Nanostructured Semi-Transparent TiO2 Nanoparticle Coatings Produced by Magnetron-Based Gas Aggregation Source
Abstract
1. Introduction
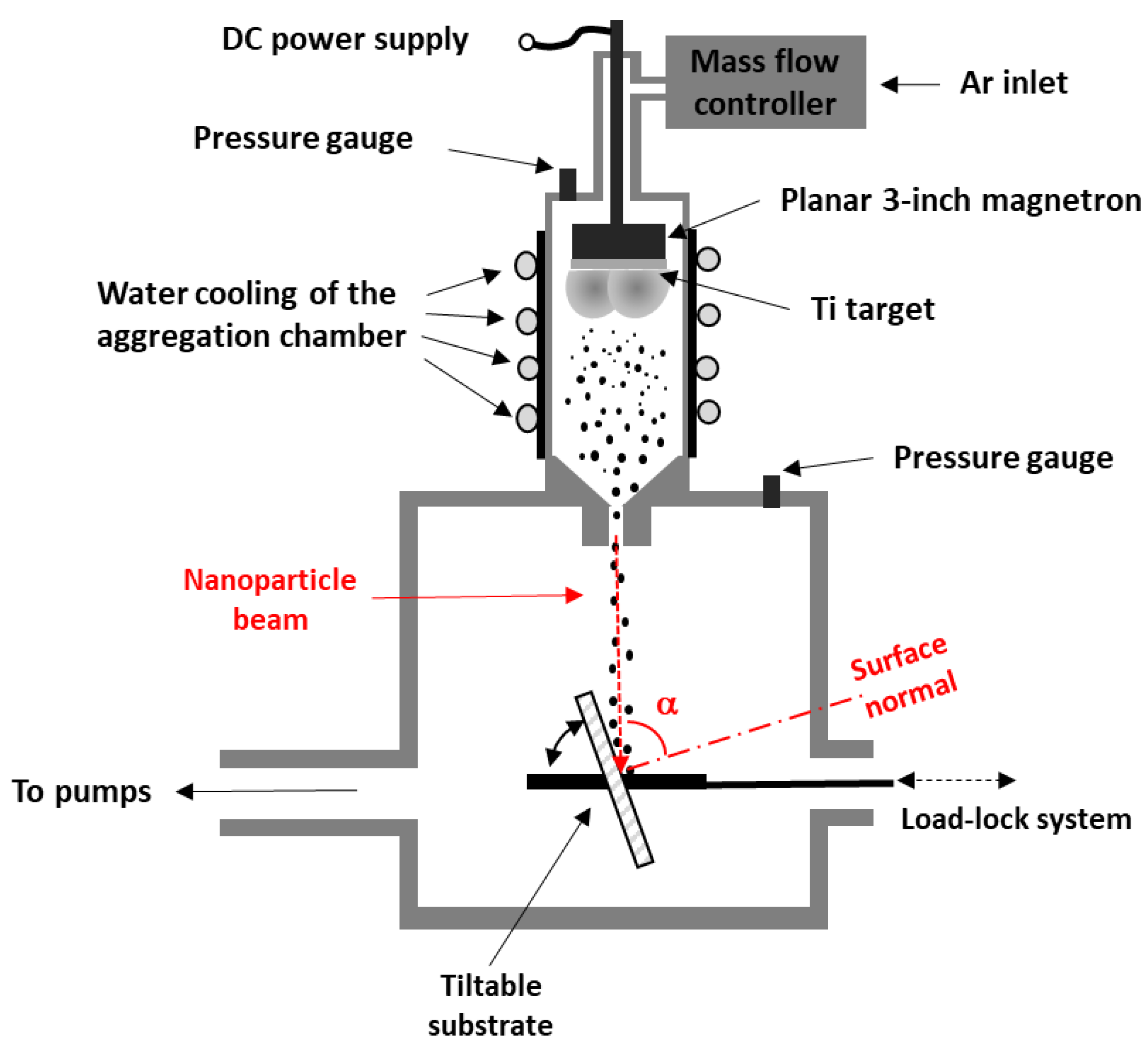
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Characterization
3. Results
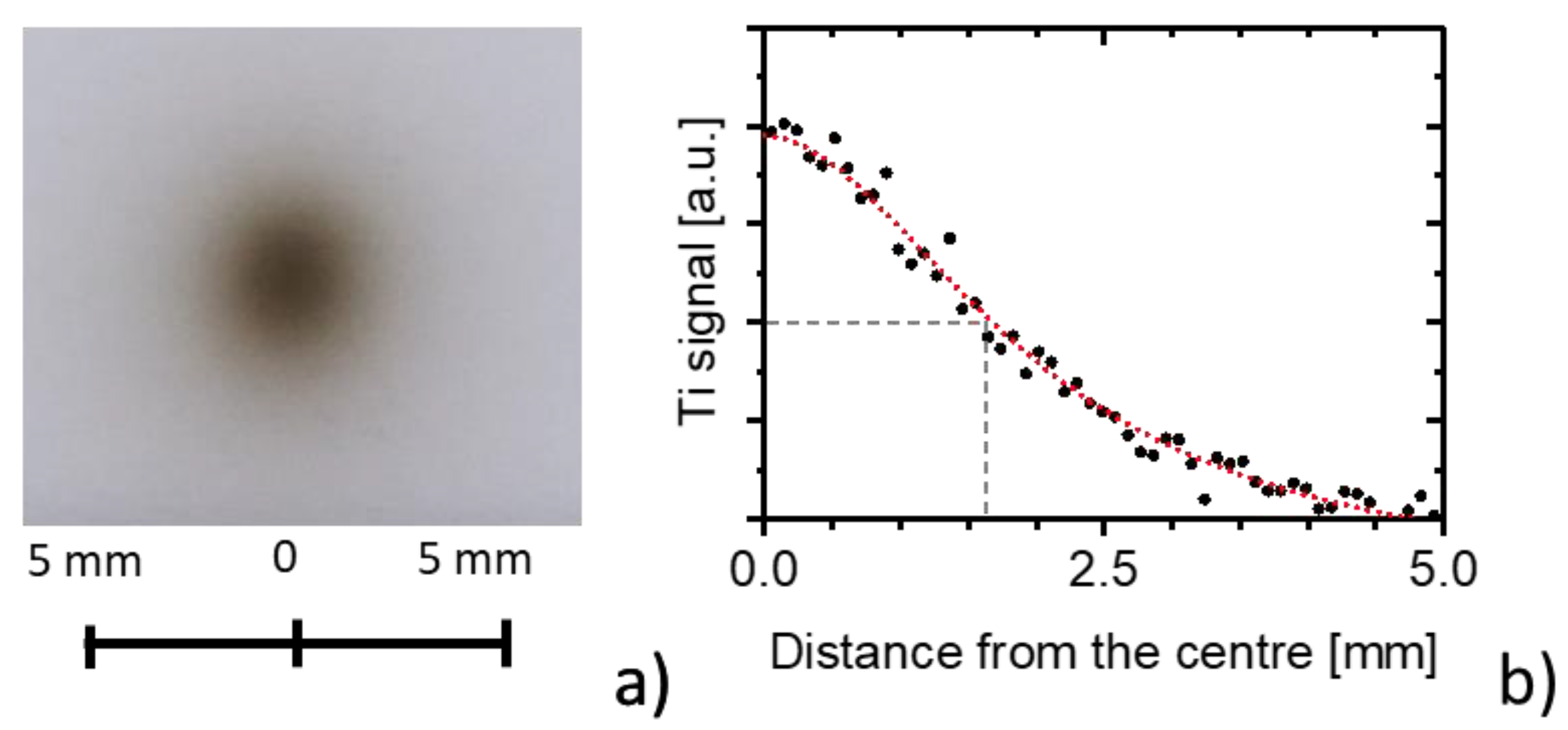
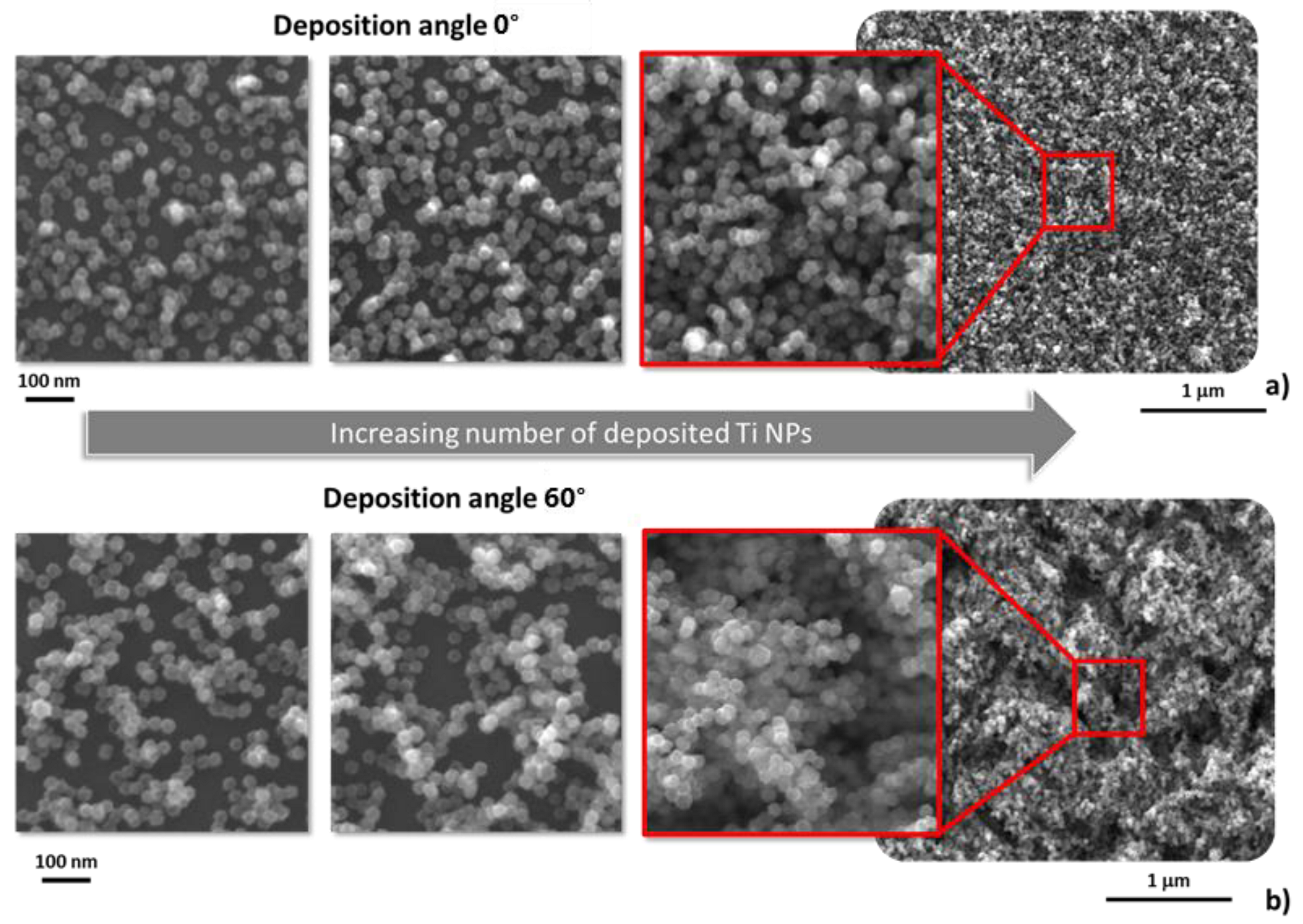
3.1. Deposition at Zero Tilting
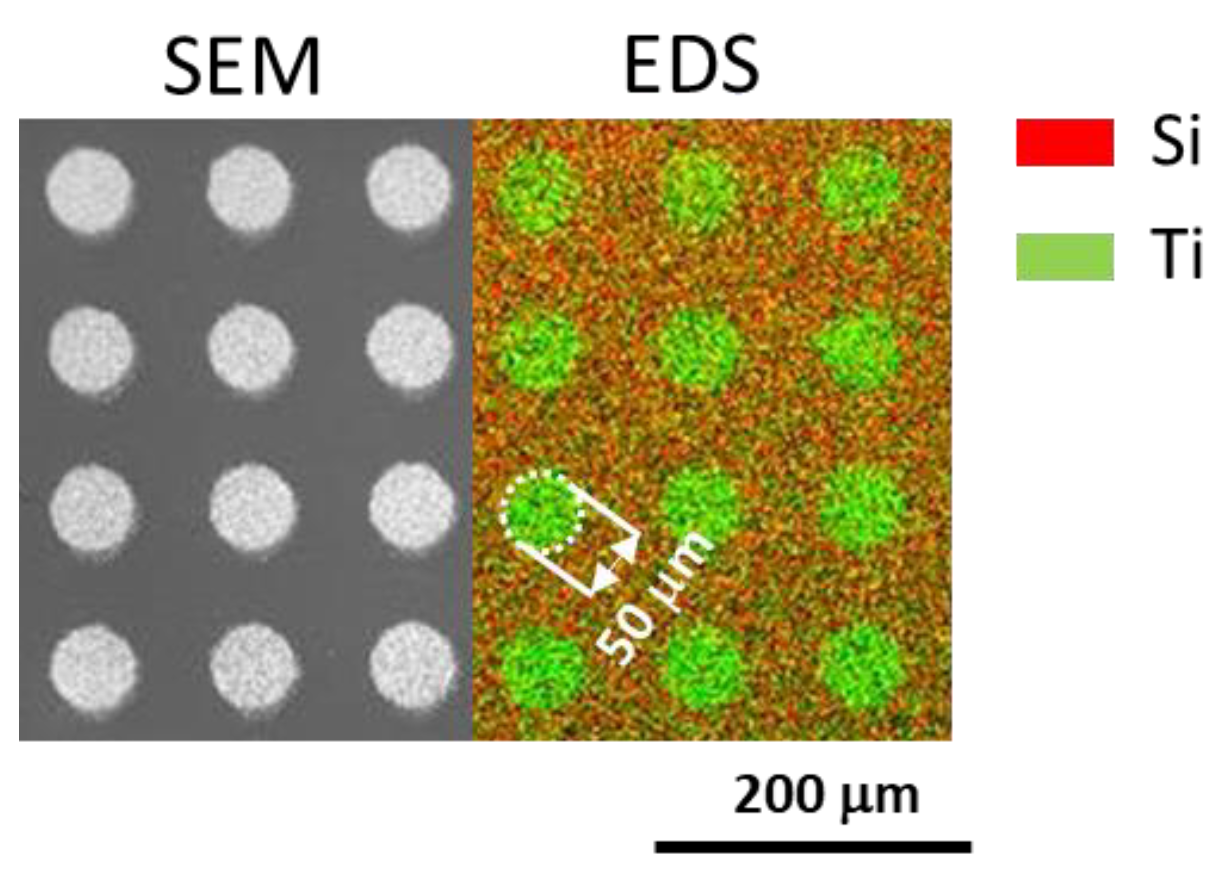
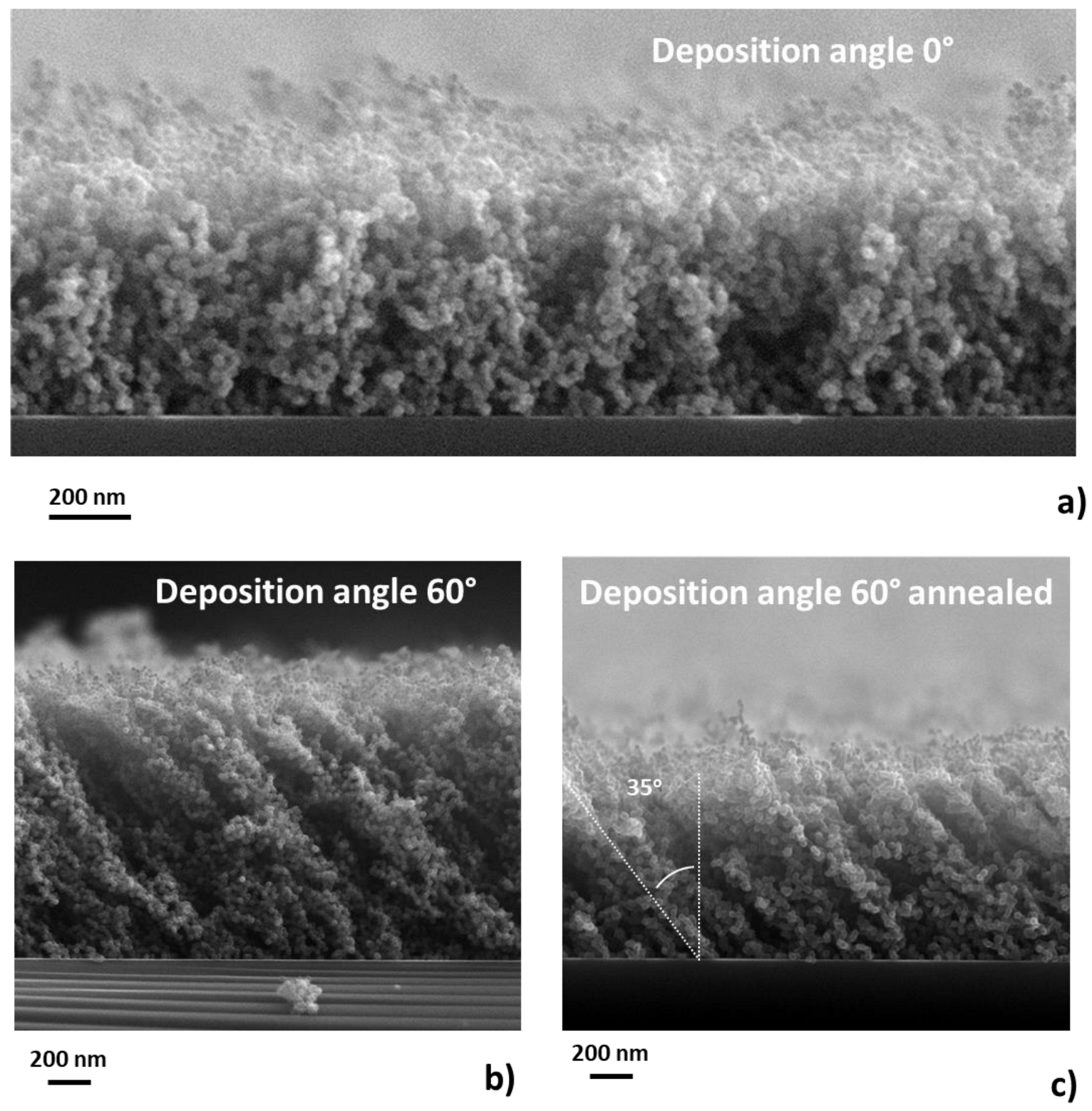
3.2. Influence of the Tilting Angle on the Morphology of Produced Nanoparticle Films
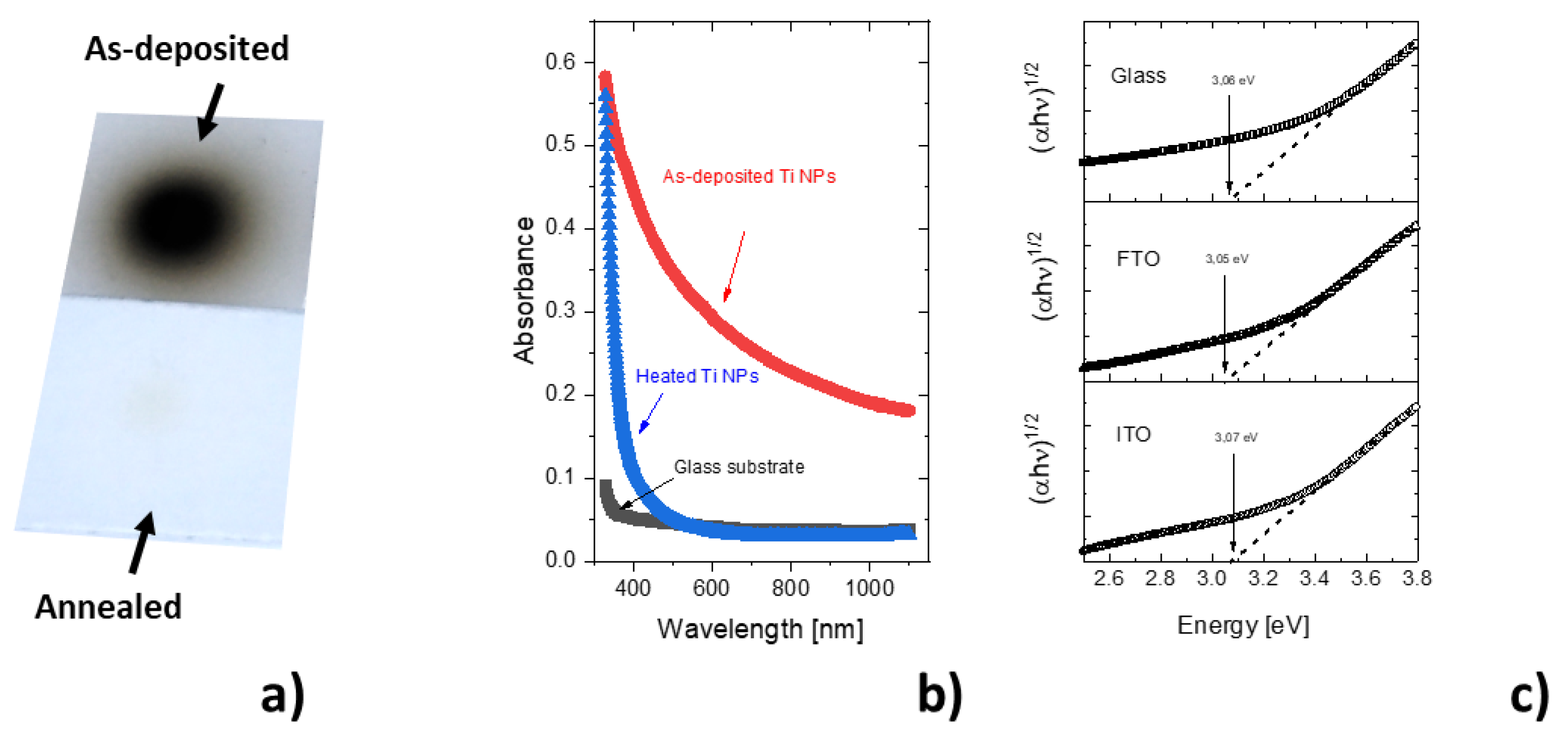
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent Advances in Syntheses, Properties and Applications of TiO2 Nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef] [PubMed]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Gas Sensors Based on Titanium Oxides (Review). Coatings 2022, 12, 699. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Navarrete, J.; Gomez, R. Synthesis, Characterization and Photocatalytic Activity of TiO2 Nanostructures: Nanotubes, Nanofibers, Nanowires and Nanoparticles. Catal. Today 2016, 266, 90–101. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, G.S.; Yuan, X.Y.; Xie, T.; Zhang, L.D. Fabrication and Optical Properties of TiO2 Nanowire Arrays Made by Sol Gel Electrophoresis Deposition into Anodic Alumina Membranes. J. Phys. Condenes. Matter 2003, 15, 2917. [Google Scholar] [CrossRef]
- Miao, L.; Tanemura, S.; Toh, S.; Kaneko, K.; Tanemura, M. Fabrication, Characterization and Raman Study of Anatase-TiO2 Nanorods by a Heating-Sol-Gel Template Process. J. Cryst. Growth 2004, 264, 246–252. [Google Scholar] [CrossRef]
- Kouao, D.S.; Grochowska, K.; Siuzdak, K. The Anodization of Thin Titania Layers as a Facile Process towards Semitransparent and Ordered Electrode Material. Nanomaterials 2022, 12, 1131. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 Nanotubes: Self-Organized Electrochemical Formation, Properties and Applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Hanif, M.B.; Sihor, M.; Liapun, V.; Makarov, H.; Monfort, O.; Motola, M. Porous vs. Nanotubular Anodic TiO2: Does the Morphology Really Matters for the Photodegradation of Caffeine? Coatings 2022, 12, 1002. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Brett, M.J. Glancing Angle Deposition: Fabrication, Properties, and Applications of Micro- and Nanostructured Thin Films. J. Vac. Sci. Technol. A 2007, 25, 1317–1335. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on Oblique Angle Deposition of Thin Films: From Fundamentals to Devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Haberland, H.; Karrais, M.; Mall, M.; Thurner, Y. Thin Films from Energetic Cluster Impact: A Feasibility Study. J. Vac. Sci. Technol. A 1992, 10, 3266–3271. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Kuzminova, A.; Kylián, O.; Biederman, H. Comparison of Magnetron Sputtering and Gas Aggregation Nanoparticle Source Used for Fabrication of Silver Nanoparticle Films. Surf. Coat. Technol. 2015, 275, 296–302. [Google Scholar] [CrossRef]
- Hanková, A.; Kuzminova, A.; Hanuš, J.; Košutová, T.; Solař, P.; Kousal, J.; Kylián, O. Nanostructured and Columnar Vanadium and Vanadium Oxides Films Synthesized by Means of Magnetron-Based Gas Aggregation Source. Surf. Coat. Technol. 2022, 431, 128015. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Qamhieh, N.; Ghamlouche, H.; Thaker, S.; El-Shaer, M. Fabrication of Size-Selected Pd Nanoclusters Using a Magnetron Plasma Sputtering Source. J. Appl. Phys. 2010, 107, 034317. [Google Scholar] [CrossRef]
- Acsente, T.; Negrea, R.F.; Nistor, L.C.; Logofatu, C.; Matei, E.; Birjega, R.; Grisolia, C.; Dinescu, G. Synthesis of Flower-like Tungsten Nanoparticles by Magnetron Sputtering Combined with Gas Aggregation. Eur. Phys. J. D 2015, 69, 60097. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Prysiazhnyi, V.; Dyčka, F.; Kylián, O.; Kúš, P.; Sezemský, P.; Štěrba, J.; Straňák, V. Gas Aggregated Ag Nanoparticles as the Inorganic Matrix for Laser Desorption/Ionization Mass Spectrometry. Appl. Surf. Sci. 2021, 541, 148469. [Google Scholar] [CrossRef]
- Gracia-Pinilla, M.; Martínez, E.; Vidaurri, G.S.; Pérez-Tijerina, E. Deposition of Size-Selected Cu Nanoparticles by Inert Gas Condensation. Nanoscale Res. Lett. 2010, 5, 180–188. [Google Scholar] [CrossRef]
- Drabik, M.; Choukourov, A.; Artemenko, A.; Polonskyi, O.; Kylian, O.; Kousal, J.; Nichtova, L.; Cimrova, V.; Slavinska, D.; Biederman, H. Structure and Composition of Titanium Nanocluster Films Prepared by a Gas Aggregation Cluster Source. J. Phys. Chem. C 2011, 115, 20937–20944. [Google Scholar] [CrossRef]
- Polonskyi, O.; Ahadi, A.M.; Peter, T.; Fujioka, K.; Abraham, J.W.; Vasiliauskaite, E.; Hinz, A.; Strunskus, T.; Wolf, S.; Bonitz, M.; et al. Plasma Based Formation and Deposition of Metal and Metal Oxide Nanoparticles Using a Gas Aggregation Source. Eur. Phys. J. D 2018, 72, 80419. [Google Scholar] [CrossRef]
- Shelemin, A.; Kylián, O.; Hanuš, J.; Choukourov, A.; Melnichuk, I.; Serov, A.; Slavínská, D.; Biederman, H. Preparation of Metal Oxide Nanoparticles by Gas Aggregation Cluster Source. Vacuum 2015, 120, 162–169. [Google Scholar] [CrossRef]
- Popok, V.N.; Jeppesen, C.M.; Fojan, P.; Kuzminova, A.; Hanuš, J.; Kylián, O. Comparative Study of Antibacterial Properties of Polystyrene Films with TiOx and Cu Nanoparticles Fabricated Using Cluster Beam Technique. Beilstein J. Nanotechnol. 2018, 9, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Košutová, T.; Horák, L.; Pleskunov, P.; Hanuš, J.; Nikitin, D.; Kúš, P.; Cieslar, M.; Gordeev, I.; Burazer, S.; Choukourov, A.; et al. Thermally-Driven Morphogenesis of Niobium Nanoparticles as Witnessed by in-Situ x-Ray Scattering. Mater. Chem. Phys. 2022, 277, 125466. [Google Scholar] [CrossRef]
- Matěj, Z.; Kužel, R.; Nichtová, L. XRD Total Pattern Fitting Applied to Study of Microstructure of TiO2 Films. Powder Diffr. 2010, 25, 125–131. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Drábik, M.; Choukourov, A.; Artemenko, A.; Kousal, J.; Polonskyi, O.; Solaå, P.; Kylián, O.; Matoušek, J.; Pešička, J.; Matolínová, I.; et al. Morphology of Titanium Nanocluster Films Prepared by Gas Aggregation Cluster Source. Plasma Processes Polym. 2011, 8, 640–650. [Google Scholar] [CrossRef]
- Henderson, M.A. A Surface Science Perspective on TiO2 Photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Baur, W.H. Atomabstände Und Bindungswinkel Im Brookit, TiO2. Acta Crystallogr. 1961, 14, 214–216. [Google Scholar] [CrossRef]
- Cromer, D.T.; Herrington, K. The Structures of Anatase and Rutile. J. Am. Chem. Soc. 1955, 77, 4708–4709. [Google Scholar] [CrossRef]
- Borghi, F.; Podestà, A.; Piazzoni, C.; Milani, P. Growth Mechanism of Cluster-Assembled Surfaces: From Submonolayer to Thin-Film Regime. Phys. Rev. Appl. 2018, 9, 044016. [Google Scholar] [CrossRef]
- Dirks, A.G.; Leamy, H.J. Columnar Microstructure in Vapor-Deposited Thin Films. Thin Solid Films 1977, 47, 219–233. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanková, A.; Kuzminova, A.; Kylián, O. Nanostructured Semi-Transparent TiO2 Nanoparticle Coatings Produced by Magnetron-Based Gas Aggregation Source. Coatings 2023, 13, 51. https://doi.org/10.3390/coatings13010051
Hanková A, Kuzminova A, Kylián O. Nanostructured Semi-Transparent TiO2 Nanoparticle Coatings Produced by Magnetron-Based Gas Aggregation Source. Coatings. 2023; 13(1):51. https://doi.org/10.3390/coatings13010051
Chicago/Turabian StyleHanková, Adéla, Anna Kuzminova, and Ondřej Kylián. 2023. "Nanostructured Semi-Transparent TiO2 Nanoparticle Coatings Produced by Magnetron-Based Gas Aggregation Source" Coatings 13, no. 1: 51. https://doi.org/10.3390/coatings13010051
APA StyleHanková, A., Kuzminova, A., & Kylián, O. (2023). Nanostructured Semi-Transparent TiO2 Nanoparticle Coatings Produced by Magnetron-Based Gas Aggregation Source. Coatings, 13(1), 51. https://doi.org/10.3390/coatings13010051







