Study on Microstructure and Oxidation Resistance Mechanism of Y-Modified NiCrAlY Coating Prepared by Pack Cementation
Abstract
1. Introduction
2. Materials and Methods
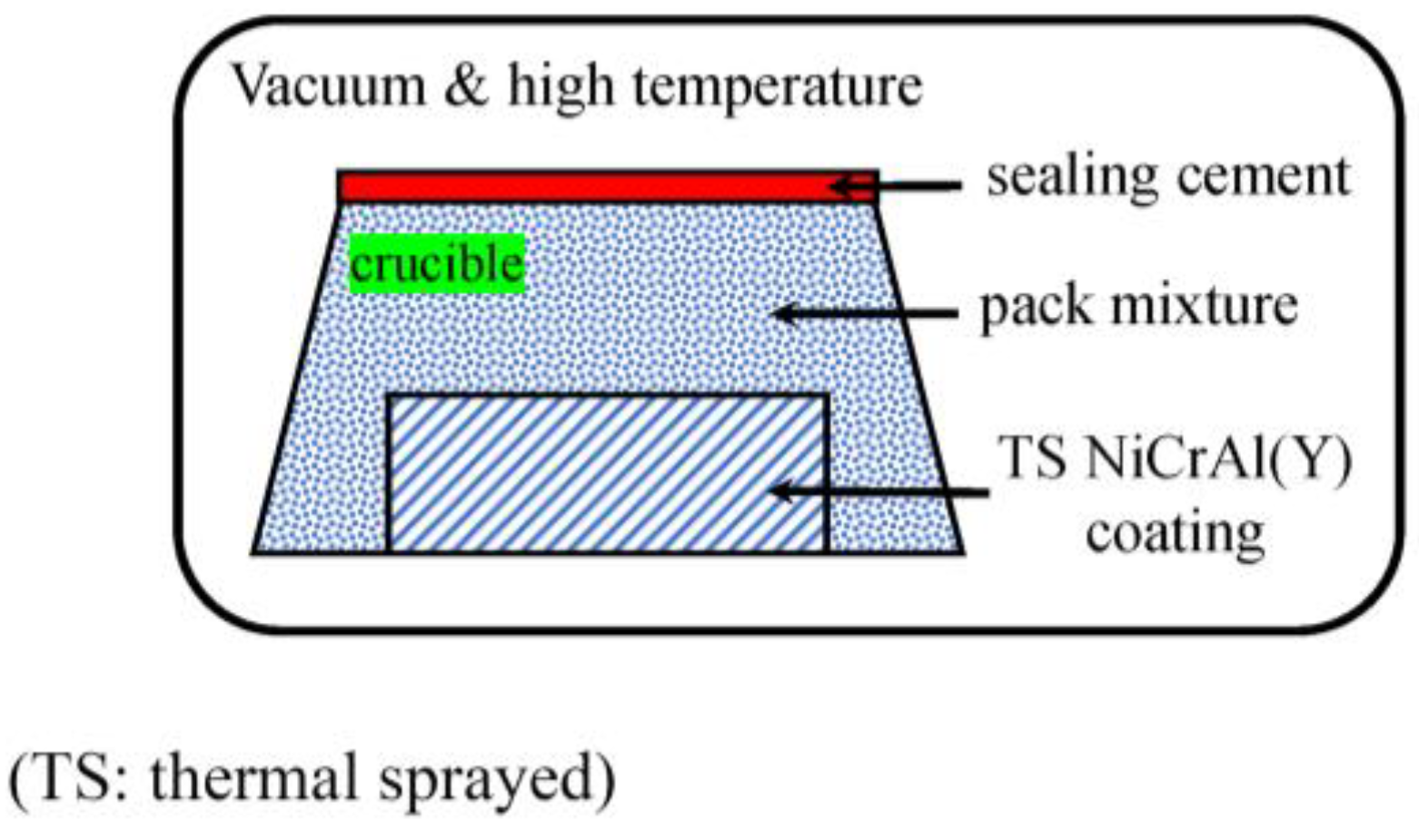
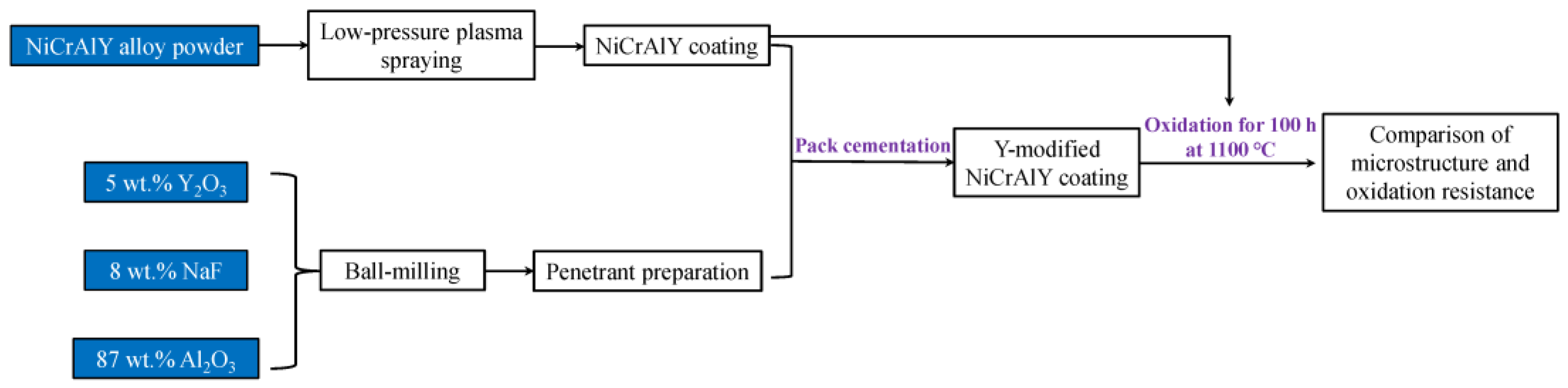
2.1. Preparation of Coatings
2.2. Oxidation Resistance Test and Characterization
3. Results and Discussion
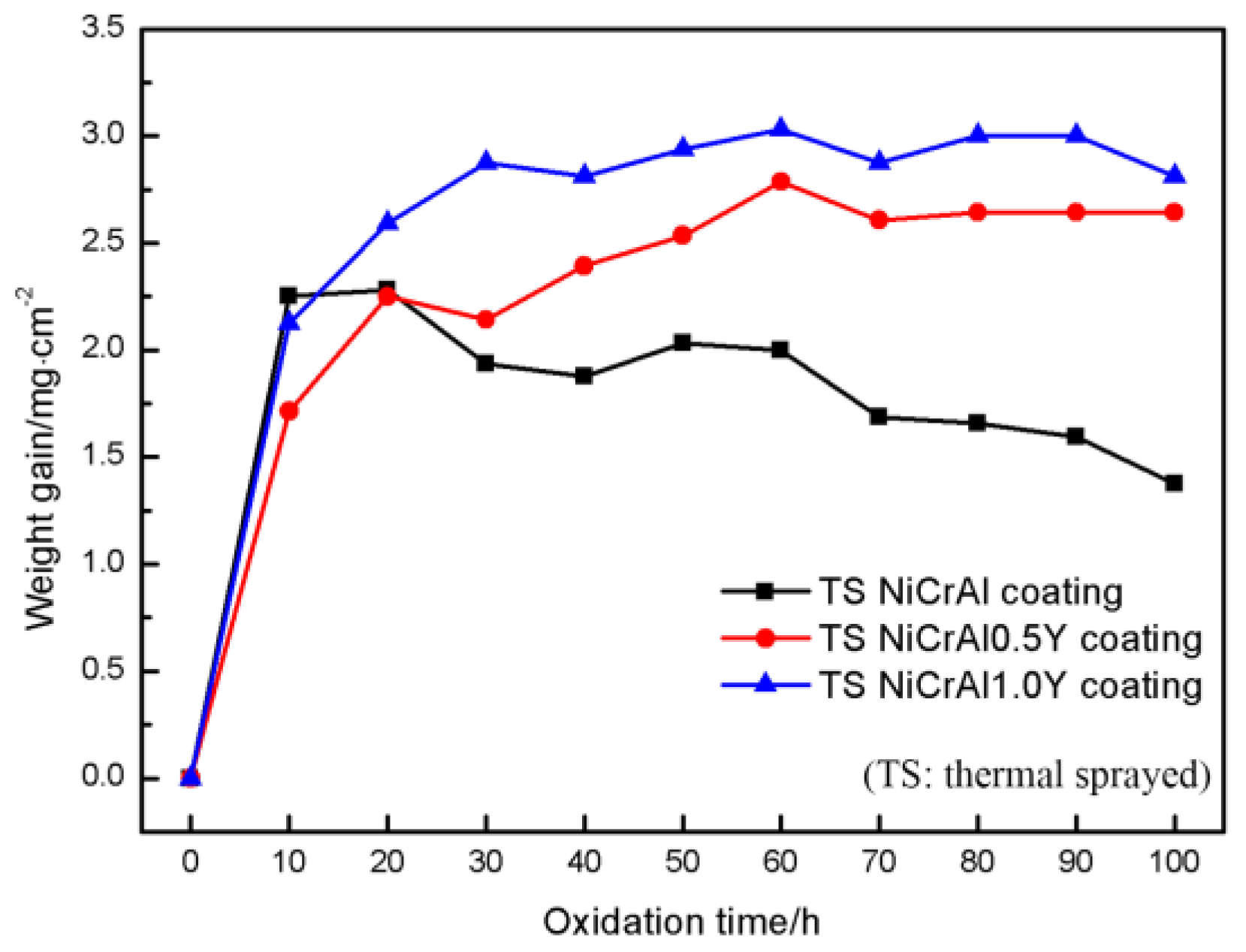
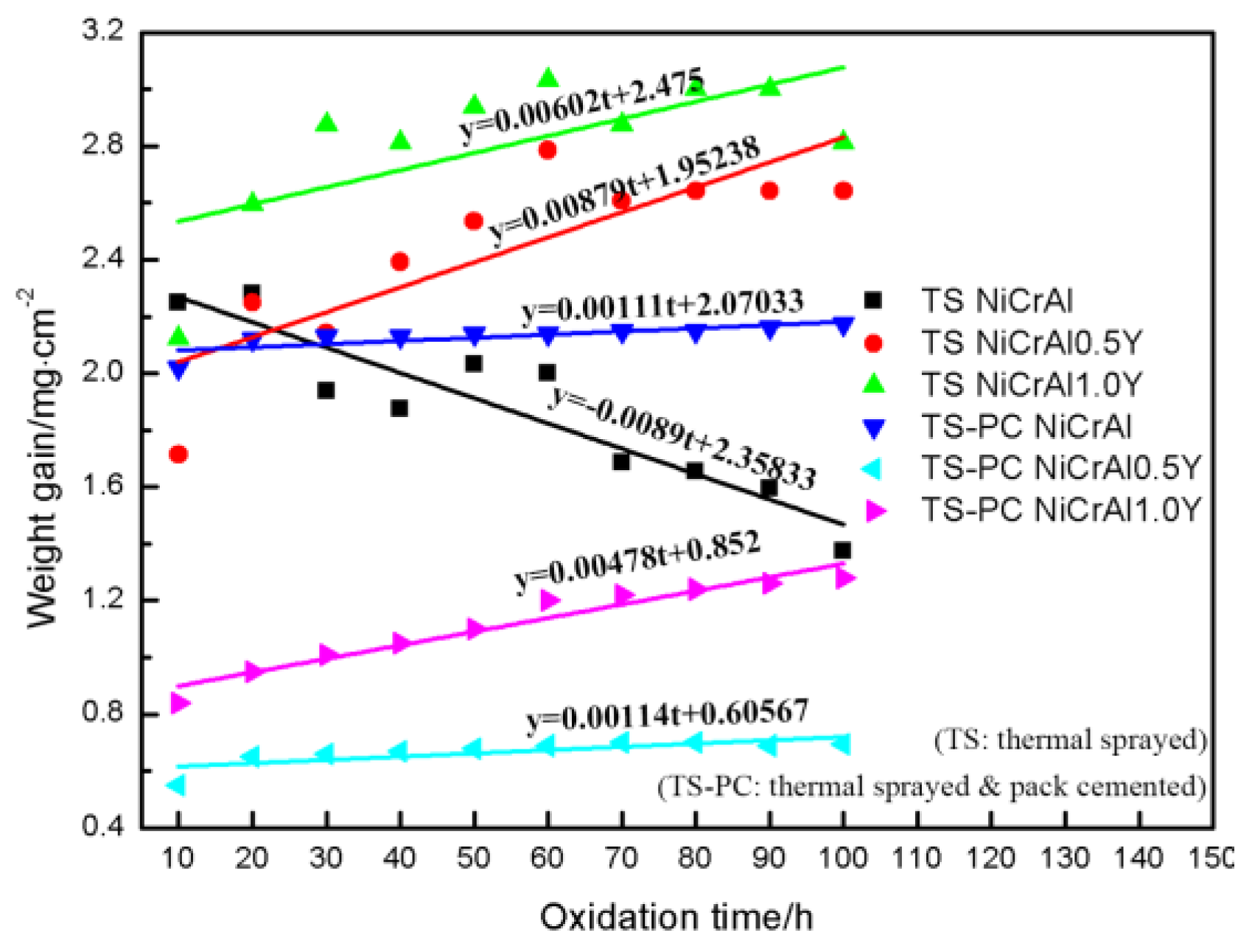
3.1. Oxidation Behavior of TS NiCrAlY Coatings
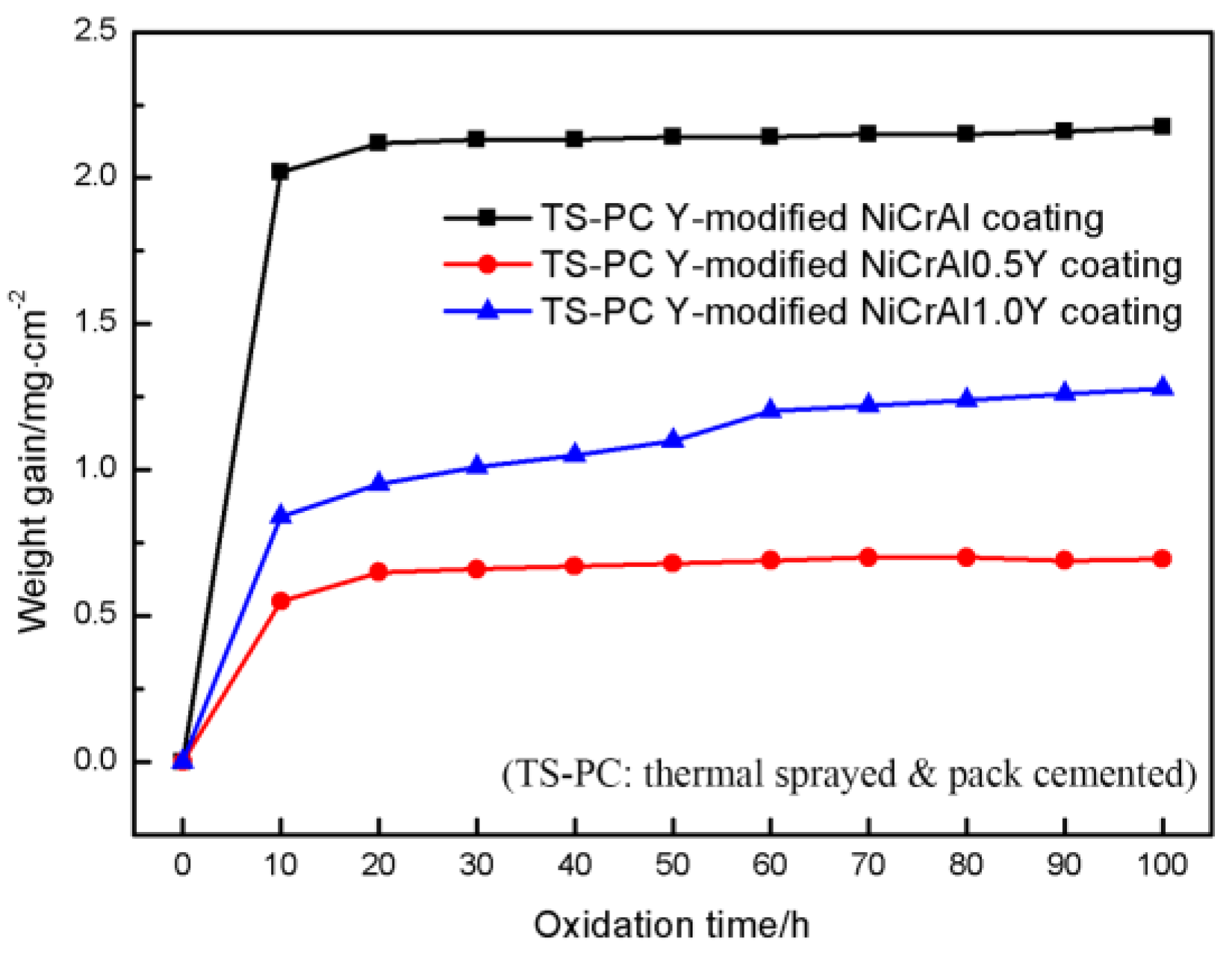
3.2. Oxidation Behavior of TS-PC Y-modified NiCrAlY Coatings
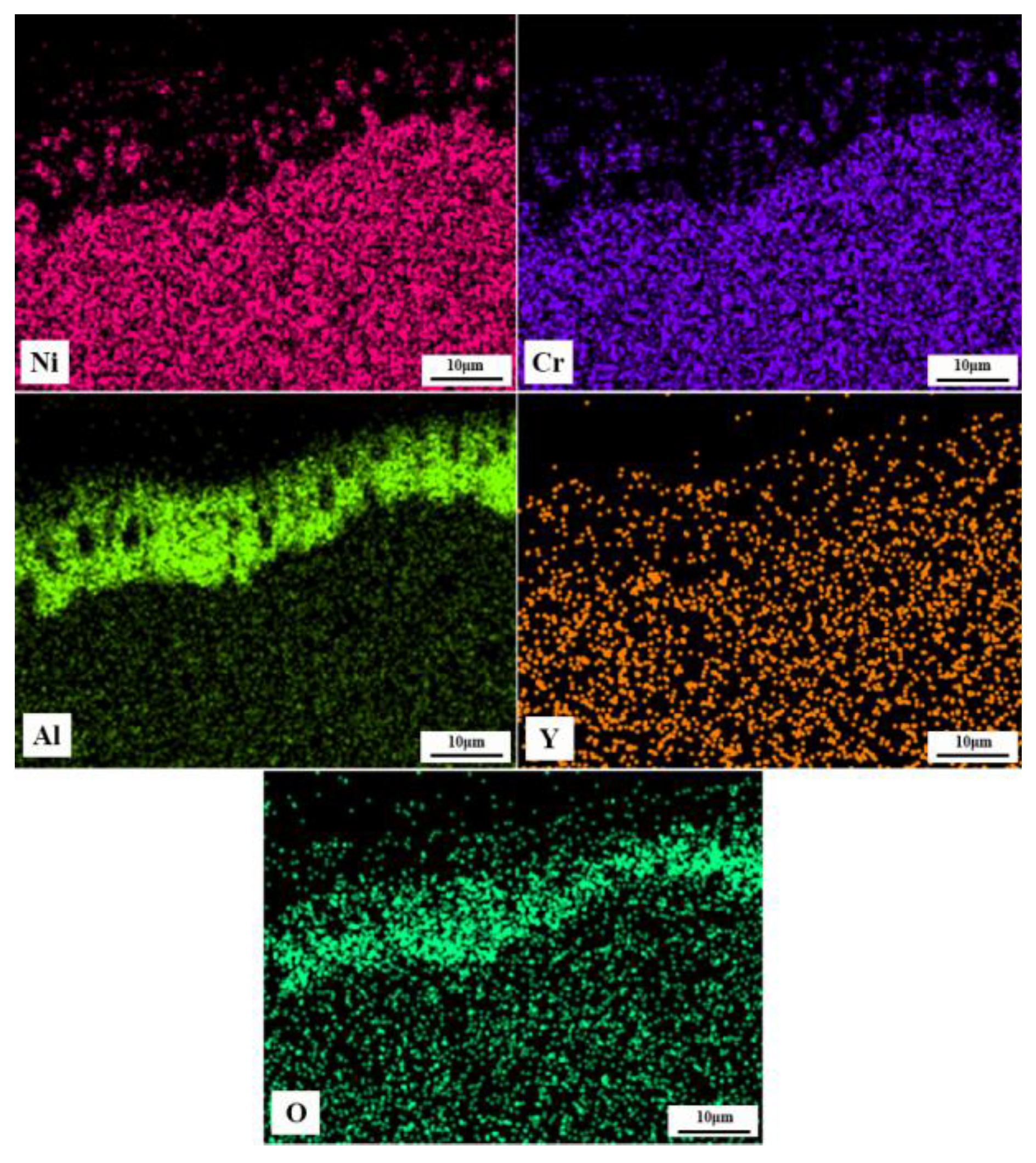
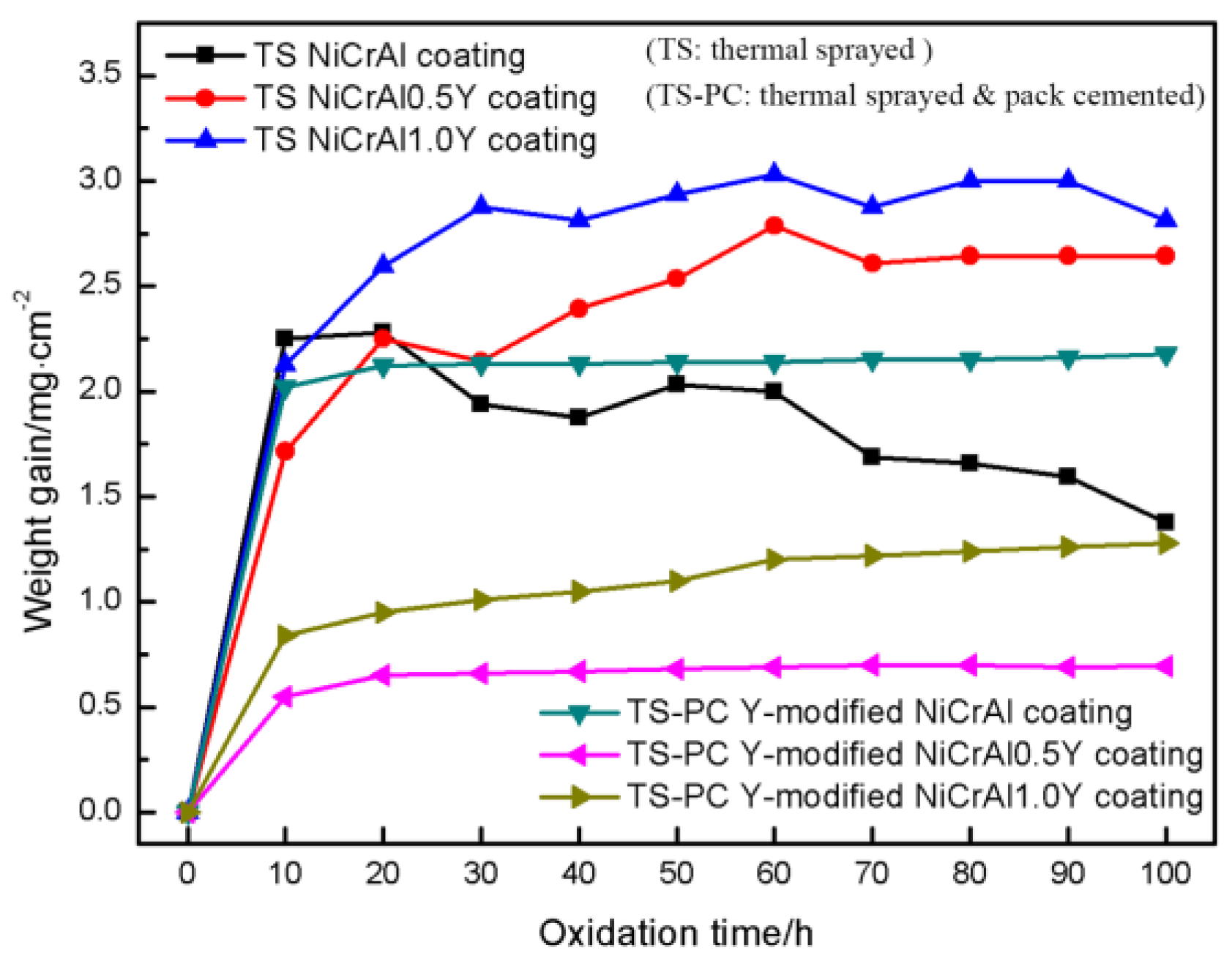
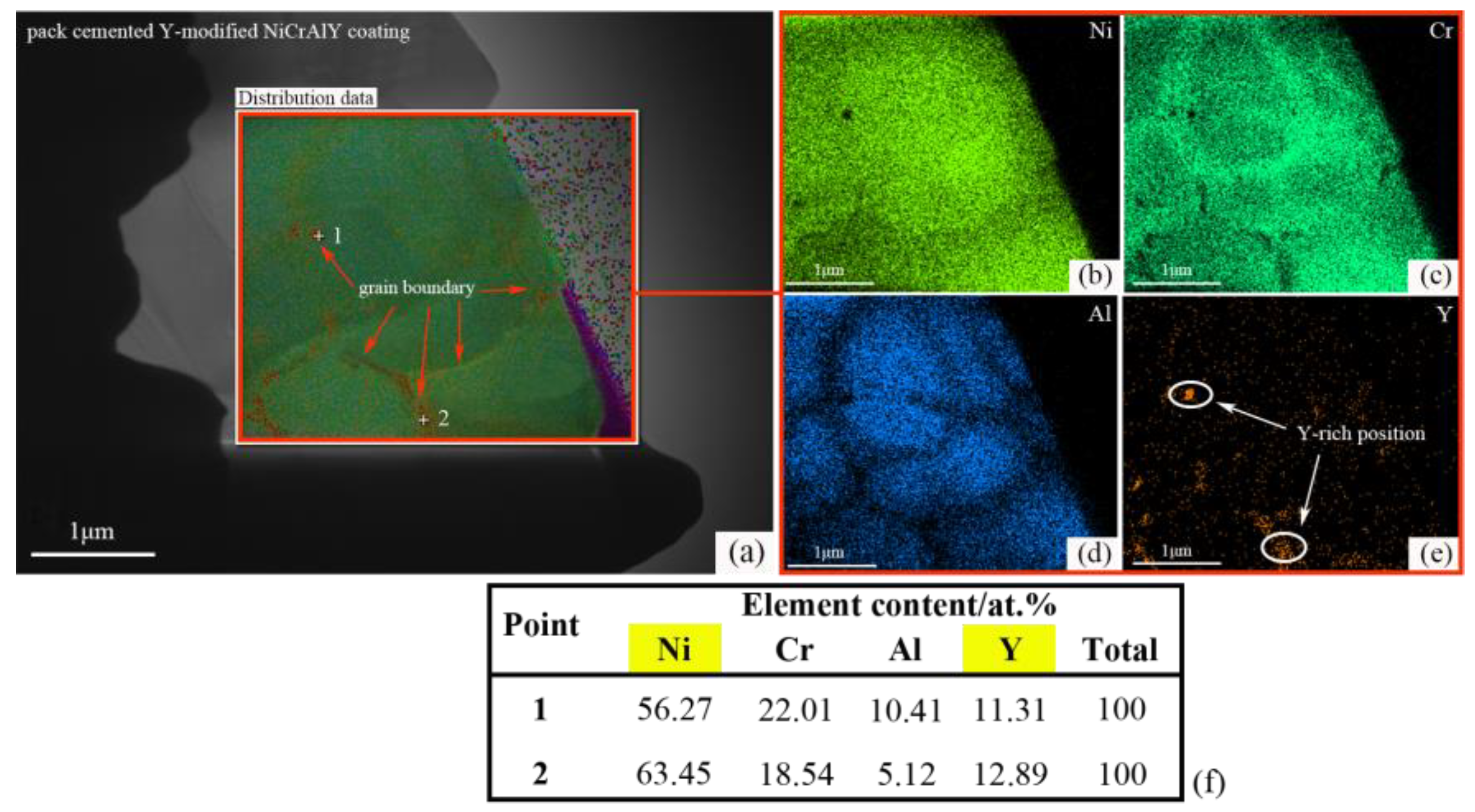
3.3. Comparison of Oxidation Resistance of Coatings and Y-modifying Mechanisms
4. Conclusions
- (1)
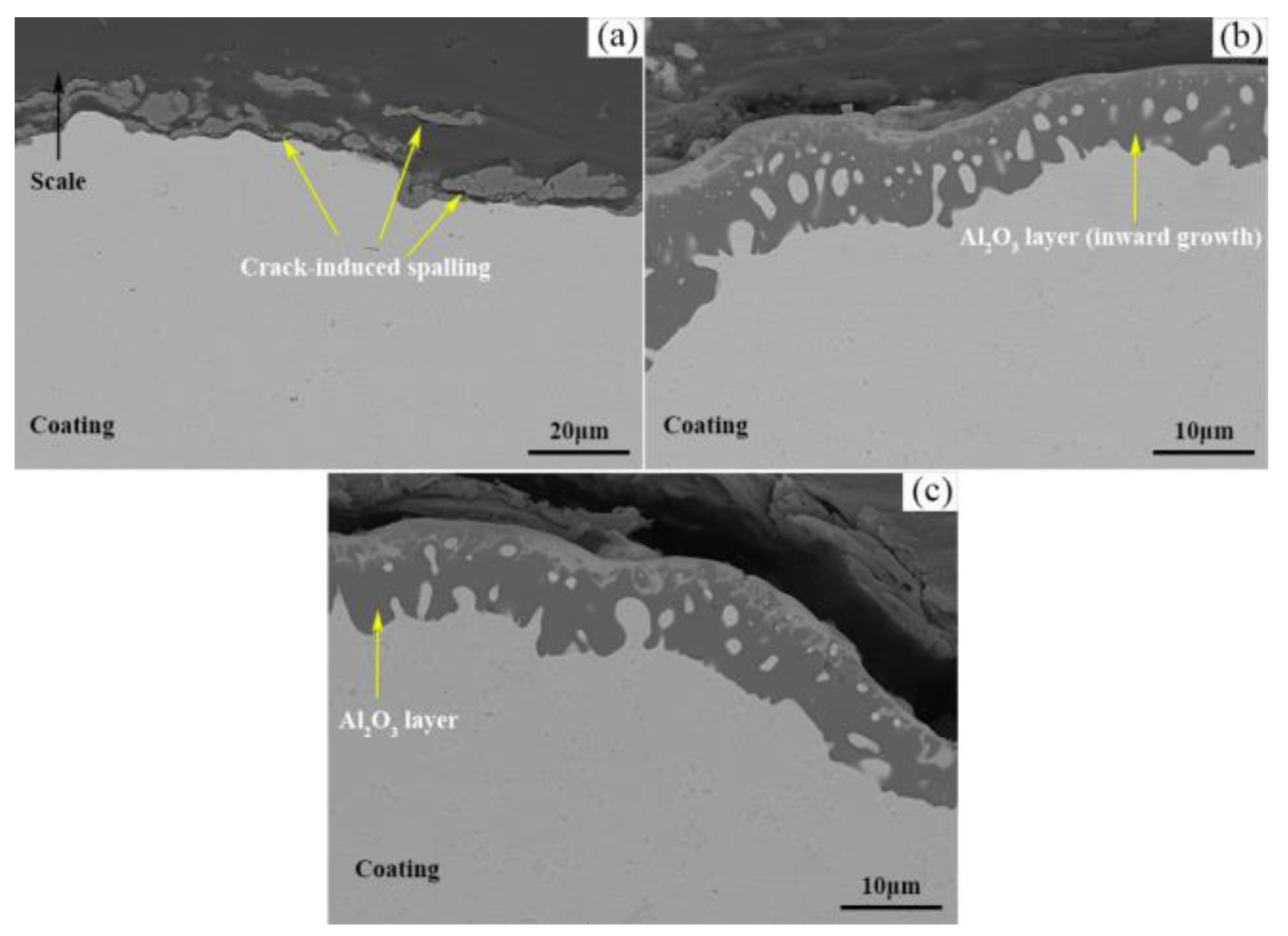
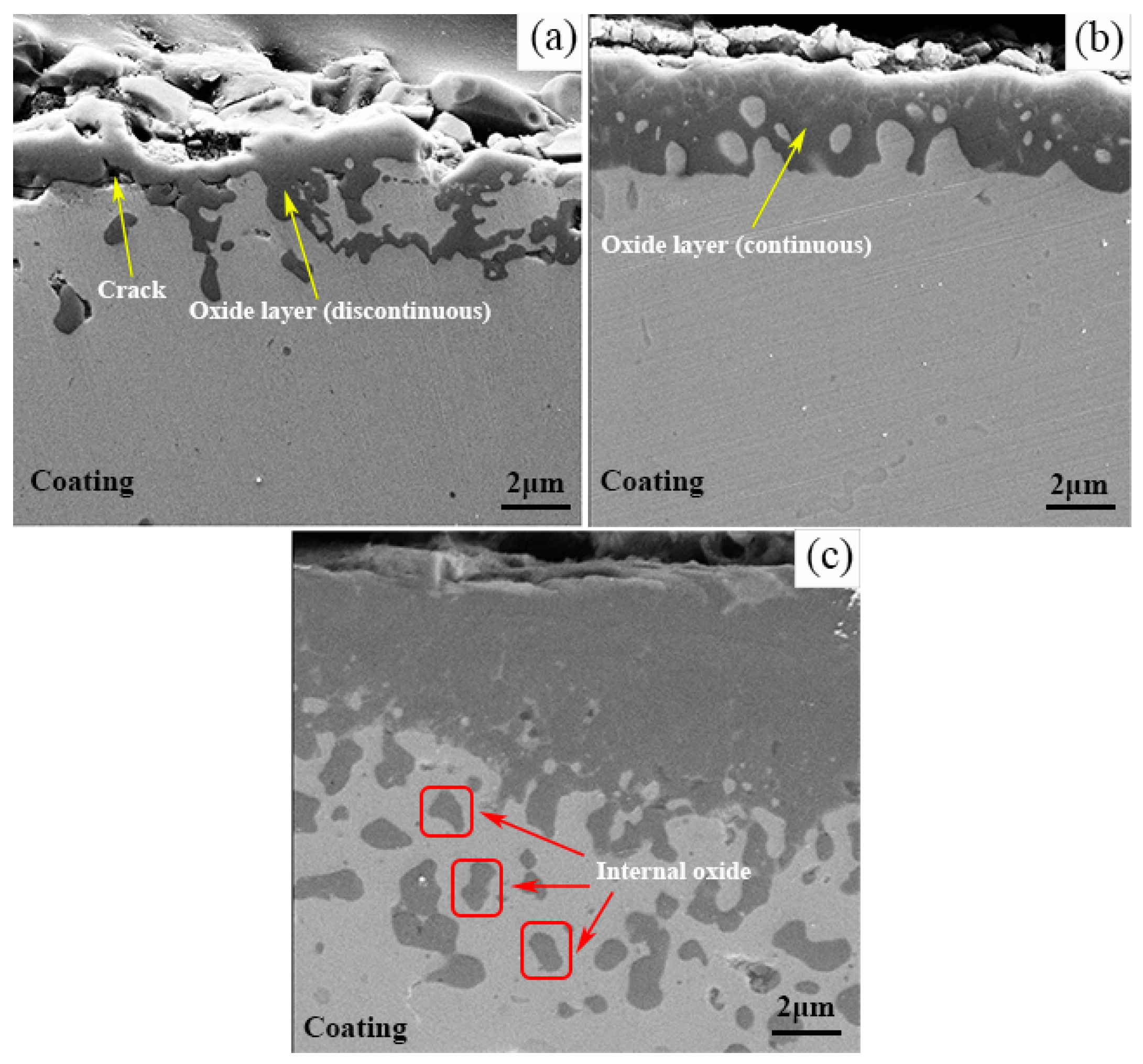
- The Al2O3 oxide film formed on the surface inhibits the further diffusion of O, and is the main oxidation resistance mechanism of NiCrAlY coating. A lot of cracks generated on the interface between Al2O3 oxide film and TS NiCrAl coating substrate due to the mismatch of the thermal and physical properties of the two, which resulted in the oxide scale falling off, and resulted in the worst oxidation resistance. The existence of Y can improve the adhesion of the oxide film and inhibit the diffusion of Al3+. However, excessive Y content will lead to the formation of complex oxides such as Y-Al-O at high temperatures, which will reduce the thermal stability of the coating under the effect of phase-change stress.
- (2)
- The oxidation resistance can be significantly improved by permeating Y into the matrix of the TS NiCrAlY coatings. The intermetallic compound Ni5Y in the TS-PC Y-modified coating is more stable, which can prolong the time for Y to generate Y-Al-O and other complex oxides. When the oxidation time at 1100 °C was 100 h, the oxidation weight gain of TS NiCrAl0.5Y and TS NiCrAl1.0Y coatings was 3.8 times and 2.2 times that of TS-PC Y-modified coatings, respectively.
- (3)
- Under the condition of containing the same amount of Y, the pack-cemented NiCrAlY coating has better oxidation resistance and hardness than the thermal-sprayed NiCrAlY coating. The oxidation resistance of the pack-cemented NiCrAlY coatings increases with the increase in Y content when it is in a range of 0~1 wt.%. When the coating was oxidized at 1100 °C for 100 h, the oxidation weight gain of the pack-cemented NiCrAl0.5Y and NiCrAl1.0Y coatings was about 1.8 and 0.9 mg/cm2, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, C.H.; Bai, C.Y.; Luo, Y.J. The structure and high temperature corrosion behavior of pack aluminized coatings on superalloy IN-738LC. Mater. Chem. Phys. 2004, 86, 258–268. [Google Scholar] [CrossRef]
- Shao, Q.; Lyu, B.H.; Yuan, J.L.; Wang, X.; Ke, M.F.; Zhao, P. Shear thickening polishing of the concave surface of high-temperature nickel-based alloy turbine blade. J. Mater. Res. Technol. 2021, 11, 72–84. [Google Scholar] [CrossRef]
- Pakseresht, A.; Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Nafchi, M.R.; Bazli, M.; Kirubaharan, K. Failure mechanisms and structure tailoring of YSZ and new candidates for thermal barrier coatings: A systematic review. Mater. Des. 2022, 222, 111044. [Google Scholar] [CrossRef]
- Jia, L.; Wen, T.P.; Tian, C.; Liu, Z.L.; Yu, J.K.; Yuan, L. Preparation and thermophysical properties of RE2CrTaO7 (Y, Sm, Dy, Yb) ceramics for thermal barrier coating applications. Ceram. Int. 2022, 48, 23814–23820. [Google Scholar] [CrossRef]
- He, J.L. Advanced MCrAlY alloys with doubled TBC lifetime. Surf. Coat. Technol. 2022, 448, 128931. [Google Scholar] [CrossRef]
- Liang, J.J.; Wei, H.; Zhu, Y.L.; Sun, X.F.; Hu, Z.Q.; Dargusch, M.S.; Yao, X.D. Influence of Re on the properties of a NiCoCrAlY coating alloy. J. Mater. Sci. Technol. 2011, 27, 408–414. [Google Scholar] [CrossRef]
- Sudiro, T.; Sano, T.; Kyo, S.; Ishibashi, O.; Nakamori, M.; Kurokawa, K. High temperature corrosion of CoNiCrAlY-Si alloys in an air-Na2SO4-NaCl gas atmosphere. Mater. Trans. 2011, 52, 433–438. [Google Scholar] [CrossRef]
- Poza, P.; Grant, P.S. Microstructure evolution of vacuum plasma sprayed CoNiCrAlY coatings after heat treatment and isothermal oxidation. Surf. Coat. Technol. 2006, 201, 2887–2896. [Google Scholar] [CrossRef]
- Pomeroy, M.J. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Lartigue-Korinek, S.; Bouchet, D.; Bleloch, A.; Colliex, C. HAADF study of the relationship between intergranular defect structure and yttrium segregation in an alumina grain boundary. Acta Mater. 2011, 59, 3519–3527. [Google Scholar] [CrossRef]
- Serrano Vergel, C.; Kwakernaak, C.; Nijdam, T.J.; Sloof, W.G. Yttrium and oxygen distributions in MCrAlY coatings after deposition, annealing and oxidation. Mater. High Temp. 2009, 26, 153–159. [Google Scholar] [CrossRef]
- Guo, J.T.; Zhou, L.Z.; Li, G.S. High temperature structural intermetallics and their strengthening-softening mechanisms. Chin. J. Nonferrous Met. 2011, 21, 1–34. [Google Scholar]
- Vasiliev, A.L.; Aindow, M.; Blackburn, M.J.; Watson, T.J. Phase stability and microstructure in devitrified Al-rich Al-Y-Ni alloys. Intermetallics 2004, 12, 349–362. [Google Scholar] [CrossRef]
- Subanovic, M.; Sebold, D.; Vassen, R.; Wessel, E.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Effect of manufacturing related parameters on oxidation properties of MCrAlY bond coats. Mater. Corros. 2008, 59, 463–470. [Google Scholar] [CrossRef]
- Toscano, J.; Vaβen, R.; Gil, A.; Subanovic, M.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Parameters affecting TGO growth and adherence on MCrAlY-bond coats for TBC’s. Surf. Coat. Technol. 2006, 201, 3906–3910. [Google Scholar] [CrossRef]
- Lau, H. Influence of yttria on the cyclic lifetime of YSZ TBC deposited on EB-PVD NiCoCrAlY bondcoats and its contribution to a modified TBC adhesion mechanism. Surf. Coat. Technol. 2013, 235, 121–126. [Google Scholar] [CrossRef]
- Gil, A.; Naumenko, D.; Vassen, R.; Toscano, J.; Subanovic, M.; Singheiser, L.; Quadakkers, W.J. Y-rich oxide distribution in plasma sprayed MCrAlY-coatings studied by SEM with a cathodoluminescence detector and Raman spectroscopy. Surf. Coat. Technol. 2009, 204, 531–538. [Google Scholar] [CrossRef]
- Gil, A.; Shemet, V.; Vassen, R.; Subanovic, M.; Toscano, J.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Effect of surface condition on the oxidation behaviour of MCrAlY coatings. Surf. Coat. Technol. 2006, 201, 3824–3828. [Google Scholar] [CrossRef]
- Wu, Y.N.; Qin, M.; Feng, Z.C.; Liang, Y.; Sun, C.; Wang, F.H. Improved oxidation resistance of NiCrAlY coatings. Mater. Lett. 2003, 57, 2404–2408. [Google Scholar] [CrossRef]
- Xu, K.D.; Ren, Z.M.; Li, C.J. Progress in application of rare metals in superalloys. Rare Met. 2014, 33, 111–126. [Google Scholar] [CrossRef]
- Qiao, M.; Zhou, C.G. Codeposition of Co-Al-Y on nickel base superalloys by pack cementation process. Corros. Sci. 2013, 75, 454–460. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Ouyang, P.; Yan, H.; Si, L. Study on Microstructure and Oxidation Resistance Mechanism of Y-Modified NiCrAlY Coating Prepared by Pack Cementation. Coatings 2023, 13, 63. https://doi.org/10.3390/coatings13010063
Zhang S, Ouyang P, Yan H, Si L. Study on Microstructure and Oxidation Resistance Mechanism of Y-Modified NiCrAlY Coating Prepared by Pack Cementation. Coatings. 2023; 13(1):63. https://doi.org/10.3390/coatings13010063
Chicago/Turabian StyleZhang, Shuting, Peixuan Ouyang, Hongjuan Yan, and Lina Si. 2023. "Study on Microstructure and Oxidation Resistance Mechanism of Y-Modified NiCrAlY Coating Prepared by Pack Cementation" Coatings 13, no. 1: 63. https://doi.org/10.3390/coatings13010063
APA StyleZhang, S., Ouyang, P., Yan, H., & Si, L. (2023). Study on Microstructure and Oxidation Resistance Mechanism of Y-Modified NiCrAlY Coating Prepared by Pack Cementation. Coatings, 13(1), 63. https://doi.org/10.3390/coatings13010063





