Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review
Abstract
:1. Introduction
2. Research Highlights on Antibacterial Surface Treatment for Orthopedic Implants
2.1. Metal Coating
2.2. Nanocomposite Coating
| Composition of Surface Coating | Anti-Bacteria Properties | Anti-Bacteria Ingredients or Mechanisms | References |
|---|---|---|---|
| Ag/HA nanocomposite coating | Inhibition of S aureus and E coli biofilm formation | Release of silver nanoparticles | [58] |
| DLC surfaces containing silver nanoparticles | Reduction the growth of S aureus and S epidermidis | Release of silver | [59,60] |
| NanoAg-EGF | Strong inhibitory actions against the five pathogenic organisms | Sustained release of nanosilver | [64] |
| Silver ion-doped ceramic nanopowder coating | An increase in resistance to bacterial colonization | Controlled release of Ag+ ions; keep the total amount of silver low | [65] |
| GC/PEG/ZnO/Ag nanocomposites | High antibacterial activity toward E. coli | Release of ZnO and Ag nanoparticles | [69] |
| Lubricated orthopedic implant surface (LOIS) | Inhibition of P. aeruginosa and MRSA biofilm formation | The self-healing property of lubricants in micro/nanostructured surfaces; repel the adhesion of various liquids | [70] |
2.3. Antibiotic Coating
3. Surface Topography Modification
| Composition of Surface Topography Structure | Anti-Bacteria Properties | Anti-Bacteria Ingredients or Mechanisms | Reference |
|---|---|---|---|
| Vancomycin-loaded Ti coatings with interconnected micro-patterned structure | Prophylaxis against MRSA infection | Micro-patterned structure inhibit bacterial adhesion and biofilm formation Bactericidal effect of vancomycin | [105] |
| Nanophase ZnO and TiO2 | Reduce S. epidermidis adhesion | ROS generation; release of Zinc ion; bacterial cell membrane damage | [109] |
| Micro-to nano-scale patterning mimicked the surface architecture of dragonfly wings | Reduce almost 50% of Pseudomonas aeruginosa cells and 20% of the Staphylococcus aureus cells adhesion | Nano-wire arrays make the surface moderately bactericidal | [116] |
| Micro-grooved surfaces | Prevent bacterial colonization | The formation of a biological seal | [117] |
| Hydrothermally grown oxide layers composed of nanoflowers, nanopetals and nanofibers | Antibacterial properties against Staphylococcus aureus and methicillin resistant Staphylococcus aureus | The interaction of bacterial cells with the nano-sized pointed morphologies | [118] |
| Polydopamine and hyaluronic acid immobilisation on vancomycin-loaded titanium nanotube | Antibacterial ability against S. aureus Inhibit the formation of bacterial biofilms | Titanium nanotubes inhibit the bacterial biofilms Bactericidal effect of vancomycin Dopamine can control drug release | [119] |
| N-halamine polymeric coating | Long-lasting renewable antibacterial efficacy | Ti-PAA-NCl can kill the key bacteria and prevent the formation of bacterial biofilm | [120] |
| An antimicrobial polymeric bilayer structure containing hollow zinc oxide nanotubes | Present the highest activity against Gram-negative bacteria | Antimicrobial effectiveness is dependent on zinc oxide concentration | [121] |
4. Covalently Grafted Bioactive Agents to the Titanium Surfaces
5. Multicomponent Coating
6. The Evaluation of Antibacterial Coating
7. Conclusions and the Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.-J.; Goodman, S.B. Identification of periprosthetic joint infection after total hip arthroplasty. J. Orthop. Transl. 2014, 3, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Van De Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Kuehl, R.; Sutter, S.T.; Morgenstern, M.; Dangel, M.; Egli, A.; Nowakowski, A.; Suhm, N.; Theilacker, C.; Widmer, A.F. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: An observational prospective study with 229 patients. Clin. Microbiol. Infect. 2019, 25, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Alt, V. Antimicrobial coated implants in trauma and orthopaedics–A clinical review and risk-benefit analysis. Injury 2017, 48, 599–607. [Google Scholar] [CrossRef]
- Shigita, S.; Tsurumi, H.; Naka, H. Antiviral Fiber, Process for Producing the Fiber, and Textile Product Comprising the Fiber. US20100086617A1, 8 April 2010. [Google Scholar]
- Zhang, P.; Zhang, Z.; Li, W. Antibacterial TiO2 coating incorporating silver nanoparticles by microarc oxidation and ion implantation. J. Nanomater. 2013, 2013, 2. [Google Scholar]
- Xu, F.F.; Imlay, J.A. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef] [Green Version]
- Stani, V.; Dimitrijevic, S.; Antic-Stankovic, J.; Mitric, M.; Jokic, B.; Plecas, I.B.; Raicevic, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2010, 77, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Furko, M.; Balázsi, C. Morphological, chemical, and biological investigation of ionic substituted, pulse current deposited calcium phosphate coatings. Materials 2020, 13, 4690. [Google Scholar] [CrossRef] [PubMed]
- Sutha, S.; Karunakaran, G.; Rajendran, V. Enhancement of antimicrobial and long-term biostability of the zinc-incorporated hydroxyapatite coated 316L stainless steel implant for biomedical application. Ceram. Int. 2013, 39, 5205–5212. [Google Scholar] [CrossRef]
- Herber, V.; Okutan, B.; Antonoglou, G.; Sommer, N.; Payer, M. Bioresorbable magnesium-based alloys as novel biomaterials in oral bone regeneration: General review and clinical perspectives. J. Clin. Med. 2021, 10, 1842. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yin, J.; Yang, J.; Li, Q.; Zheng, W.; Liu, S.; Jiang, X. The density of surface coating can contribute to different antibacterial activities of gold nanoparticles. Nano Lett. 2020, 20, 5036–5042. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.; Hoyos Nogués, M.; Gil Mur, F.J.; Pérez Antoñanzas, R. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mecha-nisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Gao, S.; Zhang, Y.-W.; Zhou, R.-B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Ak, A.; Mittal, A.; Das, A.; Sen, D.; Mariappan, C.R. Mesoporous electroactive silver doped calcium borosilicates: Structural, antibacterial and myogenic potential relationship of improved bio-ceramics-ScienceDirect. Ceram. Int. 2020, 47, 3586–3596. [Google Scholar]
- Gaul, L.E.; Staud, A.H. Clinical spectroscopy: Seventy cases of generalized argyrosis following organic and colloidal silver medication, including a biospectrometric analysis of ten cases. J. Am. Med. Assoc. 1935, 104, 1387–1390. [Google Scholar] [CrossRef]
- De La Riviere, A.B.; Dossche, K.M.; Birnbaum, D.E.; Hacker, R. First clinical experience with a mechanical valve with silver coating. J. Heart Valve Dis. 2000, 9, 123–130. [Google Scholar]
- Tweden, K.S.; Cameron, J.D.; Razzouk, A.J.; Holmberg, W.R.; Kelly, S.J. Biocompatibility of silver-modified polyester for antimicrobial protection of prosthetic valves. J. Heart Valve Dis. 1997, 6, 553–561. [Google Scholar]
- Perrelli, G.; Piolatto, G. Tentative reference values for gold, silver and platinum: Literature data analysis. Sci. Total Environ. 1992, 120, 93–96. [Google Scholar] [CrossRef]
- Evans, A.; Kavanagh, K.A. Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens. J. Med Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Williams, A. How safe is silver in wound care? J. Wound Care 2004, 13, 131–136. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Med. J. 2019, 120, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Gaetke, L.M. and C.K. Chow, Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Araya, M.; McGoldrick, M.C.; Klevay, L.M.; Strain, J.; Robson, P.; Nielsen, F.; Olivares, M.; Pizarro, F.; Johnson, L.; Poirier, K.A. Determination of an acute no-observed-adverse-effect level (NOAEL) for copper in water. Regul. Toxicol. Pharmacol. 2001, 34, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Chen, B.; Klevay, L.M.; Strain, J.; Johnson, L.; Robson, P.; Shi, W.; Nielsen, F.; Zhu, H.; Olivares, M.; et al. Confirmation of an acute no-observed-adverse-effect and low-observed-adverse-effect level for copper in bottled drinking water in a multi-site international study. Regul. Toxicol. Pharmacol. 2003, 38, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Mathys, Z.K.; White, A.R. Copper and alzheimer’s disease. Adv. Neurobiol. 2017, 18, 199–216. [Google Scholar] [PubMed]
- Liu, J.; Zhao, H.; Wang, Y.; Shao, Y.; Zhang, L.; Xing, M. Impacts of simultaneous exposure to arsenic (III) and copper (II) on inflammatory response, immune homeostasis, and heat shock response in chicken thymus. Int. Immunopharmacol. 2018, 64, 60–68. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Liu, B.; Tian, Y.; Ma, W. Effect of magnesium ion on human osteoblast activity. Braz. J. Med Biol. Res. 2016, 49. [Google Scholar] [CrossRef]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High magnesium inhibits human osteoblast differentiation in vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef]
- Purnama, A.; Hermawan, H.; Couet, J.; Mantovani, D. Assessing the biocompatibility of degradable metallic materials: State-of-the-art and focus on the potential of genetic regulation. Acta Biomater. 2010, 6, 1800–1807. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Variola, F.; Brunski, J.B.; Orsini, G.; de Oliveira, P.T.; Wazen, R.; Nanci, A. Nanoscale surface modifications of medically relevant metals: State-of-the art and perspectives. Nanoscale 2010, 3, 335–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Bacakova, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials de-veloped for bone implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [CrossRef]
- Streicher, R.M.; Schmidt, M.; Fiorito, S. Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine 2007, 2, 861–874. [Google Scholar] [CrossRef]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human os-teoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Staruch, R.; Griffin, M.F.; Butler, P. Nanoscale surface modifications of orthopaedic implants: State of the art and per-spectives. Open Orthop. J. 2016, 10, 920–938. [Google Scholar] [CrossRef]
- Guerra, R.; Lima, E.; Guzmán, A. Antimicrobial supported nanoparticles: Gold versus silver for the cases of escherichia coli and salmonella typhi. Microporous Mesoporous Mater. 2013, 170, 62–66. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yi, J.; Zhao, G.; Huang, L.; Yan, G.; Chen, Y.; Liu, P. Layer-by-layer assembly of silver nanoparticles embedded polyelectrolyte multilayer on magnesium alloy with enhanced antibacterial property. Surf. Coat. Technol. 2016, 286, 103–112. [Google Scholar] [CrossRef]
- Zhao, C.; Hou, P.; Ni, J.; Han, P.; Chai, Y.; Zhang, X. Ag-incorporated FHA coating on pure Mg: Degradation and in vitro antibacterial properties. ACS Appl. Mater. Interfaces 2016, 8, 5093–5103. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.-H.; Hsiao, Y.-P.; Hsu, C.-Y.; Lai, P.-S. Photo-crosslinked polymeric matrix with antimicrobial functions for excisional wound healing in mice. Nanomaterials 2018, 8, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Ashraf, M.A. Influence of silver-hydroxyapatite nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med. J. 2015, 64, 506–513. [Google Scholar]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N.; Kümpel, K.; Schneider, M.F.; Wixforth, A.; Gollwitzer, H.; Burgkart, R.; et al. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Stritzker, B. Plasma immersion ion implantation of polymers and silver–polymer nano composites. Surf. Coatings Technol. 2010, 204, 1875–1879. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Szalay, G.; Alt, V.; Lips, K.S. In vitro assessment of nanosilver-functionalized PMMA bone cement on primary human mesenchymal stem cells and osteoblasts. PLoS ONE 2014, 9, e114740. [Google Scholar] [CrossRef]
- Marta, M.; Cochis, A.; Kumar, A.; Arciola, C.R.; Rimondini, L.; Verné, E. Copper-Doped Bioactive Glass As Filler for PMMA-based bone cements: Morphological, mechanical, reac-tivity, and preliminary antibacterial characterization. Materials 2018, 11, 961. [Google Scholar]
- Vaiana, C.A.; Leonard, M.K.; Drummy, L.F.; Singh, K.M.; Bubulya, A.; Vaia, R.A.; Naik, R.R.; Kadakia, M.P. Epidermal growth factor: Layered silicate nanocomposites for tissue regeneration. Biomacromolecules 2011, 12, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-D.; Wang, S.-H.; Liu, R.; Zhou, C.-J.; Cao, K.; Liu, J.-Y.; Chen, Y.; Chen, F.-H. Study of the biological effectiveness of a nanosilver-epidermal growth factor sustained-release carrier. Exp. Ther. Med. 2013, 5, 1231–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kose, N.; Otuzbir, A.; Pekşen, C.; Kiremitçi, A.; Doğan, A. A silver ion-doped calcium phosphate-based ceramic nanopowder-coated prosthesis increased infection re-sistance. Clin. Orthop. Relat. Res. 2013, 471, 2532–2539. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zheng, X.; Yan, D.; Yin, G.; Liao, X.; Kang, Y.; Yao, Y.; Huang, D.; Hao, B. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 2008, 24, 4140–4144. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, 829–858. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.I. Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef]
- Chae, K.; Jang, W.Y.; Park, K.; Lee, J.; Kim, H.; Lee, K.; Lee, C.K.; Lee, Y.; Lee, S.H.; Seo, J. Antibacterial infection and immune-evasive coating for orthopedic implants. Sci. Adv. 2020, 6, eabb0025. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 85, 1115–1122. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir Acs J. Surf. Colloids 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Ashcroft, J.M.; Chen, D.; Min, G.; Kim, C.H.; Murkhejee, B.; Larabell, C.; Keasling, J.D.; Chen, F.F. Charge-associated effects of fullerene derivatives on microbial structural integrity and central metabolism. Nano Lett. 2007, 7, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; Abicht, H.; Solioz, M. Killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef] [Green Version]
- Yusof, H.M.; Rahman, N.A.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kwon, H.S.; Peng, Z.; Elder, A.; Yang, H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 2012, 6, 2157–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.; Amal, R. Cytotoxic origin of copper(II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Dayaghi, E.; Bakhsheshi-Rad, H.; Hamzah, E.; Akhavan-Farid, A.; Ismail, A.; Aziz, M.; Abdolahi, E. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: In vitro cell biology and antibacterial activity assessment. Mater. Sci. Eng. C 2019, 102, 53–65. [Google Scholar] [CrossRef]
- Coelho, C.C.; Padrão, T.; Costa, L.; Pinto, M.T.; Costa, P.C.; Domingues, V.F.; Quadros, P.A.; Monteiro, F.J.; Sousa, S.R. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Sci. Rep. 2020, 10, 19098. [Google Scholar] [CrossRef]
- Shuai, C.; Dong, Z.; He, C.; Yang, W.; Peng, S.; Yang, Y.; Qi, F. A peritectic phase refines the microstructure and enhances Zn implants. J. Mater. Res. Technol. 2020, 9, 2623–2634. [Google Scholar] [CrossRef]
- Mashino, T.; Okuda, K.; Hirota, T.; Hirobe, M.; Nagano, T.; Mochizuki, M. Inhibition of E. coli growth by fullerene derivatives and inhibition mechanism. Bioorg. Med. Chem. Lett. 1999, 9, 2959–2962. [Google Scholar] [CrossRef]
- Jensen, A.W.; Wilson, S.R.; Schuster, D.I. Biological applications of fullerenes. Bioorg. Med. Chem. 1996, 4, 767–779. [Google Scholar] [CrossRef]
- Nina, T.; Luh, T.Y.; Chou, C.K.; Chang, T.Y.; Wu, J.J.; Liu, C.C.; Lei, H.Y. In vitro action of carboxyfullerene. J. Antimicrob. Chemother. 2002, 49, 641–649. [Google Scholar]
- Manna, S.K.; Sarkar, S.; Barr, J.; Wise, K.; Barrera, E.V.; Jejelowo, O.; Ramesh, G.T. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005, 5, 1676–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, R.J.; Berry, C.; Brigmon, R. Structural and biological properties of carbon nanotube composite films. Mater. Sci. Eng. B 2005, 123, 123–129. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Mückley, T.; Hofmann, G.O. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury 2006, 37 (Suppl. S2), S95–S104. [Google Scholar] [CrossRef]
- Gulati, K.; Aw, M.S.; Findlay, D.; Losic, D. Local drug delivery to the bone by drug-releasing implants: Perspectives of nano-engineered titania nanotube arrays. Ther. Deliv. 2012, 3, 857–873. [Google Scholar] [CrossRef]
- Berretta, J.M.; Jennings, J.A.; Courtney, H.S.; Beenken, K.E.; Smeltzer, M.S.; Haggard, W.O. Blended chitosan paste for in-fection prevention: Preliminary and preclinical evaluations. Clin. Orthop. Relat. Res. 2017, 475, 1857–1870. [Google Scholar]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2018, 83, 37–54. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated in-fections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar]
- Oshima, S.; Sato, T.; Honda, M.; Suetsugu, Y.; Ozeki, K.; Kikuchi, M. Fabrication of gentamicin-loaded hydroxyapatite/collagen bone-like nanocomposite for anti-infection bone void fillers. Int. J. Mol. Sci. 2020, 21, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, E.J. Recent coating developments for combination devices in orthopedic and dental applications: A literature review. Adv. Drug Deliv. Rev. 2017, 112, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Degoutin, S.; Chai, F.; Raoul, G.; Hornez, J.C.; Martel, B.; Blanchemain, N. Gentamicin-loaded poly(lactic-co-glycolic acid) microparticles for the prevention of maxillofacial and ortho-pedic implant infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 108–116. [Google Scholar] [CrossRef]
- Stevanović, M.; Djošić, M.; Janković, A.; Nešović, K.; Kojić, V.; Stojanović, J.; Grujić, S.; Bujagić, I.M.; Rhee, K.Y.; Mišković-Stanković, V. Assessing the bioactivity of gentamicin-preloaded hydroxyapatite/chitosan composite coating on titanium substrate. ACS Omega 2020, 5, 15433–15445. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected economic burden of periprosthetic joint infection of the hip and knee in the united states. J. Arthroplast. 2020, 36, 1484–1489.e3. [Google Scholar] [CrossRef]
- Muzzio, N.E.; Pasquale, M.A.; Rios, X.; Azzaroni, O.; Llop, J.; Moya, S.E. Adsorption and exchangeability of fibronectin and serum albumin protein corona on annealed poly-electrolyte multilayers and their consequences on cell adhesion. Adv. Mater. Interfaces 2019, 6, 1900008. [Google Scholar] [CrossRef]
- Muzzio, N.E.; Gregurec, D.; Diamanti, E.; Irigoyen, J.; Pasquale, M.A.; Azzaroni, O.; Moya, S.E. Thermal annealing of polyelectrolyte multilayers: An effective approach for the enhancement of cell adhesion. Adv. Mater. Interfaces 2016, 4, 1600126. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Quere, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [Green Version]
- Pahal, S.; Gakhar, R.; Raichur, A.; Varma, M.M. Polyelectrolyte multilayers for bio-applications: Recent advancements. IET Nanobiotechnol. 2017, 11, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Jiang, X.Y.; Yamane, M.; Lee, M.; Goodman, S.B.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2015, 3, 8757–8770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maerten, C.; Jierry, L.; Schaaf, P.; Boulmedais, F. Review of electrochemically triggered macromolecular film buildup processes and their biomedical appli-cations. ACS Appl. Mater. Interfaces 2017, 9, 28117–28138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Liu, P.; Tong, D.; Ding, C.; Zhang, Z.; Xie, Y.; Tang, H.; Ji, F. Vancomycin-loaded titanium coatings with an interconnected micro-patterned structure for prophylaxis of infections: An in vivo study. RSC Adv. 2018, 8, 9223–9231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.-C.; Siedlecki, C.A. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater. 2012, 8, 72–81. [Google Scholar] [CrossRef]
- Ercan, B.; Kummer, K.M.; Tarquinio, K.M.; Webster, T.J. Decreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation. Acta Biomater. 2011, 7, 3003–3012. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreasedStaphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78A, 595–604. [Google Scholar] [CrossRef]
- Desrousseaux, C.; Cueff, R.; Aumeran, C.; Garrait, G.; Mailhot-Jensen, B.; Traoré, O.; Sautou, V. Fabrication of acrylonitrile-butadiene-styrene nanostructures with anodic alumina oxide templates, characterization and biofilm development test for staphylococcus epidermidis. PLoS ONE 2015, 10, e0135632. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J. Laser Surface Treatment of Bio-Implant Materials; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef]
- Jin, L.; Guo, W.; Xue, P.; Gao, H.; Zhao, M.; Zheng, C.; Zhang, Y.; Han, D. Quantitative assay for the colonization ability of heterogeneous bacteria on controlled nanopillar structures. Nanotechnology 2015, 26, 55702. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Geeganagamage, N.M.; Baulin, V.; Vongsvivut, J.; Tobin, M.J.; Luque, P.; Crawford, R.J.; Ivanova, E.P. The susceptibility of Staphylococcus aureus CIP 65.8 and Pseudomonas aeruginosa ATCC 9721 cells to the bactericidal action of nanostructured Calopteryx haemorrhoidalis damselfly wing surfaces. Appl. Microbiol. Biotechnol. 2017, 101, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Viela, F.; Navarro-Baena, I.; Jacobo-Martín, A.; Hernández, J.J.; Boyano-Escalera, M.; Osorio, M.R.; Rodríguez, I. Nano-engineering safer-by-design nanoparticle based moth-eye mimetic bactericidal and cytocompatible polymer surfaces. RSC Adv. 2019, 8, 22606–22616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.; Juodkazis, S.; Crawford, R.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [Green Version]
- Guillem-Marti, J.; Delgado, L.; Godoy-Gallardo, M.; Pegueroles, M.; Herrero, M.; Gil, F.J. Fibroblast adhesion and activation onto micro-machined titanium surfaces. Clin. Oral Implant. Res. 2012, 24, 770–780. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Garcia, C.B.; Hameed, P.; Manivasagam, G.; Webster, T.J. Hydrothermal treatment of etched titanium: A potential surface nano-modification technique for enhanced biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102016. [Google Scholar] [CrossRef]
- He, R.; Sui, J.; Wang, G.; Wang, Y.; Xu, K.; Qin, S.; Xu, S.; Ji, F.; Zhang, H. Polydopamine and hyaluronic acid immobilisation on vancomycin-loaded titanium nanotube for prophylaxis of implant infections. Colloids Surf. B Biointerfaces 2022, 216, 112582. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef]
- Dicastillo, C.L.; Patiño Vidal, C.; Falcó, I.; Sánchez, G.; Márquez, P.; Escrig, J. Antimicrobial bilayer nanocomposites based on the incorporation of as-synthetized hollow zinc oxide nanotubes. Nanomaterials 2020, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-step preparation of antifouling polysulfone ultrafiltration membranes via modification by a cationic polyelectrolyte based on polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Vasita, R.; Shanmugam, K. Improved biomaterials for tissue engineering applications: Surface modification of polymers. Curr. Top. Med. Chem. 2008, 8, 341–353. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Olivier, A.; Meyer, F.; Raquez, J.-M.; Damman, P.; Dubois, P. Surface-initiated controlled polymerization as a convenient method for designing functional polymer brushes: From self-assembled monolayers to patterned surfaces. Prog. Polym. Sci. 2012, 37, 157–181. [Google Scholar] [CrossRef]
- Gooding, J.J.; Ciampi, S. The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011, 40, 2704–2718. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar]
- Vacanti, J.P.; Langer, R. Tissue engineering. Lancet 1993, 354, S132–S134. [Google Scholar]
- Vasita, R.; Katti, D.S. Growth factor-delivery systems for tissue engineering: A materials perspective. Expert Rev. Med. Devices 2006, 3, 29–47. [Google Scholar] [CrossRef]
- Alcheikh, A.; Pavon-Djavid, G.; Helary, G.; Petite, H.; Migonney, V.; Anagnostou, F. PolyNaSS grafting on titanium surfaces enhances osteoblast differentiation and inhibits Staphylococcus aureus ad-hesion. J. Mater. Sci. Mater. Med. 2013, 24, 1745–1754. [Google Scholar] [CrossRef]
- Helary, G.; Noirclere, F.; Mayingi, J.; Migonney, V. A new approach to graft bioactive polymer on titanium implants: Improvement of MG 63 cell differentiation onto this coating. Acta Biomater. 2009, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.M.; Falentin-Daudré, C.; Blanquaert, D.; Thomas, D.; Granja, P.L.; Migonney, V. Role of protein environment and bioactive polymer grafting in the S. epidermidis response to titanium alloy for biomedical applications. Mater. Sci. Eng. C 2014, 45, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Willcox, M.D.; Ho KK, K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its invivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Rasul, R.; Cole, N.; Balasubramanian, D.; Chen, R.; Kumar, N.; Willcox, M. Interaction of the antimicrobial peptide melimine with bacterial membranes. Int. J. Antimicrob. Agents 2010, 35, 566–572. [Google Scholar] [CrossRef]
- Chouirfa, H.; Evans, D.M.; Bean, P.; Saleh-Mghir, A.; Crémieux, C.A.; Castner, G.D.; Migonney, V. Grafting of bioactive polymers with various architectures: A versatile tool for preparing antibacterial in-fection and biocompatible surfaces. Acs Appl. Mater. Interfaces 2018, 10, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Gao, T.; Zhang, N.; He, J.; Wu, F. Covalent immobilization of DJK-5 peptide on porous titanium for enhanced antibacterial effects and restrained inflammatory osteoclastogenesis. Colloids Surf. B Biointerfaces 2021, 202, 111697. [Google Scholar] [CrossRef] [PubMed]
- Peyre, J.; Humblot, V.; Méthivier, C.; Berjeaud, J.M.; Pradier, C.M. Co-Grafting of Amino–Poly(ethyleneglycol)and Magainin I on a TiO2 surface: Tests of antifoulingand antibac-terial activities. J. Phys. Chem. B 2012, 116, 13839–13847. [Google Scholar] [CrossRef]
- Stavrakis, A.I.; Zhu, S.; Hegde, V.; Loftin, A.H.; Ashbaugh, A.G.; Niska, J.A.; Bernthal, N.M. In vivo efficacy of a “smart” antimicrobial implant coating. J. Bone Joint. Surg. Am. 2016, 98, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xi, Y.; Bai, J.; Jiang, Z.; Wang, S.; Zhang, H.; Dai, W.; Chen, C.; Gou, Z.; Yang, G.; et al. Covalent grafting of hyperbranched poly-L-lysine on Ti-based implants achieves dual functions of antibacteria and promoted osteointegration in vivo. Biomaterials 2021, 269, 120534. [Google Scholar] [CrossRef]
- Nie, B.; Ao, H.; Long, T.; Zhou, J.; Tang, T.; Yue, B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: An in vivo study. Colloids Surf. B Biointerfaces 2017, 150, 183–191. [Google Scholar] [CrossRef]
- Nie, B.; Ao, H.; Zhou, J.; Tang, T.; Yue, B. Biofunctionalization of titanium with bacitracin immobilization shows potential for anti-bacteria, osteogenesis and reduction of macrophage inflammation. Colloids Surf. B Biointerfaces 2016, 145, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for orthopedic applications: An overview of surface modification to improve biocompatibility and prevent bacterial biofilm formation. Science 2020, 23, 101745. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Waseem, A.; Karpukhina, N.; Mohsin, S. Strontium- and zinc-containing bioactive glass and alginates scaffolds. Bioengineering 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malagurski, I.; Levic, S.; Mitric, M.; Pavlovic, V.; Dimitrijevic-Brankovic, S. Bimetallic alginate nanocomposites: New antimicrobial biomaterials for biomedical application. Mater. Lett. 2017, 212, 32–36. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Suárez-González, D.; Barnhart, K.; Saito, E.; Vanderby, R.; Hollister, S.J.; Murphy, W.L. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J. Biomed. Mater. Res. Part A 2010, 95A, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz, M.; Alizadeh, P. On the role of alginate coating on the mechanical and biological properties of 58S bioactive glass scaffolds. Int. J. Biol. Macromol. 2020, 167, 947–961. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Cai, Y.; Ji, H.; Zhou, G.; Zhao, X.; Zhang, M. In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 90, 1083–1091. [Google Scholar] [CrossRef]
- Mahmoud, E.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Moskalewicz, T.; Warcaba, M.; Łukaszczyk, A.; Kot, M.; Kopia, A.; Hadzhieva, Z.; Boccaccini, A.R. Electrophoretic deposition, microstructure and properties of multicomponent sodium alginate-based coatings incorporated with graphite oxide and hydroxyapatite on titanium biomaterial substrates-ScienceDirect. Appl. Surf. Sci. 2021, 575, 151688. [Google Scholar] [CrossRef]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.A.; Goldmann, W.H.; Boccaccini, A.R. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants. Surf. Coat. Technol. 2019, 381, 125138. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, Z.; Yin, C.; Zhang, C.; Lin, G.; Li, Q. Controllable drug release and simultaneously carrier decomposition of SiO2-drug composite nanoparticles. J. Am. Chem. Soc. 2013, 135, 5709–5716. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, X.; Yao, Y.; Luo, J.; Tang, Q.; Wu, H.; Lin, S.; Han, C.; Wei, Q.; Chen, L. Electrophoretic deposition of chitosan/gelatin coatings with controlled porous surface topography to enhance initial osteoblast adhesive responses. J. Mater. Chem. B 2016, 4, 7584–7595. [Google Scholar] [CrossRef]
- Pantović Pavlović, M.R.; Stanojevic, B.P.; Pavlovic, M.M.; Mihailovic, M.D.; Stevanovic, J.S.; Panic, V.V.; Ignjatovic, N.L. Anodizing/anaphoretic electrodeposition of nano-calcium phosphate/chitosan lactate multifunctional coatings on titanium with advanced corrosion resistance, bioactivity, and antibacterial properties. ACS Biomater. Sci. Eng. 2021, 7, 3088–3102. [Google Scholar] [CrossRef]
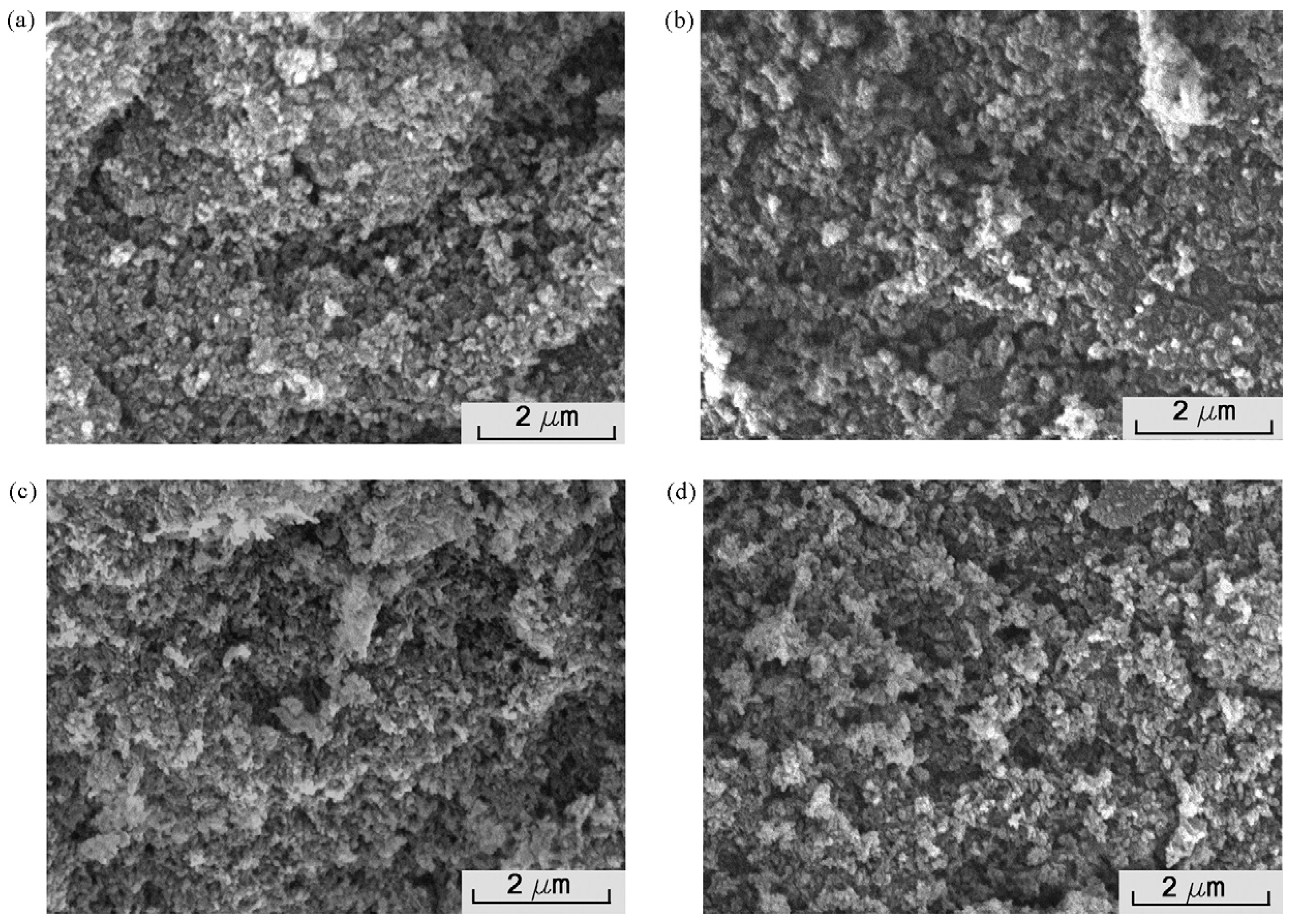
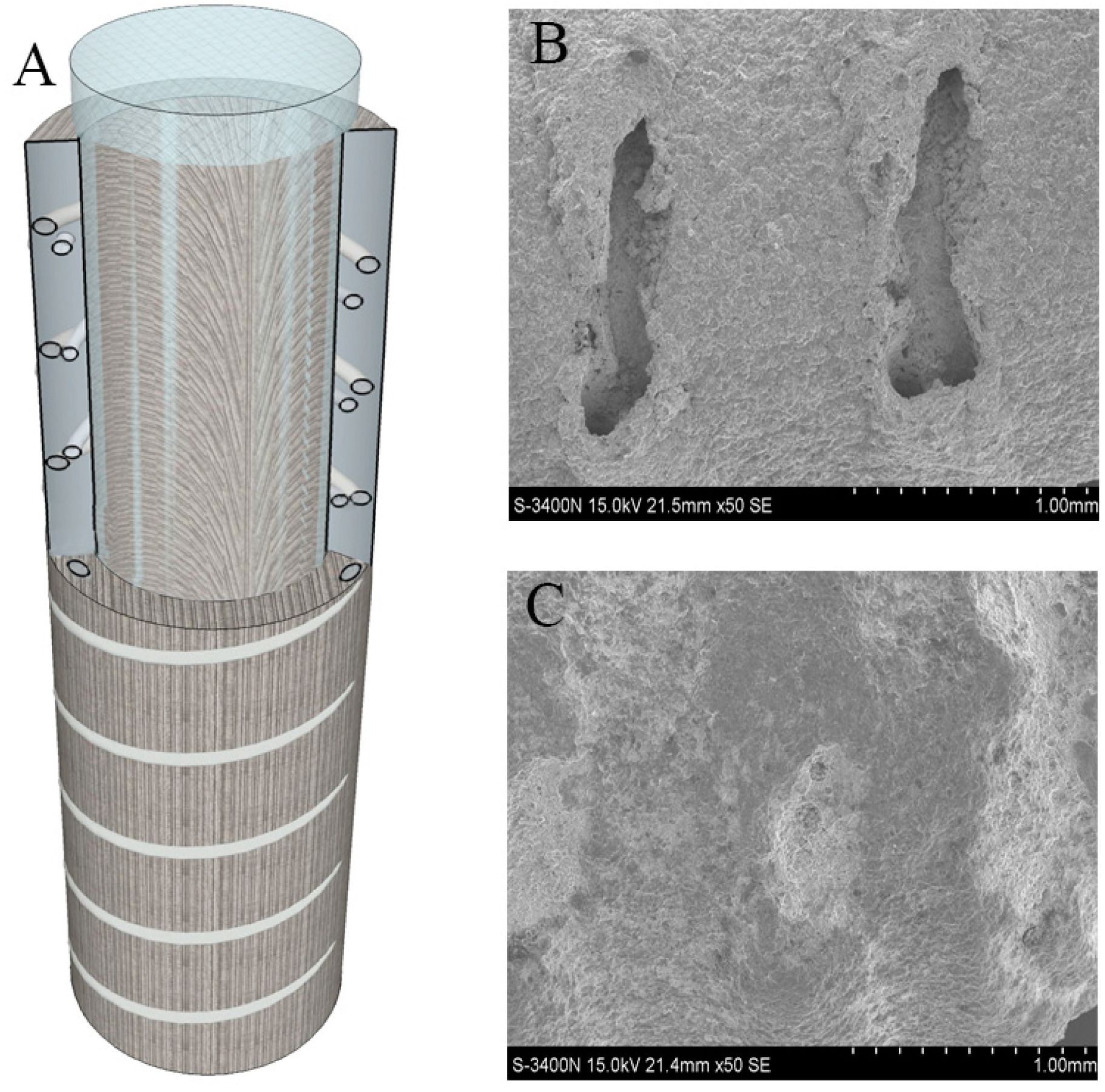
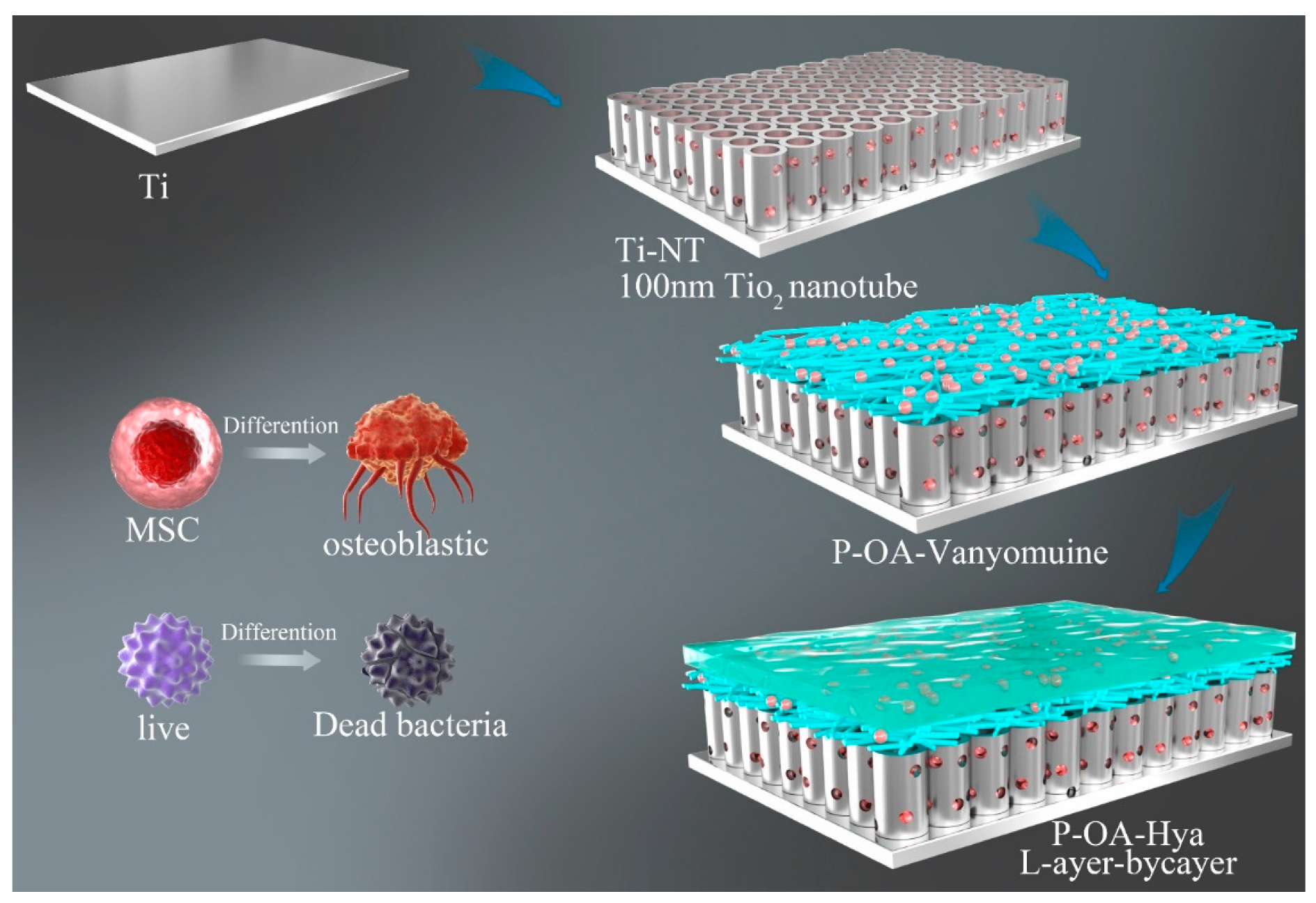

| Antibacterial Strategies | Preparation Technologies | References |
|---|---|---|
| Metal coating | Metal coating could be prepared using the pulsed current electro-chemical deposition technique, the spin coating method, etc. | [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] |
| Nanocomposite coating | Several studies have employed nanotechnology (plasma immersion ion implantation (PIII), the electrospray method, sol-cast transformation and so on) to construct nanoscale surfaces. | [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86] |
| Antibiotic coating | The drugs included conventional antibiotics, such as gentamicin, amoxicillin, carbeni-cillin, vancomycin, and cephalothin, which were incorporated in sustained-release an-tibiotic delivery devices by the electrospinning and the elec-trophoretic deposition process (EPD). | [87,88,89,90,91,92,93,94,95] |
| Surface topography modification | surface modification refers to the modification of the thin layer on the surface of implants at the atomic, molecular, or geomorphological level by anodiza-tion, layer-by-layer modification, electrodeposition, etc. | [96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] |
| Covalently grafted bioactive agents to the titanium surfaces | With incorporating antibiotics, proteins, and AMPs with different functions into implant design, bioactive surfaces have been synthesized to facilitate specific biological responses. | [122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143] |
| Multicomponent coating | EPD, which is a coating technology, makes it possible for the co-deposition of different types of materials to develop multicomponent coatings on metallic substrates. | [144,145,146,147,148,149,150,151,152,153,154,155,156,157,158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, J.; Liu, S.; Chen, M.; Zhang, H. Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review. Coatings 2022, 12, 1125. https://doi.org/10.3390/coatings12081125
Sui J, Liu S, Chen M, Zhang H. Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review. Coatings. 2022; 12(8):1125. https://doi.org/10.3390/coatings12081125
Chicago/Turabian StyleSui, Junhao, Shu Liu, Mengchen Chen, and Hao Zhang. 2022. "Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review" Coatings 12, no. 8: 1125. https://doi.org/10.3390/coatings12081125
APA StyleSui, J., Liu, S., Chen, M., & Zhang, H. (2022). Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review. Coatings, 12(8), 1125. https://doi.org/10.3390/coatings12081125






