Trend of Bioactive Molecules and Biomaterial Coating in Promoting Tendon—Bone Healing
Abstract
1. Introduction
2. Stem Cell Therapy
3. Cytokine Therapy
4. Exosomes
5. Scaffolds Based on Biomaterial Coating
5.1. Polymer Materials
5.2. Hydrogels
5.3. Decellularised Tendon Scaffold
5.4. Composite Material
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Huang, Z.; Liu, W.; Liu, Y.; Qian, H.; Lei, T.; Hua, L.; Hu, Y.; Zhang, Y.; Lei, P. Electrospun polycaprolactone/hydroxyapatite/ZnO films as potential biomaterials for application in bone-tendon interface repair. Coll. Surf. B Biointerfaces 2021, 204, 111825. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Wong, O.T. Tendon stem cells: Experimental and clinical perspectives in tendon and tendon-bone junction repair. Muscle Ligaments Tendons J. 2012, 2, 163–168. [Google Scholar]
- Pennisi, E. Tending tender tendons. Science 2002, 295, 1011. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.; Ebert, J.; Joss, B.; Bhabra, G.; Ackland, T.; Wang, A. Exercise Rehabilitation in the Non-Operative Management of Rotator Cuff Tears: A Review of The Literature. Int. J. Sports Phys Ther. 2016, 11, 279–301. [Google Scholar]
- Sammer, D.M.; Chung, K.C. Advances in the healing of flexor tendon injuries. Wound Repair Regen. 2014, 22, 25–29. [Google Scholar] [CrossRef]
- Oh, L.S.; Wolf, B.R.; Hall, M.P.; Levy, B.A.; Marx, R.G. Indications for rotator cuff repair: A systematic review. Clin. Orthop. Relat. Res. 2007, 455, 52–63. [Google Scholar] [CrossRef]
- Moffat, K.L.; Sun, W.H.; Pena, P.E.; Chahine, N.O.; Doty, S.B.; Ateshian, G.A.; Hung, C.T.; Lu, H.H. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc. Natl. Acad. Sci. USA 2008, 105, 7947–7952. [Google Scholar] [CrossRef]
- Apostolakos, J.; Durant, T.J.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The enthesis: A review of the tendon-to-bone insertion. Muscle Ligaments Tendons J. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Lu, H.H.; Thomopoulos, S. Functional attachment of soft tissues to bone: Development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 2013, 15, 201–226. [Google Scholar] [CrossRef]
- Lazarides, A.L.; Alentorn-Geli, E.; Choi, J.H.; Stuart, J.J.; Lo, I.K.; Garrigues, G.E.; Taylor, D.C. Rotator cuff tears in young patients: A different disease than rotator cuff tears in elderly patients. J. Shoulder Elb. Surg. 2015, 24, 1834–1843. [Google Scholar] [CrossRef]
- Hexter, A.T.; Pendegrass, C.; Haddad, F.; Blunn, G. Demineralized Bone Matrix to Augment Tendon-Bone Healing: A Systematic Review. Orthop. J. Sports Med. 2017, 5, 2325967117734517. [Google Scholar] [CrossRef]
- Woo, S.L.; Buckwalter, J.A. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987. J. Orthop. Res. 1988, 6, 907–931. [Google Scholar] [CrossRef]
- Matyas, J.R.; Anton, M.G.; Shrive, N.G.; Frank, C.B. Stress governs tissue phenotype at the femoral insertion of the rabbit MCL. J. Biomech. 1995, 28, 147–157. [Google Scholar] [CrossRef]
- Tibor, L.; Chan, P.H.; Funahashi, T.T.; Wyatt, R.; Maletis, G.B.; Inacio, M.C. Surgical Technique Trends in Primary ACL Reconstruction from 2007 to 2014. J. Bone Jt. Surg. Am. 2016, 98, 1079–1089. [Google Scholar] [CrossRef]
- Ernstbrunner, L.; Borbas, P.; Rohner, M.; Brun, S.; Bachmann, E.; Bouaicha, S.; Wieser, K. Biomechanical analysis of arthroscopically assisted latissimus dorsi transfer fixation for irreparable posterosuperior rotator cuff tears-Knotless versus knotted anchors. J. Orthop. Res. 2021, 39, 2234–2242. [Google Scholar] [CrossRef]
- Houck, D.A.; Kraeutler, M.J.; McCarty, E.C.; Bravman, J.T. Fixed- Versus Adjustable-Loop Femoral Cortical Suspension Devices for Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis of Biomechanical Studies. Orthop. J. Sports Med. 2018, 6, 2325967118801762. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Genin, G.M.; Galatz, L.M. The development and morphogenesis of the tendon-to-bone insertion—What development can teach us about healing. J. Musculoskelet. Neuronal Interact. 2010, 10, 35–45. [Google Scholar]
- Dodwell, E.R.; Lamont, L.E.; Green, D.W.; Pan, T.J.; Marx, R.G.; Lyman, S. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am. J. Sports Med. 2014, 42, 675–680. [Google Scholar] [CrossRef]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Jt. Surg. Am. 2004, 86, 219–224. [Google Scholar] [CrossRef]
- Harryman, D.T., 2nd; Mack, L.A.; Wang, K.Y.; Jackins, S.E.; Richardson, M.L.; Matsen, F.A., 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J. Bone Jt. Surg. Am. 1991, 73, 982–989. [Google Scholar]
- Wang, J.; Xu, J.; Song, B.; Chow, D.H.; Yung, P.S.; Qin, L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017, 63, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Bunker, D.L.; Ilie, V.; Ilie, V.; Nicklin, S. Tendon to bone healing and its implications for surgery. Muscles Ligaments Tendons J. 2014, 4, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Oguma, H.; Murakami, G.; Takahashi-Iwanaga, H.; Aoki, M.; Ishii, S. Early anchoring collagen fibers at the bone-tendon interface are conducted by woven bone formation: Light microscope and scanning electron microscope observation using a canine model. J. Orthop. Res. 2001, 19, 873–880. [Google Scholar] [CrossRef]
- Killian, M.L.; Cavinatto, L.M.; Ward, S.R.; Havlioglu, N.; Thomopoulos, S.; Galatz, L.M. Chronic Degeneration Leads to Poor Healing of Repaired Massive Rotator Cuff Tears in Rats. Am. J. Sports Med. 2015, 43, 2401–2410. [Google Scholar] [CrossRef]
- Pancholi, N.; Gregory, J.M. Biologic Augmentation of Arthroscopic Rotator Cuff Repair Using Minced Autologous Subacromial Bursa. Arthrosc. Tech. 2020, 9, e1519–e1524. [Google Scholar] [CrossRef]
- Tomita, F.; Yasuda, K.; Mikami, S.; Sakai, T.; Yamazaki, S.; Tohyama, H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy 2001, 17, 461–476. [Google Scholar] [CrossRef]
- Newsham-West, R.; Nicholson, H.; Walton, M.; Milburn, P. Long-term morphology of a healing bone-tendon interface: A histological observation in the sheep model. J. Anat. 2007, 210, 318–327. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Zhang, E.; Zhang, R.; Cai, D.; Zhu, S.; Ran, J.; Bunpetch, V.; Cai, Y.; Heng, B.C.; et al. Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 2017, 56, 129–140. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med. Sci. Monit. 2020, 26, e923328. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 2019, 17, 211. [Google Scholar] [CrossRef]
- Nourissat, G.; Diop, A.; Maurel, N.; Salvat, C.; Dumont, S.; Pigenet, A.; Gosset, M.; Houard, X.; Berenbaum, F. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS ONE 2010, 5, e12248. [Google Scholar] [CrossRef]
- Tan, Q.; Lui, P.P.Y.; Rui, Y.F.; Wong, Y.M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng. Part A 2012, 18, 840–851. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Geburek, F.; Roggel, F.; Van Schie, H.T.M.; Beineke, A.; Estrada, R.; Weber, K.; Hellige, M.; Rohn, K.; Jagodzinski, M.; Welke, B.; et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: A controlled experimental trial. Stem Cell Res. 2017, 8, 129. [Google Scholar] [CrossRef]
- Carvalho, A.D.M.; Badial, P.R.; Álvarez, L.E.C.; Yamada, A.L.M.; Borges, A.S.; Deffune, E.; Hussni, C.A.; Alves, A.L.G. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: A randomized controlled trial. Stem Cell Res. 2013, 4, 85. [Google Scholar] [CrossRef]
- Ahrberg, A.B.; Horstmeier, C.; Berner, D.; Brehm, W.; Gittel, C.; Hillmann, A.; Josten, C.; Rossi, G.; Schubert, S.; Winter, K.; et al. Effects of mesenchymal stromal cells versus serum on tendon healing in a controlled experimental trial in an equine model. BMC Musculoskelet. Disord. 2018, 19, 230. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.; Moreno, B.; de Blas, I.; Sanz, A.; Vazquez, F.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Veter-J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Ueyama, H.; Okano, T.; Orita, K.; Mamoto, K.; Ii, M.; Sobajima, S.; Iwaguro, H.; Nakamura, H. Local transplantation of adipose-derived stem cells has a significant therapeutic effect in a mouse model of rheumatoid arthritis. Sci. Rep. 2020, 10, 3076. [Google Scholar] [CrossRef]
- Puissant-Lubrano, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef]
- Mora, M.V.; Antuña, S.A.; Arranz, M.G.; Carrascal, M.T.; Barco, R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury 2014, 4, S22–S27. [Google Scholar] [CrossRef]
- Kaizawa, Y.; Franklin, A.; Leyden, J.; Behn, A.W.; Tulu, U.S.; Leon, D.S.; Wang, Z.; Abrams, G.D.; Chang, J.; Fox, P.M. Augmentation of chronic rotator cuff healing using adipose-derived stem cell-seeded human tendon-derived hydrogel. J. Orthop. Res. 2019, 37, 877–886. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Smith, C.A.; Ferrer, G.A.; Novaretti, J.V.; Pauyo, T.; Chao, T.; Hirsch, D.; Beaudry, M.F.; Herbst, E.; Tuan, R.S.; et al. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J. Shoulder Elb. Surg. 2019, 28, 654–664. [Google Scholar] [CrossRef]
- Roberts, J.H.; Halper, J. Growth Factor Roles in Soft Tissue Physiology and Pathophysiology. Adv. Exp. Med. Biol. 2021, 348, 139–159. [Google Scholar]
- Wang, R.; Xu, B.; Xu, H.-G. Up-Regulation of TGF-β Promotes Tendon-to-Bone Healing after Anterior Cruciate Ligament Reconstruction using Bone Marrow-Derived Mesenchymal Stem Cells through the TGF-β/MAPK Signaling Pathway in a New Zealand White Rabbit Model. Cell Physiol. Biochem. 2017, 41, 213–226. [Google Scholar] [CrossRef]
- Cheng, P.; Han, P.; Zhao, C.; Zhang, S.; Wu, H.; Ni, J.; Hou, P.; Zhang, Y.; Liu, J.; Xu, H.; et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials 2016, 81, 14–26. [Google Scholar] [CrossRef]
- Setiawati, R.; Utomo, D.N.; Rantam, F.; Ifran, N.N.; Budhiparama, N.C. Early Graft Tunnel Healing After Anterior Cruciate Ligament Reconstruction with Intratunnel Injection of Bone Marrow Mesenchymal Stem Cells and Vascular Endothelial Growth Factor. Orthop. J. Sports Med. 2017, 5, 2325967117708548. [Google Scholar] [CrossRef]
- Petersen, W.; Pufe, T.; Zantop, T.; Tillmann, B.; Mentlein, R. Hypoxia and PDGF have a synergistic effect that increases the expression of the angiogenetic peptide vascular endothelial growth factor in Achilles tendon fibroblasts. Arch. Orthop. Trauma. Surg. 2003, 123, 485–488. [Google Scholar] [CrossRef]
- Han, L.; Hu, Y.-G.; Jin, B.; Xu, S.-C.; Zheng, X.; Fang, W.-L. Sustained BMP-2 release and platelet rich fibrin synergistically promote tendon-bone healing after anterior cruciate ligament reconstruction in rat. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8705–8712. [Google Scholar] [PubMed]
- Abate, M.; Di Gregorio, P.; Schiavone, C.; Salini, V.; Tosi, U.; Muttini, A. Platelet Rich Plasma in Tendinopathies: How to Explain the Failure. Int. J. Immunopathol. Pharmacol. 2012, 25, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; McCann, P.; Colliver, J.; Breidahl, B.; Ackland, T.; Svard, U. Do Postoperative Platelet-Rich Plasma Injections Accelerate Early Tendon Healing and Functional Recovery After Arthroscopic Supraspinatus Repair? A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, e56. [Google Scholar] [CrossRef][Green Version]
- Chen, R.; Zhu, H.; Gu, X.; Xiang, X. Effects of Platelet-Rich Plasma on Tendon-Bone Healing After Anterior Cruciate Ligament Reconstruction. Orthop. Surg. 2021, 14, 88–95. [Google Scholar] [CrossRef]
- Chellini, F.; Tani, A.; Zecchi-Orlandini, S.; Sassoli, C. Influence of Platelet-Rich and Platelet-Poor Plasma on Endogenous Mechanisms of Skeletal Muscle Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 683. [Google Scholar] [CrossRef]
- Kia, C.; Baldino, J.; Bell, R.; Ramji, A.; Uyeki, C.; Mazzocca, A. Platelet-Rich Plasma: Review of Current Literature on its Use for Tendon and Ligament Pathology. Curr. Rev. Musculoskelet. Med. 2018, 11, 566–572. [Google Scholar] [CrossRef]
- Kida, Y.; Morihara, T.; Matsuda, K.-I.; Kajikawa, Y.; Tachiiri, H.; Iwata, Y.; Sawamura, K.; Yoshida, A.; Oshima, Y.; Ikeda, T.; et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J. Shoulder Elb. Surg. 2013, 22, 197–205. [Google Scholar] [CrossRef]
- De Gasperi, R.; Hamidi, S.; Harlow, L.M.; Ksiezak-Reding, H.; Bauman, W.A.; Cardozo, C.P. Denervation-related alterations and biological activity of miRNAs contained in exosomes released by skeletal muscle fibers. Sci. Rep. 2017, 7, 12888. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Xu, T.; Xu, M.; Bai, J.; Lin, J.; Yu, B.; Liu, Y.; Guo, X.; Shen, J.; Sun, H.; Hao, Y.; et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology 2019, 71, 57–65. [Google Scholar] [CrossRef]
- Huang, Y.; He, B.; Wang, L.; Yuan, B.; Shu, H.; Zhang, F.; Sun, L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res. Ther. 2020, 11, 496. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Perrini, C.; Tasquier, R.; Deregibus, M.C.; Camussi, G.; Pascucci, L.; Marini, M.G.; Corradetti, B.; Bizzaro, D.; De Vita, B.; et al. Equine Amniotic Microvesicles and Their Anti-Inflammatory Potential in a Tenocyte Model In Vitro. Stem Cells Dev. 2016, 25, 610–621. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Cui, Q.; Han, P.; Yang, S.; Shi, M.; Zhang, T.; Zhang, Z.; Li, Z. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res. Ther. 2020, 11, 402. [Google Scholar] [CrossRef]
- Dai, X.; Heng, B.C.; Bai, Y.; You, F.; Sun, X.; Li, Y.; Tang, Z.; Xu, M.; Zhang, X.; Deng, X. Restoration of electrical microenvironment enhances bone regeneration under diabetic conditions by modulating macrophage polarization. Bioact. Mater. 2020, 6, 2029–2038. [Google Scholar] [CrossRef]
- Han, X.; Liao, L.; Zhu, T.; Xu, Y.; Bi, F.; Xie, L.; Li, H.; Huo, F.; Tian, W.; Guo, W. Xenogeneic native decellularized matrix carrying PPARγ activator RSG regulating macrophage polarization to promote ligament-to-bone regeneration. Mater. Sci. Eng. C 2020, 116, 111224. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Song, W.; Yu, W.; He, Y. Adipose Stem Cell-Derived Exosomes Decrease Fatty Infiltration and Enhance Rotator Cuff Healing in a Rabbit Model of Chronic Tears. Am. J. Sports Med. 2020, 48, 1456–1464. [Google Scholar] [CrossRef]
- Mao, Z.; Fan, B.; Wang, X.; Huang, X.; Guan, J.; Sun, Z.; Xu, B.; Yang, M.; Chen, Z.; Jiang, D.; et al. A Systematic Review of Tissue Engineering Scaffold in Tendon Bone Healing in vivo. Front. Bioeng. Biotechnol. 2021, 9, 621483. [Google Scholar] [CrossRef]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef]
- Han, F.; Zhang, P.; Chen, T.; Lin, C.; Wen, X.; Zhao, P. A LbL-Assembled Bioactive Coating Modified Nanofibrous Membrane for Rapid Tendon-Bone Healing in ACL Reconstruction. Int. J. Nanomed. 2019, 14, 9159–9172. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Al-Sadi, O.; Ertel, W.; Lohan, A. Decellularized Tendon Extracellular Matrix—A Valuable Approach for Tendon Reconstruction? Cells 2012, 1, 1010–1028. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.D.L.; Da Silva, C.G.; Barreto, L.S.D.S.; Leite, K.R.M.; Tamaoki, M.J.S.; Ferreira, L.M.; De Almeida, F.G.; Faloppa, F. A new decellularized tendon scaffold for rotator cuff tears—Evaluation in rabbits. BMC Musculoskelet. Disord. 2020, 21, 689. [Google Scholar]
- Liu, G.-M.; Pan, J.; Zhang, Y.; Ning, L.-J.; Luo, J.-C.; Huang, F.-G.; Qin, T.-W. Bridging Repair of Large Rotator Cuff Tears Using a Multilayer Decellularized Tendon Slices Graft in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, S.; Tang, Y.; Li, X.; Cao, Y.; Hu, J.; Lu, H. Effect of book-shaped acellular tendon scaffold with bone marrow mesenchymal stem cells sheets on bone-tendon interface healing. J. Orthop. Transl. 2021, 26, 162–170. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Lin, Y.-C.; Chen, C.-Y.; Hsieh, Y.-Y.; Liaw, C.-K.; Huang, S.-W.; Tsuang, Y.-H.; Chen, C.-H.; Lin, F.-H. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as collagenase carrier for tendon-bone healing in a rabbit model. Polymers 2020, 12, 436. [Google Scholar] [CrossRef]
- Kaizawa, Y.; Leyden, J.; Behn, A.W.; Tulu, U.S.; Franklin, A.; Wang, Z.; Abrams, G.; Chang, J.; Fox, P.M. Human Tendon-Derived Collagen Hydrogel Significantly Improves Biomechanical Properties of the Tendon-Bone Interface in a Chronic Rotator Cuff Injury Model. J. Hand Surg. 2019, 44, 899.e1–899.e11. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhou, L.; Bi, Z.; Shi, M.; Wang, D.; Li, Q. Fabrication of a novel Three-Dimensional porous PCL/PLA tissue engineering scaffold with high connectivity for endothelial cell migration. Eur. Polym. J. 2021, 161, 110834. [Google Scholar] [CrossRef]
- Han, L.; Fang, W.-L.; Jin, B.; Xu, S.-C.; Zheng, X.; Hu, Y.-G. Enhancement of tendon-bone healing after rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9075–9084. [Google Scholar]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D printing of HA/PCL composite tissue engineering scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications. Nanomaterials 2019, 9, 590. [Google Scholar] [CrossRef]
- Moran, J.M.; Pazzano, D.; Bonassar, L.J. Characterization of Polylactic Acid-Polyglycolic Acid Composites for Cartilage Tissue Engineering. Tissue Eng. 2003, 9, 63–70. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Liu, W.; Shan, Q.; Buonocore, S.D.; Cui, L. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast. Reconstr. Surg. 2002, 110, 1280–1289. [Google Scholar]
- Guo, J.; Ning, C.; Liu, X. Bioactive calcium phosphate silicate ceramic surface-modified PLGA for tendon-to-bone healing. Colloids Surf. B Biointerfaces 2018, 164, 388–395. [Google Scholar] [CrossRef]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.-D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef]
- Foraida, Z.I.; Kamaldinov, T.; Nelson, D.; Larsen, M.; Castracane, J. Elastin-PLGA hybrid electrospun nanofiber scaffolds for salivary epithelial cell self-organization and polarization. Acta Biomater. 2017, 62, 116–127. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Heath, D.E. A review of decellularized extracellular matrix biomaterials for regenerative engineering applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Chen, X. Injectable hydrogels for tendon and ligament tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1598. [Google Scholar] [CrossRef]
- Gaffney, L.; Wrona, E.A.; Freytes, D.O. Potential synergistic effects of stem cells and extracellular matrix scaffolds. ACS Biomater. Sci. Eng. 2017, 4, 1208–1222. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; de Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Youngstrom, D.W.; Barrett, J.G. Engineering tendon: Scaffolds, bioreactors, and models of regeneration. Stem Cells Int. 2016, 2016, 3919030. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- He, B.; Zhu, Q.; Chai, Y.; Ding, X.; Tang, J.; Gu, L.; Xiang, J.; Yang, Y.; Zhu, J.; Liu, X. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: A prospective, multicentre controlled clinical trial. J. Tissue Eng. Regen. Med. 2015, 9, 286–295. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, J.; Cao, X.; Chen, C.; Li, S.; Guo, D.; Zhang, J.; Liu, P.; Shi, F.; Tang, B. Composite skin grafting with human acellular dermal matrix scaffold for treatment of diabetic foot ulcers: A randomized controlled trial. J. Am. College Surgeons 2016, 222, 1171–1179. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.-X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef]
- Woon, C.Y.; Pridgen, B.C.; Kraus, A.; Bari, S.; Pham, H.; Chang, J. Optimization of human tendon tissue engineering: Peracetic acid oxidation for enhanced reseeding of acellularized intrasynovial tendon. Plastic Reconstr. Surgery 2011, 127, 1107–1117. [Google Scholar] [CrossRef]
- Whitlock, P.W.; Seyler, T.M.; Parks, G.D.; Ornelles, D.A.; Smith, T.L.; van Dyke, M.E.; Poehling, G.G. A novel process for optimizing musculoskeletal allograft tissue to improve safety, ultrastructural properties, and cell infiltration. JBJS 2012, 94, 1458–1467. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber Scaffolds with Gradations in Mineral Content for Mimicking the Tendon-to-Bone Insertion Site. Nano Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Erisken, C.; Kalyon, D.M.; Wang, H. Functionally graded electrospun polycaprolactone and beta-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials 2008, 29, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, T.; Paiva, M.; Marques, A.T.; Lopes, M.A. Potential of Graphene-Polymer Composites for Ligament and Tendon Repair: A Review. Adv. Eng. Mater. 2020, 22, 2000492. [Google Scholar] [CrossRef]
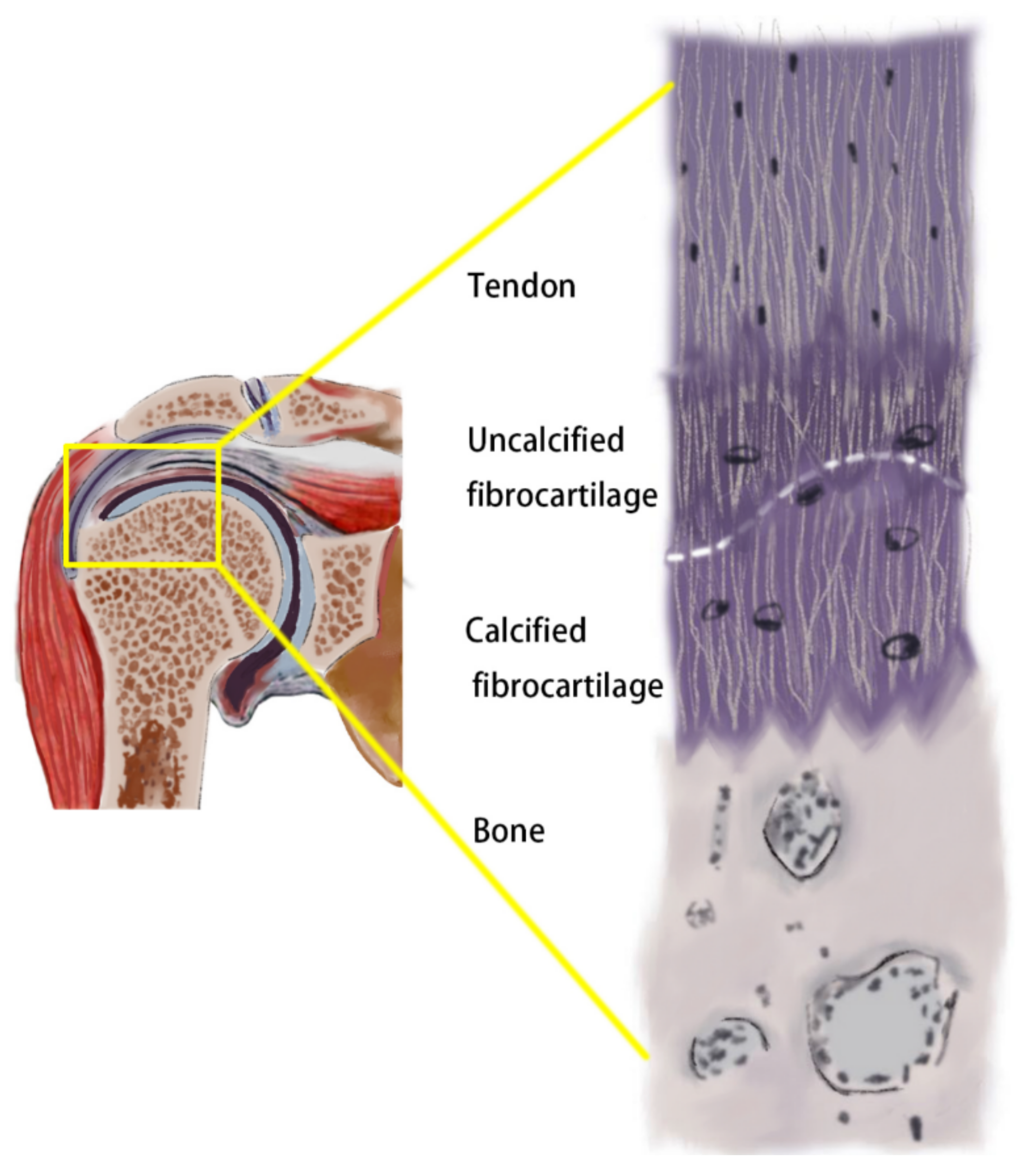
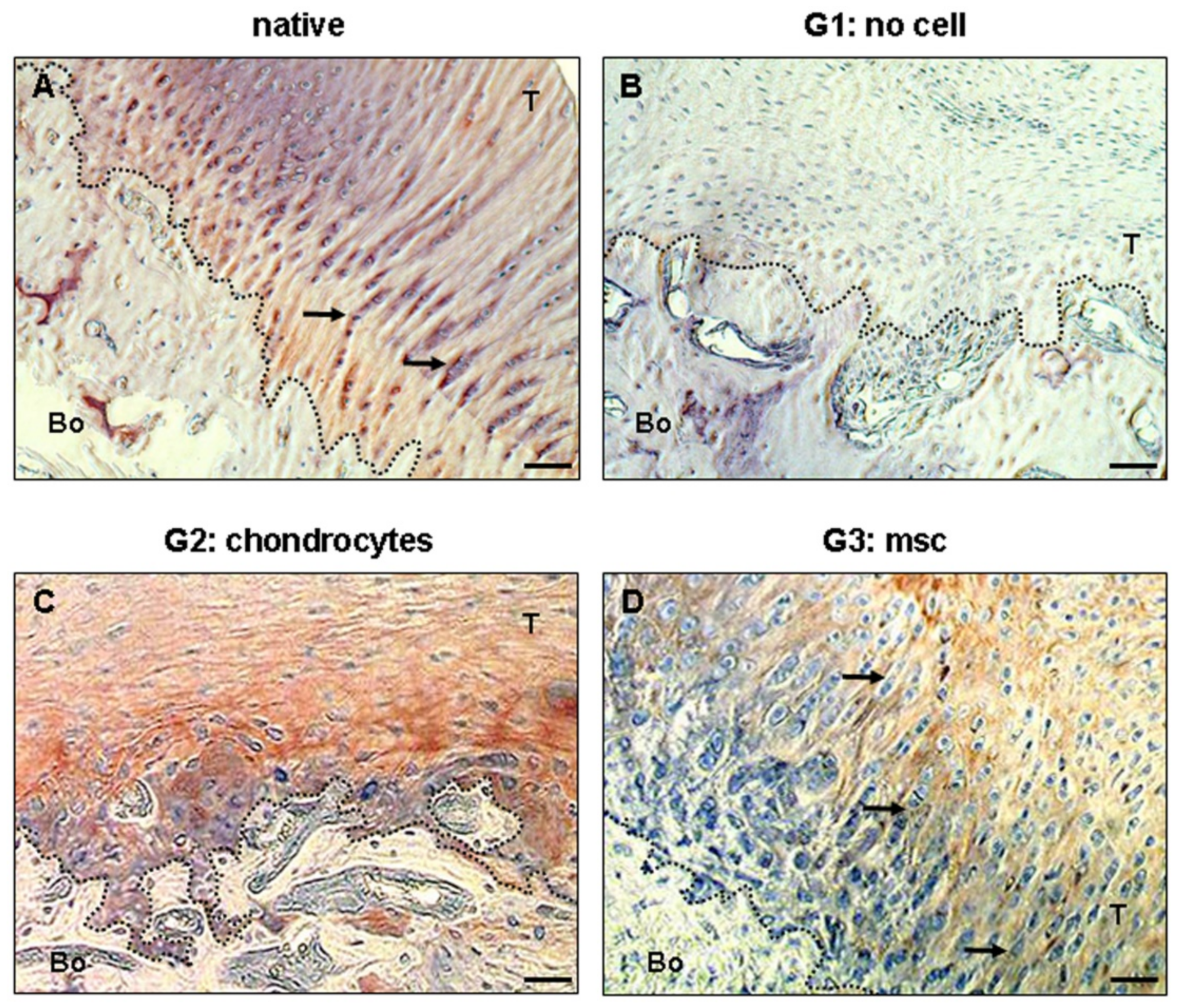

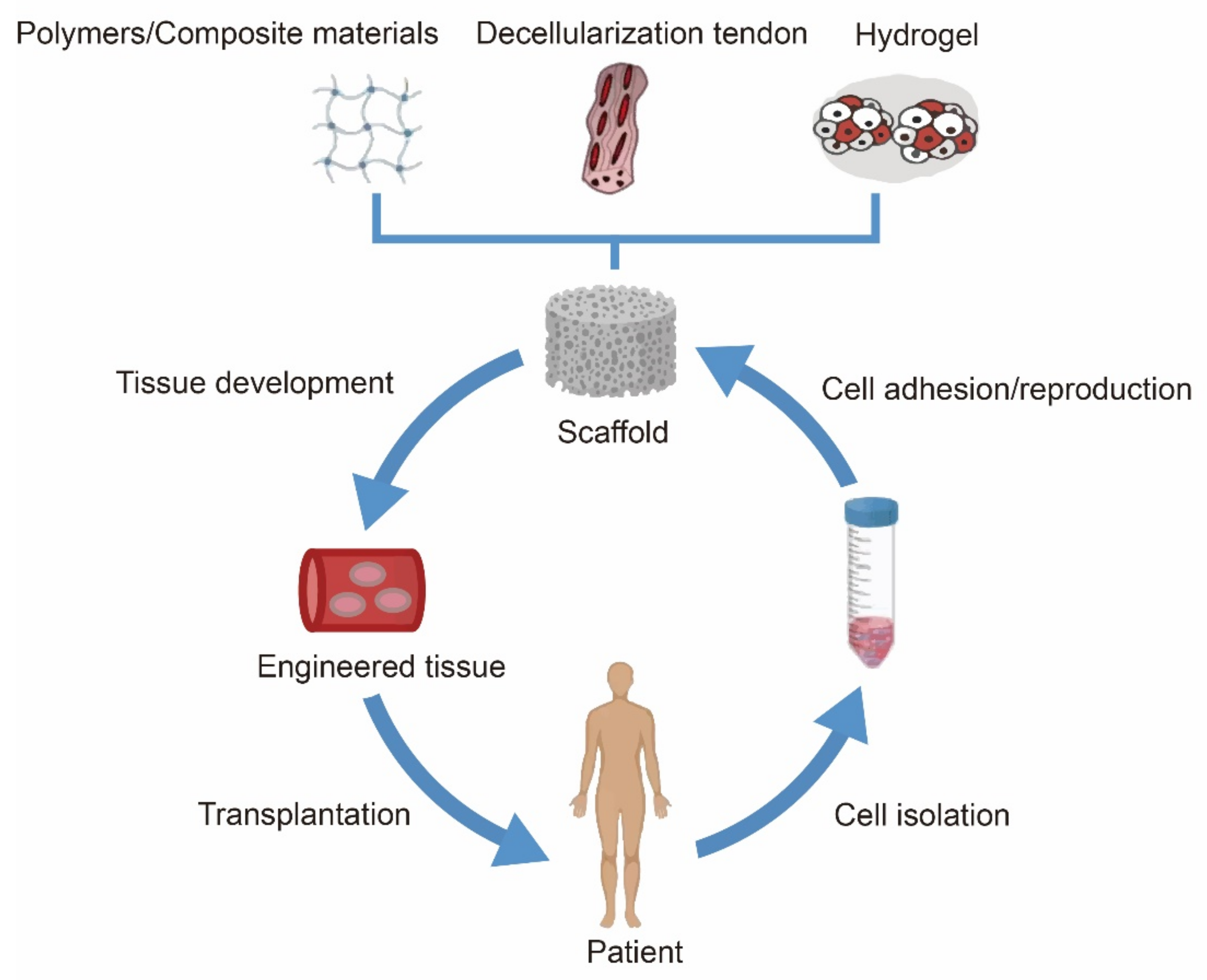
| Objective of the Study | Author | Tendon | Type of Study | Model |
|---|---|---|---|---|
| BMSCs | Shi et al. [32] | Achilles tendon | In vivo | Mouse |
| BMSCs | Shi et al. [33] | Patellar tendon | In vivo | Mouse |
| TDSCs | Tan et al. [35] | Patellar tendon | In vitro | Rat |
| TSPCs | Bi et al. [36] | Patellar tendon and human hamstring tendon | In vivo and in vitro | Human and Mouse |
| AT-MSCs | Geburek et al. [37] | Superficial digital flexor tendon | In vivo | Horse |
| adMSC | Carvalho et al. [38] | Superficial digital flexor tendon | In vivo | Horse |
| adMSC | Ahrberg et al. [39] | Superficial digital flexor tendon | In vivo | House |
| BM-MSCs and AT-MSCs | Romero et al. [40] | Superficial digital flexor tendon | In vivo | House |
| ASCs | Mora et al. [43] | Supraspinatus tendon | In vivo | Rat |
| ASCs | Kaizawa et al. [44] | Supraspinatus tendon | In vivo | Rat |
| ADSCs | Rothrauff et al. [45] | Supraspinatus and infraspinatus tendons | In vivo | Rat |
| Objective of the Study | Author | Tendon | Type of Study | Model |
|---|---|---|---|---|
| TGF-β | Wang et al. [47] | Anterior cruciate ligament | In vivo | Rabbit |
| VEGF | Setiawati et al. [49] | Anterior cruciate ligament | In vivo | Rabbit |
| PDGF | Petersen et al. [50] | Achilles tendons | In vivo | Rat |
| PRP | Abate et al. [53] | Supraspinatus tendon | In vivo | Human |
| Objective of the Study | Author | Tendon | Type of Study | Model |
|---|---|---|---|---|
| Tenocyte-derived exosomes | Xu et al. [61] | Achilles tendon | In vitro | Rat |
| Bone marrow mesenchymal stem cell-derived exosomes | Huang et al. [62] | Supraspinatus tendon | In vivo | Rat |
| Tendon stem cell-derived exosomes | Zhang et al. [64] | Achilles tendon | In vivo | Rat |
| Adipose stem cell-derived exosomes | Wang et al. [67] | Supraspinatus tendon | In vivo | Rabbit |
| Bone marrow stromal cell-derived exosomes | Shi et al. [32] | Achilles tendon | In vivo | Mouse |
| Objective of the Study | Author | Tendon | Type of Study | Model |
|---|---|---|---|---|
| PCL and CS/HA | Han et al. [70] | Anterior cruciate ligament | In vitro | Rabbit |
| Decellularised tendon scaffold | Schulze-Tanzil et al. [71] | Achilles tendon | In vitro | Rabbit |
| Decellularised tendon scaffold | de Lima Santos et al. [72] | Gastrocnemius muscle tendons | In vitro | Rabbit |
| Decellularised tendon scaffold | Liu et al. [73] | Infraspinatus tendons | In vitro | Rabbit |
| Decellularised tendon scaffold | Zhou et al. [74] | Patella-patellar tendon | In vitro | Rabbit |
| Hydrogels (chitosan/gelatin/β-glycerol phosphate) | Huang et al. [75] | Anterior cruciate ligament | In vitro | Rabbit |
| Hydrogels (an injectable, thermoresponsive, type-I collagen-rich, decellularised human tendon-derived) | Kaizawa et al. [76] | Supraspinatus tendon | In vitro | Rabbit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Yang, C. Trend of Bioactive Molecules and Biomaterial Coating in Promoting Tendon—Bone Healing. Coatings 2022, 12, 1143. https://doi.org/10.3390/coatings12081143
Fu Z, Yang C. Trend of Bioactive Molecules and Biomaterial Coating in Promoting Tendon—Bone Healing. Coatings. 2022; 12(8):1143. https://doi.org/10.3390/coatings12081143
Chicago/Turabian StyleFu, Zhiwei, and Chunxi Yang. 2022. "Trend of Bioactive Molecules and Biomaterial Coating in Promoting Tendon—Bone Healing" Coatings 12, no. 8: 1143. https://doi.org/10.3390/coatings12081143
APA StyleFu, Z., & Yang, C. (2022). Trend of Bioactive Molecules and Biomaterial Coating in Promoting Tendon—Bone Healing. Coatings, 12(8), 1143. https://doi.org/10.3390/coatings12081143






