Abstract
Saturation diffusion with chromium has not been adequately studied among all the surface thermochemical treatment (STCT) processes of steels. Especially, the complex saturation behavior when several elements are added directly for chemical treatment needs to be systematically studied. This work aims at determining the effect of V, Mo, and Co on the parameters of chromium thermal saturation diffusion (thickness, phase composition, microstructure, and microhardness) of the surface layer in X35CrNi2-3 steel. The process was carried out at a temperature of 1000 °C for 24 h. The results showed that complex structural chromium plating together with the addition of strong carbide-forming elements (V and Mo) has a significant effect on the phase composition of the fabricated layer, where the formation of VC and Mo2C carbides significantly increases the microhardness of the samples to 2000 HV and 2500 HV, respectively. On the other hand, the addition of Co with a less carbide-forming affinity has little effect on the phase composition of the coating, and nitride compounds predominated in the microstructure similar to the single-element chromium plating. The results indicate the possibility of improving and accelerating the traditional thermal chromium plating processes and opening up new horizons for obtaining gradient coatings with superior tribological properties.
1. Introduction
A fairly large number of machine parts are made from materials with low hardness and resistance to abrasive wear, such as most non-ferrous alloys, as well as low- and medium-carbon steels. In order to extend the life of the products and prevent their immature destruction, protective coatings are applied to the parts. Various methods can be used for fabricating coatings such as electroplating [1,2], laser cladding [3,4], thermal spraying [5,6,7,8], and sintering slurry-based ceramic coatings [9]. A good alternative to these coating methods is surface thermochemical treatment (STCT), in which the chemical composition, structure, and properties of the surface layer of the treated metals are changed [10]. STCT has a number of advantages, in particular, the surface of the workpiece is directly involved in chemical reactions and diffusion saturation, thus resulting in excellent adhesion of the fabricated layer to the matrix material. Moreover, STCT technology is quite simple and does not require complex and expensive equipment.
To impart strength and hardness to the surface layer of steel, STCT techniques such as nitriding [11,12,13,14], carburizing [15,16,17], nitrocarburizing [18,19,20], oxidation [21], and boriding [22,23,24] are used.
Thermal saturation diffusion with chromium is a less common STCT method [25,26,27,28], but one of its obvious advantages is the improvement of not only strength but also the corrosion resistance of the treated surface in various media [10]. The mechanism of diffusion chromium plating is described in detail elsewhere [29]. According to the presented data, the transfer of chromium from the filling to the steel surface is carried out through the gas phase. The process is carried out at temperatures of 900–1100 °C for 3–4 h to achieve better diffusion of chromium from the surface into the matrix. Researchers also paid attention to the effect of the carbon content of steel, the higher its content, the more likely the formation of chromium carbides in the layer obtained after STCT; 0.1 wt. % is indicated as a threshold carbon value [29]. Further studies [30,31,32] showed that diffusion chromium plating of steel with a carbon concentration higher than 0.3 wt. % can be attributed to “hard” chromium plating (hard chromization process), in which the formation of iron and chromium carbides/nitridesis an integral part of the process, providing high strength for the formed layer. It has been indicated that the presence of titanium, niobium, tungsten, molybdenum, and vanadium in the steel grade can prevent the formation of chromium carbides in the resulting layer [29]. Moreover, other authors suggested that the addition of strong carbide-forming elements directly into the composition of the mixture for diffusion chromium plating may reduce the likelihood of the formation of chromium carbides due to the formation of carbides of these elements, but they did not conduct experimental studies [29].
Thus, most of the conducted works on diffusion saturation with chromium are focused on the effect of carbon concentration of steel, and the platting parameters such as temperature and holding time. The complex saturation with various elements when they are added directly to the composition of the mixture for chemical treatment has not yet been systematically studied. At the moment there is a need to establish the relationship between the composition of the mixture for STCT and the characteristics of the layer obtained by diffusion saturation. This is the main scientific novelty of the present study.
Therefore, the aim of this work is to determine the effect of V, Mo, and Co on the parameters of chromium thermal diffusion saturation (thickness, phase composition, microstructure, and microhardness) of the surface layer in X35CrNi2-3 steel. From the practical point of view, the present work finds out the conditions for the formation of gradient coatings with increased hardness.
2. Materials and Methods
To carry out the STCT treatment, a commercial medium-carbon low-alloy steel of the X35CrNi2-3 types (manufactured by PJSC “ChMK”, Chelyabinsk, Russia) was selected as substrate. Its composition is given in Table 1. The initial FeV (manufactured by PJSC “ChEMK”, Chelyabinsk, Russia) and Cr, Co, Mo powders (manufactured JSC “POLEMA”, Tula, Russia) were ground and calibrated by dry sifting on sieves. The composition of the precursor elemental mixtures is also given in Table 1.

Table 1.
Chemical composition of steel and the components of saturating mixtures, wt. %.
Thermal diffusion saturation of chromium steel samples with dimensions of 10 mm × 15 mm × 24 mm was carried out by using four different powder mixtures. One mixture did not contain any additional additives, two of the mixtures contained stronger carbide-forming elements compared to chromium (V and Mo), and the latter contains a less carbide-forming element (Co). The first experiment (without the introduction of additives) was carried out in a mixture of 47 wt. % chromium powder fraction with particle sizes less than 150 µm, 50 wt. % Al2O3 fractions (0.5–2.0 mm), and 3 wt. % NH4Cl. The remaining three experiments were carried out with mixtures composed of 42 wt. % Cr powder, 5 wt. % additive (either crushed FeV, molybdenum powder, or cobalt powder), 50 wt. % Al2O3, and 3 wt. % NH4Cl. The particle sizes of FeV, Co, and Mo powders did not exceed 140 µm.
The components of the saturating mixture for each of the experiments were thoroughly mixed in a vibrating mill and then loaded into a metal container. The steel sample was also placed in the container. The container was sealed with a graphite seal and installed in a Nabertherm 41/H chamber furnace (Nabertherm GmbH, Lilienthal, Germany). Saturation was carried out at a temperature of 1000 °C for 24 h. At the end of the exposure time, the container was removed from the oven and cooled in air. The steel sample was kept in the container until it was completely cooled.
The temperature of the chromium thermal diffusion saturation was selected according to the literature [29,30,31,32,33]. Moreover, it has been reported that insufficiently high temperatures lead to the formation of a discontinuous and very thin layer [32]. A relatively longer holding time compared to that used in the literature [29,30,31,32,34] was employed. However, the authors of [33] also determined the time of the saturation process to be about 23 h during the STCT. Such an increase in the holding time was associated with the use of chromium powder in the mixture, rather than crushed ferrochrome.
The microstructure was studied using a JSM-7001F scanning electron microscope (SEM) (JEOL, Tokyo, Japan) equipped with an energy dispersive spectrometer (EDS; Oxford INCA, Abingdon, UK) for qualitative and quantitative X-ray microanalysis. X-ray diffraction (XRD) was performed on an Ultima IV diffractometer (Rigaku, Tokyo, Japan) by using Cu Kα radiation. A number of different phases were calculated by Rietveld refinement. The microhardness of the coating and substrate was measured using an FM-800 microhardness tester (Future-Tech Corp., Tokyo, Japan) at a load of 300 g for a holding time of 10 s.
3. Results and Discussion
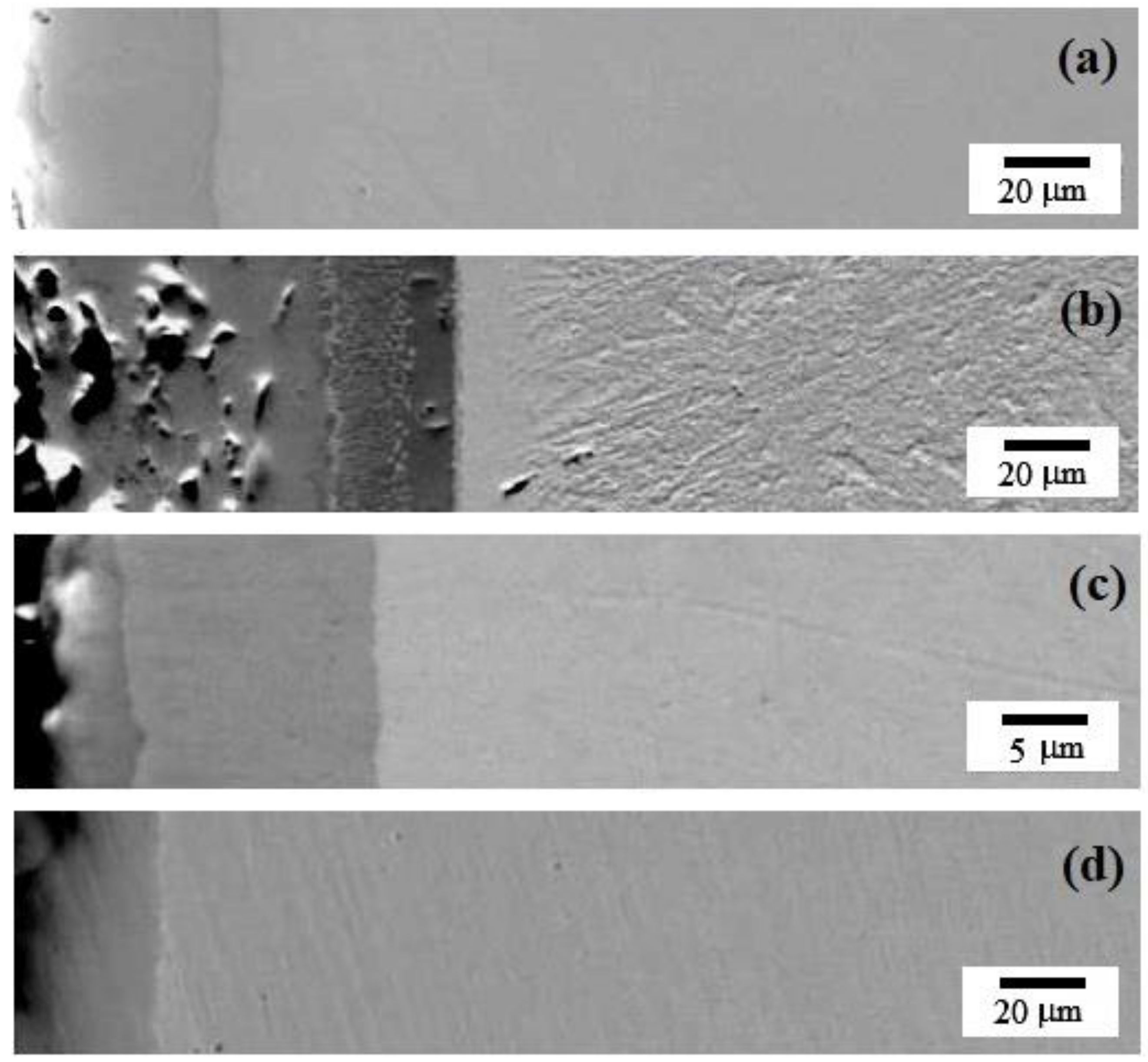
Figure 1 and Table 2 show the transverse sections of the samples after STCT to determine the thickness and uniformity of the resulting layer. For the sample prepared by thermal diffusion chromium plating using a mixture without additives, the thickness of the external layer was about 25 µm (see Figure 1a). The thickness of the coating was about 50–55 µm for the sample containing 5% ferrovanadium (see Figure 1b), and 15 µm (see Figure 1c,d) for the sample containing either molybdenum powder or cobalt powder. The thickness of the obtained layer without additives is comparable with the thickness of 20–30 µm reported here [30], but less than the thickness of about 30–40 µm for chromium-saturated areas [33].
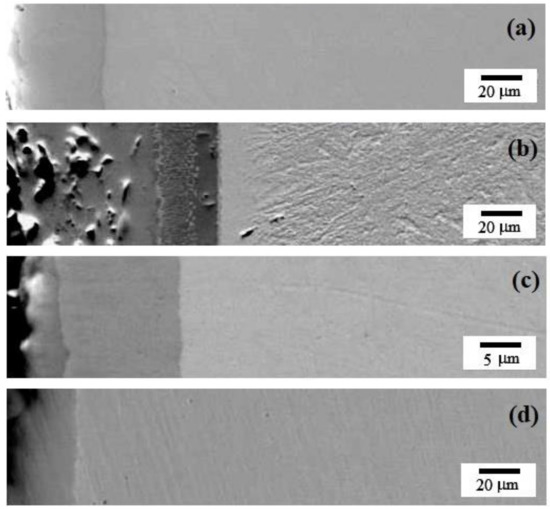
Figure 1.
Backscattered electrons SEM images of the resulting layer after thermal diffusion chromium plating of the sample: (a) without additives; (b) with 5% FeV; (c) with 5% Mo; (d) with 5% Co.

Table 2.
Dimension of the coating and percentage of the phases in different coatings.
Thus, at the initial stage, it can be stated that the use of a ferroalloy (in particular, FeV) as an additive to the mixture for carrying out STCT can accelerate the process and increase the thickness of the resulting layer. Powders of pure metals exhibit less chemical activity, and therefore the thickness of the obtained layer would be smaller. However, the behavior needs to be further investigated in the future.
According to Figure 1a,d, for the specimens fabricated by using either chromium powder alone or Cr + 5% Co, the resulting layer is homogeneous. For the samples fabricated by using a mixture containing strong carbide-forming agents (see Figure 1b,c) the resulting layer is inhomogeneous, for the sample with Cr + 5% Mo, two components are visible (thicknesses of 3 μm and 12 μm), and for the sample with Cr + 5% FeV, the STCT layer consists of three sublayers with thicknesses (from the edge of the sample deep into the surface) of 37 µm, 10 µm, and 7 µm, which indicates a possible gradient nature of the resulting layers.
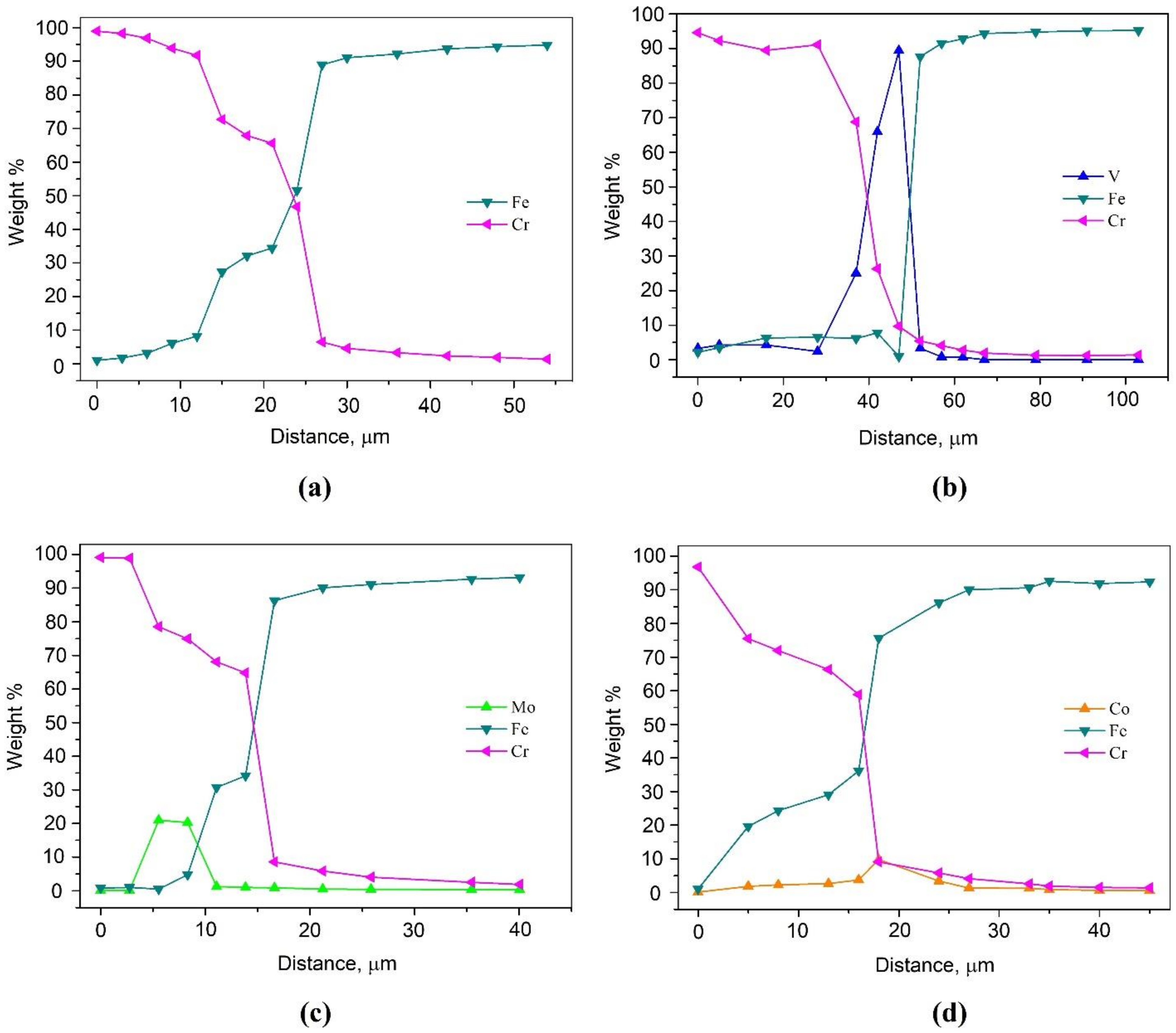
To determine the distribution of elements, a chemical analysis was carried out using EDS from the surface into the interior of the samples and the corresponding graphs were plotted; the results are shown in Figure 2. Additionally, this analysis made it possible to determine the depth of diffusion of chromium into the base metal (Table 2). It can be seen that the depth of diffusion of the main saturating element into the metal substrate varies in samples fabricated by using different additives. For the sample with the addition of Co and Mo, the depth of chromium diffusion is commensurate or even higher than that in the single-component saturation process. On the other hand, the addition of FeV hinders the diffusion of chromium, due to its binding into complex carbides. These values are also confirmed by the results of optical microscopy.
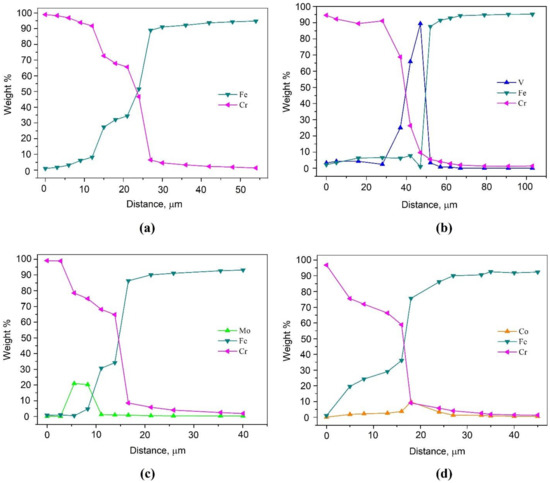
Figure 2.
EDS line scan showing the distribution of the elements across the resulting layer after thermal diffusion chromium plating of the sample: (a) without additives; (b) with 5% FeV; (c) with 5% Mo; (d) with 5% Co.
It can be noted that in all three cases with additional elements introduced into the mixture for STCT, these elements are present in the layer obtained after treatment, and an increased concentration of additives is noted not on the surface of the sample, but already at a certain depth. For the sample with additionally introduced vanadium, its concentration increases at a depth of 25 μm from the surface and drops at the boundary with steel; for the sample with 5% Mo, the increase and decrease in the molybdenum concentration occurs within the STCT layer from 3 to 11 µm across the layer thickness; for the sample with 5% Co, its slight increase can be noted directly at the interface with steel.
Of all these samples, only the sample with vanadium has a concentration above 50 wt. % (at a depth of 42 μm from the surface, the concentration of vanadium is even 90 wt. %), which indicates its high chemical activity during the process, comparable to or even superior to chromium. The authors of [35] also point out the possibility of joint transfer of metals through the gas phase; moreover, using thermodynamic calculations, they proved the probability of the formation of chlorides of several metals and their further coprecipitation on the surface of the treated steel.
It should also be noted that the data obtained for samples with vanadium and molybdenum confirm the gradient nature of the diffusion layers. The existing transition zones with mutual diffusion of chromium and iron, observed on the graphs (see Figure 2), provide excellent adhesion of such coatings to the substrate, which coincides with the observed microstructure (see Figure 1), wherein the region of the STCT boundary there are no chips, cracks or pores in the layer with the matrix material. A similar microstructure, which ensures the adhesion of the coating to steel due to diffusion processes, can also be observed in laser cladding [36,37].
Using data from Table 1 and Table 2, and Figure 2, we can calculate the diffusion rate of chromium ions based on Fick’s second law. The calculation method is described in detail elsewhere [38]. Expression for calculation
where D is diffusion rate (cm2/s), τ is holding time (sec, in our case 86,400 s), x is diffusion layer thickness (cm), c is chromium concentration in steel (%), and c0 is the chromium concentration in diffusion layer (%). Function [38] on the graph constructed according to empirical data. For sample without additives c/c0 = 0.042, x = 25 × 10–4 cm, D = 9.92 × 10–12 cm2/s. For sample with 5% FeV c/c0 = 0.027, x = 38 × 10–4 cm, D = 1.63 × 10–11 cm2/s. For sample with 5% Mo c/c0 = 0.029, x = 15 × 10–4 cm, D = 2.71 × 10–12 cm2/s. For sample with 5% Co c/c0 = 0.032, x = 15 × 10–4 cm, D = 3.10 × 10–12 cm2/s. Thus, the diffusion rate of Cr ions actually increases with the addition of ferrovanadium.
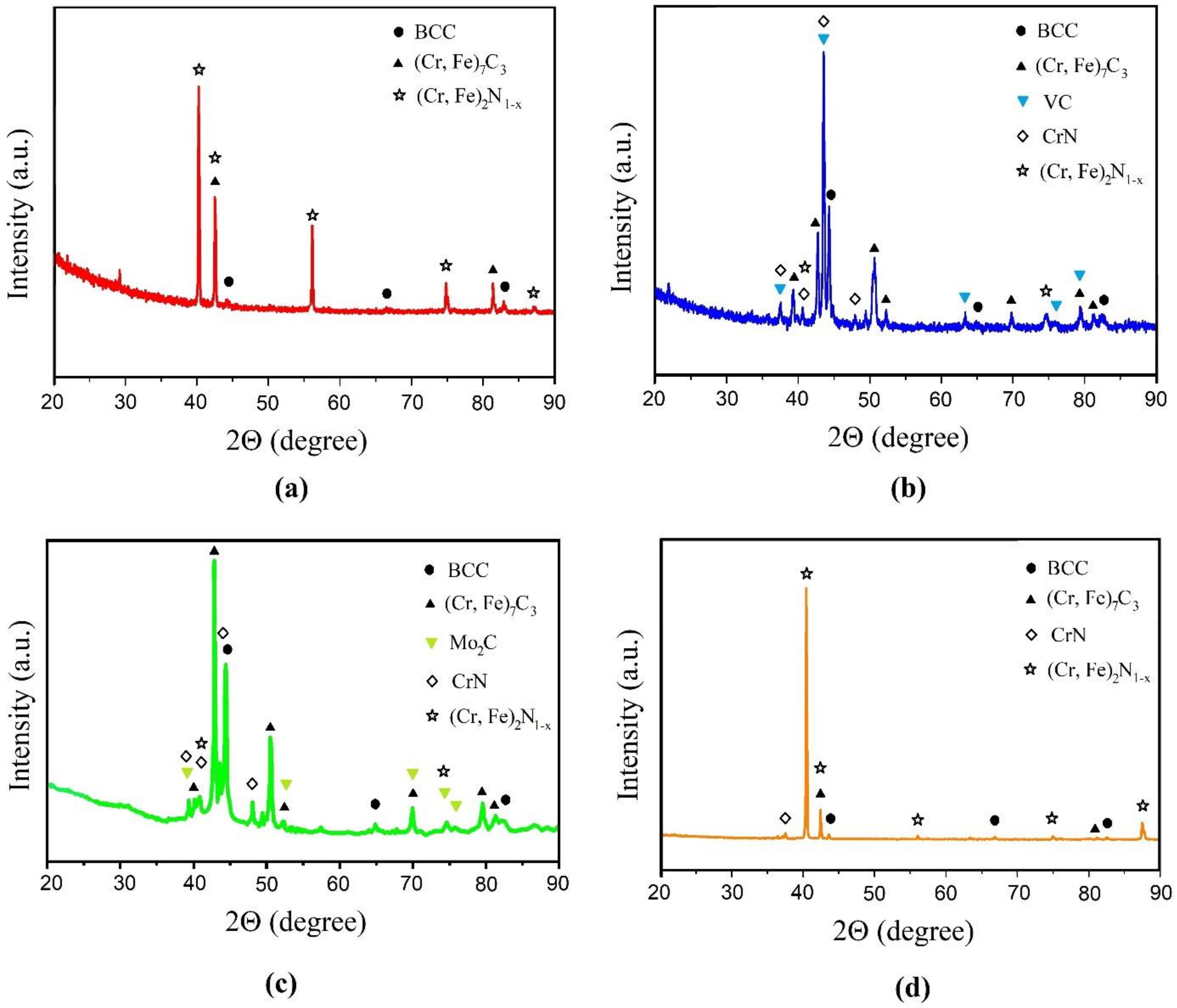
Figure 3 shows the results of the X-ray phase analysis carried out from the surface of the samples after STCT. As follows from the data obtained, for the Cr and Cr + 5% Co samples, the main phases formed are (Cr, Fe)2N1−x and CrN nitrides, (Cr, Fe)7C3 carbide, and a trace amount of chromium-based solid solution (BCC), which coincides with the results of [30,31,32]. When V and Mo are added to the mixture for STCT, the picture changes significantly: the proportion of carbide (Cr, Fe)7C3 increases, while VC appears in the layer for the sample with the addition of vanadium, and Mo2C appears for the sample with the addition of molybdenum. The nitride peaks become less intense, while the peaks for the solid solution indicate that its fraction in the phase composition of the STCT layers increases. Thus, carbide-forming additives significantly change the phase composition of the layers during thermal diffusion saturation with chromium. The percentage of phases for each sample is given in Table 2.
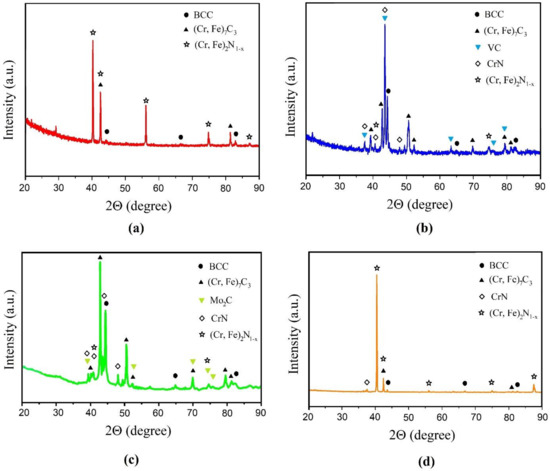
Figure 3.
XRD of the surface after thermal diffusion chromium plating of the sample: (a) without additives; (b) with 5% FeV; (c) with 5% Mo; (d) with 5% Co.
Table 3 shows data on the change in the Gibbs free energy of the formation of chromium, molybdenum, and vanadium carbides, as well as chromium nitrides at 1000 °C (according data from [39,40,41]). According to Table 3, the formation of chromium carbide Cr7C3 and chromium nitride Cr2N can compete with each other, as can be seen in the diffraction pattern for the sample without additives (see Figure 3a). Moreover, a similar picture can be observed for the Cr + 5% Co sample (see Figure 3d), since the formation of cobalt carbide is thermodynamically unlikely. At the same time, the formation of molybdenum Mo2C and vanadium VC carbides is thermodynamically preferable to chromium compounds, which is confirmed by X-ray phase analysis data. Some amount of carbide and chromium nitrides in Figure 3b,c can be explained by the small number of additives introduced (only 5%). It can be assumed that with an increase in their percentage, chromium carbides will practically not be formed. This assumption is supported by the data of the authors of [42], according to which, at the mass ratio in the used mixture FeCr/FeV = 35/65, after diffusion saturation, the layer consisted almost exclusively of vanadium carbides.

Table 3.
Data on the change in the Gibbs free energy of the formation of carbides and nitrides at 1000 °C.
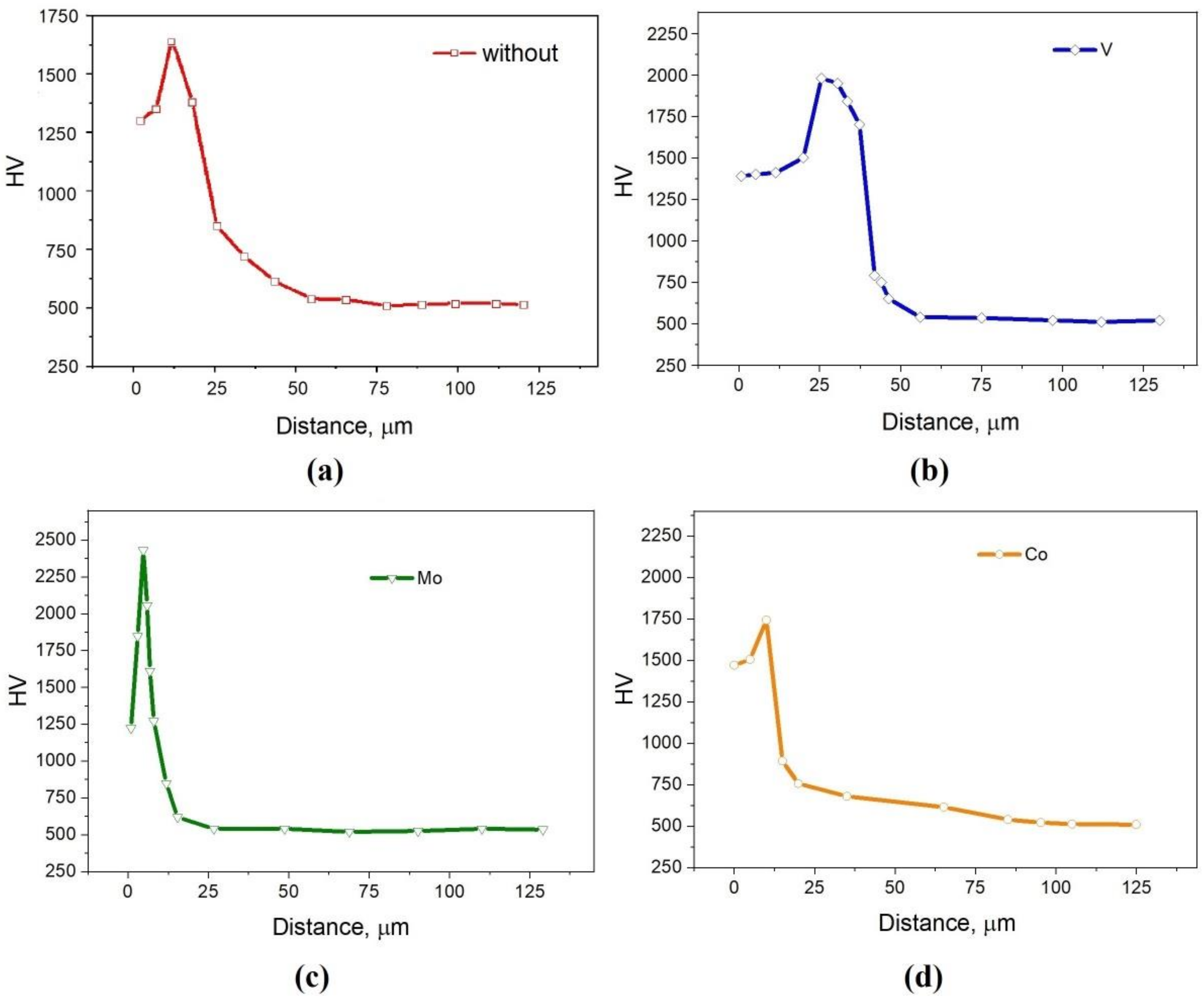
Figure 4 shows the results of measuring the microhardness from the surface into the interior of the samples. It should be noted that the maximum hardness does not fall on the outer layers of the coating, but is slightly deeper, which is typical for gradient coatings. For a sample without additives and with 5% Co, the maximum microhardness is about 1700–1750 HV. Such values are comparable with 1657–1878 HV [30] and 1400–1600 HV [32] for layers obtained by diffusion saturation with only chromium (without additional additives). For a sample with 5% FeV, the maximum microhardness is 2000 HV, which is comparable with the values of 2130–2163 HV presented in [42] for FeCr/FeV saturation. The layer with 5%Mo showed the highest hardness with a maximum of 2500 HV, which makes molybdenum one of the most promising additions for Cr plating.
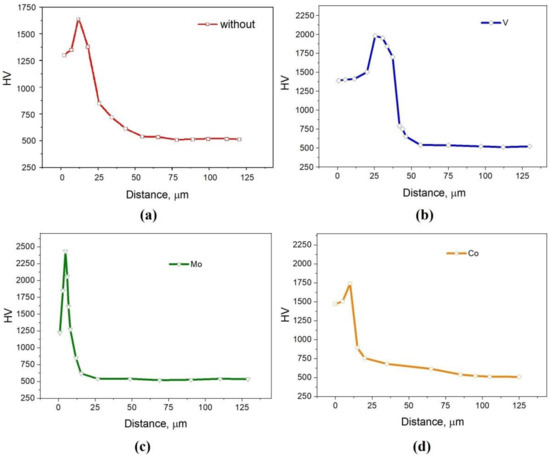
Figure 4.
The microhardness values from the surface into the interior of the sample: (a) without additives; (b) with 5% FeV; (c) with 5% Mo; (d) with 5% Co.
4. Conclusions
Based on the data obtained, the following conclusions can be drawn:
1. Vanadium and molybdenum are promising additives to be used for chromium thermal diffusion saturation since they facilitate the formation of hard carbide-containing coating (2000–2500 HV) on structural steel.
2. When strong carbide-forming elements (V, Mo) are used as additives, the fabricated coating contains mainly VC and Mo2C carbides, respectively. For one-component chromium plating and when a weak carbide-forming additive (Co) is used, the outer coating is enriched mainly with nitrides.
3. The choice of additive in the complex chromium plating has a significant effect on the dimensions of both external diffusion coatings and the diffusion depth of the main saturating element (Cr).
4. The use of ferroalloy powders (in particular, FeV) as an additive to the mixture for carrying out STCT can accelerate the process and increase the thickness of the resulting outer layer to 50–55 µm. Pure metal powders, on the other hand, show less chemical activity; as a result, the thickness of the resulting layer decreased from 25 µm for the sample without additives to 15 µm for the coating fabricated with molybdenum and cobalt additives. This observation requires further research.
Author Contributions
Conceptualization, N.S. and I.P.; methodology, N.S.; validation, A.O.M., V.M.; formal analysis, O.S.; investigation, N.S. and O.S.; resources, I.P.; writing—original draft preparation, O.S., N.S. and A.O.M.; writing—review and editing, A.O.M.; visualization, V.M.; supervision, I.P.; project administration, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inwood, B.; Garwood, A. Electroplated coatings for wear resistance. Tribol. Int. 1978, 11, 113–119. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Chen, J.; Zhou, X.; Zong, Y. Microstructure, Wear and Corrosion Behaviors of Electrodeposited Ni-Diamond Micro-Composite Coatings. Coatings 2022, 12, 1391. [Google Scholar] [CrossRef]
- Wei, A.; Tang, Y.; Tong, T.; Wan, F.; Yang, S.; Wang, K. Effect of WC on Microstructure and Wear Resistance of Fe-Based Coating Fabricated by Laser Cladding. Coatings 2022, 12, 1209. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Pang, M. Effect of WC content on laser cladding Ni-based coating on the surface of stainless steel. Mater. Today Commun. 2022, 31, 103357. [Google Scholar] [CrossRef]
- Samodurova, M.; Shaburova, N.; Samoilova, O.; Ostovari Moghaddam, A.; Pashkeev, K.; Ul’Yanitckiy, V.; Trofimov, E. Properties of WC–10%Co–4%Cr Detonation Spray Coating Deposited on the Al–4%Cu–1%Mg Alloy. Materials 2021, 14, 1206. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mulik, R.S.; Mahapatra, M.M. Mechanical property evaluation of carbon nanotubes reinforced plasma sprayed YSZ-alumina composite coating. Ceram. Int. 2018, 44, 6980–6989. [Google Scholar] [CrossRef]
- Roy, M.; Rao, C.; Rao, D.; Sundararajan, G. Abrasive wear behaviour of detonation sprayed WC–Co coatings on mild steel. Surf. Eng. 1999, 15, 129–136. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2020, 27, 1947–1968. [Google Scholar] [CrossRef]
- Kanwal, S.; Thakare, J.G.; Pandey, C.; Singh, I.; Mahapatra, M.M. Characterization of slurry-based mullite coating deposited on P91 steel welds. J. Aust. Ceram. Soc. 2019, 55, 519–528. [Google Scholar] [CrossRef]
- Czerwinski, F. Chapter “Thermochemical Treatment of Metals” in Heat Treatment-Conventional and Novel Applications; IntechOpen: London, UK, 2012; 422p. [Google Scholar] [CrossRef]
- Wei, R.; Vajo, J.J.; Matossian, J.N.; Wilbur, P.J.; Davis, J.A.; Williamson, D.L.; Collins, G.A. A comparative study of beam ion implantation, plasma ion implantation and nitriding of AISI 304 stainless steel. Surf. Coat. Technol. 1996, 83, 235–242. [Google Scholar] [CrossRef]
- Karimzadeh, N.; Moghaddam, E.G.; Mirjani, M.; Raeissi, K. The effect of gas mixture of post-oxidation on structure and corrosion behavior of plasma nitrided AISI 316 stainless steel. Appl. Surf. Sci. 2013, 283, 584–589. [Google Scholar] [CrossRef]
- Alphonsa, J.; Raja, V.S.; Mukherjee, S. Study of plasma nitriding and nitrocarburizing for higher corrosion resistance and hardness of 2205 duplex stainless steel. Corros. Sci. 2015, 100, 121–132. [Google Scholar] [CrossRef]
- Tao, X.; Collins, T.J.; Ao, Q.; Liu, H.; Dashtbozorg, B.; Li, X.; Dong, H. Active screen plasma nitriding of Fe-24Mn-2Al-0.45C TWIP steel: Microstructure evolution and a synergistic selective oxidation mechanism. Acta Mater. 2022, 241, 118418. [Google Scholar] [CrossRef]
- Ernst, F.; Cao, Y.; Michal, G.M. Carbides in low-temperature-carburized stainless steels. Acta Mater. 2004, 52, 1469–1477. [Google Scholar] [CrossRef]
- Scheuer, C.J.; Cardoso, R.P.; Pereira, R.; Mafra, M.; Brunatto, S.F. Low temperature plasma carburizing of martensitic stainless steel. Mater. Sci. Eng. A 2012, 539, 369–372. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhang, F.; Qin, Y.; Wang, X.; Lv, B. Microstructures and properties of a novel carburizing nanobainitic bearing steel. Mater. Sci. Eng. A 2020, 777, 139086. [Google Scholar] [CrossRef]
- Sun, Y. Enhancement in corrosion resistance of austenitic stainless steels by surface alloying with nitrogen and carbon. Mater. Lett. 2005, 59, 3410–3413. [Google Scholar] [CrossRef]
- Huang, R.; Wang, J.; Zhong, S.; Li, M.; Xiong, J.; Fan, H. Surface modification of 2205 duplex stainless steel by low temperature salt bath nitrocarburizing at 430 °C. Appl. Surf. Sci. 2013, 271, 93–97. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Spies, H.-J.; Biermann, H. Use of a solid carbon precursor for DC plasma nitrocarburizing of AISI 4140 steel. Vacuum 2018, 149, 146–149. [Google Scholar] [CrossRef]
- Hoppe, S. Fundamentals and applications of the combination of plasma nitrocarburizing and oxidizing. Surf. Coat. Technol. 1998, 98, 1199–1204. [Google Scholar] [CrossRef]
- Ozbek, I.; Bindal, C. Kinetics of borided AISI M2 high speed steel. Vacuum 2011, 86, 391–397. [Google Scholar] [CrossRef]
- Ipek, M.; CelebiEfe, G.; Ozbek, I.; Zeytin, S.; Bindal, C. Investigation of Boronizing Kinetics of AISI 51100 Steel. J. Mater. Eng. Perform. 2012, 21, 733–738. [Google Scholar] [CrossRef]
- Kul, M.; Danacı, I.; Gezer, Ş.; Karaca, B. Effect of boronizing composition on hardness of boronized AISI 1045 steel. Mater. Lett. 2020, 279, 128510. [Google Scholar] [CrossRef]
- Ozdemir, O.; Sen, S.; Sen, U. Formation of chromium nitride layers on AISI 1010 steel by nitro-chromizing treatment. Vacuum 2007, 81, 567–570. [Google Scholar] [CrossRef]
- Hakami, F.; Heydarzadeh Sohi, M.; Rasizadeh Ghani, J.; Ebrahimi, M. Chromizing of plasma nitrided AISI 1045 steel. Thin Solid Films 2011, 519, 6783–6786. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U. The effect of boronizing and boro-chromizing on tribological performance of AISI 52100 bearing steels. Ind. Lubr. Tribol. 2009, 61, 146–153. [Google Scholar] [CrossRef]
- Aghaie-Khafri, M.; Abady, M.M.N. A Study of Chromo-Boronizing on DIN 1.2714 Steel by Duplex Surface Treatment. JOM 2012, 64, 694–701. [Google Scholar] [CrossRef]
- Meier, G.H.; Cheng, C.; Perkins, R.A.; Bakker, W. Diffusion chromizing of ferrous alloys. Surf. Coat. Technol. 1989, 39–40, 53–64. [Google Scholar] [CrossRef]
- Lee, J.-W.; Duh, J.-G. Evaluation of microstructures and mechanical properties of chromized steels with different carbon contents. Surf. Coat. Technol. 2004, 177–178, 525–531. [Google Scholar] [CrossRef]
- Lee, J.-W.; Wang, H.-C.; Li, J.-L.; Lin, C.-C. Tribological properties evaluation of AISI 1095 steel chromized at different temperatures. Surf. Coat. Technol. 2004, 188–189, 550–555. [Google Scholar] [CrossRef]
- Bogdanov, S.P.; Khristiuk, N.A.; Sychov, M.M. The structure of the chromium plating on steel fabricated using iodine transport. J. Phys. Conf. Ser. 2021, 1967, 012039. [Google Scholar] [CrossRef]
- Lee, S.B.; Cho, K.H.; Lee, W.G.; Jang, H. Improved corrosion resistance and interfacial contact resistance of 316L stainless-steel for proton exchange membrane fuel cell bipolar plates by chromizing surface treatment. J. Power Sources 2009, 187, 318–323. [Google Scholar] [CrossRef]
- Lu, S.D.; Wang, Z.B.; Lu, K. Enhanced chromizing kinetics of tool steel by means of surface mechanical attrition treatment. Mater. Sci. Eng. A 2010, 527, 995–1002. [Google Scholar] [CrossRef]
- Dehula, A.I.; Kharchenko, N.A.; Hovorun, T.P.; Khizhniak, V.G.; Loskutova, T.V.; Smokovych, I.Y.; Kravchenko, Y.O. Physicochemical conditions of complex diffusion saturation of metal surfaces with titanium and chromium. High Temp. Mater. Process. 2017, 21, 239–250. [Google Scholar] [CrossRef]
- Samoilova, O.; Shaburova, N.; Samodurova, M.; Pashkeev, K.; Ostovari Moghaddam, A.; Doubenskaia, M.; Sova, A.; Trofimov, E. Microstructural evolution of Al0.25CoCrFeNiCu and Al0.45CoCrFeNiSi0.45 high-entropy alloys during laser cladding. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2022, 236, 1806–1813. [Google Scholar] [CrossRef]
- Samoilova, O.; Shaburova, N.; Pashkeev, K.; Samodurova, M.; Trofimov, E. Al0.25CoCrFeNiV High Entropy Alloy Coating Deposited by Laser Cladding on Stainless Steel. Materials 2022, 15, 7058. [Google Scholar] [CrossRef]
- Umansky, Y.S.; Finkelstein, B.N.; Blanter, M.E. Physical Foundations of Metallurgy; Metallurgizdat: Moscow, Russia, 1955; 721p. [Google Scholar]
- Shatynski, S.R. The thermochemistry of transition metal carbides. Oxid. Met. 1979, 13, 105–118. [Google Scholar] [CrossRef]
- Iwai, T.; Takahashi, I.; Handa, M. Gibbs free energies of formation of molybdenum carbide and tungsten carbide from 1173 to 1573 K. Met. Mater. Trans. A 1986, 17, 2031–2034. [Google Scholar] [CrossRef]
- Ono-Nakazato, H.; Taguchi, K.; Tamura, K.; Tomatsu, Y.; Usui, T. Determination of standard gibbs energies of formation of Cr2N and CrN. Met. Mater. Trans. B 2001, 32, 1113–1118. [Google Scholar] [CrossRef]
- Ariati, M.; Narottama, P.W.; Cipto, A. Study of temperature effect on carbide layer formation behaviour of dual elements thermal reactive deposition on SUJ2 steel substrate. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).