Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of ZnO NPs
2.3. Instrumental Analysis
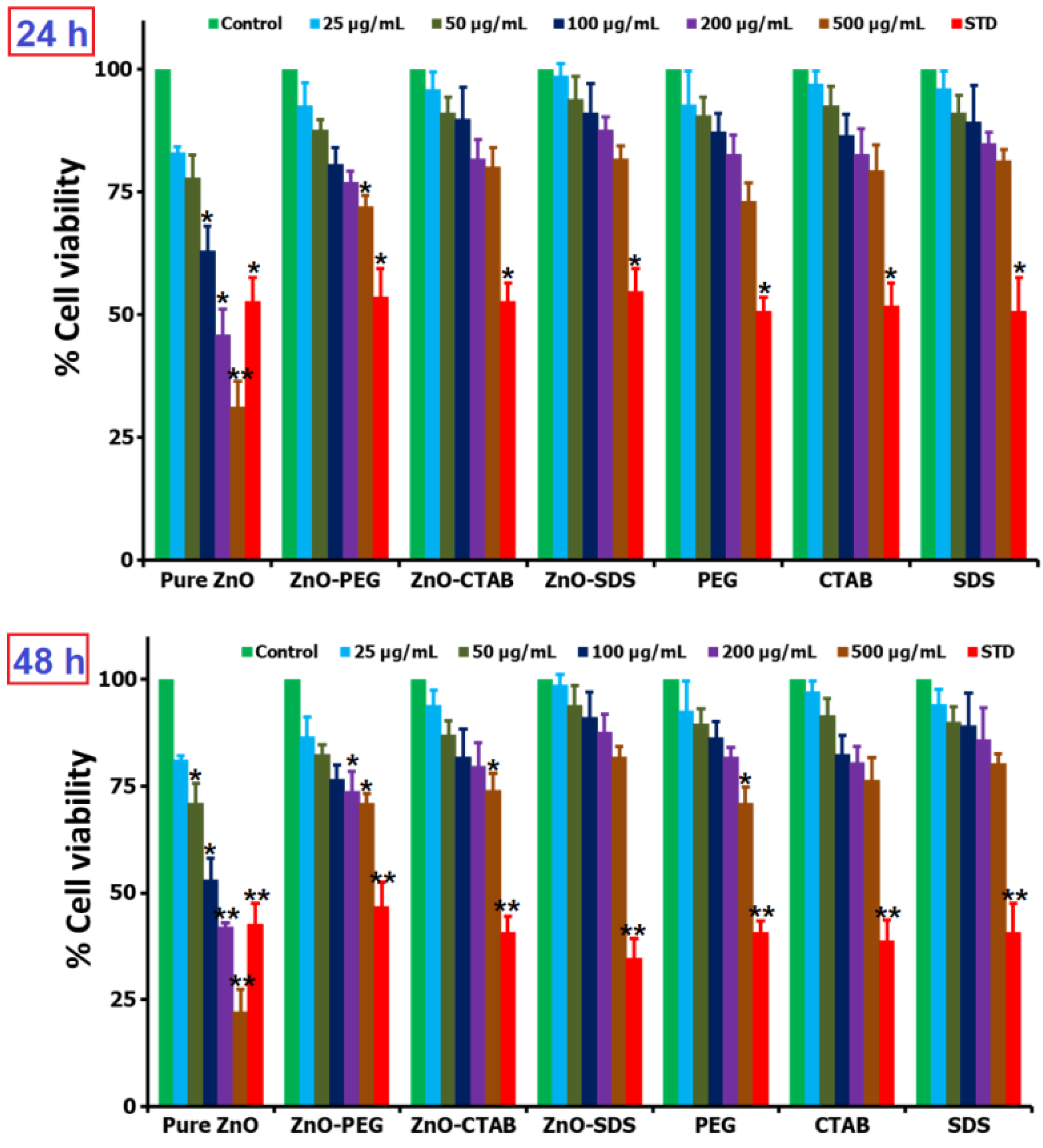
2.4. Studies of Cell Viability
2.5. Apoptosis Assay
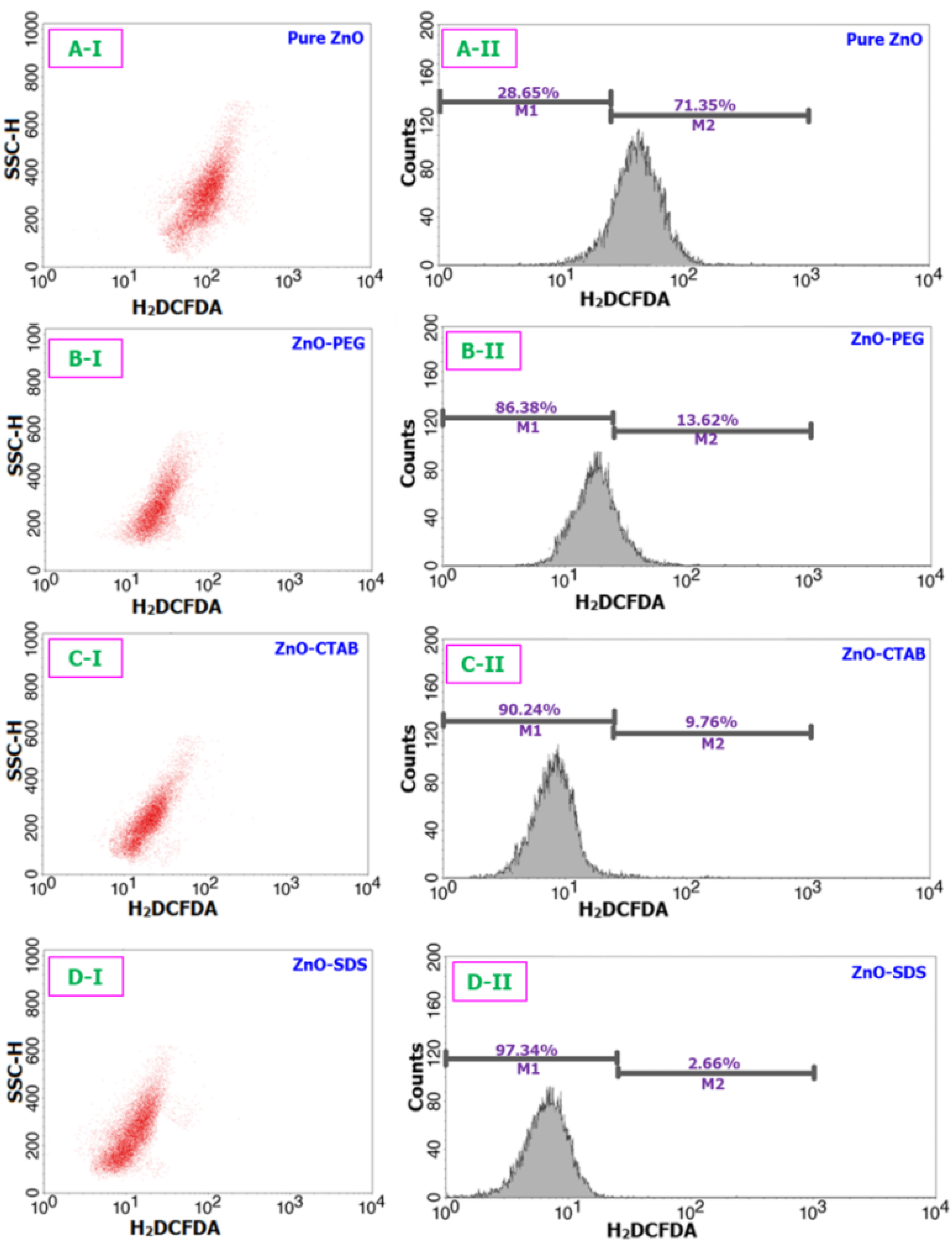
2.6. Oxidative Stress Assay
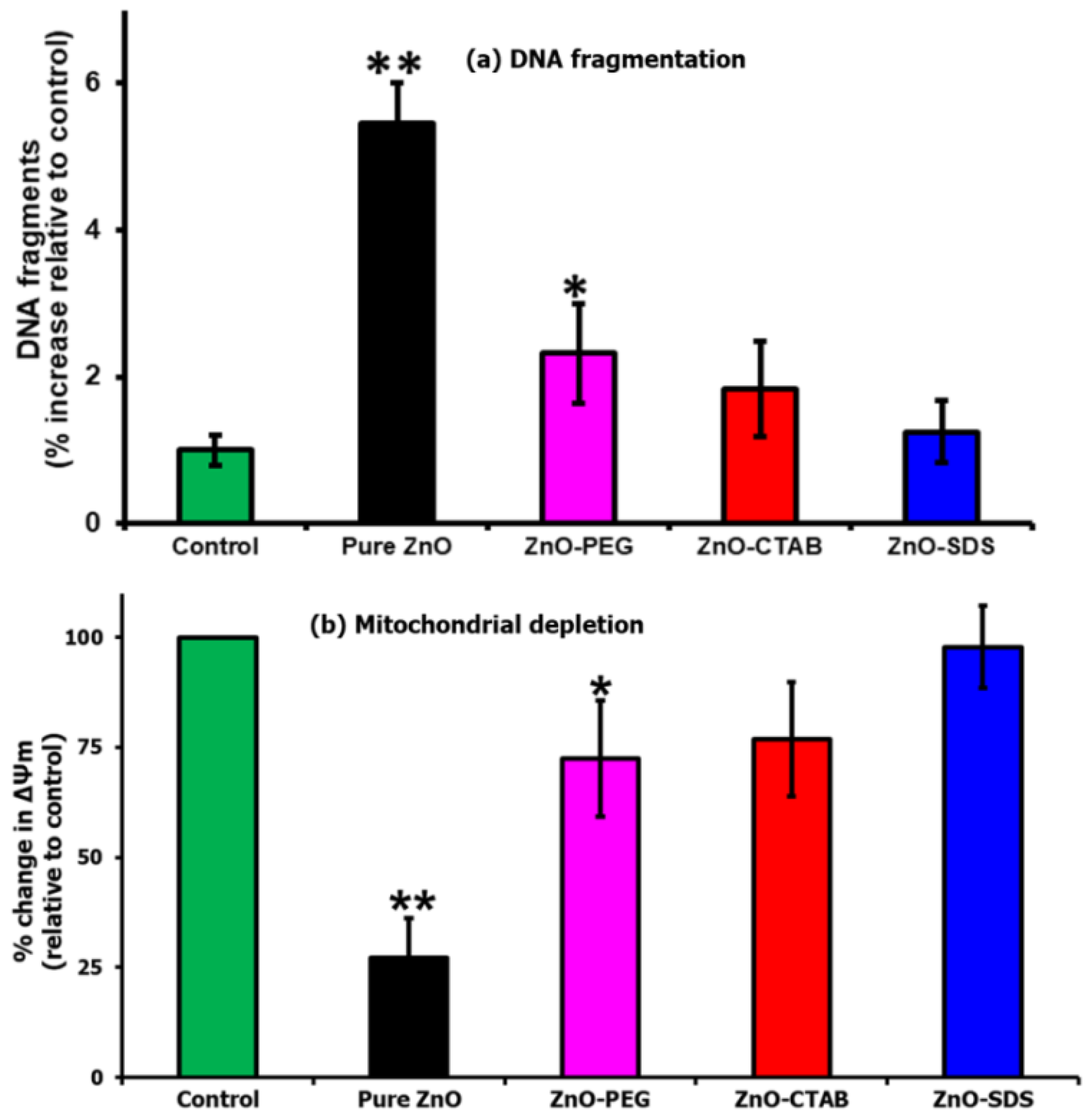
2.7. DNA Fragmentation Assay
2.8. Mitochondrial Depletion Assay
2.9. Statistical Analysis
3. Results and Discussion
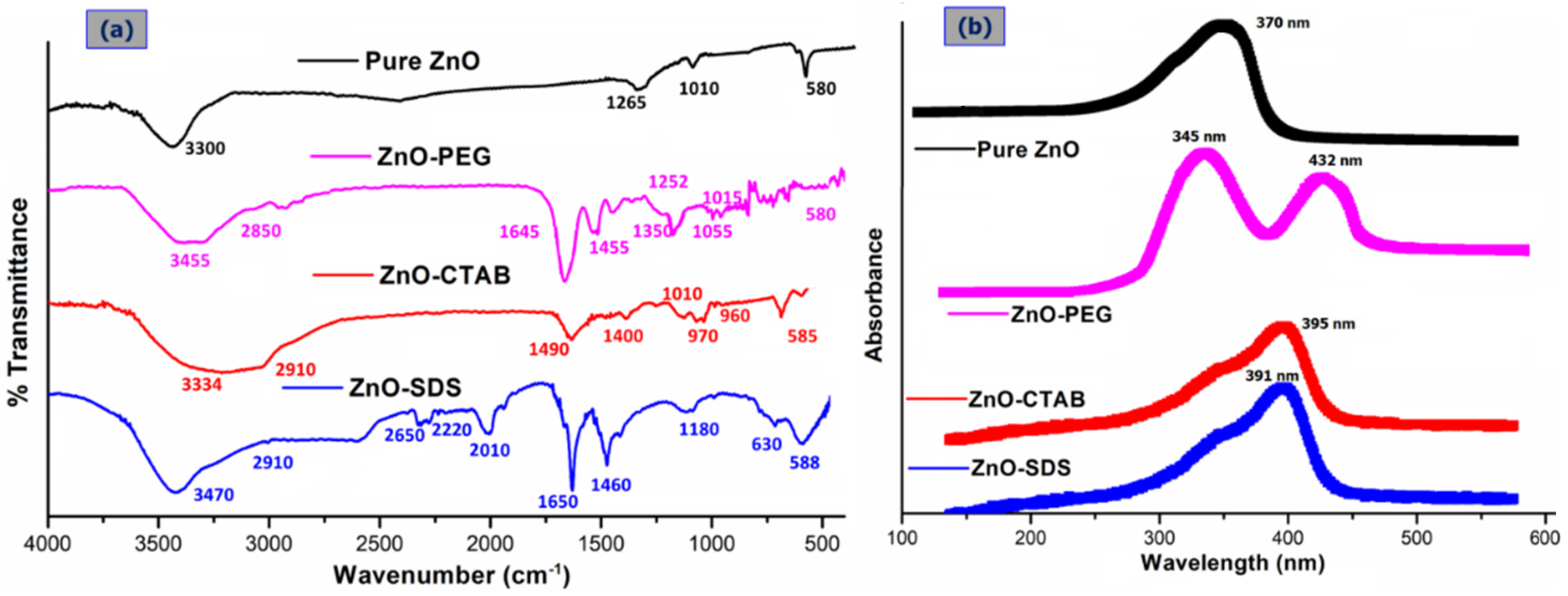
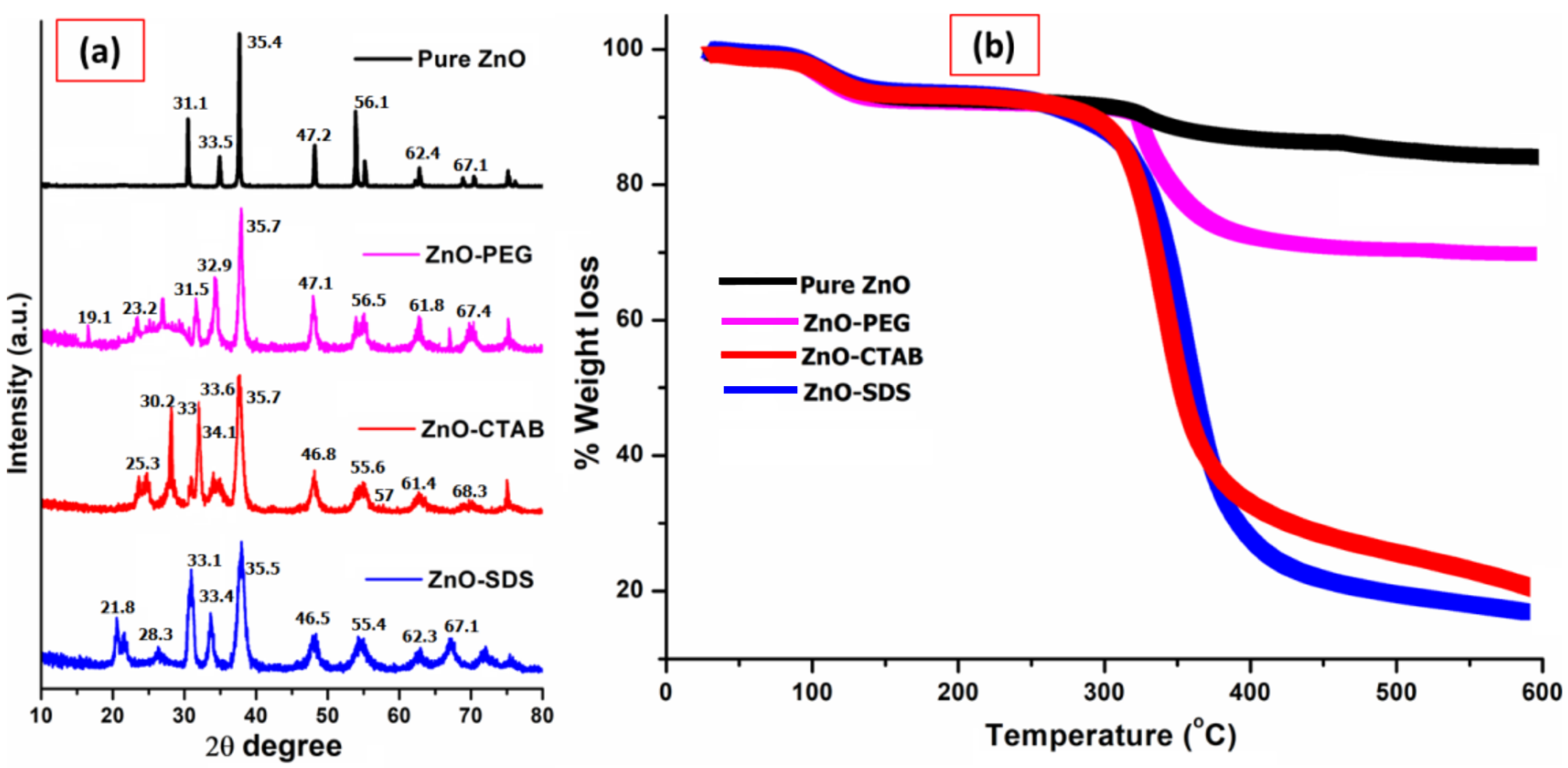
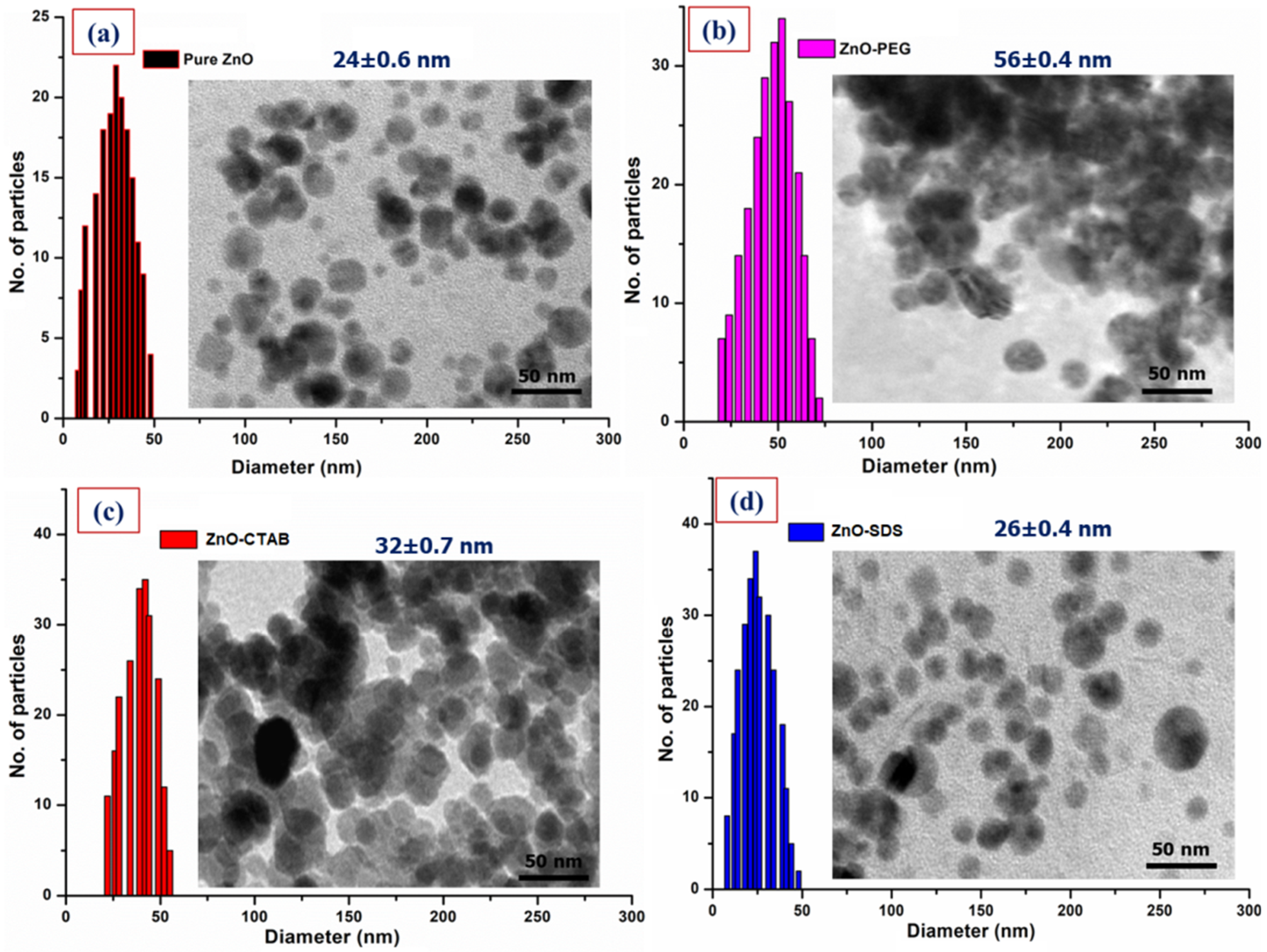
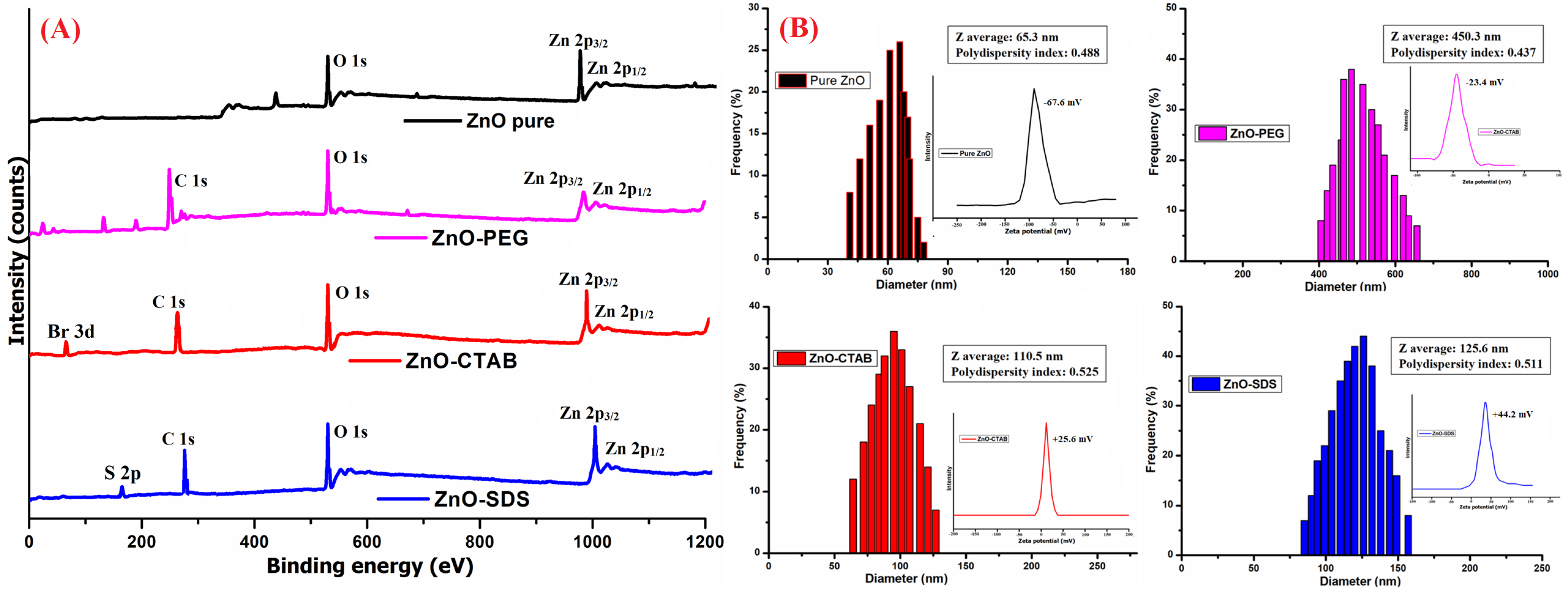
3.1. Physicochemical Characterization of Surface-Modified ZnO NPs
3.2. In Vitro Molecular Biology Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for Reducing the Toxicity of Metal and Metal Oxide NPs as Biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Wang, S.; Liu, Y.; Pope, C. Phototoxicity of zinc oxide nanoparticle conjugates in human ovarian cancer NIH: OVCAR-3 cells. J. Biomed. Nanotechnol. 2008, 4, 432–438. [Google Scholar] [CrossRef]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.A.; Turney, T.W. Reducing ZnO nanoparticle cytotoxicity by surface modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A. In Vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J. Colloid Interf. Sci. 2016, 480, 146–158. [Google Scholar] [CrossRef]
- Limo, M.J.; Ramasamy, R.; Perry, C.C. ZnO Binding Peptides: Smart Versatile Tools for Controlled Modification of ZnO Growth Mechanism and Morphology. Chem. Mater. 2015, 27, 1950–1960. [Google Scholar] [CrossRef]
- Noguera, C. Polar Oxide Surfaces. J. Phys. Condens. Matter 2000, 12, R367–R410. [Google Scholar] [CrossRef]
- Meyer, B.; Marx, D. Density-functional study of the structure and stability of ZnO surfaces. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 035403. [Google Scholar] [CrossRef]
- Diebold, U.; Koplitz, L.V.; Dulub, O. Atomic-scale properties of low-index ZnO surfaces. Appl. Surf. Sci. 2004, 237, 336–342. [Google Scholar] [CrossRef]
- Shewale, V.; Joshi, P.; Mukhopadhyay, S.; Deshpande, M.; Pandey, R.; Hussain, S.; Karna, S.P. First-principles study of nanoparticle−biomolecular interactions: Anchoring of a (ZnO)12 cluster on nucleobases. J. Phys. Chem. C 2011, 115, 10426–10430. [Google Scholar] [CrossRef]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef]
- Sola-Rabada, A.; Liang, M.-K.; Roe, M.J.; Perry, C.C. Peptide-directed crystal growth modification in the formation of ZnO. J. Mater. Chem. B 2015, 3, 3777–3788. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bin Hussein, M.Z.; Taufiq-Yap, Y.H. The Effect of Sodium Dodecyl Sulfate (SDS) and Cetyltrimethylammonium Bromide (CTAB) on the Properties of ZnO Synthesized by Hydrothermal Method. Int. J. Mol. Sci. 2012, 13, 13275–13293. [Google Scholar] [CrossRef]
- Anand, A.; Varghese, S. Role of surfactants on the stability of nano-zinc oxide dispersions. Part. Sci. Technol. 2017, 35, 67–70. [Google Scholar]
- Kunkel, M.; Schildknecht, S.; Boldt, K.; Zeyffert, L.; Schleheck, D.; Leist, M.; Polarz, S. Increasing the resistance of living cells against oxidative stress by nonnatural surfactants as membrane guards. ACS Appl. Mater. Interf. 2018, 10, 23638–23646. [Google Scholar] [CrossRef]
- Popescu, T.; Matei, C.O.; Vlaicu, I.D.; Tivig, I.; Kuncser, A.C.; Stefan, M.; Ghica, D.; Miclea, L.C.; Savopol, T.; Culita, D.C.; et al. Influence of surfactant-tailored Mn-doped ZnO nanoparticles on ROS production and DNA damage induced in murine fibroblast cells. Sci. Rep. 2020, 10, 18062. [Google Scholar] [CrossRef]
- Raisi Dehkourdi, B.; Fatahian, S.; Shahanipoor, K. Synthesis, Characterization and renal toxicity of ZnO and polyethylene glycol coated ZnO nanoparticles. Nanomed. J. 2017, 4, 55–60. [Google Scholar]
- Oleszczuk, P.; Jośko, I.; Skwarek, E. Surfactants decrease the toxicity of ZnO, TiO2 and Ni nanoparticles to Daphnia magna. Ecotoxicology 2015, 24, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Garay-Jimenez, J.C.; Young, A.; Gergeres, D.; Greenhalgh, K.; Turos, E. Methods for purifying and detoxifying sodium dodecyl sulfate–stabilized polyacrylate nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 98–105. [Google Scholar] [CrossRef]
- Singh, B.N.; Rawat, A.K.S.; Khan, W.; Naqvi, A.H.; Singh, B.R. Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa Rhamnolipids. PLoS ONE 2014, 9, e106937. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Yusof, N.A. Surface ligand influenced free radical protection of superparamagnetic iron oxide nanoparticles (SPIONs) toward H9c2 cardiac cells. J. Mater. Sci. 2014, 49, 6290–6301. [Google Scholar] [CrossRef]
- Liufu, S.; Xiao, H.; Li, Y. Investigation of PEG adsorption on the surface of zinc oxide nanoparticles. Powder Technol. 2004, 145, 20–24. [Google Scholar] [CrossRef]
- Patra, M.K.; Manoth, M.; Singh, V.K.; Gowd, G.S.; Choudhry, V.S.; Vadera, S.R.; Kumar, N. Synthesis of stable dispersion of ZnO quantum dots in aqueous medium showing visible emission from bluish green to yellow. J. Lumin. 2009, 129, 320–324. [Google Scholar] [CrossRef]
- Leila, D.; Mar, L.-G.; Fatima, B.; Abddelyamine, N.; Ali, B.; Nacereddine, H. Effect of polyethylene glycol and propyltrimethoxysilane on structural and optical properties of zinc oxide nanoparticles synthesized by sol–gel process. J. Theor. Appl. Phys. 2018, 12, 159–167. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.H.B.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar]
- Samaele, N.; Amornpitoksuk, P.; Suwanboon, S. Effect of pH on the morphology and optical properties of modified ZnO particles by SDS via a precipitation method. Powder Technol. 2010, 203, 243–247. [Google Scholar] [CrossRef]
- Prasad, V.; D’Souza, C.; Yadav, D.; Shaikh, A.J.; Vigneshwaran, N. Spectroscopic characterization of zinc oxide nanorods synthesized by solid-state reaction. Spectrochim. Acta A 2006, 65, 173–178. [Google Scholar] [CrossRef]
- Wu, L.; Wu, Y.; Lü, W. Preparation of ZnO Nanorods and optical characterizations. Phys. E 2005, 28, 76–82. [Google Scholar] [CrossRef]
- Vidyasagar, C.C.; Naik, Y.A. Naik Surfactant (PEG 400) effects on crystallinity of ZnO nanoparticles. Arab. J. Chem. 2016, 9, 507–510. [Google Scholar] [CrossRef]
- Singla, M.L.; Kumar, M. Optical characterization of ZnO nanoparticles capped with various surfactants. J. Lumin. 2009, 129, 434–438. [Google Scholar] [CrossRef]
- Jose, A.; Devi, K.S.; Pinheiro, D.; Narayana, S.L. Electrochemical synthesis, photodegradation and antibacterial properties of PEG capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 187, 25–34. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhang, Q.; Li, T. Adjusting the proportions of {0001} facets and high-index facets of ZnO hexagonal prisms and their photocatalytic activity. RSC Adv. 2017, 7, 3515. [Google Scholar] [CrossRef]
- Anandan, M.; Dinesh, S.; Krishnakumar, N.; Balamurugan, K. Improved photocatalytic properties and anti-bacterial activity of size reduced ZnO nanoparticles via PEG-assisted precipitation route. J. Mater. Sci. Mater. Electron. 2016, 27, 12517–12526. [Google Scholar] [CrossRef]
- Trino, L.D.; Dias, L.F.; Albano, L.G.; Bronze-Uhle, E.S.; Rangel, E.C.; Graeff, C.F.; Lisboa-Filho, P.N. Zinc oxide surface functionalization and related effects on corrosion resistance of titanium implants. Ceram. Int. 2018, 44, 4000–4008. [Google Scholar] [CrossRef]
- Parra, M.R.; Haque, F.Z. Haque Poly(ethylene glycol) (PEG)-assisted shape-controlled synthesis of one-dimensional ZnO nanorods. Optik 2015, 126, 1562–1566. [Google Scholar] [CrossRef]
- Rahman, M.Y.A.; Umar, A.A.; Taslim, R.; Salleh, M.M. Effect of surfactant on the physical properties of ZnO nanorods and the performance of ZnO photoelectrochemical cell. J. Exp. Nanosci. 2015, 10, 599–609. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, D.; Chang, J.; Bai, Z.; Jiang, K. Synthesis of cup-like ZnO microcrystals via a CTAB-assisted hydrothermal route. Mater. Chem. Phys. 2009, 117, 422–424. [Google Scholar] [CrossRef]
- Zou, X.; Ke, J.; Hao, J.; Yan, X.; Tian, Y. A new method for synthesis of ZnO flower-like nanostructures and their photocatalytic performance. Phys. B 2022, 624, 413395. [Google Scholar] [CrossRef]
- Eadi, S.B.; Kim, S.; Jeong, S.W. Effect of surfactant on growth of ZnO nanodumbbells and their characterization. J. Chem. 2017, 2017, 1728345. [Google Scholar] [CrossRef]
- Li, P.; Wei, Y.; Liu, H.; Wang, X.-K. Growth of well-defined ZnO microparticles with additives from aqueous solution. J. Solid State Chem. 2005, 178, 855–860. [Google Scholar] [CrossRef]
- Ahson, R.; Ahmad, R.; Afzal, N.; Mubarik, F.E. Effect of structure modifying agents on the structural, morphological and optical features of hydrothermally grown ZnO. J. Nanosci. Nanotechnol. 2020, 20, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Ataei-Pirkooh, A.; Mm Sadeghi, G.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Iqbal, M.; Riaz, S.; Zia, R.; Naseem, S. Fabrication and properties of zinc oxide thin film prepared by sol-gel dip coating method. Mater. Sci. 2015, 33, 515–520. [Google Scholar] [CrossRef]
- Rajendran, G.; Datta, S.P.; Singh, R.D.; Datta, S.C.; Vakada, M. Synthesis and characterization of ZnO nanoparticles–comparison of acetate (precursor) based methods. Inorg. Nano-Metal Chem. 2021, 52, 185–194. [Google Scholar] [CrossRef]
- Pranjali, P.; Meher, M.K.; Raj, R.; Prasad, N.; Poluri, K.M.; Kumar, D.; Guleria, A. Physicochemical and antibacterial properties of PEGylated zinc oxide nanoparticles dispersed in peritoneal dialysis fluid. ACS Omega 2019, 4, 19255–19264. [Google Scholar] [CrossRef]
- Medina, J.; Bolaños, H.; Mosquera-Sanchez, L.P.; Rodriguez-Paez, J.E. Controlled synthesis of ZnO nanoparticles and evaluation of their toxicity in Mus musculus mice. Int. Nano Lett. 2018, 8, 165–179. [Google Scholar] [CrossRef]
- Wu, C.K.; Sivashanmugan, K.; Guo, T.F.; Wen, T.C. Enhancement of inverted polymer solar cells performances using cetyltrimethylammonium-bromide modified ZnO. Materials 2018, 11, 378. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Xiang, L. Influence of sodium dodecyl sulfate on the fabrication of zinc oxide nanoparticles. Res. Chem. Intermed. 2011, 37, 281–289. [Google Scholar] [CrossRef]
- Li, D.; Hu, J.; Fan, F.; Bai, S.; Luo, R.; Chen, A.; Liu, C.C. Quantum-sized ZnO nanoparticles synthesized in aqueous medium for toxic gases detection. J. Alloys Compd. 2012, 539, 205–209. [Google Scholar] [CrossRef]
- Sun, J.-H.; Huang, J.-H.; Lan, X.-Y.; Zhang, F.-C.; Zhao, L.-Z.; Zhang, Y. Enhancing the performance of blue quantum-dot light-emitting diodes through the incorporation of polyethylene glycol to passivate ZnO as an electron transport layer. RSC Adv. 2020, 10, 23121–23127. [Google Scholar] [CrossRef]
- Singletary, M.; Hagerty, S.; Muramoto, S.; Daniels, Y.; MacCrehan, W.A.; Stan, G.; Lau, J.W.; Pustovyy, O.; Globa, L.; Morrison, E.E.; et al. PEGylation of zinc nanoparticles amplifies their ability to enhance olfactory responses to odorant. PLoS ONE 2017, 12, e0189273. [Google Scholar] [CrossRef]
- Punnoose, A.; Dodge, K.; Rasmussen, J.W.; Chess, J.; Wingett, D.; Anders, C. Cytotoxicity of ZnO Nanoparticles Can Be Tailored by Modifying Their Surface Structure: A Green Chemistry Approach for Safer Nanomaterials. ACS Sustain. Chem. Eng. 2014, 2, 1666–1673. [Google Scholar] [CrossRef]
- Marsalek, R. Particle Size and Zeta Potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef]
- Chibowski, S. Investigation of the Mechanism of Polymer Adsorption on a Metal Oxide/Water Solution Interface. Adsorpt. Sci. Technol. 1996, 14, 179–188. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Influence of particle geometry and PEGylation on phagocytosis of particulate carriers. Int. J. Pharm. 2014, 465, 159–164. [Google Scholar] [CrossRef]
- Moayedian, T.; Mosaffa, F.; Khameneh, B.; Tafaghodi, M. Combined effects of PEGylation and particle size on uptake of PLGA particles by macrophage cells. Nanomed. J. 2015, 2, 299–304. [Google Scholar]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y.; Ge, F.; Liu, S.; Xiao, H. Antagonistic effect of nano-ZnO and cetyltrimethyl ammonium chloride on the growth of Chlorella vulgaris: Dissolution and accumulation of nano-ZnO. Chemosphere 2018, 196, 566–574. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Zhang, F.; Chen, D.; Ding, W. ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res. Lett. 2014, 9, 117. [Google Scholar] [CrossRef]
- Bai, D.-P.; Zhang, X.-F.; Zhang, G.-L.; Huang, Y.-F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef]
- Ng, C.T.; Yong, L.Q.; Hande, M.P.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int. J. Nanomed. 2017, 12, 1621. [Google Scholar] [CrossRef]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J.; et al. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol.-Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]

| Surfactant | Structure |
|---|---|
| PEG | 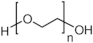 |
| CTAB | 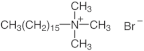 |
| SDS | 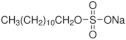 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, F.; Bwatanglang, I.B.; Al-Lohedan, H.A.; Shaik, J.P.; Al-Tilasi, H.H.; Soleiman, A.A. Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro. Coatings 2023, 13, 172. https://doi.org/10.3390/coatings13010172
Mohammad F, Bwatanglang IB, Al-Lohedan HA, Shaik JP, Al-Tilasi HH, Soleiman AA. Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro. Coatings. 2023; 13(1):172. https://doi.org/10.3390/coatings13010172
Chicago/Turabian StyleMohammad, Faruq, Ibrahim Birma Bwatanglang, Hamad A. Al-Lohedan, Jilani Purusottapatnam Shaik, Hissah Hamad Al-Tilasi, and Ahmed A. Soleiman. 2023. "Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro" Coatings 13, no. 1: 172. https://doi.org/10.3390/coatings13010172
APA StyleMohammad, F., Bwatanglang, I. B., Al-Lohedan, H. A., Shaik, J. P., Al-Tilasi, H. H., & Soleiman, A. A. (2023). Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro. Coatings, 13(1), 172. https://doi.org/10.3390/coatings13010172






