; Wang, Z.; Akhtar, K.; Yuan, A. Cyanometallic framework-derived hierarchical Co3O4-NiO/graphene foam as high-performance binder-free electrodes for supercapacitors. Chem. Eng. J. 2019, 369, 57–63. [Google Scholar] [CrossRef]
- Kishore, S.C.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Alagan, M.; Vinodh, R.; Shanmugam, M.; Lee, Y.R. Solid Waste-Derived Carbon Fibers-Trapped Nickel Oxide Composite Electrode for Energy Storage Application. Energy Fuels 2020, 34, 14958–14967. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Sakthivel, P.; Asaithambi, S.; Murugan, R.; Babu, G.A.; Yuvakkumar, R.; Ravi, G. Solvent dependent morphological modification of micro-nano assembled Mn2O3/NiO composites for high performance supercapacitor applications. Ceram. Int. 2018, 45, 4298–4307. [Google Scholar] [CrossRef]
- AshokC, S.; Thomas, N. Influence of fuel to oxidizer ratio on the supercapacitive properties of NiO synthesized by solution combustion method. Solid State Sci. 2022, 133, 107004. [Google Scholar] [CrossRef]
- Moniruzzaman; Kumar, Y.A.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-Dimensional Core-Shell Structure of Cobalt-Doped@MnO2 Nanosheets Grown on Nickel Foam as a Binder-Free Battery-Type Electrode for Supercapacitor Application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]














| Sample | Specific Capacitance, Electrolyte | Number of Used Reagents 1 | Heating Requirement (Conditions) | Direct Formation on the Substrate | Ref. |
|---|---|---|---|---|---|
| Ni(OH)2 coated carbon NP mediated hybrid three-dimensional graphene | 4667 F g−1 at 2 A g−1 in 1 M KOH | 6 | Yes (1000 °C) | Yes | [30] |
| NiMnO3/Ni(OH)2 | 2454 F g−1 at 1 A g−1 in 2 M KOH | 9 | Yes (80 °C) | Yes | [27] |
| Hollow Ni-Co LDH nanocages | 2369 F g−1 at 0.5 A g−1 in 2 M KOH | 4 | Yes (90 and 60 °C) | No | [31] |
| Ni(OH)2/ZnO | 2265 F g−1 at 1 A/g in 6M KOH | 4 | Yes (60 °C) | Yes | [26] |
| Ni/SnS2@Ni(OH)2-CC | 2090 F g−1 at 1 A g−1 in 2 M KOH | 6 | Yes (160, 100, 60 °C) | Yes | [24] |
| La decorated Ni(OH)2 nanosheets | 1510.7 F g−1 at 1 A g−1 | 7 | Yes (70 °C) | No | [33] |
| This work | 1408 F g−1 at 1 A g−1 (191.7 mA·h·g−1) in 1 M KOH | 4 | No | Yes | - |
| Hierarchical hollow nanocages of Ni-Co LDH | 1198 F g−1 at 1 A g−1 in 2 M KOH | 8 | Yes (60 °C) | No | [58] |
| Co3O4 embedded α-Co/Ni(OH)2 hollow nanocages | 1000 F g−1 at 1 A g−1 | - | - | No | [59] |
| Ni–Co LDH/STSC | 992.2 F g−1 at 1 A g−1 in 1 M KOH | 6 | Yes (600, 800, 100 °C) | No | [60] |
| NiNF@TBC | 945 F g−1 at 1 A g−1 in 1 M Na2SO4 | 4 | Yes (800, 600, 160, 110, 80, 70 °C) | No | [23] |
| CoNi-DH/NHCS | 578 C g−1 (160 mA·h·g−1) at 1 A g−1 in 1 M KOH | 14 | Yes (50, 800, 80 °C) | No | [22] |
| Nickel oxide-porous carbon composite | 811 F g−1 at 1 A g−1 in 6 M KOH | 2 | Yes (700, 80 °C) | No | [61] |
| Hierarchical Co3O4-NiO/graphene foam | 766 F g−1 at 1 A g−1 in 2 M KOH | 5 | Yes (60, 350 °C) | Yes | [62] |
| Ni-Co LDH nanosheets with RGO | 642 F g−1 at 10 A g−1 in 1 M KOH | 4 | Yes (180, 80, 550 °C) | Yes | [63] |
| Micro-nano assembled Mn2O3/NiO composite | 566.21 F g−1 at 0.5 A g−1 in 1 M KOH | 4 | Yes (120 °C) | No | [64] |
| Ni(OH)2/g-C3N4 | 463 F g−1 at 1 A g−1 in 1 M KOH | 3 | No | Yes | [32] |
| NiO@SW-CFs | 356 F g−1 at 2 A g−1 in 1 M KOH | 5 | Yes (100, 800 °C) | No | [65] |
| NiO synthesized by solution combustion method | 174.7 C/g at 10 A/g (58.9 mA·h·g−1) | 2 | Yes (50, 100, 300 °C) | No | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotok, V.; Ondrejka, P.; Mikolášek, M.; Sojková, M.; Novák, P.; Gregor, M.; Kovalenko, V.; Sukhyy, K. A Facile Two-Step PVP-Assisted Deposition of Co-Activated Nanosized Nickel Hydroxide Directly on a Substrate for Large-Scale Production of Supercapacitor Electrodes. Coatings 2023, 13, 84. https://doi.org/10.3390/coatings13010084
Kotok V, Ondrejka P, Mikolášek M, Sojková M, Novák P, Gregor M, Kovalenko V, Sukhyy K. A Facile Two-Step PVP-Assisted Deposition of Co-Activated Nanosized Nickel Hydroxide Directly on a Substrate for Large-Scale Production of Supercapacitor Electrodes. Coatings. 2023; 13(1):84. https://doi.org/10.3390/coatings13010084
Chicago/Turabian StyleKotok, Valerii, Peter Ondrejka, Miroslav Mikolášek, Michaela Sojková, Patrik Novák, Maroš Gregor, Vadym Kovalenko, and Kostyantyn Sukhyy. 2023. "A Facile Two-Step PVP-Assisted Deposition of Co-Activated Nanosized Nickel Hydroxide Directly on a Substrate for Large-Scale Production of Supercapacitor Electrodes" Coatings 13, no. 1: 84. https://doi.org/10.3390/coatings13010084
APA StyleKotok, V., Ondrejka, P., Mikolášek, M., Sojková, M., Novák, P., Gregor, M., Kovalenko, V., & Sukhyy, K. (2023). A Facile Two-Step PVP-Assisted Deposition of Co-Activated Nanosized Nickel Hydroxide Directly on a Substrate for Large-Scale Production of Supercapacitor Electrodes. Coatings, 13(1), 84. https://doi.org/10.3390/coatings13010084







