Electroless Ni-P-PTFE Composite Plating with Rapid Deposition and High PTFE Concentration through a Two-Step Process
Abstract
:1. Introduction
2. Experimental
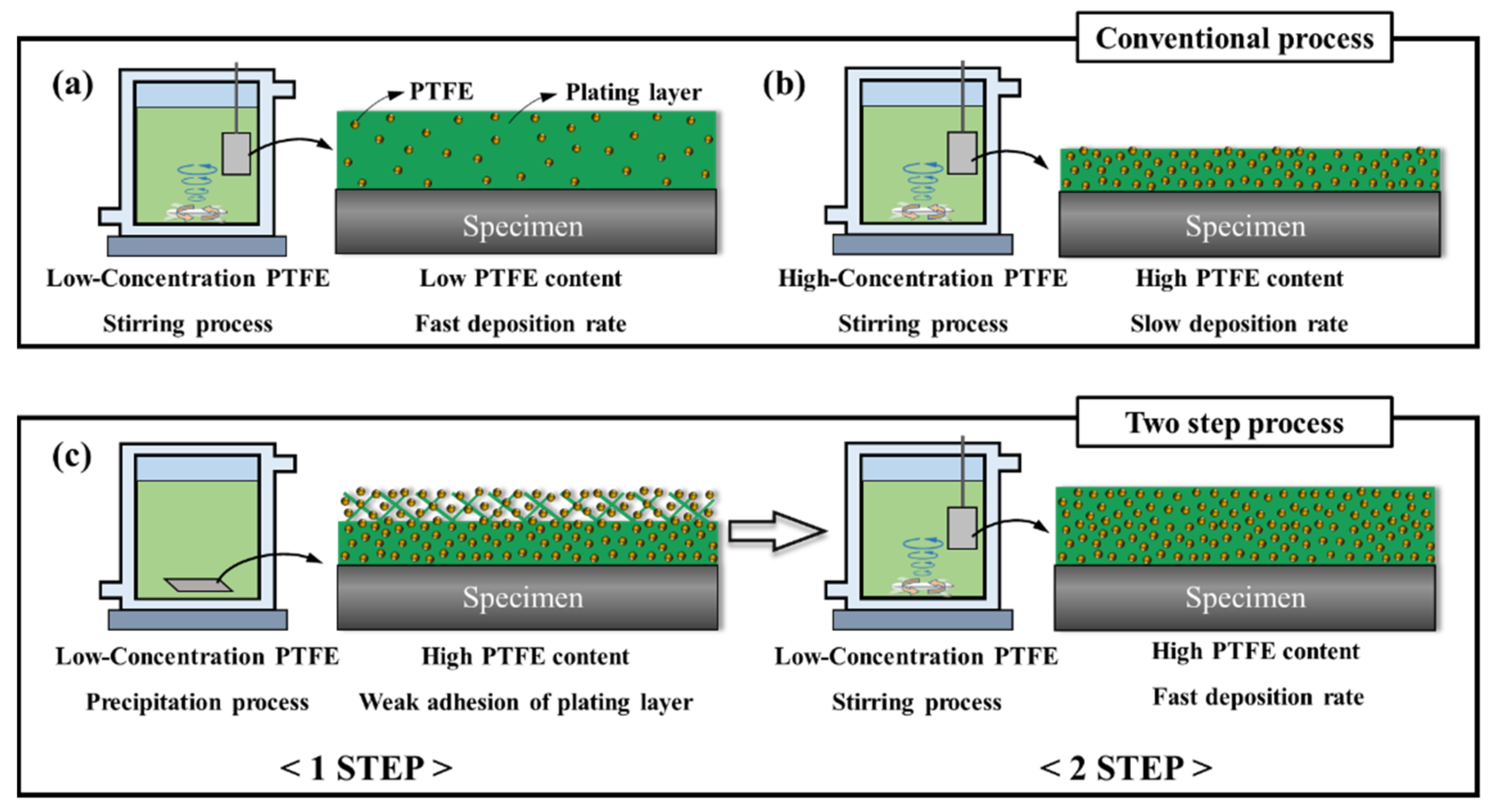
2.1. Conventional Process for Electroless Ni-P-PTFE Composite Plating
2.2. Two-Step Process for Electroless Ni-P-PTFE Composite Plating
2.3. Characterization
3. Results and Discussion
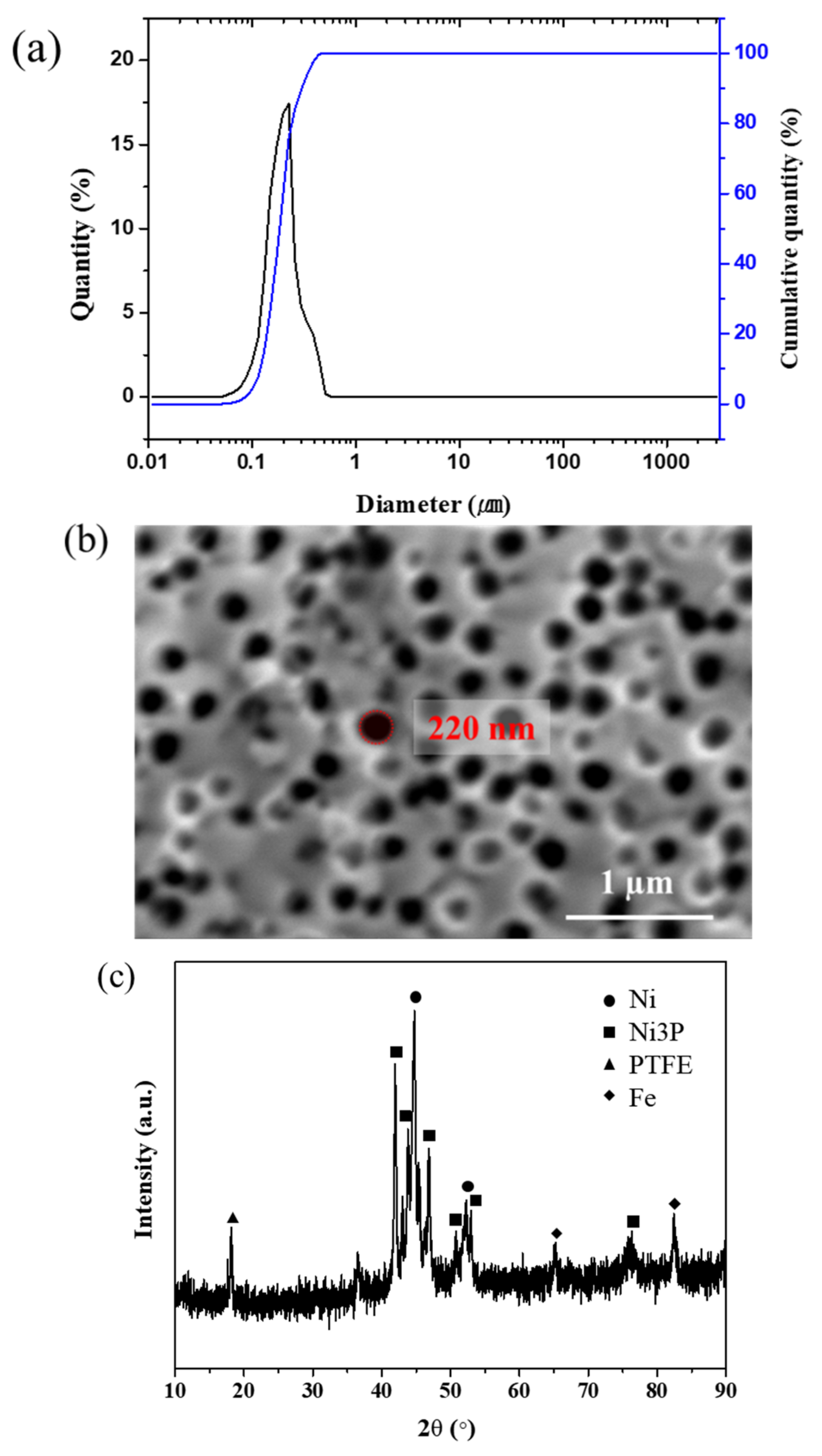
3.1. Size Distribution and Characteristics of PTFE Particles
3.2. Precipitation Characterization of Ni–PTFE Composite Plating
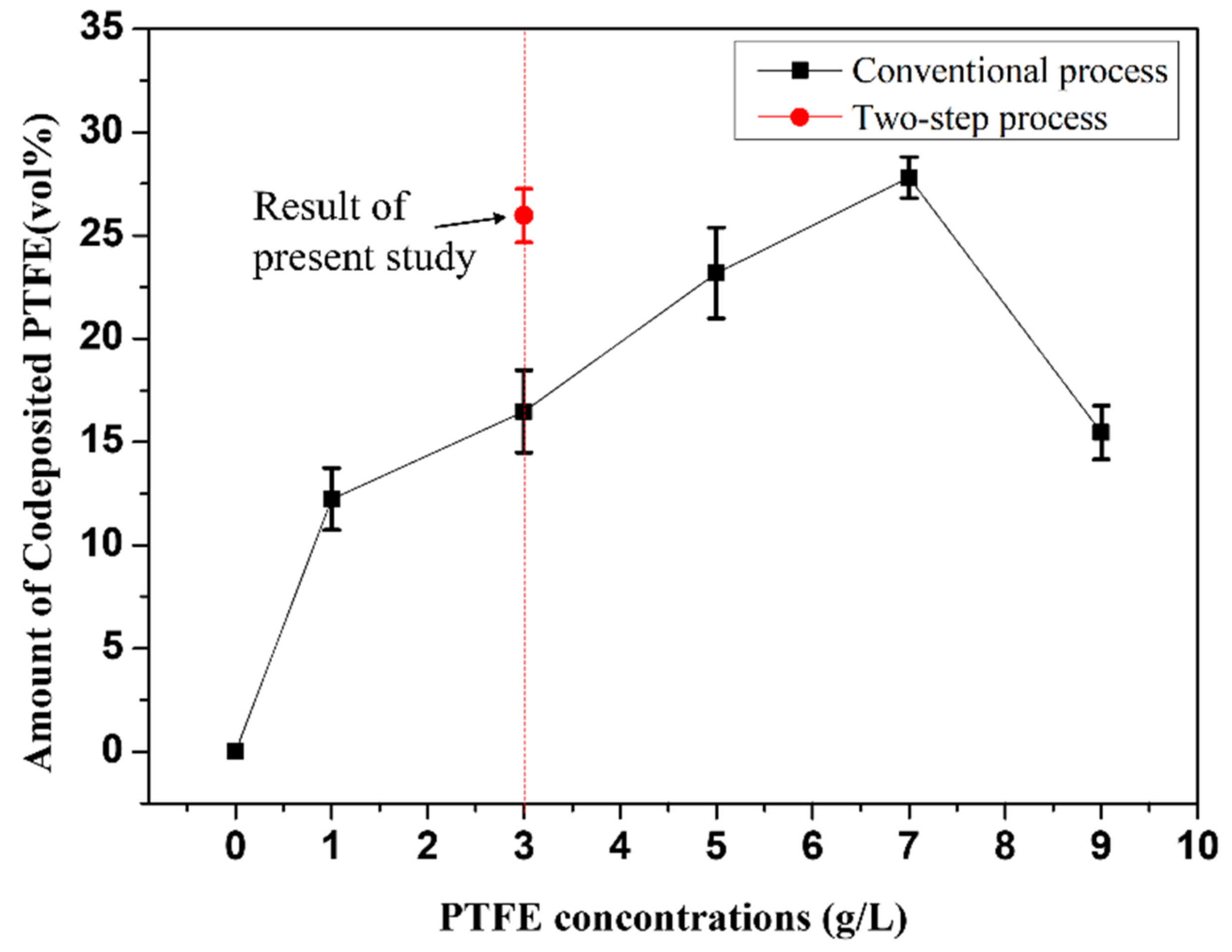
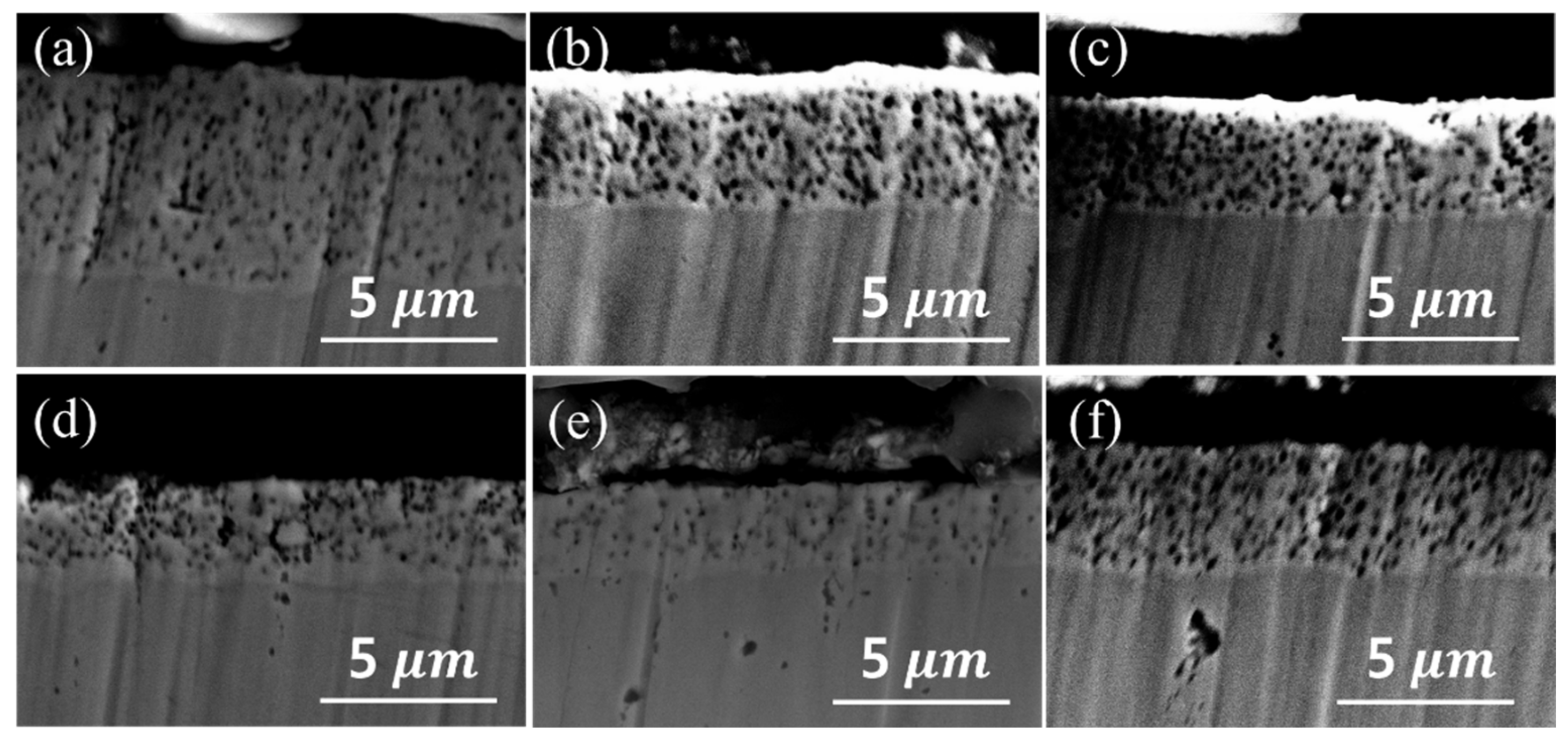
3.2.1. Plating Deposition Rate and PTFE Content of Plating Layer
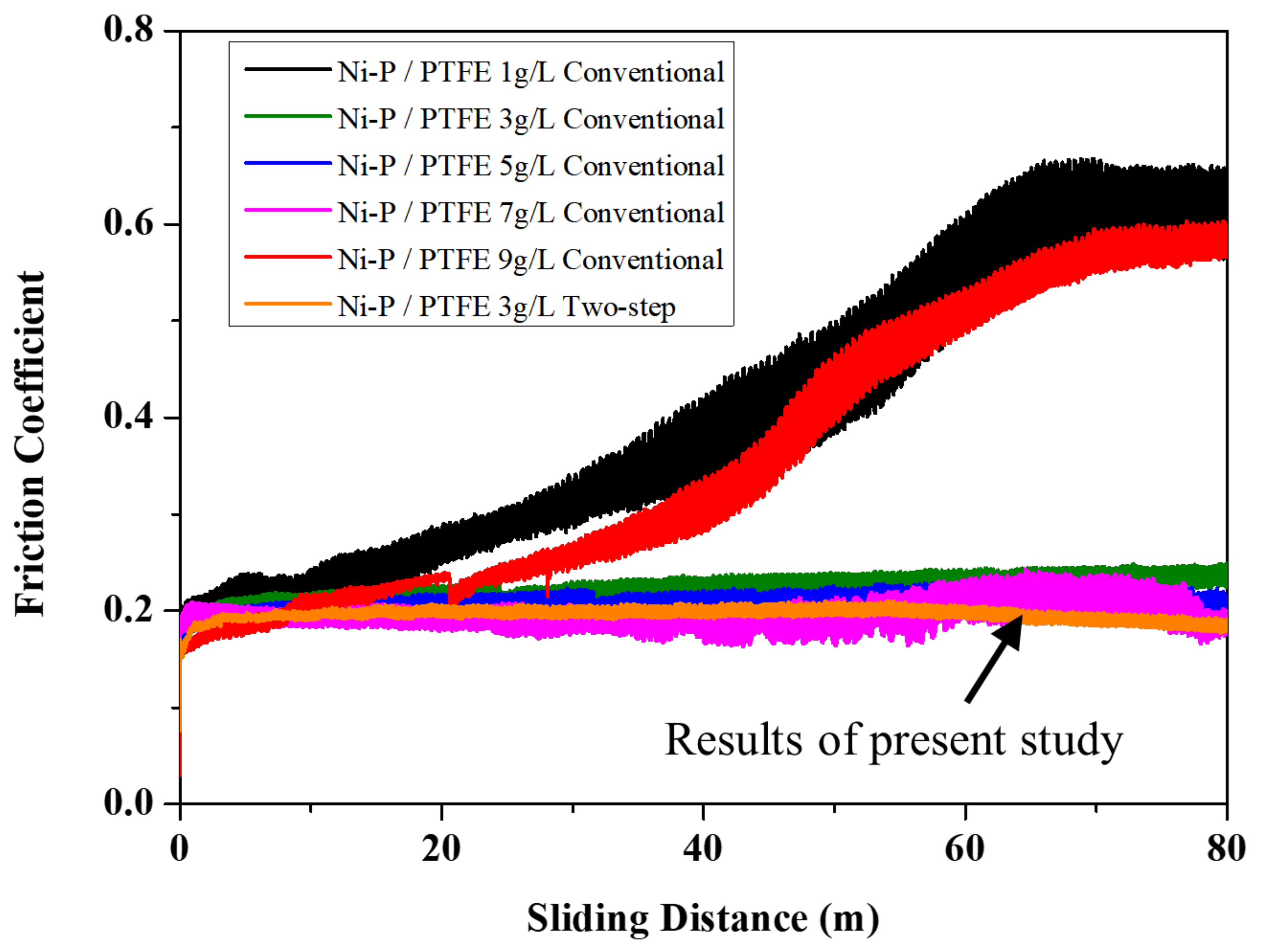
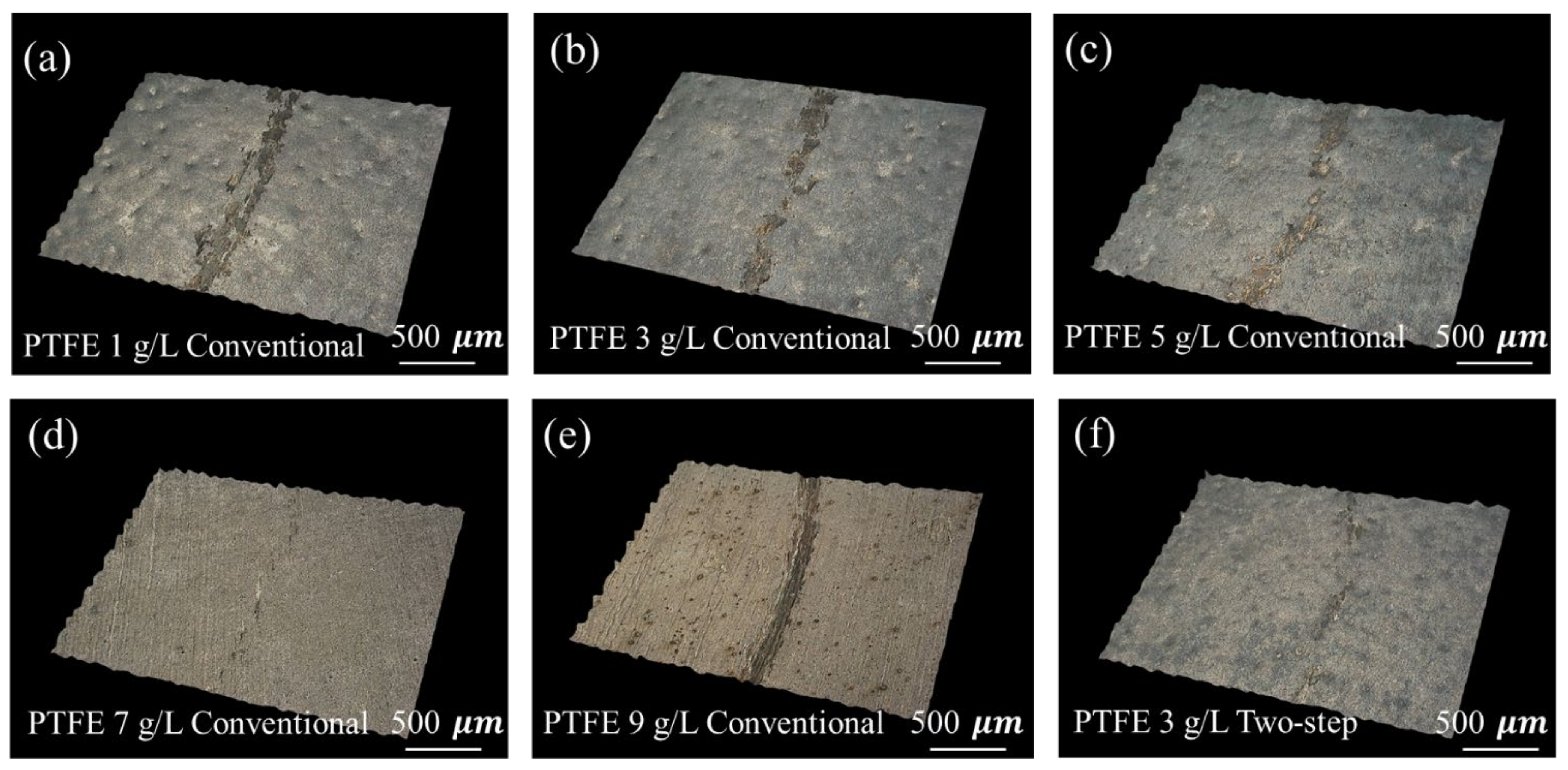
3.2.2. Friction Coefficient and Wear Resistance of Plating Layer
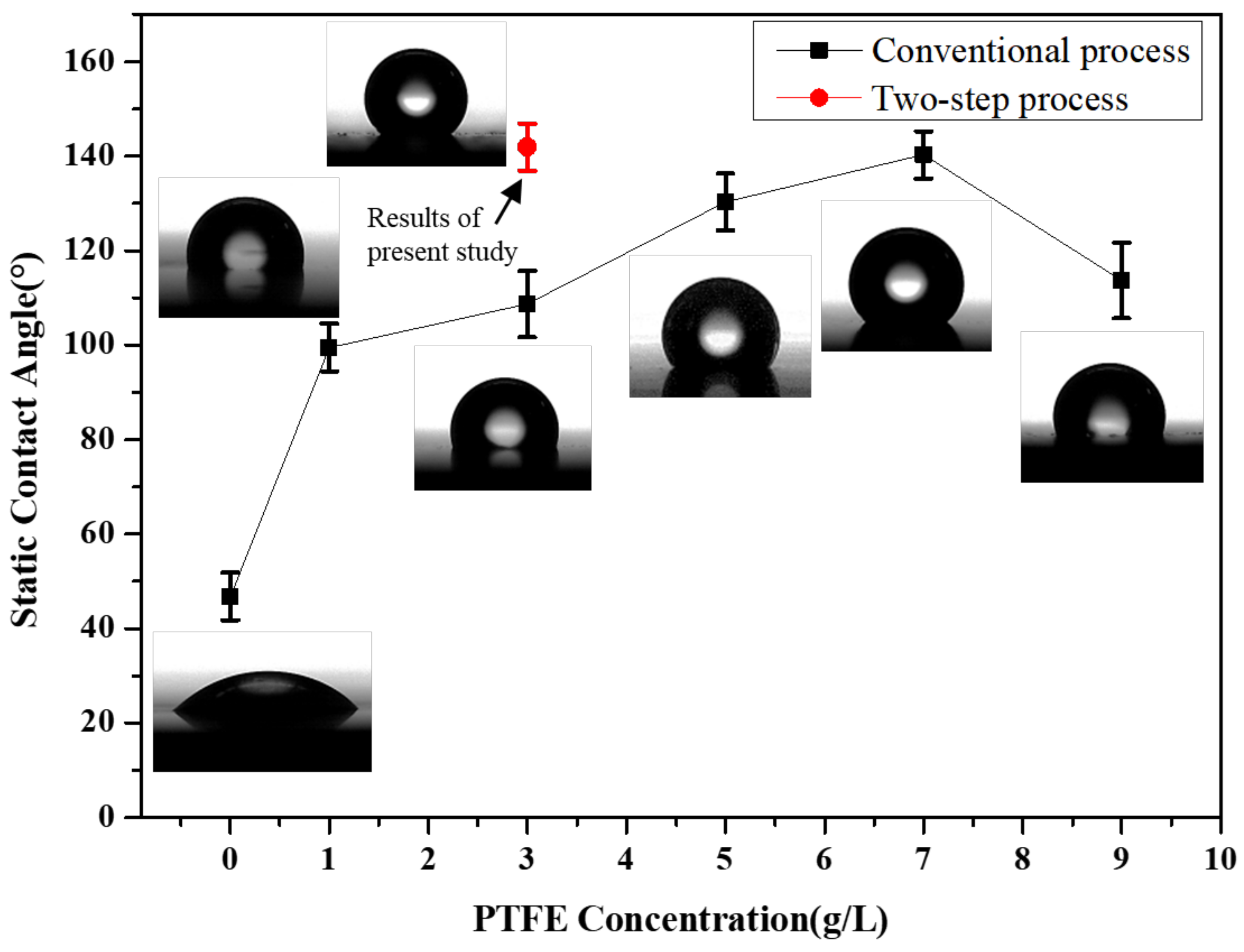
3.2.3. Static and Dynamic Contact Angle of Plating Layer
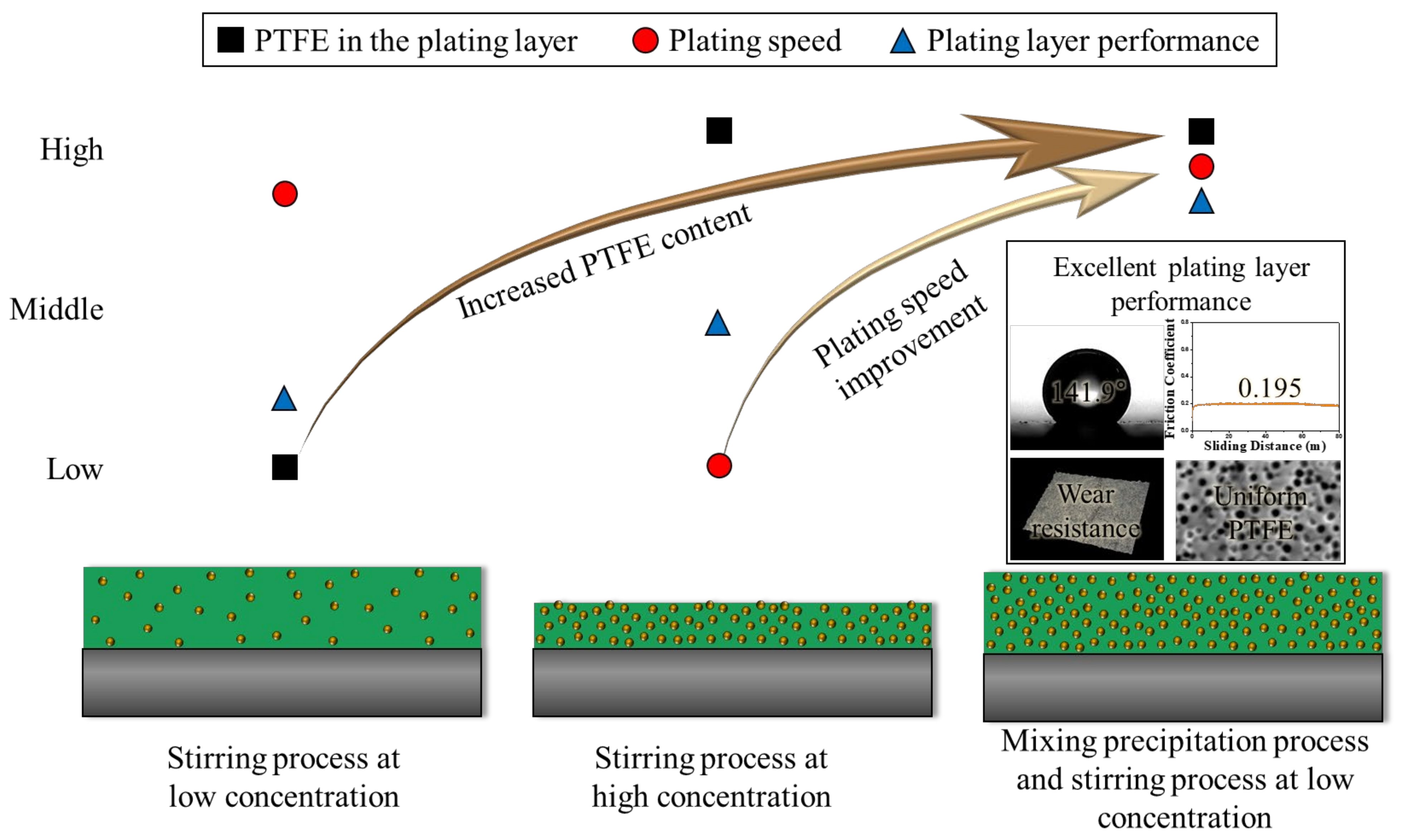
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iacovetta, D.; Tam, J.; Erb, U. Synthesis, structure, and properties of superhydrophobic nickel–PTFE nanocomposite coatings made by electrodeposition. Surf. Coat. Technol. 2015, 279, 134–141. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Vazirinasab, E.; Momen, G.; Jafari, R. Advances in the fabrication of superhydrophobic polymeric surfaces by polymer molding processes. Ind. Eng. Chem. Res. 2020, 59, 9343–9363. [Google Scholar] [CrossRef]
- Parvate, S.; Prakhar, D.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Li, Q.; Liang, H.; Song, J.; Guo, C.; Tang, J. Preparation of transparent sandwich-like superhydrophobic coating on glass with high stability and self-cleaning properties. Coatings 2022, 12, 228. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, J.; Lin, N.; Tang, B. Review on electroless plating Ni–P coatings for improving surface performance of steel. Surf. Rev. Lett. 2014, 21, 1430002. [Google Scholar] [CrossRef]
- Krishnan, K.H.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An overall aspect of electroless Ni-P depositions—A review article. Metall. Mater. Trans. A 2006, 37, 1917–1926. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- de Hazan, Y.; Werner, D.; Z’graggen, M.; Groteklaes, M.; Graule, T. Homogeneous Ni-P/Al2O3 nanocomposite coatings from stable dispersions in electroless nickel baths. J. Colloid Interface Sci. 2008, 328, 103–109. [Google Scholar] [CrossRef]
- Chang, C.-S.; Hou, K.-S.; Ger, M.-D.; Chung, C.-K.; Lin, J.-F. Effects of annealing temperature on microstructure, surface roughness, mechanical and tribological properties of Ni–P and Ni–P/SiC films. Surf. Coat. Technol. 2016, 288, 135–143. [Google Scholar] [CrossRef]
- Lee, C.-K. Comparative corrosion resistance of electroless Ni-P/nano-TiO2 and Ni-P/nano-CNT composite coatings on 5083 aluminum alloy. Int. J. Electrochem. Sci. 2012, 7, 12941–12954. [Google Scholar]
- Gao, P.; Xie, Z.; Ouyang, C.; Tao, T.; Wu, X.; Huang, Q. Electrochemical characteristics and interfacial contact resistance of Ni-P/TiN/PTFE coatings on Ti bipolar plates. J. Solid State Electrochem. 2018, 22, 1971–1981. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Corrosion and wear resistance study of Ni-P and Ni-P-PTFE nanocomposite coatings. Cent. Eur. J. Eng. 2011, 1, 234–243. [Google Scholar]
- Ger, M.D.; Hou, K.H.; Wang, L.M.; Hwang, B.J. The friction and wear of Ni–P–PTFE composite deposits under water lubrication. Mater. Chem. Phys. 2002, 77, 755–764. [Google Scholar] [CrossRef]
- Ren, L.; Cheng, Y.; Wang, Q.; Yang, J. Study on the properties of Ni-W-P coating with PTFE co-deposition. Surf. Topogr. Metrol. Prop. 2019, 7, 045009. [Google Scholar] [CrossRef]
- Sheu, H.-H.; Jian, S.-Y.; Lin, M.-H.; Hsu, C.-I.; Hou, K.-H.; Ger, M.-D. Electroless Ni-P/PTFE self-lubricating composite thin films applied for medium-carbon steel substrate. Int. J. Electrochem. Sci. 2017, 12, 5464–5482. [Google Scholar] [CrossRef]
- Boakye, G.O.; Ormsdóttir, A.M.; Gunnarsson, B.G.; Irukuvarghula, S.; Khan, R.; Karlsdóttir, S.N. The effect of polytetrafluoroethylene (PTFE) particles on microstructural and tribological properties of electroless Ni-P+ PTFE puplex coatings developed for geothermal applications. Coatings 2021, 11, 670. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P-PTFE-Al2O3 dispersion nanocomposite coating for corrosion and wear resistance. J. Mater. Eng. Perform. 2014, 23, 142–151. [Google Scholar] [CrossRef]
- Karaguiozova, Z.K. Characterisation of electroless Ni-P and electroless composite coatings Ni-P/Ni-PTFE. Int. J. Surf. Sci. Eng. 2018, 12, 496–506. Available online: https://pubs.rsc.org/en/content/articlelanding/2016/ta/c6ta01133k-fn1 (accessed on 18 December 2018). [CrossRef]
- Wan, Y.; Yu, Y.; Cao, L.; Zhang, M.; Gao, J.; Qi, C. Corrosion and tribological performance of PTFE-coated electroless nickel boron coatings. Surf. Coat. Technol. 2016, 307, 316–323. Available online: https://pubs.rsc.org/en/content/articlelanding/2013/cc/c3cc46034g-fn1 (accessed on 15 December 2016). [CrossRef]
- Mafi, I.R.; Dehghanian, C. Comparison of the coating properties and corrosion rates in electroless Ni–P/PTFE composites prepared by different types of surfactants. Appl. Surf. Sci. 2011, 257, 8653–8658. [Google Scholar] [CrossRef]
- Hu, R.; Su, Y.; Liu, Y.; Liu, H.; Chen, Y.; Cao, C.; Ni, H. Deposition process and properties of electroless Ni-P-Al2O3 composite coatings on magnesium alloy. Nanoscale Res. Lett. 2018, 13, 198. [Google Scholar] [CrossRef]
- Sharma, V.; Chotia, C.; Tarachand; Ganesan, V.; Okram, G.S. Influence of particle size and dielectric environment on the dispersion behaviour and surface plasmon in nickel nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 14096–14106. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Müller-Steinhagen, H.; Liu, G. Graded Ni–P–PTFE coatings and their potential applications. Surf. Coat. Technol. 2002, 155, 279–284. [Google Scholar] [CrossRef]
- Srinivasan, K.N.; John, S. Studies on electroless nickel–PTFE composite coatings. Surf. Eng. 2005, 21, 156–160. [Google Scholar] [CrossRef]
- Sawyer, W.G.; Freudenberg, K.D.; Bhimaraj, P.; Schadler, L.S. A study on the friction and wear behavior of PTFE filled with alumina nanoparticles. Wear 2003, 254, 573–580. [Google Scholar] [CrossRef]
- Biswas, S.K.; Kalyani Vijayan, K. Friction and wear of PTFE—A review. Wear 1992, 158, 193–211. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.-S.; Yu, Q.-Y.; Zhang, Y.-X.; Zhao, W.-J.; Zeng, Z.-X. Structure and wear resistance of high hardness Ni-B coatings as alternative for Cr coatings. Surf. Coat. Technol. 2015, 264, 80–86. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, L.; Liang, A.; Chen, L.; Zhang, J. Fabrication of a nanocrystalline Cr-C layer with excellent anti-wear performance. Mater. Lett. 2007, 61, 4107–4109. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lim, D.-S. Tribological behavior of PTFE film with nanodiamond. Surf. Coat. Technol. 2004, 188, 534–538. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Lin, C.-H.; Jian, Y.-J.; Hou, K.-H.; Ger, M.-D. The fabrication and characteristics of electroless nickel and immersion Au-polytetrafluoroethylene composite coating on aluminum alloy 5052 as bipolar plate. Surf. Coat. Technol. 2017, 313, 151–157. [Google Scholar] [CrossRef]
- Ragesh, P.; Ganesh, V.A.; Nair, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A.W. Contact angles on hydrophobic solid surfaces and their interpretation. J. Colloid Interface Sci. 1992, 148, 190–200. [Google Scholar] [CrossRef]
| Element | High-Carbon Steel (wt.%) |
|---|---|
| C | 0.70–0.80 |
| Si | 0.25–0.50 |
| Mn | 0.60–0.80 |
| P | ≤0.030 |
| S | ≤0.030 |
| Cr | 0.30–0.40 |
| Plating Process | PTFE Concentration | Advancing Contact Angle | γSL (mN/m) | ||
|---|---|---|---|---|---|
| Conventional Process | 1 g/L | 98.42 | 70.51 | 22.71 | 33.03 |
| Conventional Process | 3 g/L | 107.5 | 70.51 | 17.41 | 38.62 |
| Conventional Process | 5 g/L | 133.4 | 70.51 | 5.03 | 53.48 |
| Conventional Process | 7 g/L | 140.1 | 70.51 | 2.98 | 57.07 |
| Conventional Process | 9 g/L | 116.5 | 70.51 | 12.51 | 43.97 |
| Two-step Process | 3 g/L | 141.1 | 70.51 | 2.73 | 57.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Park, J.; Son, K.; Kim, D.; Kim, K.; Kang, M. Electroless Ni-P-PTFE Composite Plating with Rapid Deposition and High PTFE Concentration through a Two-Step Process. Coatings 2022, 12, 1199. https://doi.org/10.3390/coatings12081199
Lee M, Park J, Son K, Kim D, Kim K, Kang M. Electroless Ni-P-PTFE Composite Plating with Rapid Deposition and High PTFE Concentration through a Two-Step Process. Coatings. 2022; 12(8):1199. https://doi.org/10.3390/coatings12081199
Chicago/Turabian StyleLee, Myungwon, Junghyun Park, Kyeongsik Son, Donghyun Kim, Kwangho Kim, and Myungchang Kang. 2022. "Electroless Ni-P-PTFE Composite Plating with Rapid Deposition and High PTFE Concentration through a Two-Step Process" Coatings 12, no. 8: 1199. https://doi.org/10.3390/coatings12081199
APA StyleLee, M., Park, J., Son, K., Kim, D., Kim, K., & Kang, M. (2022). Electroless Ni-P-PTFE Composite Plating with Rapid Deposition and High PTFE Concentration through a Two-Step Process. Coatings, 12(8), 1199. https://doi.org/10.3390/coatings12081199







